Abstract
OBJECTIVE—Diabetic nephropathy clusters in families, suggesting that genetic factors play a role in its pathogenesis. We investigated whether similar clustering exists for proliferative retinopathy in families with two or more siblings with type 1 diabetes.
RESEARCH DESIGN AND METHODS—The FinnDiane Study has characterized 20% (4,800 patients) of adults with type 1 diabetes in Finland. In 188 families, there were at least two siblings with type 1 diabetes. Ophthalmic records were obtained for 369 of 396 (93%) and fundus photographs for 251 of 369 (68%) patients. Retinopathy was graded based on photographs and/or repeated ophthalmic examinations using the Early Treatment of Diabetic Retinopathy grading scale.
RESULTS—Mean age at onset of diabetes was 14.3 ± 10.2 years, and mean duration was 25.9 ± 11.8 years. Proliferative retinopathy was found in 115 of 369 patients (31%). The familial risk of proliferative retinopathy was estimated in 168 of 188 sibships, adjusted for A1C, duration, and mean blood pressure. Proliferative retinopathy in the probands (48 of 168) was associated with an increased risk (odds ratio 2.76 [95% CI 1.25- 6.11], P = 0.01) of proliferative retinopathy in the siblings of probands (61 of 182). The heritability of proliferative retinopathy was h2 = 0.52 ± 0.31 (P < 0.05).
CONCLUSIONS—We found a familial clustering of proliferative retinopathy in patients with type 1 diabetes. The observation cannot be accounted for by conventional risk factors, suggesting a genetic component in the pathogenesis of proliferative retinopathy in type 1 diabetes.
Diabetic nephropathy and proliferative retinopathy are severe microvascular complications of diabetes. Diabetic nephropathy clusters in families, suggesting that genetic factors play a role in the pathogenesis of this complication (1). However, there is not yet evidence for a similar clustering of proliferative retinopathy in families with type 1 diabetes.
After 20 years of diabetes, almost all patients with type 1 diabetes and 58% of patients with type 2 diabetes show signs of retinopathy. When retinopathy worsens, severe visual loss eventually threatens 5–10% of patients (2). The most severe form of retinopathy is proliferative retinopathy, and most of the patients with this complication will become blind after 5–10 years without treatment (3). The prevalence of proliferative retinopathy varies between 13 and 50% after 15–20 years of diabetes duration in patients needing insulin (2,4).
The prevalence of any retinopathy is strongly related to duration and glucose exposure (2,4). Furthermore, poor glycemic control increases the incidence and progression of retinopathy (5,6). Nevertheless, glycemic exposure seems to explain only a part of the risk of proliferative retinopathy (7). In patients with type 2 diabetes, high blood pressure increases the incidence of retinopathy (8). It is noteworthy that proliferative retinopathy is associated with diabetic nephropathy, a complication that is at least in part genetically determined (2). Such an association suggests that familial factors may also contribute to the development of proliferative retinopathy.
Therefore, the aim of this study was to elucidate whether there is a familial clustering of proliferative retinopathy in patients with longstanding type 1 diabetes and to estimate the degree of familiality by calculating the heritability of proliferative retinopathy.
RESEARCH DESIGN AND METHODS
The present study was undertaken as part of the ongoing FinnDiane Study (Finnish Diabetic Nephropathy Study), a nationwide multicenter project with the aim of identifying genetic and environmental risk factors for diabetic complications in type 1 diabetes. The protocol is in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Helsinki University Central Hospital.
The FinnDiane Study has, to date, recruited 4,800 patients with type 1 diabetes. All patients who visit any of the participating 92 hospitals and health care centers are given an opportunity to take part in the FinnDiane Study. The response rate has been 78% (9). Although FinnDiane is not a population-based study in the strict sense, the distribution of the patients closely follows the distribution of the general population in Finland. As a part of the baseline visit, patients answered the question of whether any of their close relatives had type 1 diabetes, as defined by having an age at onset of 40 years or less and insulin treatment initiated within 1 year of the diagnosis. With these criteria, 188 families with at least two siblings with type 1 diabetes were found (Table 1). All of the siblings were contacted, and those siblings who agreed to take part signed a consent form and were characterized at a FinnDiane center. Data on medication, cardiovascular status, diabetic complications, hypertension, and cardiovascular disease were obtained using a standardized questionnaire, which was completed by the patient's attending physician. Blood pressure was measured twice in the sitting position using a mercury sphygmomanometer after a rest of at least 10 min. Anthropometric data, such as height and weight, were recorded, and blood was drawn for the laboratory measurements, including A1C.
