Abstract
OBJECTIVE—Autoimmune diabetes in the nonobese diabetic (NOD) mouse model results from a breakdown of T-cell tolerance caused by impaired tolerogenic dendritic cell development and regulatory T-cell (Treg) differentiation. Re-establishment of the Treg pool has been shown to confer T-cell tolerance and protection against diabetes. Here, we have investigated whether murine thymic stromal lymphopoietin (TSLP) re-established tolerogenic function of dendritic cells and induced differentiation and/or expansion of Tregs in NOD mice and protection against diabetes.
RESEARCH DESIGN AND METHODS—We examined the phenotype of TSLP-conditioned bone marrow dendritic cells (TSLP-DCs) of NOD mice and their functions to induce noninflammatory Th2 response and differentiation of Tregs. The functional relevance of TSLP and TSLP-DCs to development of diabetes was also tested.
RESULTS—Our results showed that bone marrow dendritic cells of NOD mice cultured in the presence of TSLP acquired signatures of tolerogenic dendritic cells, such as an absence of production of pro-inflammatory cytokines and a decreased expression of dendritic cell costimulatory molecules (CD80, CD86, and major histocompatibility complex class II) compared with LPS-treated dendritic cells. Furthermore, TSLP-DCs promoted noninflammatory Th2 response and induced the conversion of naïve T-cells into functional CD4+CD25+Foxp3+ Tregs. We further showed that subcutaneous injections of TSLP for 6 days or a single intravenous injection of TSLP-DCs protected NOD mice against diabetes.
CONCLUSIONS—Our study demonstrates that TSLP re-established a tolerogenic immune response in NOD mice and protects from diabetes, suggesting that TSLP may have a therapeutic potential for the treatment of type 1 diabetes.
Dendritic cells are professional antigen-presenting cells (APCs) that have the potential to induce immune response and T-cell tolerance (1). Immature or semimature tolerogenic dendritic cells have been shown to induce and maintain peripheral T-cell tolerance, whereas terminally differentiated mature dendritic cells induce the development of effector T-cells (1). Tolerogenic dendritic cells (tDCs) produce interleukin (IL)-10 and have impaired abilities to synthesize IL-12p70 and indolamine 2,3-dioxygenase and to activate T-cells in vitro (2). Conditioning dendritic cells with granulocyte macrophage–colony-stimulating factor (GM-CSF) (3), IL-10, and/or transforming growth factor-β (TGF-β) (4,5) as well as 1,25-dihydroxyvitamin D3 (6) has been shown to promote tDCs that induce Th2 response and/or differentiation of CD4+CD25+Foxp3+ regulatory T-cells (Tregs). When injected in mice, tDCs were able to suppress acute graft-versus-host disease (7) and autoimmunity (8). Recently, we and others have shown that injections of GM-CSF prevented the development of autoimmune diseases by increasing the number of semimature tDCs and by inducing Treg differentiation (9–11).
Tregs arise during the normal process of T-cell maturation in the thymus, and their differentiation can be induced in the periphery by conversion of CD4+CD25−Foxp3− into CD4+CD25+Foxp3+ Tregs (12–14). Tregs are crucial for suppressing autoimmune responses and maintaining peripheral immunological tolerance (15). The influence of Tregs in maintaining T-cell tolerance is strongly supported by the observations of the development of autoimmune syndromes in mice lacking Tregs and by the findings that defects in Foxp3 gene expression in humans and mice lead to autoimmune syndromes in early life (16,17). In agreement with these observations, prevention of rheumatoid arthritis, inflammatory bowel disease, and type 1 diabetes has been achieved by reconstitution of autoimmune-prone mice with Tregs (18).
Autoimmune diabetes in the nonobese diabetic (NOD) mouse model results from a breakdown of T-cell tolerance due to impaired development of tDCs and Treg differentiation (19,20). In addition, bone marrow–derived dendritic cells (BM-DCs) of NOD mice were shown to express abnormal levels of costimulatory molecules under pro-inflammatory conditions and increased capacity to secrete IL-12p70 and to stimulate CD4+ and CD8+ T-cells (21–23). Consequently, the capacity of dendritic cells in NOD mice to sustain the pool and suppressive function of Tregs is altered, which leads to progression of type 1 diabetes (24,25).
Thymic stromal lymphopoietin (TSLP) was first identified in conditioned medium supernatants of the mouse thymic stromal cell line Z210R.1 (26). TSLP, a member of the IL-7 cytokine family, is preferentially expressed by epithelial cells mainly in the lung, skin, and gut (27,28). Recently, clues for a function of TSLP in humans came from two observations. TSLP was found to be selectively expressed by thymic epithelial cells of Hassall's corpuscles, and TSLP-activated dendritic cells (TSLP-DCs) induced differentiation of CD4+Foxp3− thymocytes into CD4+Foxp3+ Tregs (29). Recently, Jiang et al. (30) have reported that TSLP produced by mouse medullary thymic epithelial cells contribute to Foxp3+ expression and Treg maturation. In addition, Lee et al. (31) have shown that TSLP triggered the conversion of thymic Foxp3−CD4+ T-cells into Foxp3+ T-cells in a dendritic cell–independent manner.
Here, we have investigated whether murine TSLP-DCs and TSLP induce differentiation and/or expansion of Tregs in the NOD mouse model and protection against diabetes. We found that TSLP-DCs acquire signatures of tDCs and induce the conversion of naïve T-cells into functional Tregs. We have further shown that subcutaneous injections of TSLP or a single intravenous injection of TSLP-DCs protects NOD mice against diabetes. Our data are the first to report that TSLP induces a tolerogenic immune response and protects against diabetes in NOD mice.
