Abstract
OBJECTIVE—Proinflammatory cytokines contribute to systemic low-grade inflammation and insulin resistance. Tumor necrosis factor (TNF)-α impedes insulin signaling in insulin target tissues. We determined the role of inhibitor of nuclear factor-κB kinase (IKK)β in TNF-α–induced impairments in insulin signaling and glucose metabolism in skeletal muscle.
RESEARCH DESIGN AND METHODS—Small interfering RNA (siRNA) was used to silence IKKβ gene expression in primary human skeletal muscle myotubes from nondiabetic subjects. siRNA gene silencing reduced IKKβ protein expression 73% (P < 0.05). Myotubes were incubated in the absence or presence of insulin and/or TNF-α, and effects of IKKβ silencing on insulin signaling and glucose metabolism were determined.
RESULTS—Insulin increased glucose uptake 1.7-fold (P < 0.05) and glucose incorporation into glycogen 3.8-fold (P < 0.05) in myotubes from nondiabetic subjects. TNF-α exposure fully impaired insulin-mediated glucose uptake and metabolism. IKKβ siRNA protected against TNF-α–induced impairments in glucose metabolism, since insulin-induced increases in glucose uptake (1.5-fold; P < 0.05) and glycogen synthesis (3.5-fold; P < 0.05) were restored. Conversely, TNF-α–induced increases in insulin receptor substrate-1 serine phosphorylation (Ser312), Jun NH2-terminal kinase phosphorylation, and extracellular signal–related kinase-1/2 mitogen-activated protein kinase (MAPK) phosphorylation were unaltered by siRNA-mediated IKKβ reduction. siRNA-mediated IKKβ reduction prevented TNF-α–induced insulin resistance on Akt Ser473 and Thr308 phosphorylation and phosphorylation of the 160-kDa Akt substrate AS160. IKKβ silencing had no effect on cell differentiation. Finally, mRNA expression of GLUT1 or GLUT4 and protein expression of MAPK kinase kinase kinase isoform 4 (MAP4K4) was unaltered by IKKβ siRNA.
CONCLUSIONS—IKKβ silencing prevents TNF-α–induced impairments in insulin action on Akt phosphorylation and glucose uptake and metabolism in human skeletal muscle.
Subclinical inflammation plays a role in the etiology of peripheral insulin resistance in type 2 diabetes (1–6). Correlative studies in type 2 diabetic patients link excessive levels of the proinflammatory cytokine tumor necrosis factor (TNF)-α in adipose tissue (7), blood plasma (8–10), and skeletal muscle (11) with whole-body insulin resistance (12,13). In vivo studies provide evidence that an acute infusion of TNF-α into healthy humans rapidly induces skeletal muscle insulin resistance by disrupting insulin signaling to GLUT4 translocation without altering the hepatic glucose production (1). Thus, the identification and characterization of TNF-α–sensitive targets may provide insight into mechanism(s) for the development of peripheral insulin resistance in response to inflammatory stress.
TNF-α is principally secreted by activated macrophages in response to infection and stress. Two unique membrane receptors, TNF-α receptor (TNFR)1 and TNFR2, mediate the biological effects of TNF-α (14). The TNFR1 receptor subtype transduces TNF-α signaling to the transcription factor nuclear factor-κB (NF-κB) (14). TNF-α–induced activation of NF-κB is mediated by inhibitor of nuclear factor-κB kinase (IKK)β, a serine kinase involved in the phosphorylation and subsequent degradation of inhibitor of nuclear factor-κB (IκB), which leads to the activation of the transcription factor NF-κB (15–18). IKKβ has been implicated in the development of insulin resistance (19). High doses of salicylates target and inhibit IKKβ to sensitize insulin signaling and reverse the associated pathogenic characteristics including hyperglycemia, hyperinsulinemia, and dyslipidemia in diabetic mice (3,20). The proposed mechanism by which salicylates sensitize insulin signaling to glucose uptake involves inhibition of IKKβ (3,20) and enhanced insulin signaling to metabolic end points (21). Collectively, these data implicate a role for IKKβ-mediated events in the pathogenesis of insulin resistance in obesity and type 2 diabetes.
TNF-α directly induces insulin resistance in primary human skeletal muscle cells (22). TNF-α–mediated insulin resistance can be rescued via small interfering RNA (siRNA)-mediated silencing of mitogen-activated protein kinase kinase kinase kinase isoform 4 (MAP4K4) (22), an upstream regulator of Jun NH2-terminal kinase (JNK) and extracellular signal–related kinase (ERK)1/2 (p42/44 mitogen-activated protein kinase [MAPK]) kinase (22–24). In addition to mediating insulin resistance through the activation of JNK, TNF-α exerts negative effects on insulin signaling through the serine/threonine kinase IKKβ in cultured immortalized cells (25,26) and in animal models of type 2 diabetes (3). Here, we test the hypothesis that IKKβ is an intermediate kinase for TNF-α action on insulin-mediated glucose uptake in primary human skeletal muscle cells. We provide evidence that TNF-α–induced insulin resistance on signal transduction at the level of Akt is prevented by siRNA-mediated silencing of IKKβ. We also show that the TNF-α–induced impairments on insulin-mediated glucose uptake and glycogen synthesis in primary human myotubes are prevented by IKKβ inhibition. Thus, strategies to inhibit IKKβ expression in human skeletal muscle may prevent insulin resistance associated with inflammatory stress.