TABLE 1.
Structure of sibships
| Siblings with type 1 diabetes in the same family | Families | Patients with type 1 diabetes | Ophthalmic data available | Patients with proliferative retinopathy |
|---|---|---|---|---|
| 2 siblings | 171 | 342 | 323 | 101 |
| 3 siblings | 14 | 42 | 38 | 13 |
| 4 siblings | 3 | 12 | 8 | 1 |
| Total | 188 | 396 | 369 | 115 |
We were able to obtain ophthalmic records for 369 of 396 (93%) patients. Photographs were available for 251 of 369 (68%) of these patients, and records of repeated fundus examinations performed by a specialist in ophthalmology for 332 of 369 (90%) patients. Both were available for 217 of 369 (59%) patients. Those patients with images available (68%) had been photographed on a median of three separate occasions (interquartile range [IQR] 1–5). For 34 of 369 (9%) patients, the only source of information was the screening photographs taken at the local health canters. A diabetologist's evaluation of the fundi was the only source of information for only 3 of 369 (1%) of the patients, all of whom had mild diabetic retinopathy. All available patient data were used to score the severity and progression of retinopathy, a procedure handled by an ophthalmologist unaware of the demographic data and the presence or absence of other complications. The Early Treatment of Diabetic Retinopathy (ETDRS) grading scale was used, where 10 represents no retinopathy; 61 and upward, proliferative retinopathy; and 80, advanced retinopathy (10). Patients without photographs were assigned a most probable estimate of ETDRS score based on the descriptions of repeated fundus examinations. The eye with the more severe retinopathy was used to define the severity of retinopathy of the patient.
Patient's nephropathy status was classified according to their urinary albumin excretion rate (AER) in at least two of three overnight or 24-h urine collections. Normal AER was defined as an AER <20 μg/min or AER <30 mg/24 h, microalbuminuria as an AER ≥20 and <200 μg/min or ≥30 and <300 mg/24 h, macroalbuminuria as an AER ≥200 μg/min or ≥300 mg/24 h, and end-stage renal disease as when the patient required dialysis or renal transplantation.
Statistical analysis.
The siblings were ranked by age, and the oldest sibling was designated as the proband of each sibship. There were four twin pairs from four different families, and the sibling with the longest duration of diabetes was chosen as the proband in these particular sibships. Two twin pairs were monozygotic as determined by microsatellite markers (ABI MD-10 V2.5; Applied Biosystems, Foster City, CA).
Data are presented as means ± SD for continuous, normally distributed variables and median and IQR for non–normally distributed variables. Means ± SE are given for heritability estimates. Unadjusted intrafamilial associations were estimated by calculating intraclass correlations (ICCs) for sibpairs. The FCOR program of the SAGE software package (Case Western Reserve University, Cleveland, OH) was used with a uniform weighting scheme, giving equal weights for each sibship regardless of the number of sibpairs within the sibships (11). The correlations between ordinal ETDRS scores and nephropathy status were also calculated with the FCOR program using the same weighting scheme. Mean differences in current age and duration of diabetes between probands and siblings were calculated using a linear mixed model (Table 2).
TABLE 2.