RESEARCH DESIGN AND METHODS
NOD/Ltj mice were from The Jackson Laboratories (Bar Harbor, ME). 8.3-NOD mice obtained from Dr. P. Santamaria (Microbiology and Infectious Diseases, University of Calgary, Alberta, Canada) have been described previously (32). The mice were housed under pathogen-free conditions, in accordance with the guidelines of the local institutional animal care committee.
Antibodies and reagents.
Anti–CD8α-PE (clone 53–6.7), anti–CD4-fluorescein isothiocyanate (FITC)/biotin/APC (clone GK1.5), anti–CD25-FITC (clone 7D4), anti–CD11b-FITC (clone M1/70), anti–CD11c-FITC/biotin (clone HL3), anti–CD80-biotin (clone 16-10A1), anti–CD86-biotin (clone GL1), and anti–I-Ag7-biotin (clone 10-3.6) antibodies, and streptavidin-PerCP were from Becton Dickinson (San Jose, CA). Anti–Foxp3-FITC/PE (FJK-16s) and anti-Rat IgG2a (eBR2a) antibodies were from eBiosciences (San Diego, CA). Anti-CD3 antibody (clone 145-2C11) was from Dr. P. Santamaria. The NRP-A7 peptide, a mimotope of the endogenous IGRP peptide that is recognized by the TCR of 8.3 CD8+ T-cells, and tumor-derived negative control peptide (TUM) were from C. Servis (Biochemistry Institute, Lausanne University, Switzerland). Murine recombinant TSLP (lot no. ELR0307011) was from R&D Systems (Minneapolis, MN).
Treatment and dendritic cells transfer.
Female NOD/Ltj mice were injected subcutaneously with recombinant mouse TSLP (500 ng · 200 μl−1 · mouse−1 · day−1) or PBS for 6 days. In dendritic cell transfer experiments, 3-week-old female NOD/Ltj mice received one intravenous injection of TSLP-DCs or LPS-DCs (5 × 106 cells/mouse). Diabetes was monitored by a urine glucose test using Uristix (Bayer, Minneapolis, MN), and blood glucose was measured with an Advantage Accu-Check glucometer (Roche Diagnostics, Indianapolis, IN). The animals were considered diabetic after two positive Uristix readings or when blood glucose was >15 mmol/l.
T-cell isolation.
CD4+ T-cell subpopulations and CD8+ T-cells were purified using antibody-coated magnetic beads from Miltenyi Biotec (Bergish Gladbach, Germany).
Generation of BM-DCs.
BM-DCs were generated with GM-CSF and IL-4 as previously described (33). On day 7, dendritic cells were left unstimulated (immature DCs [iDCs]) or exposed (48 h) to 1 μg/ml LPS (Sigma-Aldrich, St. Louis, MO) or 20 ng/ml TSLP (R&D Systems).
Proliferation assays and cytokine quantification.
CD8+ T-cells (2 × 104 lymphocytes/well) were incubated with 1 μg/ml NRP-A7 peptide–or 1 μg/ml TUM peptide–pulsed irradiated dendritic cells (5 × 103 cells/well) for 3 days at 37°C. CD4+ T-cells (2 × 104 lymphocytes/well) were incubated with a combination of 5 μg/ml anti-CD3 and 20 units/ml IL-2 in the presence of irradiated dendritic cells (5 × 103 cells/well) under similar conditions. Supernatants were collected 48 h later for cytokine quantification using ELISA kits (R&D Systems). Cultures were pulsed with 1 μCi [3H]thymidine/well during the last 18 h and radioactivity was counted.
Foxp3 expression.
Foxp3 staining was assessed by intracellular staining using FITC–anti-mouse/rat staining kit (eBiosciences, San Diego, CA) (11). Cells were analyzed using the CellQuest (BD Biosciences) or the FCS express V3 software (De Novo Software, Los Angeles, CA).
CFSE-based killing assay.
Killing assays were adapted from the technique of Jedema et al. (34). Briefly, RMAS-Kd cells (preincubated at 26°C overnight) were labeled with CFSE, washed, and resuspended (5 × 104 cells/ml) in lymphocyte complete medium. Carboxy fluorescein diacetate succinimidyl ester (CFSE)-labeled RMAS-Kd cells (5 × 103 cells · 100 μl−1 · well−1) were pulsed with NRP-A7 or TUM (1 μg/ml) and used as target cells. Effector 8.3-CD8+ T-cells were added at 1:2, 1:4, and 1:10 target:effector ratios. The plates were incubated at 37°C for 6 h and analyzed by fluorescence-activated cell sorter (FACS). The percentage of survival was calculated as follows: % survival = [number of viable CFSE+ target cells (t = 6 h)]/[number viable CFSE+ target cells (t = 0)] × 100.
T-cell and BM-DC co-cultures.
BM-DCs were cultured for 48 h in the presence of LPS or TSLP, extensively washed, and resuspended in fresh medium. Dendritic cells were then co-cultured with total splenic T-cells, purified CD4+CD25− T-cells, or purified CD4+CD25+ T-cells in round-bottom anti-CD3–coated 96-well culture plates in LCM containing 20 units/ml IL-2. Cultures were done in triplicate at a 1:4 dendritic cell:T-cell ratio.
Suppression assays.
Purified CD4+CD25− T-cells cultured in the presence of iDCs, LPS-DCs, or TSLP-DCs for 3 or 7 days were co-cultured with purified 8.3-CD8+ T-cells at a 1:1 ratio in the presence of 1 μg/ml peptide-pulsed APCs (105 irradiated splenocytes/well) for 3 days at 37°C as described previously (11). Cells were pulsed with 1 μCi [3H]thymidine/well during the last 18 h and radioactivity was counted.
Histopathology.
Pancreata were fixed in formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin. Islet insulitis was scored as described previously (11).
Statistical analyses.
Student's t test and χ2 tests were used to determine the statistical significance, which was set at the 95% confidence level.
RESULTS
TSLP-DCs display a tolerogenic phenotype.
