Abstract
OBJECTIVE—Epidemiological and experimental studies have led to the hypothesis of fetal origin of adult diseases, suggesting that some adult diseases might be determined before birth by altered fetal development. We have previously demonstrated in the rat that in utero exposure to maternal diabetes impairs renal development leading to a reduction in nephron number. Little is known on the long-term consequences of in utero exposure to maternal diabetes. The aim of the study was to assess, in the rat, long-term effects of in utero exposure to maternal diabetes on blood pressure and renal function in adulthood.
RESEARCH DESIGN AND METHODS—Diabetes was induced in Sprague-Dawley pregnant rats by streptozotocin on day 0 of gestation. Systolic blood pressure, plasma renin activity, and renal function were measured in the offspring from 1 to 18 months of age. High-salt diet experiments were performed at the prehypertensive stage, and the abundance of tubular sodium transporters was evaluated by Western blot analysis. Kidney tissues were processed for histopathology and glomerular computer-assisted histomorphometry.
RESULTS AND CONCLUSIONS—We demonstrated that in utero exposure to maternal diabetes induces a salt-sensitive hypertension in the offspring associated with a decrease in renal function in adulthood. High-salt diet experiments show an alteration of renal sodium handling that may be explained by a fetal reprogramming of tubular functions in association or as a result of the inborn nephron deficit induced by in utero exposure to maternal diabetes.
Diabetes has recently assumed an epidemic proportion. Estimates suggest that worldwide, 30 million women of reproductive age will suffer from diabetes by 2030. Data from birth certificates indicate that some form of maternal diabetes complicates ∼3% of pregnancies in the U.S. (1,2). Preexisting diabetes complicates pregnancy at a rate of 1–3 per 1,000 births and increases the rates of obstetric complications, stillbirth, perinatal mortality, congenital malformations, and macrosomia compared with the background population (3,4). Gestational diabetes is also associated with substantial rates of maternal and perinatal complications. Diabetic-associated malformations result from developmental defects occurring in early organogenesis (5). They include caudal regression syndrome and urogenital abnormalities, which can be as severe as renal agenesis (6). Besides these numerous epidemiological data concerning perinatal outcome of pregnancy of diabetic women, little is known about the long-term consequences of in utero exposure to maternal diabetes in adulthood (7–9).
Fetal programming refers to the observation that an adverse environmental stimulus experienced in utero during the critical period of development of organogenesis can induce long-term effects on developing organism (10,11). These structural and functional effects predispose the offspring to several diseases in adulthood, i.e., hypertension and cardiovascular diseases. Many epidemiological studies have clearly confirmed the seminal works of Barker and Bagby (10), which evidenced the inverse relationship between a low birth weight as a marker of intrauterine stress and the risk of developing cardiovascular disease and hypertension. Brenner and colleagues (12) proposed that the inborn nephron deficit associated with this low birth weight predisposes offspring to impaired renal sodium excretion and to increased susceptibility to hypertension. More recently, several studies have also suggested a similar association between low birth weight and chronic kidney disease (12).
We have previously shown that offspring of streptozotocin-induced diabetic rats have an impaired nephrogenesis with a reduction of 30% of nephron number (13–15). Our model is characterized by moderate levels of maternal hyperglycemia and by normal gestation and delivery with healthy pups without intrauterine growth retardation or congenital malformation.
Therefore, the first aim of this work was to determine in our rat model whether maternal diabetes programs adult hypertension in the offspring and to address the implication of impaired renal sodium excretion in this process. The second aim of this study was to determine whether inborn nephron deficit alters renal function and to identify glomerular hypertrophy and injury as factors of progression of renal diseases.
RESEARCH DESIGN AND METHODS
Animals and nephron counting.
Pregnant Sprague-Dawley rats, weighing 250–300 g, were made diabetic on day 0 of gestation by a single intraperitoneal injection of streptozotocin (Sigma, Saint Quentin-Fallavier, France) (35 mg/kg body weight in 0.4 mol/l citrate buffer, pH 4.5). The diabetic state was checked by measuring the plasma glucose concentration (Accuchek, Roche, France). Only pregnant females whose plasma glucose ranged between 15 and 20 mmol/l were included in the study. This diabetic status was confirmed every 2 days until delivery. As previously shown, this protocol results in a 30% reduction of the nephron number (13).
On the day of delivery, the newborn rats were weighed. Each litter was then reduced to 10 pups. The present study was restricted to male offspring. All the animals were maintained in a temperature- and light-controlled room at 21°C with a 12-h light cycle. They had free access to food (UAR Laboratory, Villemoison sur Orge, France) and tap water.
Seventy-six rats issued from 16 control mothers (control mother offspring) and 74 rats issued from 16 diabetic mothers (diabetic mother offspring) were used in this study. Six 1-month-old animals issued from three different litters of each group were removed, weighed, and prepared for nephron counting by acid maceration method as previously described (13). The remaining animals were followed from 1 to 18 months of life for blood pressure or for functional or histological renal evaluation. Some of them, seven and six rats issued from two different litters of control mother offspring and diabetic mother offspring groups, respectively, were followed after 18 months of life to determine the effect of in utero exposure to maternal diabetes on long-term survival. All experiments were conducted in accordance with the institutional guidelines and the recommendations for the care and use of laboratory animals put forward by the French Ministry of Agriculture.
Design of the experimental protocol from 1 to 18 months.
In each group, we have assessed the following in rats of 1 to 18 months of age: systolic blood pressure, renal function and proteinuria, plasma renin activity (PRA), renal renin expression (RRE), glomerular histomorphometry, and renal histology.