Clinical characteristics and concordance within sibships (intraclass correlations) for the siblings with type 1 diabetes
| Variable | n (396) | Mean ± SD | Range | ICC (95% CI) |
|---|---|---|---|---|
| Age at onset (years) | 387 | 14.3 ± 10.2 | 0.2–40.0 | 0.15 (0.02–0.21) |
| Duration of diabetes at the latest ophthalmic examination (years) | 369 | 25.9 ± 11.8 | 1.0–55.4 | 0.39 (0.26–0.45) |
| Difference of duration between probands and siblings (years) | 2.4 ± 1.3* | |||
| Current age (years) | 387 | 40.2 ± 11.5 | 14.5–69.3 | 0.69 (0.61–0.73) |
| Age difference between proband and siblings (years) | 4.9 ± 1.2* | |||
| A1C (%) | 380 | 8.5 ± 1.5 | 5.0–13.8 | 0.22 (0.09–0.29) |
| Systolic pressure (mmHg) | 378 | 135 ± 18 | 95–215 | 0.20 (0.07–0.27) |
| Diastolic pressure (mmHg) | 378 | 80 ± 9 | 50–113 | 0.10 (−0.04 to 0.16) |
| MAP (mmHg) | 378 | 100 ± 12 | 72–140 | 0.18 (0.04–0.25) |
| BMI (kg/m2) | 354 | 25.3 ± 3.9 | 16.2–43.0 | 0.21 (0.07–0.28) |
| Total cholesterol (mmol/l) | 341 | 4.9 ± 1.0 | 1.9–12.0 | 0.21 (0.06–0.30) |
| HDL cholesterol (mmol/l) | 341 | 1.6 ± 0.52 | 0.2–4.3 | 0.33 (0.18–0.40) |
| Current smoking | 369 | Yes 86 (23%) | 0.15 (0.01–0.22) | |
| No 83 (77%) | ||||
| ETDRS score | 169 | 40 (IQR 20–62)† | 10–80 | 0.37 (0.24–0.43) |
| PDR | Yes 115 (31%) | 0.28 (0.14–0.35) | ||
| No 254 (69%) | ||||
| Nephropathy status | 328 | Normoalbuminuria 202 (54%) | 0.26 (0.06–0.36) | |
| Microalbuminuria 51 (14%) | ||||
| Macroalbuminuria 50 (14%) | ||||
| End-stage renal disease 25 (7%) | ||||
Data are
SE or
median value (188 sibships, men/women 202/167) unless otherwise indicated. MAP, mean arterial pressure; PDR, proliferative retinopathy.
To study familial aggregation of proliferative retinopathy or any retinopathy, three complementary analyses were used. First, the presence or absence of proliferative retinopathy or any retinopathy in the proband was estimated as a risk factor for the corresponding condition in the other siblings. The familial risks were estimated with logistic regression models, adjusted for conventional risk factors, and fitted with generalized estimating equations using exchangeable correlation structure to account for correlations within sibships (12). Second, to measure the degree of concordance within sibships, the ICC of durations of diabetes to the diagnosis of proliferative retinopathy was calculated in the 29 sibships in which two siblings had proliferative retinopathy (Fig. 1). Third, the heritability (h2) of proliferative retinopathy was estimated by a liability threshold model as implemented in the SOLAR software (SOLAR, version 4.0.7; Southwest Foundation for Biomedical Research, San Antonio, TX) with A1C, mean arterial pressure, sex, and duration of diabetes as covariates. The liability threshold model is an extension of the variance components model to dichotomous traits, such as proliferative retinopathy (13). In the variance components model, the overall phenotypic variation is partitioned into individual variance components due to polygenic effects (multiple unmeasured genes under an additive variance), covariates (e.g., duration, sex, A1C, and blood pressure), and random environmental effects. The estimated h2 is defined as the ratio of the genetic variance component to the residual phenotypic variance and is an estimate of the familiality of the trait. The significance of the genetic component was determined by a likelihood ratio test. All other statistical calculations except the ICCs and the variance component models were performed with SPSS 15.0 (SPSS, Chicago, IL).
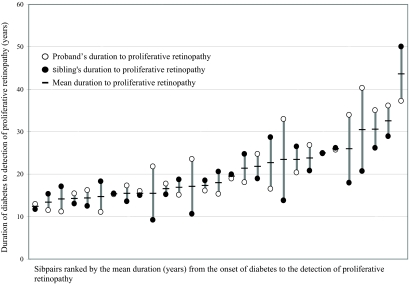
FIG. 1.
Concordance within sibship (ICC) of survival time without proliferative retinopathy in the 29 sibpairs in which both members had proliferative retinopathy (ICC 0.47 [95% CI 0.14–0.71], P = 0.004).