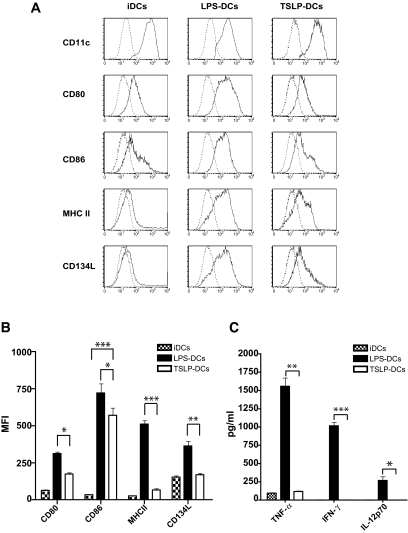
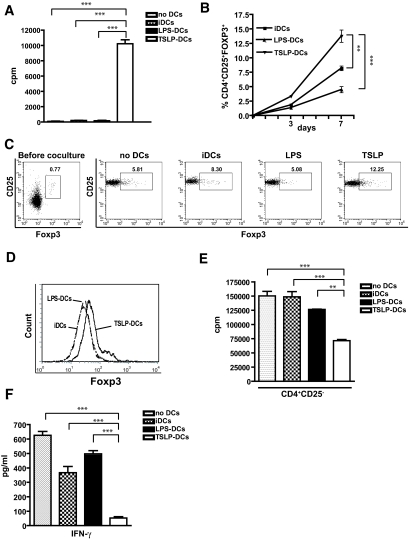
We first examined the effect of TSLP on the phenotype of BM-DCs generated with GM-CSF and IL-4. As expected, nonstimulated BM-DCs expressed low levels of CD80, CD86, and major histocompatibility complex (MHC) class II molecules, a characteristic of iDCs (Fig. 1A), and further stimulation with LPS (LPS-DCs) increased levels of CD80, CD86, and MHC class II (Fig. 1A), a characteristic of fully mature dendritic cells. Interestingly, iDCs exposed to TSLP (TSLP-DCs) expressed levels of CD80, CD86, and MHC II intermediate between those expressed by iDCs and fully mature LPS-DCs (Fig. 1A and B). The phenotype observed in the case of TSLP-DCs was characteristic of semimature dendritic cells. Furthermore, TSLP-DCs expressed low levels of OX40L (CD134) than LPS-DCs (Fig. 1A and B). Together, these results suggested that TSLP-DCs acquired a semimature phenotype.
FIG. 1.
TSLP-DCs display tolerogenic properties. A: BM-DCs (1 × 105 cells/well) were cultured for 2 days in the absence of stimulus (iDCs) or in the presence of 1 μg/ml LPS (LPS-DCs) or 20 ng/ml TSLP (TSLP-DCs). Dendritic cells were stained with isotype control antibodies (dashed line) or with specific antibodies against CD11c, CD80, CD86, I-Ag7, and CD134L (bold line) and analyzed by FACS. B: Mean fluorescence intensities (MFI) were quantified in each case. C: Quantification of TNF-α, IFN-γ, and IL-12p70 in the supernatants of BM-DCs cultured in absence of stimulus or in the presence of LPS or TSLP. Data are shown as the average ± SD of four independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
We next quantified tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and IL-12p70 in the supernatants of iDCs, LPS-iDCs, and TSLP-DCs. Consistent with previous studies (21,35), we found that LPS-DCs of NOD mice produced high amounts of TNF-α, IFN-γ, and IL-12p70, whereas TSLP-DCs produced low or barely detectable amounts of IFN-γ, TNF-α, and IL-12p70 (Fig. 1C). These data suggested that, in contrast to fully mature LPS-DCs of NOD mice, TSLP-DCs adopt a semimature phenotype and halt their production of inflammatory cytokines.
TSLP-DCs induce antigen-specific CD8+ T-cells to proliferate and to differentiate into cytotoxic T-cells.
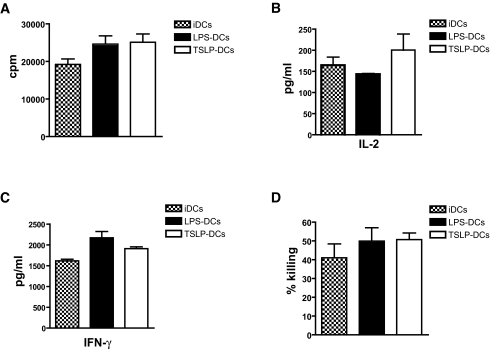
The capacity of TSLP-DCs to activate and differentiate NRP-A7–reactive 8.3-CD8+ T-cells into cytotoxic T-cells was compared with iDCs and LPS-DCs. Data showed no differences in the antigen-specific proliferation of 8.3-CD8+ T-cells in the presence of iDCs, LPS-DCs, or TSLP-DCs (Fig. 2A). To determine whether TSLP-DCs induced naïve 8.3-CD8+ T-cells to produce Tc1 or Tc2 cytokines, we quantified IL-2, IFN-γ, and IL-4. There were no differences in the amounts of IL-2 and IFN-γ produced by 8.3-CD8+ T-cells primed with iDCs, LPS-DCs, or TSLP-DCs (Fig. 2B and C). In addition, no IL-4 was detected under all experimental conditions (data not shown). To investigate whether TSLP-DCs affected differentiation of naïve 8.3-CD8+ T-cells into cytotoxic T-cells, we tested the capacity of 8.3-CD8+ T-cells cultured with iDCs or LPS-DCs or TSLP-DCs to kill RMAS-Kd cells (34). Similar levels of cytotoxic activity were detected in 8.3-CD8+ T-cells primed with NRP-A7–pulsed iDCs, LPS-DCs, or TSLP-DCs (cytolytic activities were 41.0 ± 7.45, 50.0 ± 7.04, and 50.74 ± 3.45%, respectively) (Fig. 2D).