RESEARCH DESIGN AND METHODS
Skeletal muscle biopsies were obtained with informed consent from nondiabetic individuals (seven male and two female) scheduled for abdominal surgery. The mean age and BMI of the subjects were 57 ± 2 years and 25.8 ± 0.9 kg/m2, respectively. None of the subjects had any known metabolic disorders, and all presented with normal fasting plasma glucose values (5.5 ± 0.3 mmol/l). The protocols in this study were approved by the ethics committee at Karolinska Institutet.
DMEM (Dulbecco's modified Eagle's medium), DMEM F-12, fetal bovine serum, penicillin, streptomycin, and Fungizone were obtained from Invitrogen (Stockholm, Sweden). Recombinant human TNF-α and general laboratory reagents were obtained from Sigma (St. Louis, MO), PD98059 was obtained from Calbiochem (La Jolla, CA), and radioactive reagents were purchased from Amersham (Uppsala, Sweden).
Cell culture and siRNA transfection.
Satellite cells were isolated from muscle biopsies and cultured as previously described (27). IKKβ-specific siRNA oligos were purchased from Ambion (Austin, TX). Myotubes transfected with oligos against a scrambled sequence (scrambled siRNA) were used as a control. Myotubes were transfected using Lipofectamine 2000 (Invitrogen, Sweden) as previously described (28).
Western blot analysis.
Cells were harvested and protein concentration was determined using the Pierce method. After protein determination, aliquots of lysates were mixed with 4× Laemmli-sample buffer, and proteins were separated by SDS-PAGE. Proteins were visualized by enhanced chemiluminescence and quantified by densitometry (29). TNF-α–induced degradation of IκBα was analyzed in lysates by immunoblot analysis with anti-IκBα (Cell Signaling, Beverly, MA). Signaling parameters were investigated using phosphospecific antibodies against Akt (Ser473), Akt (Thr308), ERK1/2, p42/44 MAPK kinase Thr202/Tyr204, stress-activated protein kinase/JNK (Thr183/185), glycogen synthase kinase (GSK)-3β (Ser9), and phospho-Akt Substrate (RXRXXS/T) (Cell Signaling). Antibodies against phospho–insulin receptor substrate (IRS)-1 Ser312 and total IRS-1 were purchased from Upstate (Charlottesville, VA). Antibodies against total IKKβ, Akt, and ERK were from Cell Signaling. Mitogen-activated protein kinase kinase (MEK) isoform 4 (MAP2K4) and MAP4K4 antibodies were obtained from Abgent (San Diego, CA), glyceraldehyde-3-phosphate dehydrogenase from Santa Cruz Biotechnology (Santa Cruz, CA), and Desmin from Abcam (Cambridge, U.K.).
Glucose uptake and metabolism.
Differentiated primary human muscle myotubes were exposed to saline or TNF-α (20 ng/ml) for 2 h. Thereafter, myotubes were incubated in the absence or presence of insulin (120 nmol/l), and 2-deoxyglucose uptake or glucose incorporation into glycogen was determined, as previously described (29). Total protein concentration was assessed using the Bradford method (Bio-Rad, Richmond, CA). Data are reported as nmol · mg−1 · min−1.
Statistics.
Data are presented as means ± SEM. Statistical differences were determined by Student's t test or ANOVA using Fisher's least significant differences test for post hoc determination.
RESULTS
IKKβ silencing in myotubes.
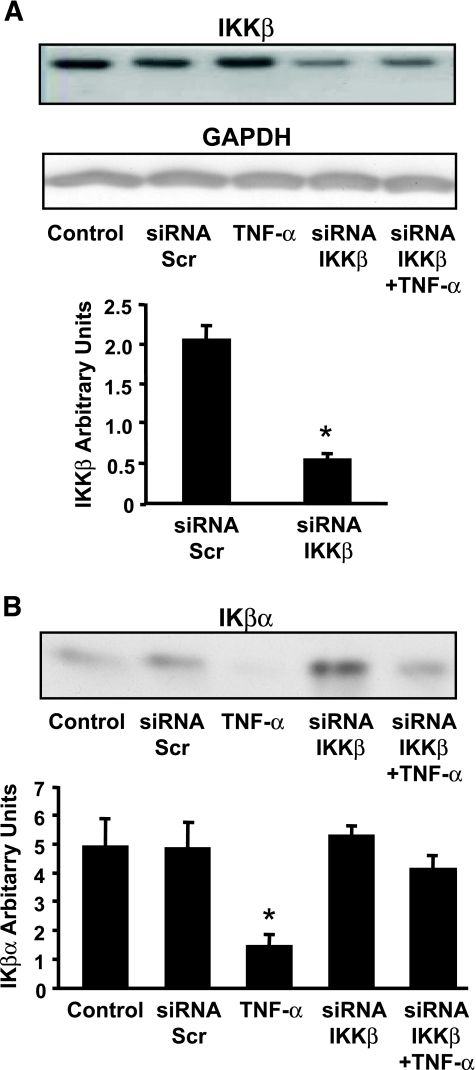
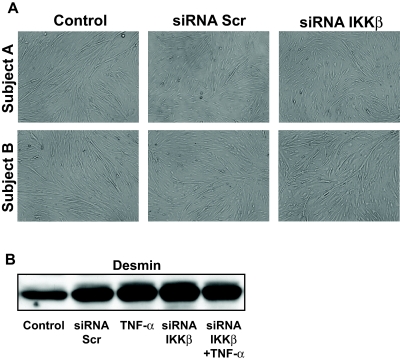
The effect of IKKβ gene silencing on IKKβ protein expression was determined in primary cultured human myotubes. Two days after induction of the myotube differentiation program, cells were transfected with siRNA against IKKβ. Expression of IKKβ was unaltered in cells exposed to a scrambled siRNA sequence or following 2 h of incubation with TNF-α (Fig. 1A). siRNA against IKKβ reduced protein expression 73%, (Fig. 1A) (P < 0.05) and mRNA expression by 55% (P < 0.01) (data not shown) compared with cells transfected with scrambled siRNA. Following TNF-α exposure (2 h, 20 ng/ml), protein content of Iκβα was reduced (Fig. 1B), indicating that Iκβα was targeted for degradation after cytokine exposure. siRNA against IKKβ increased the basal expression of Iκβα and blunted the TNF-α–mediated reduction. Myotube growth and morphology was unaltered after IKKβ silencing (Fig. 2A). Expression of the myogenic protein desmin following 6 days of differentiation (4 days after transfection) was unaltered between myotubes treated with siRNA against a scrambled sequence or IKKβ (Fig. 2B).