RESULTS
Table 1 depicts the structure of the sibships and the availability of ophthalmic data. Table 2 shows the clinical characteristics of the studied patients and unadjusted ICCs within sibships. Mean age at onset of diabetes was 14.3 ± 10.2 years. The ratio of men to women was 202:167, and mean duration of diabetes was 25.9 ± 11.8 years. The patients’ highest ETDRS scores were achieved at a median of 2.1 (IQR −10.4 to 1.0) years before the FinnDiane baseline visit. A strong positive association was found between the ETDRS score and the nephropathy status (r = 0.63, P < 0.001), which was available for 328 patients.
Proliferative retinopathy was found in 115 of 369 patients (31.1% [95% CI 26.4–35.9]). In 8 of 115 (7.0%) patients, proliferative retinopathy was discovered at their first examination by an ophthalmologist. Thus, there were no available reference points for these patients without proliferative retinopathy. The other patients (n = 107) had all had at least one ophthalmic examination at a median of 1.0 (IQR −2.2 to −0.4) years before the diagnosis. Detailed records of treatment and follow-up were available for each patient with proliferative retinopathy. Mean duration from onset of diabetes to proliferative retinopathy was 20.9 ± 7.5 years.
The familial risk of proliferative retinopathy was estimated in 182 siblings of 168 probands. Siblings of probands with proliferative retinopathy had higher unadjusted risk of proliferative retinopathy (odds ratio [OR] 4.07 [95% CI 2.06–8.07], P < 0.001) when compared with siblings of probands without proliferative retinopathy. When adjusted for duration of diabetes, A1C, and mean arterial pressure, proliferative retinopathy in the probands (48 of 168) remained a significant risk factor (2.76 [1.25–6.11], P = 0.01) for the corresponding condition in the siblings (61 of 182) (Table 3). In contrast, the absence of any retinopathy in the probands (37 of 168) was not associated with absence of any retinopathy in the siblings (39 of 182) of the probands (2.0 [0.82–5.10], P = 0.13). The absence of any retinopathy was associated with lower A1C (0.77 [0.59–0.99], P = 0.04) and shorter duration of diabetes (0.85 [0.78–0.92], P < 0.001) but not with blood pressure (1.1 [0.98–1.06], P = 0.30) or sex (0.96 [0.36–2.53], P = 0.93).
TABLE 3.
Familial risk of proliferative retinopathy
| Variable | OR (95% CI) | B (SE) | P value |
|---|---|---|---|
| PDR status of proband (yes/no) | 2.76 (1.25–6.11) | 1.02 (0.40) | 0.01 |
| MAP (mmHg) | 1.03 (0.99–1.06) | 0.02 (0.17) | 0.15 |
| A1C (%) | 1.33 (1.03–1.73) | 0.29 (0.14) | 0.03 |
| Duration of diabetes (years) | 1.13 (1.08–1.17) | 0.12 (0.02) | <0.001 |
| Men | 1.12 (0.51–2.45) | 0.12 (0.40) | 0.8 |
The presence of proliferative retinopathy in the proband was used as risk factor for proliferative retinopathy in 182 siblings of 168 probands in a logistic regression analysis. MAP, mean arterial blood pressure; PDR, proliferative diabetic retinopathy.
The 29 proband-sibling pairs in which both members had proliferative retinopathy were concordant for the survival time without proliferative retinopathy (ICC 0.47 [0.14–0.71], P = 0.004) (Fig. 1). Despite a slightly shorter duration of diabetes (25.0 ± 11.8 vs. 26.8 ± 11.9 years, P = 0.15), the younger siblings had a higher prevalence of proliferative retinopathy (34.4% [66 of 192] vs. 27.7% [49 of 177], P = 0.17) and often had a shorter duration between developing diabetes and developing proliferative retinopathy (20.2 ± 7.0 vs. 21.7 ± 8.0 years, P = 0.17) (Fig. 1). To make sure these trends did not bias the estimates of familial risk, we further calculated the risk of proliferative retinopathy by either designating the probands randomly (2.88 [1.32–6.27], P = 0.01) or designating the siblings with the longest duration as probands (2.48 [1.02–6.04], P = 0.04). Thus, the selection of the oldest sibling as proband does not seem to produce a significant bias to the estimate of familial risk.