FIG. 2.
TSLP-DCs induce antigen-specific CD8+ T-cell differentiation. A: Proliferative response of naïve 8.3-CD8+ T-cells (2 × 104 lymphocytes/well) to 1 μg/ml NRP-A7 peptide–pulsed iDCs or LPS- or TSLP-irradiated dendritic cells (5 × 103 cells/well), as indicated. Background responses against the negative control peptide TUM were subtracted. Quantification of IL-2 (B) and IFN-γ (C) secreted by naïve 8.3-CD8+ T-cells cultured under these conditions. D: Cytotoxic activity of BM-DC–activated 8.3-CD8+ T-cells against 1 μg/ml NRP-A7–or 1 μg/ml TUM-pulsed CFSE-labeled RAMS-Kd target cells. The percentage of killing was determined by subtracting nonspecific (TUM) from specific (NRP-A7) killing. Data are representative of two to three independent experiments and are the average ± SD.
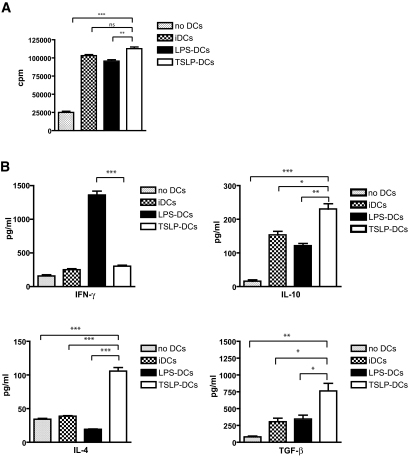
TSLP-DCs differentiate CD4+ T-cells into noninflammatory Th2 cells.
Human TSLP-DCs induce a robust expansion of CD4+ T-cells that can differentiate into noninflammatory or inflammatory Th2 cells (36–38). Therefore, we examined the ability of TSLP-DCs to polarize CD4+ T-cells into Th2 cytokine-producing cells. Proliferation and cytokine production by splenic CD4+ T-cells were determined by incubating naïve CD4+ T-cells with anti-CD3 and IL-2 in the absence or presence of allogeneic iDCs, LPS-DCs, or TSLP-DCs following the protocol of Watanabe et al. (29). Whereas, anti-CD3 and IL-2 induced moderated proliferation of CD4+ T-cells, a combination of anti-CD3 and IL-2 with iDCs, LPS-DCs, or TSLP-DCs induced 4.1-, 3.8-, and 4.5-fold increases of CD4+ T-cell proliferation, respectively (Fig. 3A). We next examined cytokine production and found that CD4+ T-cells cultured with TSLP-DCs produced significantly high amounts of Th2 cytokines, such as IL-4, and low amounts of IFN-γ, as reported previously (38). Furthermore, these cells produced more TGF-β and IL-10 than naïve CD4+ T-cells cultured with iDCs or LPS-DCs (Fig. 3B).
FIG. 3.
TSLP-DCs induce CD4+ T-cells to produce immunoregulatory cytokines. A: Proliferation of naïve CD4+ T-cells (2 × 104 lymphocytes/well) activated by a combination of 5 μg/ml anti-CD3α and 20 units/ml IL-2 in the absence of BM-DCs or in the presence of irradiated BM-DCs (5 × 103 cells/well) that had been left unstimulated (iDCs) or that that had been exposed to 1 μg/ml LPS (LPS-DCs) or 20 ng/ml TSLP (TSLP-DCs) for 48 h. B: Quantification of IFN-γ, IL-10, IL-4, and TGF-β released by CD4+ T-cells cultured under the same conditions. Data are the average ± SD and are representative of four independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
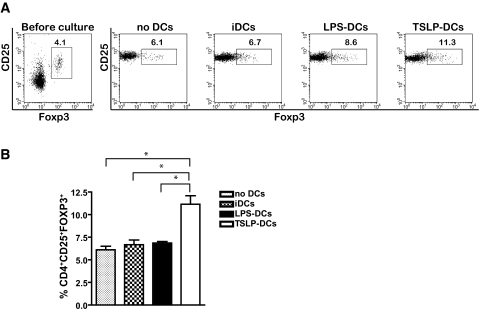
CD4+ T-cells expanded with TSLP-DCs are enriched in Tregs.
We next determined whether the increased production of IL-10 and TGF-β was due to an increased pool of Tregs within the population of CD4+ T-cells cultured in the presence of TSLP-DCs. The percentage of CD4+CD25+Foxp3+ T-cells present in CD4+ T-cells expanded with a combination of anti-CD3 and IL-2, in the absence or presence of iDCs, LPS-DCs, or TSLP-DCs, was analyzed by FACS (Fig. 4). Results showed that after 7 days of culture, CD4+ T-cells activated in the absence of dendritic cells or in the presence of iDCs or LPS-DCs contained 6.12 ± 0.55, 6.68 ± 0.73, and 6.86 ± 0.23% of CD4+CD25+Foxp3+ T-cells, respectively (Fig. 4A and B). Of significance, CD4+ T-cells co-activated in the presence TSLP-DCs contained nearly twice as much Foxp3+CD4+ T-cells (11.25 ± 1.33%, P < 0.05) as CD4+ T-cells co-activated in the presence of iDCs or LPS-DCs (Fig. 4A and B).
FIG. 4.
TSLP-DCs augment the percentage of Tregs in vitro. A: Total splenic CD4+ T-cells were cultured for 7 days in the presence of 5 μg/ml anti-CD3α and 20 units/ml IL-2 alone or combined with iDCs, LPS-DCs, or TSLP-DCs. At day 7, the cells were labeled with anti-CD4, anti-CD25, and anti-Foxp3 and analyzed by FACS. FACS data are representative of one of three independent experiments. B: Quantification of the percentages of CD4+CD25+Foxp3+ cells of CD4+ splenic T-cells under similar conditions. Data are shown as the mean ± SD of three independent experiments. *P < 0.05.