FIG. 1.
Protein expression of IKKβ and IκBα. A: Protein expression of IKKβ was unaltered in cells exposed to a scrambled siRNA sequence, a 2-h incubation with TNF-α (lanes 1–3). siRNA against IKKβ reduced protein expression by 73% (lanes 4 and 5). Glyceraldehyde-3-phosphate dehydrogenase (GADPH) expression was determined to control for equal loading. B: IκBα was measured after siRNA-mediated depletion of IKKβ. Results are means ± SE. *P < 0.05 vs. transfected scrambled siRNA (Scr) control (n = 4).
FIG. 2.
Myotube growth. A: Morphological appearance of human skeletal muscle myotube formation after introduction of scrambled siRNA or siRNA against IKKβ. Photomicrographs are shown at 10× magnification for myotubes at day 6 of differentiation (4 days after introduction of siRNA). Results are from two separate experiments. B: Representative immunoblot showing the differentiation marker desmin.
Effect of IKKβ silencing on glucose uptake and metabolism.
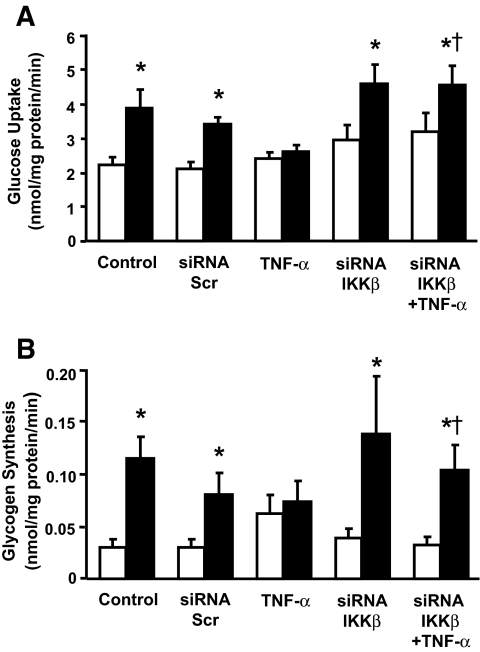
We have reported that an acute TNF-α exposure impairs insulin-stimulated glucose uptake and glycogen synthesis in cultured human skeletal muscle (22). Here, we determined whether TNF-α–induced insulin resistance is prevented by IKKβ silencing. Four days after transfection, glucose uptake (Fig. 3A) and incorporation into glycogen (Fig. 3B) was assessed under basal and insulin-stimulated conditions in the presence or absence of TNF-α. In control cells or cells transfected with scrambled siRNA, insulin (120 nmol/l) increased glucose uptake 1.7-fold (P < 0.05) and glycogen synthesis 3.8-fold (P < 0.05). Basal glucose transport and incorporation to glycogen was unaltered in cells exposed to TNF-α. However, TNF-α pretreatment reduced insulin action on glucose uptake (P < 0.05) and incorporation to glycogen (P < 0.05). Silencing of IKKβ had no effect on either basal or insulin-stimulated glucose metabolism. Importantly, siRNA-mediated silencing of IKKβ prevented the inhibitory effect of TNF-α on insulin-mediated glucose metabolism.
FIG. 3.
Effect of siRNA-mediated silencing of IKKβ on glucose uptake and glycogen synthesis. Glucose uptake (A) and glycogen synthesis (B) were measured in differentiated primary skeletal muscle myotubes under basal conditions (□) and after insulin stimulation (▪). Results are means ± SE. *P < 0.05 vs. respective basal cells. †P < 0.05 vs. TNF-α–and insulin-stimulated untransfected cells (n = 4).
Effect of IKKβ silencing on Akt phosphorylation.
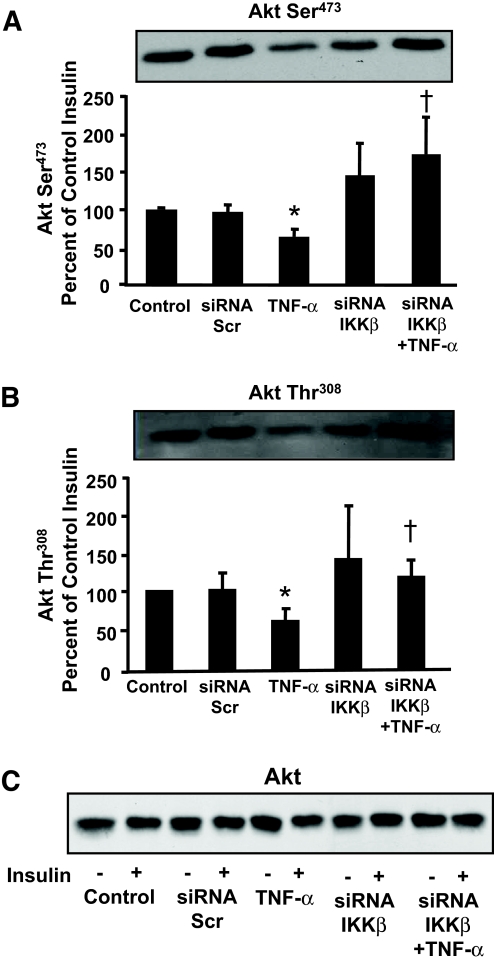
Insulin increased Akt Ser473 and Thr308 phosphorylation in primary cultures of human skeletal muscle (Fig. 4A and B, respectively). Insulin action on Akt was unaltered in cells transfected with scrambled siRNA. TNF-α impaired insulin action on Akt Ser473 and Thr308 phosphorylation (P < 0.05). IKKβ silencing had no effect on basal (data not shown) or insulin-stimulated Akt Ser473 or Thr308 phosphorylation but prevented the TNF-α–induced impairment. Protein expression of Akt was unaltered in response to insulin, TNF-α, or gene silencing (Fig. 4C).