Systolic blood pressure was measured during 3 successive days in conscious rats by a noninvasive method, tail-cuff plethysmography, using a blood pressure monitor (blood pressure recorder 8005 Narco BioSystems). Before the effective measurements, rats were trained by placing them in restrainers and pressing their tail several times. Blood pressure records were made several consecutives times on quiet animals in a silent ambience at constant temperature under a heating apparatus. Measurements were always performed by the same person.
Renal function was estimated by creatinine clearance and proteinuria. For that purpose, rats were individually housed in metabolic cages (Techniplast, Exton, PA) with free access to food and demineralized water. After 3 days of adaptation, food and water intakes were measured every 24-h period, and the urine was collected throughout the protocol during 3 other consecutive days. Urine that was spontaneously excreted during each 24-h period was collected to determine daily urinary creatinine and sodium and protein excretions. At the end of the 3-day experimental study, blood samples were collected for creatinine and PRA measurements.
Kidneys and hearts from 1- to 18-month-old control mother offspring and diabetic mother offspring rats were removed, weighed separately, and fixed with formalin for either histology or histomorphometry.
High-salt diet protocol.
Six 3-month-old rats issued from two different litters from control mother offspring and diabetic mother offspring groups were individually housed in metabolic cages and fed with normal-salt diet (0.3% NaCl). Blood pressure and urinary sodium excretion were measured. The rats were then moved on a high-salt diet (3% NaCl), and the urinary sodium excretions were measured daily during 3 days. Food intake was measured every 24-h period. After 7 days, systolic blood pressure was determined, and kidneys were then removed for sodium transporter abundances evaluation.
Analytical procedures.
Plasma and urinary analyses were performed by standard methods using a Konelab 20 analyzer (Thermo Electron, Courtaboeuf, France) to determine sodium, creatinine, and protein concentrations. Urinary creatinine concentration was measured by high-phase liquid chromatography (Dionex DX-500; Dionex, Voisins le Bretonneux, France). Analysis of PRA was performed from aortic blood for 1-, 6-, and 18-month-old rats by radioimmunoassay (GammaCoat Plasma Renin Activity 125I RIA kit; DiaSorin, Stillwater, MN). Plasma glucose concentrations were determined immediately after sampling by the glucose-oxidase method using a glucose analyzer (Beckman Instruments, Fullerton, CA).
Histology.
Histological analysis was performed on kidney samples taken at 1, 3, 6, 12, 18, and 23 months of age for both groups. Tissues were fixed in formalin, embedded in paraffin, and cut in 4-μm-thick transversal sections. Kidney sections were routinely stained with hematein-eosin, Masson's trichrome, and silver staining. Semiquantitative evaluations of glomerulosclerosis, interstitial fibrosis, tubular atrophy, and vascular lesions were performed in kidney sections from both groups in a blinded fashion as previously described (16).
Computer-assisted morphometric analysis.
Computer assisted-morphometric analysis was performed on kidney samples obtained at 1, 3, 6, 12, and 18 months for both groups, as previously described (17,18). For each animal, the total glomerular surface area (TGA) was expressed as the mean of the values measured in 50 randomly sampled glomeruli (25 in the superficial cortex and 25 in the juxtamedullary cortex). The TGA, limited by the internal edge of Bowman's capsule, was determined in 1- to 18-month-old rats. In addition, the total area of capillary lumens (TCL) and total mesangial surface area (TMA) were measured in both groups at 6- and 18-month-old rats. The TCL-to-TGA and TMA-to-TGA ratios were then calculated for each glomerulus and expressed as the mean of 50 measured glomeruli for each animal.
Immunohistochemistry.
Immunohistochemistry with anti-renin polyclonal antibody was performed on kidney sections with a three-step avidin-biotin immunoperoxidase method with prior antigen unmasking procedure as previously described (19). Renin immunoreactivity was then evaluated in a blinded fashion using a semiquantitative scoring system as previously described (19).
Preparation of membrane fractions and Western blot analysis.
Kidneys of both groups were removed from the anesthetized rats and cut into 5-mm slices. To identify Na transporters, membrane fractions from the cortex and the outer medulla (inner stripe) were prepared, and Western blot analysis was performed as previously described (20,21) (primary antibodies used were anti-NHE3, 1:1,000; anti-NCC, 1:50,000; anti-BSC1, 1:5,000; anti-α ENaC, 1:3,000; anti-βENaC, 1:20,000; anti-γ ENaC, 1:2,000; and anti-Na+/K+ ATPase, 1:20,000). After incubation with the appropriate peroxidase-conjugated secondary antibodies, blots were washed, and luminol-enhanced chemiluminescence (ECL; Perkin Elmer Life Science Products, Boston, MA) was used to visualize bound antibodies before exposure to Hyperfilm ECL (Amersham, Arlington Heights, IL). The autoradiography was digitized with the use of a laser scanner (Epson Perfection 1650; Epson), and quantification of each band was performed by densitometry using NIH Image software. Densitometric values were normalized to the mean for the control group that was defined as 100%.
Statistical analysis.
All results are expressed as the mean ± SE values. Statistical analysis was performed by unpaired and paired Student's t test or by ANOVA when appropriate. Statistical significance was defined as P < 0.05.
RESULTS
Gestational and delivery conditions and follow-up of rats exposed in utero to maternal diabetes.
Glycemia of the 16 diabetic mothers was constant throughout gestation and fourfold higher than in the 16 controls (5.27 ± 0.34 vs. 21.6 ± 0.12 mmol/l). Gestation occurred normally, and the rats delivered spontaneously at term (21 days of gestation). The number of pups per litter was similar in both groups. At birth, pups appeared healthy with similar birth weight compared with controls. As previously described (13), in our model, in utero exposure to maternal diabetes induced nephron deficit in the offspring. At 1 month, the reduction of nephron number was ∼30% in rats issued from diabetic mothers compared with controls (35,133 ± 507 vs. 25,600 ± 570, respectively, P < 0.0001, n = 6 in each group).