TSLP-DCs induced expansion and differentiation of CD4+CD25+Foxp3+ Tregs in vitro.
We next investigated whether the increased number of Tregs in splenic CD4+ T-cells co-activated in the presence of TSLP-DCs resulted from an expansion of Tregs and/or the Treg differentiation. Purified CD4+CD25+ (Foxp3+) and CD4+CD25− (Foxp3−) were cultured with anti-CD3 and IL-2 in the absence of dendritic cells or presence of iDCs, LPS-DCs, or TSLP-DCs. The expansion of CD4+CD25+Foxp3+ T-cells was determined by [3H]thymidine incorporation assay. Stimulation with anti-CD3 and IL-2 induced marginal proliferation of CD4+CD25+ T-cells, whereas co-activation with iDCs or LPS-DCs weakly increased proliferation (Fig. 5A). In contrast, TSLP-DCs induced robust proliferation (Fig. 5A), as previously reported (29).
FIG. 5.
TSLP-DCs induce the expansion of Tregs and the conversion of naïve CD4+CD25− T-cells into CD4+CD25+Foxp3+ T-cells. A: Proliferative response of purified CD4+CD25+ T-cells (2 × 104 lymphocytes/well) exposed to a combination of 5 μg/ml anti-CD3α and 20 units/ml IL-2 and irradiated iDCs or LPS- or TSLP-DCs (5 × 103 cells/well). B: Purified CD4+CD25− T-cells were incubated with a combination of 5 μg/ml anti-CD3α and 20 units/ml IL-2 and iDCs or LPS- or TSLP-DCs at a 1:4 ratio of T-cells:BM-DCs. The percentages of CD4+CD25+Foxp3+ T-cells were determined at days 3 and 7. Data represent the mean ± SD of two to three independent experiments. C and D: Representative FACS analysis of the percentages of splenic CD4+CD25+Foxp3+ T-cells and Foxp3 expression in splenic CD4+CD25− T-cells after 7 days of cultures under similar conditions. E and F: Splenic CD4+CD25− T-cells (1 × 105 cells/well) were first cultured in the presence of anti-CD3α, IL-2, and conditioned BM-DCs. On day 7, the cells were collected, washed, and added to 8.3-CD8+ T-cells (2 × 104 lymphocytes/well) exposed to 1 μg/ml NRP-A7–or 1 μg/ml TUM-pulsed irradiated splenic APCs (105 cells/well). The proliferative response of 8.3-CD8+ T-cells was determined by [3H]thymidine incorporation, and the production of IFN-γ was quantified by ELISA. **P < 0.01 and ***P < 0.001.
The role of TSLP-DCs in de novo differentiation of CD4+CD25+Foxp3+ Tregs was investigated in the same allogeneic assay using purified naïve CD4+CD25− T-cells of NOD mice. The combination of anti-CD3 and IL-2 did not induce expression of Foxp3+ (data not shown). At day 3, TSLP-DCs induced a moderate (3.32 ± 0.18%) but significant (P < 0.01) higher percentage of Foxp3+CD4+ T-cells compared with cells cultured in the presence of iDCs or LPS-DCs (1.80 ± 0.11 and 1.38 ± 0.09%, respectively) (Fig. 5B). Of importance, the percentage of Foxp3+CD4+ T-cells further increased in the presence of TSLP-DCs (13.78 ± 1.84%) in comparison with cultures performed in the presence of iDCs (8.23 ± 0.38%) or LPS-DCs (4.54 ± 0.91%), after 7 days of culture (Fig. 5B–D). Foxp3+-differentiated CD4+ T-cells expressed high levels of CD25, CTLA4, GITR, and CD62L (data not shown).
To confirm that CD4+CD25+Foxp3+ Tregs induced by TSLP-DCs were functional Tregs, we investigated the capacity of these cells to inhibit the proliferation and IFN-γ production of diabetogenic 8.3-CD8+ T-cells in vitro (11). Results showed that CD4+CD25−Foxp3− T-cells activated with anti-CD3 and IL-2 in the absence of dendritic cells or in the presence of iDCs or LPS-DCs did not suppress the proliferation of 8.3-CD8+ T-cells (Fig. 5E). In marked contrast, CD4+CD25−Foxp3− T-cells activated with anti-CD3 and IL-2 in the presence of TSLP-DCs decreased the proliferation of 8.3-CD8+ T-cells by 50% (Fig. 5E). The suppressive effect of converted cells was also observed on IFN-γ release (Fig. 5F). These results indicated that TSLP-DCs acquired the capacity to induce the expansion of Tregs and de novo differentiation of CD4+CD25+Foxp3+ Tregs by conversion of CD4+CD25−Foxp3− T-cells and/or expansion of CD4+CD25−Foxp3+ T-cells.
TSLP increases the number of Tregs in NOD mice.