FIG. 4.
Akt Ser473 and Thr308 phosphorylation. Representative immunoblot showing Akt Ser473 (A) and Akt Thr308 (B) phosphorylation in response to insulin and TNF-α. C: Total Akt protein expression. Graphs are summarized data (mean ± SE). *P < 0.05% vs. untransfected control cells. †P < 0.05 vs. TNF-α–stimulated untransfected cells (n = 9).
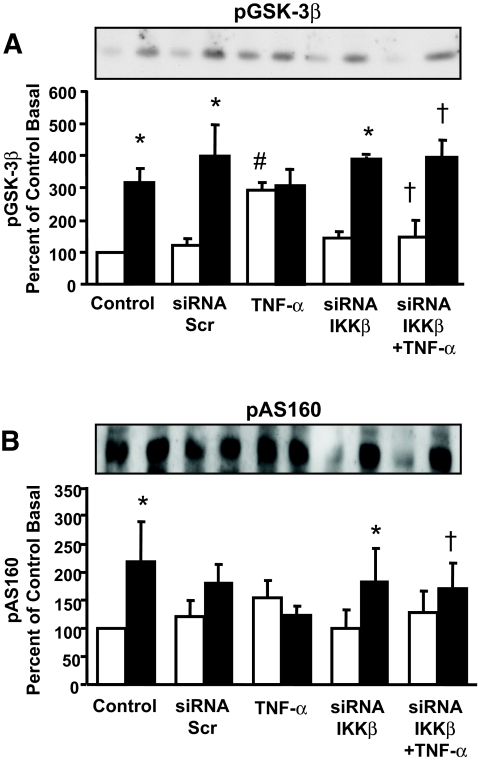
Effect of IKKβ silencing on GSK3β and AS160 phosphorylation.
To further evaluate the effect of IKKβ on insulin signaling, phosphorylation of the Akt substrates GSK3β and AS160 was determined. Insulin increased phosphorylation of GSK3β Ser9 (Fig. 5A) and AS160 (Fig. 5B) in primary cultures of human skeletal muscle. These responses were unaltered in cells transfected with scrambled siRNA. TNF-α treatment increased GSK3β Ser9 phosphorylation to the same extent as insulin but had no effect on insulin-mediated phosphorylation. Silencing of IKKβ had no effect on basal or insulin-stimulated GSK3β Ser9 and prevented the TNF-α–induced increase in GSK3β phosphorylation. TNF-α treatment prevented the insulin-mediated increase in AS160 phosphorylation, and silencing of IKKβ restored this insulin effect.
FIG. 5.
GSK-3β and AS160 phosphorylation. Representative immunoblot showing pGSK3β Ser9 (A) and Akt substrate AS160 (B) phosphorylation in response to insulin or TNF-α under basal conditions (□) and after insulin stimulation (▪). Graphs are summarized data (mean ± SE). *P < 0.05 vs. respective basal. †P < 0.05 vs. TNF-α–stimulated untransfected cells. #P < 0.05 vs. control basal (GSK3β, n = 3; AS160, n = 4).
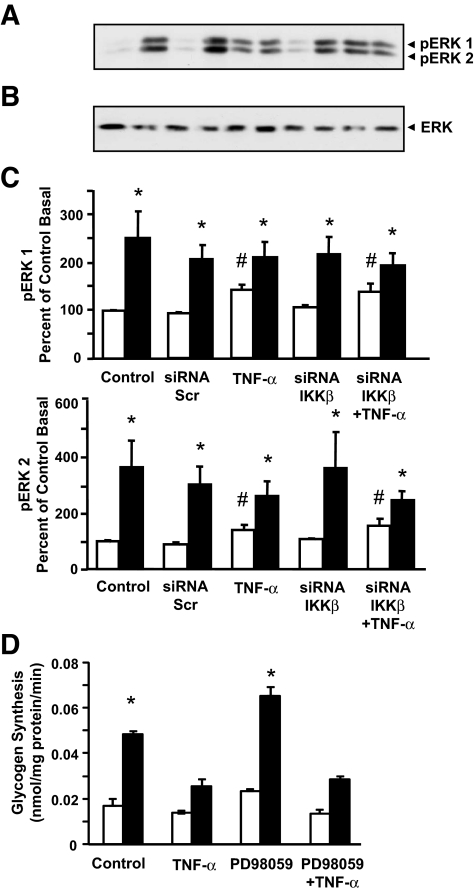
Effect of IKKβ silencing on ERK 1/2 MAPK phosphorylation.