The heritability of proliferative retinopathy was h2 = 0.52 ± 0.31 (P < 0.05) in a sample of 362 of 396 patients. The sex of the patient was left out from the variance component model as being nonsignificant (P > 0.1). The proportion of variance attributable to all covariates (A1C, duration, and blood pressure) was 0.23 (Kullback-Leibler R2).
DISCUSSION
This study shows an increased risk (OR 2.76 [95% CI 1.25–6.11], P = 0.01) of proliferative retinopathy in siblings of probands with proliferative retinopathy in type 1 diabetes. Such a familial clustering was supported by the estimated heritability of proliferative retinopathy h2 = 0.52 ± 0.31 (P < 0.05). Notably, this degree of familiality is similar to the previously reported clustering for diabetic nephropathy in type 1 patients and suggests that genes may play a major role also for the development of severe retinopathy. In previous studies regarding diabetic nephropathy, ∼50% of the risk could not be attributed to the familial clustering of conventional risk factors (14). Similarly, genetic risk factors may explain 50% of the risk of proliferative retinopathy.
Diabetic retinopathy continues to progress even after improvement of glycemic control (5,15). The more severe the retinopathy is, the longer the delay before a beneficial effect of improved glycemic control is observed (5). Thus, retinopathy appears to have an inherent momentum of progression that by time leads to an almost linear increase in the incidence of proliferative retinopathy (2). Further proof of familiality is the conspicuous concordance of the survival times within sibships (Fig. 1). Taken together, these findings may be consistent with an altered expression of one or more critical genes induced by hyperglycemia.
Previous studies have been able to show familial clustering of severe nonproliferative retinopathy in families with type 2 diabetes (16,17) and in families with a mixture of both type 1 and type 2 patients (18). However, no studies thus far have given estimates for the familial risk of proliferative retinopathy. Despite the familiality of nonproliferative retinopathy, attempts to find evidence for an involvement of any major loci in diabetic retinopathy have turned out inconclusive. Three genome-wide scans have offered suggestive evidence of linkage, though on a number of different chromosomes in patients with type 2 diabetes (19–21). The associations to various biologically relevant candidate genes have been extraordinarily difficult to replicate (22). Such results are typical for multifactorial diseases with only a moderate familiality. The genetic component of diabetic retinopathy is likely to be polygenic and does not exclude the importance of interacting environmental risk factors in the determination of the ultimate risk. Some environmental risk factors such as blood pressure and A1C also appear to be determined at least in part by genetic factors. In this study, a single A1C measurement was a significant risk factor in both the logistic regression analysis and the heritability calculation despite the patients’ highest ETDRS scores occurring at a median of 2.1 (IQR −10.4 to 1.0) years before the date of the A1C measurement in the FinnDiane Study. This could be a reflection of the predictive value of biological, between-individual variations in A1C, distinct from the mean blood glucose (23). In fact, it has been noted that a single A1C measurement offers a fair estimate of the glycemic control during the previous 10 years (24). Another significant risk factor in this study was blood pressure. Increased blood pressure is considered to be a multifactorial trait with an estimated genetic contribution in the range of 30–50% (25). The present study may be limited by the fact that the longitudinal changes in these risk factors could not be recorded, leaving possible residual intrafamilial correlations unaccounted for. However, it has been shown in a simulation study that familial clustering of two additive environmental risk factors only leads to a slight excess in the clustering of a disease among the siblings (26). Therefore, it is unlikely that the degree of familiality observed here is the result of familial clustering of glycemic control and blood pressure alone.
Microalbuminuria is a known predictor for the development of proliferative retinopathy in patients with type 1 diabetes (27). There is controversy as to whether this association is due to hyperglycemia or whether nephropathy is truly an independent risk factor for proliferative retinopathy. It has been observed that after renal transplantation or initiation of dialysis, visual function stabilizes (28). The stabilization could also be due to other factors, such as lower blood pressure during renal replacement therapy (29). A strong positive association between the severity of retinopathy and the severity of nephropathy was noted in this study (r = 0.63, P < 0.001), which supports the hypothesis that there are common predisposing factors behind these two microvascular complications.