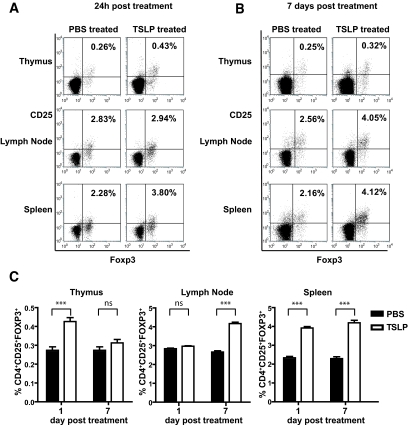
The findings that TSLP instructed dendritic cells to acquire tolerogenic properties in vitro prompted us to investigate the effect of TSLP on the development of Tregs in NOD mice. TSLP (500 ng · mouse−1 · day−1) was injected subcutaneously in the nucchal area for 6 days. The frequency of thymic and peripheral CD4+CD25+Foxp3+ T-cells was determined 24 h and 7 days after the last injection. Results showed increased percentages of CD4+CD25+Foxp3+ T-cells of TSLP-treated mice compared with control in the thymus (0.43 ± 0.03 vs. 0.27 ± 0.03%) and the spleen (3.92 ± 0.13 vs. 2.34 ± 0.12%) at 24 h (Fig. 6A and C). There were no changes in lymph nodes. At day 7, the percentage of CD4+CD25+Foxp3+ T-cells in the thymus of TSLP-injected NOD mice decreased to the levels of control mice (0.31 ± 0.03 and 0.27 ± 0.03%, respectively). The percentage of Tregs was significantly increased in lymph nodes (4.17 ± 0.14%) and spleen (4.19 ± 0.23%) compared with control (2.66 ± 0.11 and 2.30 ± 0.16%, respectively) (Fig. 6B and C), indicating that TSLP promoted the pool of thymic and peripheral Tregs in NOD mice.
FIG. 6.
TSLP increases the number of Tregs in NOD mice. A and B: NOD mice were injected subcutaneously with PBS (control) or TSLP (500 ng/animal) for 6 consecutive days. Thymus, pooled lymph nodes (mesenteric lymph node, pancreatic lymph node, brachial, inguinal, and axillary lymph nodes), and spleen were analyzed for the presence of Tregs 24 h and 7 days after treatment, as indicated. Cells were gated on CD4+ T-cells, and percentages indicate the proportion of cells that were CD4+CD25+Foxp3+. Data are representative of one of five independent experiments. C: Percentages of CD4+CD25+Foxp3+ T-cells in different organs in PBS- or TSLP-treated mice (five mice in each group). Data represent the average ± SD. ***P < 0.001.
TSLP-DCs prevent diabetes development in NOD mice.
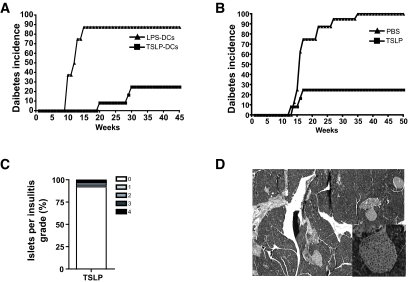
The in vitro and in vivo studies described above led us to assess first the capacity of TSLP-DCs to prevent diabetes development in NOD mice. Therefore, 3-week-old NOD mice received a single injection of LPS-DCs (control) or TSLP-DCs and were monitored for diabetes. Diabetes occurred in 87% of control mice at 12 weeks, whereas only 25% of TSLP-DC–injected mice developed diabetes starting at 18 weeks (Fig. 7A). The TSLP-DC–induced protection was maintained up to 45 weeks (P < 0.001; Fig. 7A).
FIG. 7.
TSLP and TSLP-DCs prevent diabetes development in NOD mice. A: NOD mice (12 animals/group) were injected intravenously at 3 weeks of age with LPS-DCs (5 × 106 cells/animal) or TSLP-DCs (5 × 106 cells/animal) and monitored for diabetes development. B: NOD mice (12 animals/group) were treated with PBS or TSLP (500 ng/animal) for 6 consecutive days and monitored for diabetes development. C: Pancreata of TSLP-treated NOD mice (n = 6, 40–50 islets/mouse) were scored for insulitis. D: Representative microphotographs of hematoxylin-eosin–stained pancreatic sections of TSLP-treated NOD mice.
TSLP treatment inhibits diabetes development in NOD mice.
To investigate the capacity of TSLP to protect against diabetes, 3-week-old NOD mice were treated each day with a single subcutaneous injection of PBS (control) or TSLP (500 ng/mouse) for 6 days and followed for diabetes development. Results showed that at 35 weeks, almost all control animals had developed diabetes, whereas 75% of TSLP-treated mice were diabetes free (blood glucose 5–7 mmol/l) for at least 50 weeks (P < 0.001; Fig. 7A), did not show any signs of side effects, and were fertile. Histopathological analysis of pancreata of these animals showed that 93.7% of islets lacked lymphocytic infiltration (Fig. 7B). A representative field from diabetes-free TSLP-treated NOD mice is shown (Fig. 7C). In contrast, the majority of pancreata from control NOD mice were devoid of islets (data not shown).
DISCUSSION
We report the ability of murine TSLP to activate dendritic cells of NOD mice and to blunt their pro-inflammatory potential. TSLP-DCs induced the conversion of naïve T-cells of NOD mice into Tregs in vivo and protected NOD mice from diabetes. We showed for the first time that injection of TSLP into NOD mice led to an increased number of Tregs in the thymus followed by increased peripheral Tregs numbers and conferred a significant protection against diabetes.