Insulin increased ERK 1/2 MAPK phosphorylation (P < 0.05) in primary cultures of human skeletal muscle (Fig. 6A–C). Insulin action on ERK 1/2 MAPK was unaltered in cells transfected with scrambled siRNA. TNF-α exposure increased basal ERK 1/2 MAPK phosphorylation (P < 0.05), although to a lesser extent than observed in response to insulin. TNF-α exposure did not modify insulin action on ERK 1/2 MAPK phosphorylation. IKKβ silencing had no effect on ERK 1/2 MAPK phosphorylation either in the absence or presence of insulin and/or TNF-α. Total ERK expression was unaltered in response to TNF-α exposure or siRNA gene silencing. To further explore the role of ERK in the mediation of TNF-α–induced impairments in insulin-mediated glucose metabolism, we determined the effect of the MEK inhibitor PD98059 on glycogen synthesis in response to insulin and TNF-α (Fig. 6D). Exposure of primary cultures to PD98059 reduced insulin and TNF-α–mediated ERK1/2 phosphorylation (data not shown). However, this inhibitor had no effect on glycogen synthesis in response to insulin or TNF-α exposure.
FIG. 6.
Effect of siRNA-mediated silencing of IKKβ on ERK1/2 MAPK phosphorylation. A: Representative immunoblot of ERK1/2 MAPK phosphorylation. B: Total ERK-2 protein expression. C: Summarized results for ERK1/2 MAPK under basal (□) or insulin-stimulated (▪) conditions. (n = 5). D: Glycogen synthesis was measured in differentiated primary skeletal muscle myotubes following 1 h of exposure to 50 μmol/l PD98059. Results are means ± SE from experiment carried out in triplicate. *P < 0.05 vs. respective basal. #P < 0.05 vs. control basal.
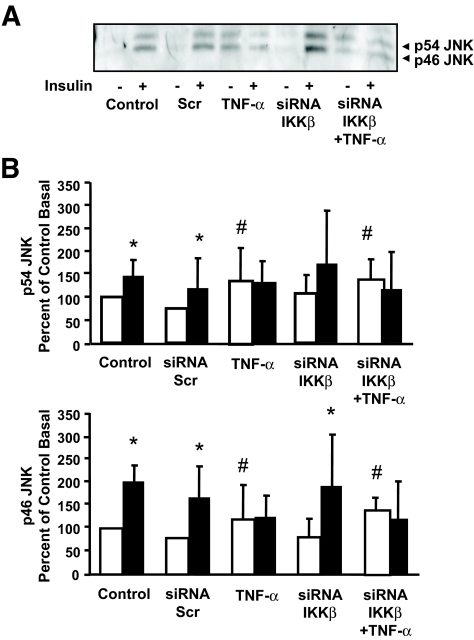
Effect of IKKβ silencing on JNK phosphorylation.
Insulin increased p54 and p46 JNK phosphorylation (P < 0.05) in primary cultures of human skeletal muscle (Fig. 7). Insulin action on p54 and p46 JNK was unaltered in cells transfected with scrambled siRNA. Similar to results noted for ERK1/2 MAPK, TNF-α exposure increased basal p54 and p46 JNK phosphorylation, and IKKβ silencing had no effect on JNK phosphorylation either in the absence or presence of insulin and/or TNF-α.
FIG. 7.
Effect of siRNA-mediated silencing of IKKβ on JNK phosphorylation. A: Representative immunoblot showing p54/p46 JNK phosphorylation. B: Summarized results for p54 and p46 JNK phosphorylation under basal (□) or insulin-stimulated (▪) conditions. Results are mean ± SE. *P < 0.05 vs. respective basal. #P < 0.05 vs. control basal (n = 3).
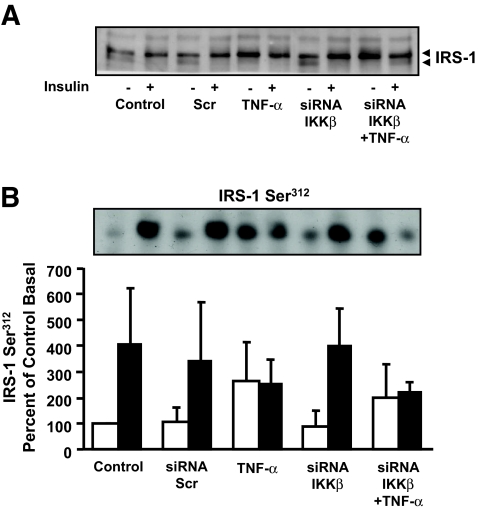
Effect of IKKβ silencing on IRS-1 gel mobility or Ser312 phosphorylation.
TNF-α exposure is associated with increased phosphorylation of IRS-1 on Ser312. Thus, we determined whether IKKβ plays a role in TNF-α–mediated IRS-1 Ser312 phosphorylation in primary human myotubes. Insulin and TNF-α increased IRS-1 Ser312 phosphorylation (Fig. 8A), and this response was unaltered in cells transfected with siRNA against either a scrambled sequence or IKKβ. To evaluate the total phosphorylation status of IRS-1, a gel mobility shift assay was performed. Insulin and TNF-α induced an upward mobility shift in IRS-1 migration in SDS-PAGE (Fig. 8B), indicating increased phosphorylation. The effect of insulin on IRS-1 mobility was unaltered in cells transfected with scrambled siRNA. IKKβ silencing had no effect on the mobility shift of IRS-1 in response to either insulin or TNF-α exposure. Thus, IKKβ does not appear to mediate TNF-α–induced phosphorylation events on IRS-1.
FIG. 8.