As shown in Table 1, mean body weight progressively increased with age and was similar in both groups from the newborn period until 3 months. However, at 6 and 12 months of age, it was significantly higher in the diabetic mother offspring group than in the control mother offspring group. Kidney and heart weights (relative to body weight) decreased as a function of age but were not significantly different in both groups. The same normal blood glucose levels were observed in control mother offspring and diabetic mother offspring groups (5.90 ± 0.23 vs. 5.61 ± 0.70 mmol/l in 6-month-old rats from both groups, n = 6 from three litters).
TABLE 1.
Body, kidney, and heart weights at death
| Age (months)
|
ANOVA
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | 18 | Age | Group | Interaction | ||
| Body weight (g) | CMO | 164 ± 5 | 435 ± 16 | 518 ± 10 | 596 ± 17 | 730 ± 22 | |||
| n | 8 | 6 | 9 | 9 | 9 | ||||
| DMO | 161 ± 24 | 441 ± 18 | 568 ± 20* | 723 ± 17† | 731 ± 22 | P < 0.0001 | P = 0.0002 | P = 0.0009 | |
| n | 8 | 6 | 9 | 9 | |||||
| Kidney weight (mg)/body weight (g) | CMO | 5.2 ± 0.3 | 3.1 ± 0.1 | 2.4 ± 0.4 | 2.3 ± 0.3 | 2.9 ± 0.1 | |||
| n | 8 | 6 | 9 | 9 | 9 | ||||
| DMO | 4.4 ± 0.2 | 2.7 ± 0.3 | 3.3 ± 0.1 | 2.2 ± 0.1 | 2.9 ± 0.1 | P < 0.0001 | P = 0.5673 | P = 0.0021 | |
| n | 8 | 6 | 9 | 9 | 9 | ||||
| Heart weight (mg)/body weight (g) | CMO | 5.6 ± 0.3 | 3.9 ± 0.2 | 2.8 ± 0.4 | 3.9 ± 0.1 | 3.2 ± 0.9 | |||
| n | 8 | 6 | 9 | 9 | 9 | ||||
| DMO | 5.5 ± 0.4 | 3.2 ± 0.3 | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.0 ± 0.2 | P < 0.0001 | P = 0.6038 | P = 0.0919 | |
| n | 8 | 6 | 9 | 9 | 9 | ||||
Data are means ± SE for n animals issued from two or three litters. CMO, control mother offspring; DMO, diabetic mother offspring. Two-way ANOVA with age and group effects. Comparisons are made between CMO and DMO at the same age (unpaired t test,
P < 0.05;
P < 0.001).
Effects of in utero exposure to maternal diabetes on blood pressure
Systolic blood pressure.
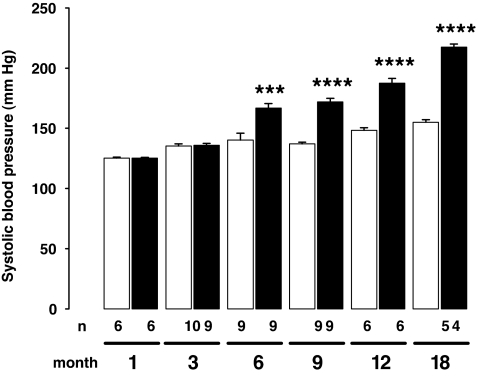
Rats exposed in utero to maternal diabetes demonstrated higher blood pressure in adulthood (Fig. 1). Although the systolic blood pressure of 1- and 3-month-old rats was similar in both groups, it was significantly increased from 6 months of age in diabetic mother offspring compared with control mother offspring. Hypertension lasted and further increased during the observation period.
FIG. 1.
Effect of maternal diabetes on blood pressure levels in the offspring. Systolic blood pressure significantly increased from 6 months in the offspring from diabetic mothers (diabetic mother offspring group, ▪) compared with control mother offspring group (□). Results are expressed as means ± SE, n = 4–10 animals in each group, issued from two or three litters. Two-way ANOVA: age effect, P < 0.0001; group effect, P < 0.0001; age × group interaction, P < 0.0001; unpaired t test between age-matched groups, ***P < 0.001, ****P < 0.0001.
PRA and RRE.
PRA and RRE are given in Table 2. At the prehypertensive stage (1- and 3-month-old rats), PRA and RRE were not significantly different in the two groups. From 6 months of age (hypertensive stage), a significant decrease of PRA was observed in diabetic mother offspring, when compared with their respective age-matched control mother offspring. With aging, the reduction of PRA and RRE was present in both groups.
TABLE 2.
Plasmatic renin activity and kidney renin immunoreactivity index
| Age (months)
|
ANOVA
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | 18 | Age | Group | Interaction | ||
| Plasma renin activity (μg · ml−1 · h−1) | CMO | 215 ± 16.6 | 175 ± 34 | 163 ± 20.7 | 162 ± 12.6 | 104 ± 5.2 | |||
| n | 7 | 7 | 7 | 9 | 5 | ||||
| DMO | 216 ± 31.3 | 107 ± 13.4 | 104 ± 11.8* | 140 ± 8.3 | 91.1 ± 23.3 | P < 0.0001 | P = 0.021 | P = 0.4022 | |
| n | 8 | 5 | 8 | 8 | 4 | ||||
| Renal renin expression (arbitrary unit) | CMO | 51.9 ± 2.4 | 54.4 ± 6.1 | 63.7 ± 6.3 | 50.2 ± 5.1 | 29 ± 3 | |||
| n | 8 | 7 | 7 | 9 | 5 | ||||
| DMO | 58.9 ± 4.2 | 48.8 ± 2.9 | 37.8 ± 2.9† | 38.2 ± 4.6 | 25.7 ± 2.3 | P < 0.0001 | P = 0.083 | P = 0.021 | |
| n | 8 | 5 | 9 | 8 | 4 | ||||
Data are means ± SE for n animals issued from two or three litters. CMO, control mother offspring; DMO, diabetic mother offspring. Two-way ANOVA with age and group effects. Comparisons are made between CMO and DMO at the same age (unpaired t test,
P < 0.05;
P < 0.01).