In humans and mice, stimuli such as CD40L or TLR ligands, LPS, and poly I:C strongly upregulate the expression of CMH II, CD80, CD86, and CD40 in dendritic cells. These stimuli induce the maturation of dendritic cells, which produce large amounts of inflammatory cytokines, such as IL-12, TNF-α, IL-1β, and IL-6, which induce Th1 differentiation. However, unlike CD40L and TLR ligands, human TSLP induces the upregulation of dendritic cells maturation markers without stimulating the production of inflammatory cytokines (39). Studies in mice have indicated that TSLP acts directly on early B- and T-cell development (40,41) and on dendritic cell maturation (42). Using BM-DCs of NOD mice, we show here that TSLP promoted a phenotype and a cytokine profile consistent with the signature of tolerogenic semimature dendritic cells reported to be involved in initiating and maintaining T-cell tolerance (43). Unlike LPS-DCs, TSLP-DCs of NOD mice displayed less mature phenotypes and switched off their production of inflammatory cytokines (IL-12p70, TNF-α, and IFN-γ) known to induce a Th1 response. Consistent with our results, TSLP blunted the production of IL-12p70, TNF-α, IFN-γ, or mRNA encoding IL-12 by human dendritic cells and type 1 IFN family members that induce Th1 differentiation (29,44,45). Recent studies have shown that TSLP-DCs induced the expansion of CD4+ and CD8+ T-cells and the differentiation of naïve CD4+ T-cells toward Th2 cells that produced IL-4, IL-13, and TNF-α but not IL-10 and IFN-γ (38,45). In addition, TSLP-DCs have been reported to prime CD8+ T-cells into IL-5–and IL-13–producing effector cells exhibiting poor cytotoxic activity (46). Here, TSLP-DCs of NOD mice induced antigen-specific CD8+ T-cell proliferation and differentiation into cytotoxic T-cells. In addition, TSLP-DCs primed CD4+ T-cells to proliferate and to differentiate into noninflammatory Th2 cells that produced large amounts of IL-4 and IL-10, as reported previously (37). Interestingly, TSLP-DCs promoted CD4+ T-cells to produce large amounts of TGF-β, a cytokine required for Foxp3 gene expression during Treg development (47). These data suggested that TSLP-DC–primed CD4+ T-cells contained a high proportion of Foxp3+ Tregs. When splenic CD4+ T-cells were cultured in the presence of TSLP-DCs, the percentage of Foxp3+ Tregs was significantly increased compared with CD4+ T-cells primed with LPS-DCs or iDCs. The increase in the pool of Tregs was not only due to the expansion of existing Tregs but also to the conversion of CD4+Foxp3− T-cells into Tregs. Our data were in agreement with a previous study that showed the capacity of TSLP-DCs to induce differentiation of Foxp3− T-cells into Tregs and their expansion using a similar allogeneic culture assay (29). Moreover, newly formed Tregs displayed characteristics of naturally occurring Tregs (15), such as expression of high levels of CD62L, CTLA-4, and GITR and suppression of proliferation of CD8+ T-cells and IFN-γ production.
In NOD mice, dendritic cell development from myeloid progenitors is impaired and is associated with abnormal levels of expression of costimulatory molecules and increased capacity to secrete IL-12p70 and to stimulate CD4+ and CD8+ T-cells (21–23). The findings that TSLP restored tolerogenic functions of BM-DCs of NOD mice were further extended in in vivo experiments. Results showed that a single injection of TSLP-DCs in young NOD mice was sufficient to induce a significant protection against diabetes, whereas LPS-DC–treated NOD mice developed accelerated diabetes. This protection resulted from an increased pool of Tregs that exhibited increased suppressive functions and contained high proportion of Foxp3high T-cells (G.B., S.G., G.D., A.A., unpublished data).
Recently, OX40 engagement of Foxp3− T-cells has been shown to suppress the induction of Foxp3 driven by antigen or exogenous TGF-β (48). Here, the conversion of Foxp3− to Foxp3+ CD4+ T-cells could be explained by a reduced expression of OX40L on TSLP-DCs. This possibility was supported by the findings of enhanced induction of Foxp3+ T-cells when the OX40L/OX40 signaling pathway was blocked (G.B., S.G., G.D., A.A., unpublished data). Moreover, the involvement of the OX40/OX40L pathway in diabetes was consistent with previous reports of diabetes protection in NOD mice treated with anti-OX40L antibodies and in OX40L−/− NOD mice (49,50). These data suggested the important role of OX40/OX40L interaction in the differentiation of Tregs and the protection against diabetes observed here in TSLP-DCs transferred NOD mice.
Naturally occurring Tregs arise from thymus and are exported to the peripheral lymphoid organs to contribute to peripheral tolerance. In NOD mice, the breakdown of T-cell tolerance is associated with quantitative and qualitative decreases in the pool of CD4+CD25+ Tregs (24,25). In view of these observations, several studies have shown that adoptive transfer of Tregs (51) or reestablishment of Treg pool using anti-CD3 or GM-CSF treatment (11,52) were effective in the restoration of T-cell tolerance and consequent protection from diabetes. Here, subcutaneous injections of TSLP in NOD mice led to increased number of Tregs in the thymus and, subsequently, in the peripheral organs (spleen and lymph node), confirming the capacity of TSLP to promote Treg differentiation in vivo. Although TSLP appears to act on dendritic cells in the human system and on dendritic cells and T-cells in the murine system (39), increased Treg pool in the thymus may result from a direct effect of TSLP on T-cells or on dendritic cells. The underlying mechanisms of increased Tregs have not been fully elucidated. The involvement of dendritic cells with tolerogenic properties in TSLP-protected NOD mice is supported by in vitro data. Furthermore, FITC skin painting experiments clearly showed that TSLP injection mobilized skin dendritic cells to the thymus, suggesting involvement of such dendritic cells in thymic Treg differentiation (data not shown). Whereas Tregs were increased in the spleen and lymph nodes 7 days after injection, the pool of thymic Tregs decreased to the levels observed in control mice. These data suggested that positively selected thymic Tregs were exported to the periphery and contributed to the Treg pool required for efficient induction and maintenance of T-cell tolerance in NOD mice. However, induction of Treg differentiation in the periphery by immigrant TSLP-conditioned skin dendritic cells cannot be excluded. This possibility is under current investigations in our laboratories. The induction of tolerogenic function of dendritic cells and increased Tregs in TSLP-treated mice could unveil potential therapeutic treatments of autoimmune diabetes.
In conclusion, our study showed for the first time that the existing default of tolerance in autoimmune diabetes in NOD mice could be restored by TSLP through induction of tolerogenic dendritic cells, resulting in Treg differentiation and promotion of noninflammatory IL-10–producing Th2 cells that are a hallmark immune response involved in the prevention of autoimmune diabetes.