Retinal photography has been reported to be the most sensitive screening method for diabetic retinopathy. The sensitivity is in excess of 80% in detecting proliferative retinopathy (30). Ophthalmoscopy has less sensitivity but, conversely, higher specificity. It provides good results in the hands of trained professionals such as ophthalmologists and diabetologists (30). A high percentage of patients with diabetes in Finland are undergoing regular fundus photography. The national guidelines for the screening of diabetic retinopathy were already published in 1992 and updated in 2006 (31) emphasizing fundus photography as the preferable screening method (32). The majority of the patients in this study had attended several screening examinations. Many were examined and treated by ophthalmologists, and eventually 68% had fundus photographs available. Even with these repeated examinations, the absence or presence of any retinopathy was not found to cluster within families. This could reflect the fact that almost all patients with long enough duration of type 1 diabetes will eventually develop some degree of retinopathy, thus making the detection of familial clustering more difficult.
The FinnDiane Study is not by definition a population-based study, which may limit the generalizability of the results. However, selection bias is unlikely because the geographic distribution of FinnDiane patients closely follows the distribution of the genetically homogeneous general population and also because we could obtain as much as 93% of the ophthalmic data in the sibships. Furthermore, the treatment of diabetes and its complications is fairly uniform across Finland. The prevalence of proliferative retinopathy in this study (31.1% [95% CI 26.4–35.9]) corresponds to the prevalence (32.1% [29.2–35.0]) in an independent sample of 1,001 patients with a 24.2 ± 11.7 duration of diabetes in the FinnDiane Study and to the prevalence (37.5% [34.6–40.4]) in a previous population-based study of type 1 diabetes patients with similar duration of diabetes in Finland (33).
In conclusion, this study found a familial clustering of proliferative retinopathy in patients with type 1 diabetes that cannot be accounted for by conventional risk factors. This suggests a significant genetic component in the pathogenesis of proliferative retinopathy.
Supplementary Material
Acknowledgments
This study was supported by the Folkhälsan Research Foundation, the Wilhelm and Else Stockmann Foundation, the Finnish Eye Foundation, the Eye and Tissue Bank Foundation, and a special governmental grant for health sciences research (TYH 3263). SAGE Software is supported by a U.S. Public Health Service Resource Grant (RR03655) from the National Center for Research Resources.
We acknowledge all of the physicians and nurses at each center participating in the collection of patients (see online appendix, available at http://dx.doi.org/db07-1495).
Published ahead of print at http://diabetes.diabetesjournals.org on 28 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Seaquist ER, Goetz FC, Rich S, Barbosa J: Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320: 1161–1165, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Klein R: The epidemiology of diabetic retinopathy: findings from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Int Ophthalmol Clin 27: 230–238, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Deckert T, Simonsen SE, Poulsen JE: Prognosis of proliferative retinopathy in juvenile diabetics. Diabetes 16: 728–733, 1967 [DOI] [PubMed] [Google Scholar]
- 4.Rossing K, Jacobsen P, Rossing P, Lauritzen E, Lund-Andersen H, Parving HH: Improved visual function in IDDM patients with unchanged cumulative incidence of sight-threatening diabetic retinopathy. Diabetes Care 21: 2007–2015, 1998 [DOI] [PubMed] [Google Scholar]
- 5.DCCT Group: Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy: The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 342: 381–389, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33): UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 7.DCCT Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus: The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 317: 703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 9.Fagerudd J, Forsblom C, Pettersson-Fernholm K, Groop PH: Implementation of guidelines for the prevention of diabetic nephropathy. Diabetes Care 27: 803–804, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL, III, Knatterud GL: Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci 39: 233–252, 1998 [PubMed] [Google Scholar]