Effect of high-salt diet on blood pressure and urinary sodium excretion.
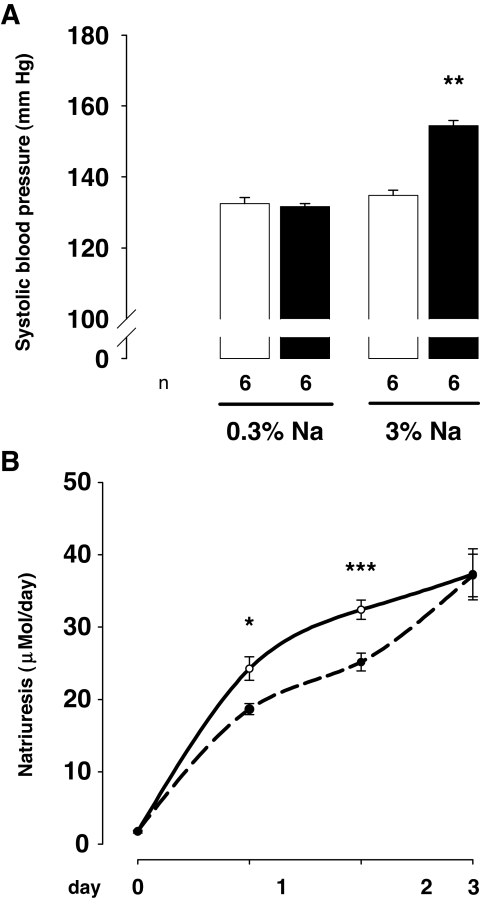
To study the response to a chronic alteration in sodium balance, both groups of rats were given a high-salt diet (3% NaCl diet instead of 0.3%) (Fig. 2). Means of the 24-h diet intake for the 3 days of sodium excretion measurement were similar in the two groups (28.32 ± 0.83 vs. 28.03 ± 1.1 g in control mother offspring and diabetic mother offspring group, respectively).
FIG. 2.
Effect of high-sodium diet on systolic blood pressure and on sodium urinary excretion. A: Systolic blood pressure was measured in 3-month-old rats under 0.3% sodium diet (0.3% Na) and after 7 days of sodium-rich diet (3% Na) in control mother offspring (□) and diabetic mother offspring (▪). Sodium-rich diet induced a significant increase of systolic blood pressure in 3-month-old diabetic mother offspring rats (n = 6 in each group, issued from two litters, paired t test: **P < 0.01) and remains ineffective on systolic blood pressure of control mother offspring rats. B: Natriuresis was followed for 3 days after the onset of high-sodium diet in the same groups of animals (control mother offspring, plain line; diabetic mother offspring, hatched line). Sodium urinary excretion was impaired in diabetic mother offspring rats compared with control mother offspring rats (n = 6 in each group, issued from two litters). Two-way ANOVA: time effect, P < 0.0001; group effect, P < 0.05; time × group interaction, P = 0.09; and unpaired t test in age-matched animals, *P < 0.05, ***P < 0.001.
Systolic blood pressure was similar in the two groups on normal-sodium diet (0.3%). High-salt diet (3%) induced a raise of systolic blood pressure in the diabetic mother offspring group (respectively, 131.7 ± 0.8 vs. 154.5 ± 3.2 mmHg; paired t test, P < 0.01) and had no effect on blood pressure in the control mother offspring group.
As expected, high-sodium diet led to a significant increase of urinary sodium excretion in both groups. However, this increase was significantly delayed in diabetic mother offspring compared with control mother offspring, accounting for a larger positive sodium balance.
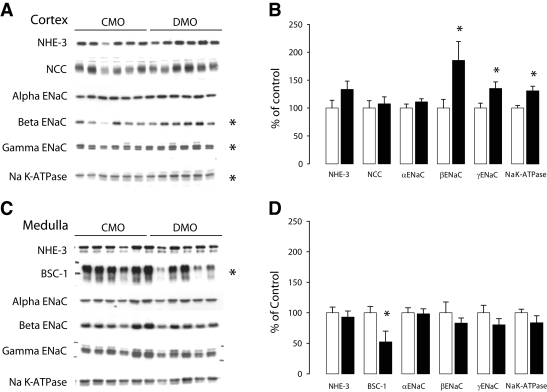
Effect of high-salt diet on relative abundance of renal sodium transporters.
We assessed the effects of a high-salt diet on sodium transporter protein by semiquantitative immunoblots of membrane fractions from the cortex and outer medulla (inner stripe) obtained from the diabetic mother offspring and control mother offspring kidney of 3-month-old rats (Fig. 3). In the cortex, both β- and γ-ENaC subunits were significantly upregulated in diabetic mother offspring compared with control mother offspring, whereas α-ENaC protein abundance was unchanged. Na/K ATPase protein abundance was also significantly upregulated in diabetic mother offspring. In the medulla, the high-salt diet led to a decrease in BSC1 protein abundance in the diabetic mother offspring group compared with the control mother offspring group. Protein levels of α-, β-, and γ-ENaC were unaffected.