Effect of siRNA-mediated silencing of IKKβ on IRS-1 Ser312 phosphorylation. Representative immunoblot showing IRS-1 migration on SDS-PAGE (A) or IRS-1 Ser312 phosphorylation (B). IRS-1 Ser312 phosphorylation results under basal (□) and insulin-stimulated (▪) conditions are reported as means ± SE percent relative to basal untransfected controls. Arrow highlights mobility shift (A) (n = 4).
Effect of IKKβ silencing on mRNA and protein expression.
Gene silencing of IKKβ did not alter either GLUT1 or GLUT4 mRNA (105 ± 31 or 87 ± 12%, respectively, as compared with cells transfected with a scrambled sequence). Because we have recently reported that MAP4K4 siRNA prevents TNF-α–induced insulin resistance (22), protein expression of MAP4K4 and the upstream kinase MAP2K4 was also assessed in cells transfected with siRNA against a scrambled sequence or IKKβ. Protein content of either MAP4K4 or MAP2K4 was unaltered in myotubes transfected with scrambled or IKKβ siRNA (data not shown).
DISCUSSION
IKKβ is a core component of the oligomeric IKK complex, which consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit IKKγ, or NEMO (16,18,30,31). The two catalytic subunits share ∼50% amino acid sequence homology, whereas the regulatory subunit does not contain a recognizable catalytic domain (21). Despite the overall sequence identity between IKKα and IKKβ, these subunits have specialized roles as mediators of cellular stress. For example, deletion of IKKα (IKKα−/−) causes limb deformities (31), whereas deletion of IKKβ (IKKβ−/−) is lethal in mouse embryos (32). Of the two catalytic subunits, IKKβ is considered to be the main regulator of NF-κB function because of its higher activity (∼30-fold) toward IκBα (17,33). Recent evidence underscores a central role of IKKβ in insulin sensitivity and type 2 diabetes pathogenesis (2,3,20,21,34,35). High doses of salicylates, which repress IKKβ activity, reverse hyperglycemia, hyperinsulinemia, and dyslipidemia in obese rodents by increasing insulin sensitivity (2,3,20,21). Consequently, we determined the role of IKKβ in insulin action on glucose uptake using siRNA-mediated gene silencing. We provide evidence that IKKβ plays a role in TNF-α–induced impairments in insulin action on Akt signaling and glucose uptake and metabolism in human skeletal muscle.
TNF-α has a biphasic response to NF-κB, which is required for cytokine-induced skeletal muscle damage and wasting/cachexia (36,37). Cytokines activate the IKK complex, leading to phosphorylation of IκBα on Ser32 and Ser36. Phosphorylation of these residues causes polyubiquitination and subsequent degradation of IκBα by proteosomes (16). The breakdown of IκBα leads to the release and nuclear translocation of NF-κB, where it binds to target genes and drives the expression of cytokines, including TNF-α, interleukin-6, and interleukin-1β (38). Here, we report that a 2-h TNF-α exposure of cultured myotubes decreased Iκβα protein content, indicating that Iκβα is targeted for degradation after cytokine exposure. This finding is consistent with the first transient phase of TNF-α–mediated activation of NF-κB (37). The siRNA-mediated reduction of IKKβ prevented the TNF-α effect on Iκβα degradation. Skeletal muscle wasting/cachexia, due to accelerated protein degradation through ubiquitin-dependent proteolysis, occurs in a manner analogous to IKKβ-mediated degradation of IκBα. Genetic inhibition of the IKKβ/NF-κB/MuRF1 pathway or through pharmacological therapeutics, such as salicylates to inhibit IKKβ activity, rescues the skeletal muscle-wasting phenotype (37,39,40). In the current study, skeletal muscle morphology was not altered by either TNF-α exposure or siRNA-mediated gene silencing of IKKβ. However, our experiments were limited to a short-term (first phase) TNF-α exposure, and we did not directly test whether IKKβ siRNA would modify culture growth in the presence of a long-term (second phase) TNF-α exposure.
TNF-α directly induces skeletal muscle insulin resistance in vivo in healthy humans (1) and rodents (41,42) and in vitro in cultured myotubes (22). Although the role of TNF-α in the development of skeletal muscle insulin resistance in type 2 diabetic patients remains unresolved, current evidence suggests that IKKβ may be an intermediate kinase through which TNF-α and other inflammatory processes induce skeletal muscle insulin resistance (5,6,35). IKKβ activation has been closely linked to the development and pathogenesis of insulin resistance (20,21). Pharmacological inhibition of IKKβ activity improves insulin-mediated glucose metabolism (3,20,21), even in insulin-resistant obese, nondiabetic individuals (43). Increased expression of IKKβ has been noted in omental fat from obese humans, potentially contributing to differential roles of omental and subcutaneous fat in the pathophysiology of obesity (44).
Studies linking IKKβ and skeletal muscle insulin resistance in rodents have yielded conflicting results. Pharmacological IKKβ inhibition ameliorated insulin resistance and upregulated plasma levels of adiponectin in KKAy mice fed a high-fat diet (45). Heterozygous IKKβ+/− mice fed a high-fat diet or intergressed on an obese ob/ob mice background were protected against the development of insulin resistance (3). Conversely, mice with either skeletal muscle–specific IKKβ knockout or a separate cohort of heterozygous IKKβ+/− were not protected against gold thioglucose–induced obesity or dietary-induced metabolic abnormalities (46). The reason for the differences noted between these animal models is unknown, but the differences could be strain specific or related to undefined experimental differences.