Acknowledgments
This work was supported by a grant from the Juvenile Diabetes Foundation International. G.B. is the recipient of a fellowship from Association de Langue Française pour l'Étude du Diabète et des Maladies Métaboliques. S.G. is the recipient of a PhD scholarship from Fonds de la Recherche en Santé du Québec (FRSQ) and the Canadian Institutes of Health Research. M.M. and C.G. are the recipients of a summer studentship from Diabète Québec. A.A. is a Canadian Diabetes Association New Investigator and a recipient of a Chercheur Boursier Junior 2 from the FRSQ.
We thank Dr. P. Santamaria for the gifts of reagents and mice. We also thank the personnel of the Animal facilities of the University of Sherbrooke for care of the mice and technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 13 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Lanzavecchia A, Sallusto F: The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol 13: 291–298, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Morelli AE, Thomson AW: Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 7: 610–621, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM: Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol 30: 1813–1822, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Riedl E, Strobl H, Majdic O, Knapp W: TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol 158: 1591–1597, 1997 [PubMed] [Google Scholar]
- 5.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH: Induction of tolerance by IL-10-treated dendritic cells. J Immunol 159: 4772–4780, 1997 [PubMed] [Google Scholar]
- 6.Penna G, Adorini L: 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164: 2405–2411, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T: Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity 18: 367–379, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM: CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 199: 1467–1477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangi E, Vasu C, Cheatem D, Prabhakar BS: IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol 174: 7006–7013, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Vasu C, Dogan RN, Holterman MJ, Prabhakar BS: Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol 170: 5511–5522, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A: Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol 179: 3638–3647, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S: Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Gavin MA, Rudensky AY: Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Ziegler SF: FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol 7: 305–310, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S: Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22: 531–562, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME: X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27: 18–20, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD: The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27: 20–21, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Leung BP: CD4+CD25+ regulatory T cells in health and disease. Clin Exp Pharmacol Physiol 33: 519–524, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA: B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12: 431–440, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Anderson MS, Bluestone JA: The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23: 447–485, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Boudaly S, Morin J, Berthier R, Marche P, Boitard C: Altered dendritic cells (DC) might be responsible for regulatory T cell imbalance and autoimmunity in nonobese diabetic (NOD) mice. Eur Cytokine Netw 13: 29–37, 2002 [PubMed] [Google Scholar]
- 22.Serreze DV, Gaskins HR, Leiter EH: Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol 150: 2534–2543, 1993 [PubMed] [Google Scholar]
- 23.Poligone B, Weaver DJ Jr, Sen P, Baldwin AS Jr, Tisch R: Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol 168: 188–196, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Berzins SP, Venanzi ES, Benoist C, Mathis D: T-cell compartments of prediabetic NOD mice. Diabetes 52: 327–334, 2003 [DOI] [PubMed] [Google Scholar]
- 25.You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, Waldmann H, Bach JF, Chatenoud L: Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes 54: 1415–1422, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A: A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 22: 321–328, 1994 [PubMed] [Google Scholar]
- 27.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R, Kastelein RA, Bazan JF: Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol 167: 336–343, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Zeuthen LH, Fink LN, Frokiaer H: Epithelial cells prime the immune response to an array of gut-derived commensals towards a tolerogenic phenotype through distinct actions of thymic stromal lymphopoietin and transforming growth factor-beta. Immunology 123: 197–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ: Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436: 1181–1185, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Jiang Q, Su H, Knudsen G, Helms W, Su L: Delayed functional maturation of natural regulatory T cells in the medulla of postnatal thymus: role of TSLP. BMC Immunol 7: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JY, Lim YM, Park MJ, Min SY, Cho ML, Sung YC, Park SH, Kim HY, Cho YG: Murine thymic stromal lymphopoietin promotes the differentiation of regulatory T cells from thymic CD4(+)CD8(−)CD25(−) naive cells in a dendritic cell-independent manner. Immunol Cell Biol 86: 206–213, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P: Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 186: 1663–1676, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM: Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A 90: 3038–3042, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jedema I, van der Werff NM, Barge RM, Willemze R, Falkenburg JH: New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood 103: 2677–2682, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Feili-Hariri M, Morel PA: Phenotypic and functional characteristics of BM-derived DC from NOD and non-diabetes-prone strains. Clin Immunol 98: 133–142, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, de Waal Malefyt R, Liu YJ: Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol 5: 426–434, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M: Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol 6: 507–514, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ: Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity 24: 827–838, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YH, Malefyt Rde W, Omori M, Zhou B, Ziegler SF: TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 25: 193–219, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Ray RJ, Furlonger C, Williams DE, Paige CJ: Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol 26: 10–16, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ: A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med 200: 159–168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF: Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6: 1047–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Lutz MB, Schuler G: Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23: 445–449, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt RD, Bazan F, Kastelein RA, Liu YJ: Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 3: 673–680, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ: TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 202: 1213–1223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, de Waal-Malefyt R, Liu YJ: Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med 197: 1059–1063, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM: Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198: 1875–1886, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.So T, Croft M: Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol 179: 1427–1430, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Pakala SV, Bansal-Pakala P, Halteman BS, Croft M: Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. Eur J Immunol 34: 3039–3046, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Martin-Orozco N, Chen Z, Poirot L, Hyatt E, Chen A, Kanagawa O, Sharpe A, Mathis D, Benoist C: Paradoxical dampening of anti-islet self-reactivity but promotion of diabetes by OX40 ligand. J Immunol 171: 6954–6960, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Piccirillo CA, Tritt M, Sgouroudis E, Albanese A, Pyzik M, Hay V: Control of type 1 autoimmune diabetes by naturally occurring CD4+CD25+ regulatory T lymphocytes in neonatal NOD mice. Ann N Y Acad Sci 1051: 72–87, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L: TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9: 1202–1208, 2003 [DOI] [PubMed] [Google Scholar]