FIG. 3.
Cortical and medullar renal sodium transporter abundance profile in sodium-rich diet. Effect of dietary NaCl loading for 10 days on major renal sodium transporter protein abundance in diabetic mother offspring and control mother offspring kidney. Semiquantitative immunoblots of membrane fractions from the cortex (A) and outer medulla (inner stripe) (C) obtained from diabetic mother offspring and their control mother offspring. For each blot, each lane was loaded with a homogenate from a different rat (n = 6 for both control mother offspring and diabetic mother offspring in cortex; n = 5 for control mother offspring and n = 6 for diabetic mother offspring in the medulla). Densitometric analyses revealed a significant increase in β- and γ-EnaC and Na/K ATPase abundance in diabetic mother offspring compared with control mother offspring in the cortex (B) and a significant decrease in BSC1 in the outer medulla (D). The results are expressed as the percentage of the control values. Bars represent means ± SE (unpaired t test between diabetic mother offspring and control mother offspring groups, *P < 0.05).
Finally, the protein levels of NHE3 and NCC were not different in diabetic mother offspring receiving a high-salt diet compared with control mother offspring either in the cortex or in the medulla.
Renal effects of in utero exposure to maternal diabetes
Renal function and proteinuria.
Table 3 reports the follow-up of proteinuria and glomerular filtration rate (GFR) estimated by the creatinine clearance, in 1- to 18-month-old rats of both groups. The creatinine clearance progressively increased with age in both groups and was significantly lower in diabetic mother offspring compared with control mother offspring. GFR was reduced by ∼10% in 3-month-old rats and by 30% from the 6- to 18-month period. Proteinuria levels increased in both groups with aging and were significantly higher in diabetic mother offspring.
TABLE 3.
Creatinine clearance and proteinuria
| Age (months)
|
ANOVA
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 9 | 12 | 18 | Age | Group | Interaction | ||
| Creatinine clearance (ml/min) | CMO | 0.76 ± 0.03 | 1.33 ± 0.04 | 2.21 ± 0.13 | 2.72 ± 0.20 | 3.02 ± 0.23 | 2.99 ± 0.27 | |||
| n | 5 | 5 | 6 | 6 | 6 | 4 | ||||
| DMO | 0.76 ± 0.12 | 1.21 ± 0.10 | 1.66 ± 0.08* | 1.75 ± 0.06† | 2.20 ± 0.18‡ | 2.04 ± 0.22 | P < 0.0001 | P < 0.001 | P = 0.0069 | |
| n | 6 | 4 | 6 | 4 | 5 | 4 | ||||
| Proteinuria (mg/day) | CMO | 4.52 ± 0.31 | 11.0 ± 0.45 | 12.5 ± 1.20 | 14.8 ± 2.08 | 18.3 ± 2.75 | 26.5 ± 2.53 | |||
| n | 5 | 5 | 6 | 6 | 6 | 4 | ||||
| DMO | 4.77 ± 0.82 | 14.7 ± 0.04* | 18.4 ± 1.34‡ | 23.3 ± 3.92 | 15.3 ± 1.55 | 57.2 ± 24.4 | P < 0.0001 | P = 0.0112 | P = 0.054 | |
| n | 6 | 4 | 6 | 4 | 5 | 4 | ||||
Data are means ± SE of n rats issued from two or three litters. CMO, control mother offspring; DMO, diabetic mother offspring. Two-way ANOVA with age and group effects. Comparisons are made between CMO and DMO at the same age (unpaired t test,
P < 0.001;
P < 0.01;
P < 0.05).
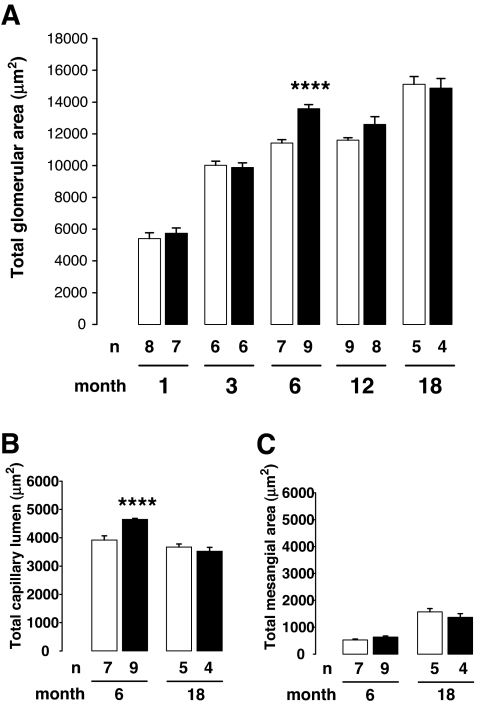
Glomerulus histomorphometry.
To address the question of a structural glomerular adaptation to the reduction of nephron number, a glomerular histomorphometry study was performed in kidneys taken from 1- to 18-month-old control mother offspring and diabetic mother offspring rats (Fig. 4). In each age-group, no significant differences were observed between glomeruli measured in either superficial or deep cortex areas between control mother offspring and diabetic mother offspring kidney rats (data not shown). TGA significantly increased with advancing age. TGA was not significantly different between the two groups, although it was transitorily increased in 6-month-old diabetic mother offspring rats. TCL was significantly increased in the 6-month-old diabetic mother offspring compared with the control mother offspring. TCL/TGA was similar in both groups. At 18 months of age, TMA and TMA/TGA were similar in diabetic mother offspring and control mother offspring groups.
FIG. 4.