TNF-α exposure leads to activation of two separate transcription factor–signaling pathways, namely the IKKβ and JNK pathways, which are linked to proinflammatory responses associated with obesity and insulin resistance (47). Here, we show that siRNA-mediated gene silencing of IKKβ prevented TNF-α–induced insulin resistance on glucose uptake and metabolism in cultured myotubes, with a concomitant increase in phosphorylation of Akt (at Ser473, Thr308), and AS160. Inhibition of ERK signaling using pharmacological inhibitor PD98059 did not alter TNF-α–mediated reduction in insulin-stimulated glycogen synthesis. Furthermore, the TNF-α–mediated activation of ERK and JNK was unaffected by the siRNA-mediated reduction of IKKβ. Additionally, the siRNA-mediated reduction of IKKβ did not prevent TNF-α–induced IRS-1 serine phosphorylation on Ser312 or the IRS-1 mobility shift as determined by SDS-PAGE. This is in contrast to some reports indicating that inhibition of IKKβ prevents IRS-1 Ser307 (equivalent to human IRS-1 Ser312) phosphorylation in cultured HEPG2, 3T3-L1 adipocytes, or embryonic kidney cells (25,26,48) but is consistent with other observations that IKKβ is disassociated from IRS-1 phosphorylation (Steven E. Shoelson, Joslin Diabetes Center, Boston, MA, personal communication). Our results indicate that the TNF-α effect on IRS-1 serine phosphorylation is primarily mediated via parallel IKKβ independent pathways, such as JNK.
IKKβ silencing fully restored the TNF-α–mediated reduction of insulin-stimulated glucose metabolism, despite modest impairments in insulin signaling at the level of Akt. However, TNF-α exposure was associated with a profound impairment in insulin-stimulated AS160 phosphorylation, which was restored following IKKβ silencing. Since AS160 is a critical step in the processes involved from insulin signaling to glucose transport, this may explain the enhanced glucose metabolism in IKKβ-depleted myotubes. Our results may also indicate that a relatively small pool of total Akt is critical for signaling to AS160, and this pool may be highly sensitive to TNF-α, possibly due to cellular localization.
In primary cultures of human muscle, TNF-α exposure enhanced phosphorylation of GSK3β. This finding is consistent with a finding of a previous study in HEK293 cells and mouse embryonic fibroblasts (49), where TNF-α–mediated activation of ERK was partly dependent on GSK3β phosphorylation. Conversely, TNF-α exposure has also been shown to prevent insulin-stimulated phosphorylation of GSK3β in HEPG2 cells (50). Here, we report that IKKβ silencing prevents the TNF-α–mediated increase in GSK3β phosphorylation; however, the functional consequence requires further investigation. Although GSK3β Ser9 phosphorylation has been linked to a reduction in the constitutive ability of GSK3 to phosphorylate Ser641 of glycogen synthase, it does not completely abolish the ability of GSK3 to phosphorylate glycogen synthase (51). Thus, further studies are warranted to establish whether GSK3 activity is modulated.
MAP4K4 is a member of the NCK interacting kinase family (23). NCK interacting kinase family kinases are potent activators of the IKK complex and activators of ERK and JNK (34), which suggests that MAP4K4 is likely to be an upstream regulator of IKKβ. Here, we show that siRNA-mediated silencing of IKKβ had no effect on expression of MAP4K4, excluding a negative feedback on this upstream kinase. We have previously reported that the siRNA-mediated reduction of MAP4K4 rescues TNF-α–mediated insulin resistance in primary human skeletal muscle cultures (22), consistent with our present findings for IKKβ. Although a reduction of either MAP4K4 or IKKβ prevents the effect of TNF-α on insulin-mediated glucose uptake and metabolism, the reduction of MAP4K4 also prevented IRS-1 serine phosphorylation and signaling to ERK 1/2 and JNK. In contrast, siRNA silencing of IKKβ did not alter TNF-α signaling to either IRS-1 Ser312, JNK, or ERK phosphorylation. These results are consistent with the hypothesis that downstream signals from MAP4K4 diverge toward an ERK/JNK pathway that mediates effects on IRS-1 serine phosphorylation and an IKKβ pathway that mediates effects on glucose uptake and metabolism.
In summary, targeted deletion of IKKβ using siRNA prevents TNF-α–mediated insulin resistance on Akt and AS160 phosphorylation and glucose uptake and metabolism in human skeletal muscle. These results underscore IKKβ as a potential therapeutic target to prevent peripheral insulin resistance.
Acknowledgments
This study was supported by grants from the Swedish Research Council, the Swedish Medical Association, the Novo-Nordisk Foundation, the Swedish Diabetes Association, the Swedish Animal Welfare Agency, the Hedlund Foundation, the Strategic Research Foundation, and the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS, contract no. LSHM-CT-2004-512013 EUGENEHEART, and contract no. LSHM-CT-2004-512013 EUGENE2).