- 11.Keen KJ, Elston RC: Robust asymptotic sampling theory for correlations in pedigrees. Stat Med 22: 3229–3247, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Hanley JA, Negassa A, Edwardes MD, Forrester JE: Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 157: 364–375, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Duggirala R, Williams JT, Williams-Blangero S, Blangero J: A variance component approach to dichotomous trait linkage analysis using a threshold model. Genet Epidemiol 14: 987–992, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Krolewski AS, Fogarty DG, Warram JH: Hypertension and nephropathy in diabetes mellitus: what is inherited and what is acquired? Diabetes Res Clin Pract 39 (Suppl.): S1–S14, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Engerman RL, Kern TS: Progression of incipient diabetic retinopathy during good glycemic control. Diabetes 36: 808–812, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Hallman DM, Huber JC Jr, Gonzalez VH, Klein BE, Klein R, Hanis CL: Familial aggregation of severity of diabetic retinopathy in Mexican Americans from Starr County, Texas. Diabetes Care 28: 1163–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Rema M, Saravanan G, Deepa R, Mohan V: Familial clustering of diabetic retinopathy in South Indian type 2 diabetic patients. Diabet Med 19: 910–916, 2002 [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group: Clustering of long-term complications in families with diabetes in the Diabetes Control and Complications Trial. Diabetes 46: 1829–1839, 1997 [PubMed] [Google Scholar]
- 19.Hallman DM, Boerwinkle E, Gonzalez VH, Klein BE, Klein R, Hanis CL: A genome-wide linkage scan for diabetic retinopathy susceptibility genes in Mexican Americans with type 2 diabetes from Starr County, Texas. Diabetes 56: 1167–1173, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC: Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes: Pima Diabetes Genes Group. Diabetes 47: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Looker HC, Nelson RG, Chew E, Klein R, Klein BE, Knowler WC, Hanson RL: Genome-wide linkage analyses to identify loci for diabetic retinopathy. Diabetes 56: 1160–1166, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Uhlmann K, Kovacs P, Boettcher Y, Hammes HP, Paschke R: Genetics of diabetic retinopathy. Exp Clin Endocrinol Diabetes 114: 275–294, 2006 [DOI] [PubMed] [Google Scholar]
- 23.McCarter RJ, Hempe JM, Gomez R, Chalew SA: Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 27: 1259–1264, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Barnas U, Schmidt A, Illievich A, Kiener HP, Rabensteiner D, Kaider A, Prager R, Abrahamian H, Irsigler K, Mayer G: Evaluation of risk factors for the development of nephropathy in patients with IDDM: insertion/deletion angiotensin converting enzyme gene polymorphism, hypertension and metabolic control. Diabetologia 40: 327–331, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Tanira MO, Al Balushi KA: Genetic variations related to hypertension: a review. J Hum Hypertens 19: 7–19, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Khoury MJ, Beaty TH, Liang KY: Can familial aggregation of disease be explained by familial aggregation of environmental risk factors? Am J Epidemiol 127: 674–683, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Mathiesen ER, Ronn B, Storm B, Foght H, Deckert T: The natural course of microalbuminuria in insulin-dependent diabetes: a 10-year prospective study. Diabet Med 12: 482–487, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Laatikainen L, Summanen P, Ekstrand A, Groop L: Ophthalmological follow-up of diabetic patients after kidney transplantation. Ger J Ophthalmol 2: 24–27, 1993 [PubMed] [Google Scholar]
- 29.Watanabe Y, Yuzawa Y, Mizumoto D, Tamai H, Itoh Y, Kumon S, Yamazaki C: Long-term follow-up study of 268 diabetic patients undergoing haemodialysis, with special attention to visual acuity and heterogeneity. Nephrol Dial Transplant 8: 725–734, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson A, McIntosh A, Peters J, O'Keeffe C, Khunti K, Baker R, Booth A: Effectiveness of screening and monitoring tests for diabetic retinopathy: a systematic review. Diabet Med 17: 495–506, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Summanen P, Kallioniemi V, Komulainen J, Lamminen H, Levanen H, Orhanen E, Tulokas S, Virtamo T, von Wendt G: Diabetic retinopathy: current care guideline. Duodecim 123: 421–422, 2007 [PubMed] [Google Scholar]
- 32.Retinopathy Working Party: A protocol for screening for diabetic retinopathy in Europe. Diabet Med 8: 263–267, 1991 [PubMed] [Google Scholar]
- 33.Virtamo T, Summanen P, Tuomilehto J, Laatikainen L: Diabetic retinopathy and visual impairment in juvenile onset diabetics in Finland (Abstract). 11th Meeting of the European Association for the Study of Diabetic Eye Complications (EASDEC). Paris, France. May 18–20, 2001. Diabete Metab 27 (Suppl.): 2, 2001 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.