Effect of maternal diabetes on glomerular morphometry. A: TGA increased with aging in control mother offspring group (□) and in diabetic mother offspring group (▪). Results are expressed as means ± SE, n = 4–9 animals in each group, issued from two to three litters (two-way ANOVA: age effect, P < 0.0001; group effect, P < 0.01; age × group interaction, P < 0.01). TGA was significantly increased in 6-month-old diabetic mother offspring rats compared with age-matched control mother offspring rats, whereas the difference did not achieve statistical significance at 12 months. TCL (B) and TMA (C) were measured at 6 and 18 months in control mother offspring and in diabetic mother offspring. TCL was increased in 6-month-old diabetic mother offspring rats compared with control mother offspring rats (unpaired t test between age-matched groups, ****P < 0.0001).
Renal histopathology.
Renal histopathology was assessed in 1- to 18-month-old control mother offspring and diabetic mother offspring rats. Before 18 months of age, all kidneys were normal and devoid of glomerulosclerosis and interstitial fibrosis (data not shown). At 18 months of age, renal lesions were limited to focal areas of interstitial fibrosis with minimal tubular atrophy and to very few glomeruli with segmental glomerulosclerosis (Fig. 5A and B). Semiquantitative analysis showed that the extent of both glomerulosclerosis (glomerulosclerosis index: control mother offspring, 10.08 ± 1.62, n = 13 rats from three different litters vs. diabetic mother offspring, 8.49 ± 1.91, n = 9 rats from two different litters; unpaired t test; NS, arbitrary units) and tubulointerstitial lesions (Interstitial fibrosis index: control mother offspring, 5.12 ± 1.69, n = 8 rats from two different litters vs. diabetic mother offspring, 7.80 ± 4.25, n = 5 rats from three different litters; unpaired t test; NS, arbitrary units) were not different in the two groups.
FIG. 5.
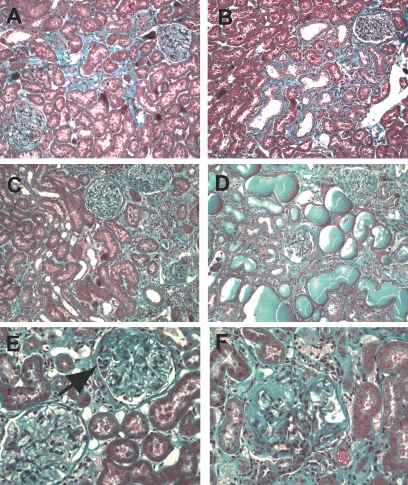
Renal histopathology in 18- and 23-month-old rats. Histopathology was performed at 18 months (A and B) and at 23 months (C–F) in kidney from control mother offspring (A, C, and E) and from diabetic mother offspring (B, D, and F). Similar focal area of interstitial fibrosis and tubular atrophy in control mother offspring (A) and diabetic mother offspring (B) 18-month-old rats. Widespread interstitial fibrosis and tubular atrophy with severe glomerulosclerosis were present in 23-month-old diabetic mother offspring rats (D and F) compared with limited alteration in control mother offspring (C and E; segmental glomerulosclerosis, arrow). Masson's trichrome. Magnification in A–D ×200; in C–F ×800. (Please see http://dx.doi.org/10.2337/db07-0780 for a high-quality digital representation of this figure.)
Survival study.
Survival study showed an increased mortality after 18 months of age in diabetic mother offspring. At 23 months, long-term survival was markedly reduced in the diabetic mother offspring group, 33.3% compared with 85.7% in the control mother offspring group. We therefore decided to kill the remaining rats to address late kidney histopathology in rats of the two groups.
A widespread interstitial fibrosis with tubular atrophy and dilatation was present in the kidneys of diabetic mother offspring rats, associated with glomerulosclerosis and glomerular cysts. A scarce interstitial fibrosis and tubular atrophy was present in control mother offspring rats (Fig. 5C–F). Glomerulosclerosis index is 7.25 ± 3.50, n = 6, in control mother offspring versus 87 and 66 in two diabetic mother offspring rats, and interstitial fibrosis index is 27.5 ± 24.3, n = 6, in control mother offspring versus 230 and 300 in two diabetic mother offspring rats. No structural changes in the intra-renal vessels were observed at any age.
DISCUSSION
The present study identifies the long-term consequences of in utero exposure to maternal diabetes in the rat and shows that in utero exposure is associated with the development of a salt-sensitive systolic hypertension and with a decrease in renal function in adulthood.
A mild to moderate increase in systolic arterial blood pressure is observed in the offspring of diabetic mothers from 6 months of age, which progressively went worse with the age. This rise in blood pressure is associated with low PRA, suggesting a salt-sensitive hypertension. We further confirmed the salt sensitivity with high-sodium diet experiments performed at prehypertensive stage: in diabetic mother offspring rats, a high-sodium diet induced an increase of systolic blood pressure and led to a shift to the right of the urinary sodium excretion curve, indicating a delayed sodium excretion. A similar salt-sensitive hypertension has been reported in a neonatal uni-nephrectomized rat model by Woods et al. (22). Together, these results are in accordance with the hypothesis of Brenner et al. (23), which states that inborn nephron deficit predisposes reduced renal sodium excretion, leading to hypertension susceptibility, especially in the setting of dietary sodium excess. Interestingly, in inbred rat models of hypertension, the relationship between nephron number and blood pressure is still a matter of debate (24–26). In addition, recently, Rocha et al. (8) and Magaton et al. (9) reported hypertension in rats issued from diabetic mothers without inborn nephron deficit. However, their model slightly differs from ours: 1) The level of maternal hyperglycemia is more pronounced in our model (we have previously shown that inborn nephron deficit correlated with the level of maternal hyperglycemia [13]); and 2) we performed direct glomerular counting with the gold-standard acid-maceration method, whereas they used an histological-derived method that is more appropriate to evaluate the density of glomeruli.