Published ahead of print at http://diabetes.diabetesjournals.org on 28 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK: Tumor necrosis factor-α induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54: 2939–2945, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Yin MJ, Yamamoto Y, Gaynor RB: The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396: 77–80, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE: Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Ruotsalainen E, Salmenniemi U, Vauhkonen I, Pihlajamaki J, Punnonen K, Kainulainen S, Laakso M: Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care 29: 2714–2720, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Shoelson SE, Lee J, Goldfine AB: Inflammation and insulin resistance. J Clin Invest 116: 1793–1801, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AFH: Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52: 812–817, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feingold KR, Grunfeld C: Role of cytokines in inducing hyperlipidemia. Diabetes 41 (Suppl. 2): S97–S101, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Mishima Y, Kuyama A, Tada A, Takahashi K, Ishioka T, Kibata M: Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with Type 2 diabetes mellitus. Diabetes Res Clin Pract 52: 119–123, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Winkler G, Lakatos P, Nagy Z, Speer G, Salamon F, Szekeres O, Kovacs M, Cseh K: Elevated serum tumor necrosis factor-alpha and endothelin 1 levels correlate with increased C-peptide concentration in android type obesity. Diabetes Care 21: 1778–1779, 1998 [PubMed] [Google Scholar]
- 11.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA: The expression of TNF alpha by human muscle: relationship to insulin resistance. J Clin Invest 97: 1111–1116, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G: Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes 52: 1799–1805, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G: Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 353: 1649–1652, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Connell MC, Macewan DJ: TNFR1-induced NF-kappaB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell Signal 19: 1238–1248, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Karin M: The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem 274: 27339–27342, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Karin M, Delhase M: The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol 12: 85–98, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Huynh QK, Boddupalli H, Rouw SA, Koboldt CM, Hall T, Sommers C, Hauser SD, Pierce JL, Combs RG, Reitz BA, Diaz-Collier JA, Weinberg RA, Hood BL, Kilpatrick BF, Tripp CS: Characterization of the recombinant IKK1/IKK2 heterodimer: mechanisms regulating kinase activity. J Biol Chem 275: 25883–25891, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Verma UN, Yamamoto Y, Prajapati S, Gaynor RB: Nuclear role of I kappa B Kinase-gamma/NF-kappa B essential modulator (IKK gamma/NEMO) in NF-kappa B-dependent gene expression. J Biol Chem 279: 3509–3515, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Shoelson SE, Lee J, Yuan M: Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord 27: S49–S52, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI: Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 109: 1321–1326, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI: Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouzakri K, Zierath JR: MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem 282: 7783–7789, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Tang X, Guilherme A, Chakladar A, Powelka AM, Konda S, Virbasius JV, Nicoloro SM, Straubhaar J, Czech MP: An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARgamma, adipogenesis, and insulin-responsive hexose transport. Proc Natl Acad Sci U S A 103: 2087–2092, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machida N, Umikawa M, Takei K, Sakima N, Myagmar BE, Taira K, Uezato H, Ogawa Y, Kariya K: Mitogen-activated protein kinase kinase kinase kinase 4 as a putative effector of Rap2 to activate the c-Jun N-terminal kinase. J Biol Chem 279: 15711–15714, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J: Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem 278: 24944–24950, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Jiang G, Dallas-Yang Q, Liu F, Moller DE, Zhang BB: Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor alpha (TNFalpha)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J Biol Chem 278: 180–186, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Al-Khalili L, Chibalin AV, Kannisto K, Zhang BB, Permert J, Holman GD, E E, Ding VDH, Zierath JR, Krook A: Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell Mol Life Sci 60: 991–998, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR: siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 4: 89–96, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Al-Khalili L, Bouzakri K, Glund S, Lönnqvist F, Koistinen HA, Krook A: Signalling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 20: 3364–3375, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Brasier AR: The NF-kappaB regulatory network. Cardiovasc Toxicol 6: 111–130, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M: Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science 284: 316–320, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM: Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 284: 321–325, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV: IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science 278: 866–869, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Hu WH, Pendergast JS, Mo XM, Brambilla R, Bracchi-Ricard V, Li F, Walters WM, Blits B, He L, Schaal SM, Bethea JR: NIBP, a novel NIK and IKK(beta)-binding protein that enhances NF-(kappa)B activation. J Biol Chem 280: 29233–29241, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N: Reduced skeletal muscle inhibitor of κBβ content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes 55: 760–767, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE: Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladner KJ, Caligiuri MA, Guttridge DC: Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem 278: 2294–2303, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Tak PP, Firestein GS: NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107: 7–11, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer HF, Goodyear LJ: Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol 103: 388–395, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE: IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Cheung AT, Ree D, Kolls JK, Fuselier J, Coy DH, Bryer-Ash M: An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology 139: 4928–4935, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE: Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4: 465–474, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Fleischman A, Shoelson SE, Bernier R, Goldfine AB: Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 31: 289–294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bashan N, Dorfman K, Tarnovscki T, Harman-Boehm I, Liberty IF, Bluher M, Ovadia S, Maymon-Zilberstein T, Potashnik R, Stumvoll M, Avinoach E, Rudich A: Mitogen-activated protein kinases, inhibitory-kappaB Kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology 148: 2955–2962, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Kamon J, Yamauchi T, Muto S, Takekawa S, Ito Y, Hada Y, Ogawa W, Itai A, Kasuga M, Tobe K, Kadowaki T: A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Comm 323: 242–248, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Rohl M, Pasparakis M, Baudler S, Baumgartl J, Gautam D, Huth M, De Lorenzi R, Krone W, Rajewsky K, Bruning JC: Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest 113: 474–481, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoelson SE, Herrero L, Naaz A: Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169–2180, 2007 [DOI] [PubMed] [Google Scholar]
- 48.de Alvaro C, Teruel T, Hernandez R, Lorenzo M: Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J Biol Chem 279: 17070–17078, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Kim JW, Lee JE, Kim MJ, Cho EG, Cho SG, Choi EJ: Glycogen synthase kinase 3beta is a natural activator of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1). J Biol Chem 278: 13995–14001, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Gupta D, Varma S, Khandelwal RL: Long-term effects of tumor necrosis factor-alpha treatment on insulin signaling pathway in HepG2 cells and HepG2 cells overexpressing constitutively active Akt/PKB. J Cell Biochem 100: 593–607, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Martin M, Rehani K, Jope RS, Michalek SM: Toll-like receptor–mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 6: 777–784, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]