Because one mechanism involved in such renal sub-optimal sodium handling may traduce a resetting of tubular sodium cotransporter expression, we then evaluated the protein abundance of Na transporters and channels in the kidney at the prehypertensive stage and under a high-salt diet. In the cortex of diabetic mother offspring rats, we found an increase of three different sodium transporter proteins: βENaC, γENaC, and Na-K ATPase. Such relative increases without decreases in other sodium transporters would be predicted to result in enhanced tubular Na reabsorption and might play a role in the development or maintenance of elevated blood pressure in these animals (27). The decrease in BSC1 in the medulla may reflect a medullary compensatory effect linked to the increase of cortical sodium transporters. An abnormal expression of Na transporters has also been reported by Manning et al. (28). In their model, maternal protein restriction is associated with low birth weight, development of hypertension at 8 weeks of age, and significant increased expression in the sodium cotransporters BSC1 and TSC (28). Together, these results strongly substantiate a perinatal reprogramming of tubular function regulation in association with or as a result of inborn nephron deficit (29).
In the present study, we also show that maternal diabetes is associated with a decreased renal function in adulthood, as assessed by creatinine clearance and proteinuria measurements. According to Brenner and colleagues (12,23), a compensatory glomerular hypertrophy and hyperfiltration occur in response to inborn nephron deficit to sustain adequate renal function. Such glomerular adaptation made at the expense of intraglomerular hypertension accelerates injury to the remaining functional glomeruli and perpetuates the ongoing glomerulosclerosis, leading to renal failure. However, in our model in which inborn nephron deficit is observed, systematic analysis of renal histology shows that both glomerulosclerosis and interstitial fibrosis are absent in diabetic mother offspring and control mother offspring groups at all stages before 18 months of age. At 18 months, because of aging (17), mild renal structural lesions occur but at the same extent in the two groups. In addition, glomerular computer-assisted histomorphometry shows a mild glomerular hypertrophy only at 6 months, at the onset of hypertension in diabetic mother offspring rats. In our model, absence of early increase of glomerular size and of sustained glomeruli hypertrophy with aging does not support a major glomerular adaptation to the inborn nephron deficit. Together, these two sets of results seem to weight against a compensatory glomerular hypertrophy and hyperfiltration as factors of alteration and worsening of the renal function until 18 months of age. Concerning the study of Rocha et al. (8), one must note that although decreased nephron number was not observed in young animals, the number of nephrons was reduced at 12 months in diabetic mother offspring rats. However, because no histological data were provided in their study to evaluate the extent of glomerulosclerosis, the mechanisms of ongoing nephron loss and its implication in the decline of renal function has not been elucidated in their model.
In the other hand, a dramatic impairment of renal histology with widespread glomerular and tubulo-interstitial lesions was observed in kidneys issued from 23-month-old diabetic mother offspring rats. Such late alterations of kidney structures compared with the early impairment of renal function could hardly be explained by the consequences of a congenital nephron deficit alone. The link between the congenital nephron deficit and the adverse effect of compensatory hyperfiltration on the remaining glomeruli leading to glomerulosclerosis has been unambiguously demonstrated (and quantitatively assessed) in models with severe reduction in the nephron number or in models with toxic renal impairment that associates both in utero glomerular reduction and tubulo-interstitial lesions (30,31). In our present model, the mild (30%) congenital nephron deficit might not be sufficient per se to induce glomerular lesions, at least during the first 18 months of life.
Another issue to consider is the kidney as a target organ of hypertension. In our model, although hypertension is present from 6 months of age, high level of systolic blood pressure is only achieved at 18 months. It is well known that the increase of blood pressure is limited and slowly increases with age in the majority of the model of perinatal programming of hypertension compared with other rat models of hypertension (32,33). This may explain why we observe the histopathological renal consequences of hypertension only in the 23-month-old rats. However, the implication of hypertension alone in the renal lesions of our model is questionable, because even at 23 months, intra-renal vascular hypertensive lesions are nearly absent at histology, and myocardial (other target organ of hypertension) histology is similar in both groups (data not shown).
This study thus identifies maternal diabetes as a novel risk factor for fetal programming of adult hypertension and impairment of renal function. Alteration of renal sodium handling, observed in our model, may be explained by a fetal resetting of tubular functions, as a consequence or in association with congenital nephron deficit.
Acknowledgments
This work was supported by a grant from the Institut National de la Recherche Medicale.
We acknowledge the technical assistance of Marie-France Belair and Martine Douheret (Institut National de la Santé et de la Recherche Médicale, Unite Mixte de Recherche S872, Centre de Recherche des Cordeliers). We also acknowledge the technical assistance of Michele Smirnoff for animal nursing and of Nelly Knobloch for secretarial assistance.
Parts of this work were presented in abstract form at the 38th annual meeting of the American Society of Nephrology, Philadelphia, Pennsylvania, 8–13 November 2005.
Published ahead of print at http://diabetes.diabetesjournals.org on 28 April 2008.
T.N. and J.-P.D.V.H. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.National Center for Health Statistics: Pregnancy complications and outcomes among women with diabetes-North Carolina. MMWR Morb Mortal Wkly Rep 42: 847–851, 1993 [PubMed] [Google Scholar]
- 2.National Center for Health Statistics: Prenatal care and pregnancies complicated by diabetes: U.S. reporting areas. MMWR Morb Mortal Wkly Rep 42: 119–122, 1993 [PubMed] [Google Scholar]
- 3.Jensen DM, Damm P, Moelsted-Pedersen L, Ovesen P, Westergaard JG, Moeller M, Beck-Nielsen H: Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 27: 2819–2823, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Casson IF, Clarke CA, Howard CV, McKendrick O, Pennycook S, Pharoah PO, Platt MJ, Stanisstreet M, van Velszen D, Walkinshaw S: Outcomes of pregnancy in insulin dependent diabetic women: results of a five year population cohort study. BMJ 315: 275–278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuhrmann K, Reiher H, Semmler K, Fischer F, Fischer M, Glockner E: Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care 6: 219–223, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Lynch SA, Wright C: Sirenomelia, limb reduction defects, cardiovascular malformation, renal agenesis in an infant born to a diabetic mother. Clin Dysmorphol 6: 75–80, 1997 [PubMed] [Google Scholar]
- 7.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF: Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 91: 3718–3724, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Rocha SO, Gomes GN, Forti AL, do Carmo Pinho Franco M, Fortes ZB, de Fatima Cavanal M, Gil FZ: Long-term effects of maternal diabetes on vascular reactivity and renal function in rat male offspring. Pediatr Res 58: 1274–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Magaton A, Gil FZ, Casarini DE, Cavanal MD, Gomes GN: Maternal diabetes mellitus: early consequences for the offspring. Pediatr Nephrol 22: 37–43, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Bagby SP: Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol 16: 2537–2544, 2005 [DOI] [PubMed] [Google Scholar]
- 11.McMillen IC, Robinson JS: Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zandi-Nejad K, Luyckx VA, Brenner BM: Adult hypertension and kidney disease: the role of fetal programming. Hypertension 47: 502–508, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M: Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48: 2240–2245, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Amri K, Freund N, Van Huyen JP, Merlet-Benichou C, Lelievre-Pegorier M: Altered nephrogenesis due to maternal diabetes is associated with increased expression of IGF-II/mannose-6-phosphate receptor in the fetal kidney. Diabetes 50: 1069–1075, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Duong Van Huyen JP, Amri K, Belair MF, Vilar J, Merlet-Benichou C, Bruneval P, Lelievre-Pegorier M: Spatiotemporal distribution of insulin-like growth factor receptors during nephrogenesis in fetuses from normal and diabetic rats. Cell Tissue Res 314: 367–379, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Karam H, Clozel JP, Bruneval P, Gonzalez MF, Menard J: Contrasting effects of selective T- and L-type calcium channel blockade on glomerular damage in DOCA hypertensive rats. Hypertension 34: 673–678, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Francois V, Heudes D, Bariety J, Bruneval P, Corman B: Glomerular capillary network of cortical nephrons is reduced in male but not in female aging rats. Mech Ageing Dev 91: 11–22, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Heudes D, Michel O, Chevalier J, Scalbert E, Ezan E, Bariety J, Zimmerman A, Corman B: Effect of chronic ANG I-converting enzyme inhibition on aging processes: I. Kidney structure and function. Am J Physiol 266: R1038–R1051, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Pietri L, Bloch-Faure M, Belair MF, Sanford LP, Doetschman T, Menard J, Bruneval P, Meneton P: Altered renin synthesis and secretion in the kidneys of heterozygous mice with a null mutation in the TGF-beta(2) gene. Exp Nephrol 10: 374–382, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Quentin F, Eladari D, Frische S, Cambillau M, Nielsen S, Alper SL, Paillard M, Chambrey R: Regulation of the Cl-/HCO3- exchanger AE2 in rat thick ascending limb of Henle's loop in response to changes in acid-base and sodium balance. J Am Soc Nephrol 15: 2988–2997, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lourdel S, Loffing J, Favre G, Paulais M, Nissant A, Fakitsas P, Creminon C, Feraille E, Verrey F, Teulon J, Doucet A, Deschenes G: Hyperaldosteronemia and activation of the epithelial sodium channel are not required for sodium retention in puromycin-induced nephrosis. J Am Soc Nephrol 16: 3642–3650, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Woods LL, Weeks DA, Rasch R: Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension 38: 337–342, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Brenner BM, Garcia DL, Anderson S: Glomeruli and blood pressure: less of one, more the other? Am J Hypertens 1: 335–347, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Skov K, Nyengaard JR, Korsgaard N, Mulvany MJ: Number and size of renal glomeruli in spontaneously hypertensive rats. J Hypertens 12: 1373–1376, 1994 [PubMed] [Google Scholar]
- 25.Fassi A, Sangalli F, Maffi R, Colombi F, Mohamed EI, Brenner BM, Remuzzi G, Remuzzi A: Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol 9: 1399–1406, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Black MJ, Briscoe TA, Constantinou M, Kett MM, Bertram JF: Is there an association between level of adult blood pressure and nephron number or renal filtration surface area? Kidney Int 65: 582–588, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA: Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Manning J, Beutler K, Knepper MA, Vehaskari VM: Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol 283: F202–F206, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kim SW, Wang W, Kwon TH, Knepper MA, Frokiaer J, Nielsen S: Increased expression of ENaC subunits and increased apical targeting of AQP2 in the kidneys of spontaneously hypertensive rats. Am J Physiol Renal Physiol 289: F957–F968, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Gilbert T, Lelievre-Pegorier M, Merlet-Benichou C: Immediate and long-term renal effects of fetal exposure to gentamicin. Pediatr Nephrol 4: 445–450, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Tendron-Franzin A, Gouyon JB, Guignard JP, Decramer S, Justrabo E, Gilbert T, Semama DS: Long-term effects of in utero exposure to cyclosporin A on renal function in the rabbit. J Am Soc Nephrol 15: 2687–2693, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Doggrell SA, Brown L: Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39: 89–105, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Vehaskari VM, Woods LL: Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 16: 2545–2556, 2005 [DOI] [PubMed] [Google Scholar]