Abstract
OBJECTIVE—The implication of innate immunity in type 1 diabetes development has long been proposed. High-mobility group box 1 (HMGB1), an evolutionarily conserved chromosomal protein, was recently recognized to be a potent innate inflammatory mediator when released extracellularly. We sought to test the hypothesis that HMGB1 acts as an innate immune mediator implicated in type 1 diabetes pathogenesis.
RESEARCH DESIGN AND METHODS—Eight- and 12-week-old NOD mice were treated with an HMGB1 neutralizing antibody once a week until 25 weeks of age and monitored for insulitis progression and diabetes onset. The underlying mechanisms of HMGB1 regulation of autoimmune response were further explored.
RESULTS—During autoimmunity, HMGB1 can be passively released from damaged pancreatic β-cells and actively secreted by islet infiltrated immune cells. Extracellular HMGB1 is potent in inducing NOD dendritic cell maturation and stimulating macrophage activation. Blockade of HMGB1 significantly inhibited insulitis progression and diabetes development in both 8- and 12-week-old NOD mice. HMGB1 antibody treatment decreased the number and maturation of pancreatic lymph node (PLN) CD11c++CD11b+ dendritic cells, a subset of dendritic cells probably associated with autoantigen presentation to naïve T-cells, but increased the number for PLN CD4+Foxp3+ regulatory T-cells. Blockade of HMGB1 also decreased splenic dendritic cell allo-stimulatory capability associated with increased tolergenic CD11c+CD8a+ dendritic cells. Interestingly, the number of CD8+interferon-γ+ (Tc1) T-cells was increased in the PLNs and spleen after blockade of HMGB1, which could be associated with retarded migration of activated autoreactive T-cells into the pancreatic islets.
CONCLUSIONS—Extracellular HMGB1 functions as a potent innate immune mediator contributing to insulitis progression and diabetes onset.
Type 1 diabetes is an autoimmune disease characterized by T-cell–mediated destruction of the insulin-secreting β-cells (1–3). It is believed that environmental risk factors interact with genetic factors to trigger the development of autoimmunity. Given the importance of innate immunity in mediating adaptive immune responses, its role in type 1 diabetes pathogenesis has long been proposed (4–7). The link between innate immunity and autoimmune diabetes is underscored by the observation that lipopolysaccharide (LPS), viral infection, or generalized activation of antigen-presenting cells (APCs) delays or prevents the establishment of peripheral tolerance (8–10). The re-discovery of toll-like receptors reacting to endogenous damage-associated molecular patterns provided additional evidence supporting a role for innate immunity in type 1 diabetes pathogenesis (11–15). Moreover, despite recent extensive studies, identification of which cells, receptors, and mediators associated with innate immunity are critical in type 1 diabetes settings is still a formidable challenge.
High-mobility group box 1 (HMGB1) is among the most evolutionarily conserved proteins in the eukaryotic kingdom (16). It was originally identified as a chromosomal protein facilitating the binding of transcription factors to their cognate DNA sequences (17). Recently, HMGB1 was re-recognized as an innate “danger signal” (alarmin) adopted by the innate immune system during evolution for mediating adaptive immune responses (18–22). Extracellular HMGB1 is potent to initiate immune responses by inducing APC activation and mediating Th1 polarization. Therefore, HMGB1 acts as a bridge that links innate and adaptive immunity. Previously, we have demonstrated a pivotal role for HMGB1 in the initiation and progression of allograft rejection in a murine cardiac transplantation model (23). In the current study, we have tested our hypothesis that HMGB1 functions as a potent innate immune mediator contributing to autoimmune progression during type 1 diabetes development. We have demonstrated that HMGB1 can be either passively released from damaged pancreatic β-cells or secreted by islet infiltrated autoreactive immune cells, such as dendritic cells. Blockade of HMGB1 in NOD mice not only prevents autoimmune progression but also delays diabetes onset. Our data provide strong evidence indicating a role for HMGB1 in autoimmune diabetes by regulation of dendritic cells, T effector cells, and regulatory T-cells (Tregs).
RESEARCH DESIGN AND METHODS
NOD/LTJ (H-2g7), C57BL/6J (H-2b), and SWR/J (H-2q) mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and housed in the specific pathogen-free facility. All studies were carried out in compliance with Medical College of Georgia Animal Care and Use Committee guidelines.
Production and purification of HMGB1 neutralizing antibodies.
Recombinant HMGB1 (rHMGB1) was expressed and purified as reported and then used to raise neutralizing antibodies in five rabbits (Covance, Denver, PA) (23). The neutralizing activity for each antibody was determined by assay of tumor necrosis factor-α (TNF-α) release after stimulating macrophages with rHMGB1. Antibodies with neutralizing effect were first affinity-purified by protein-A columns and then passed through polymyxin B beads to remove possible contaminated endotoxin.
Western blot analysis of HMGB1 passive release and active secretion.
NIT-1 cells (3 × 106) were treated either with cytokines (10 units/ml interleukin [IL]-1β, 100 units/ml TNF-α, and 100 units/ml interferon-γ [IFN-γ]; R&D Systems, Minneapolis, MN) for 72 h or with UV light (1 min) or 4 μmol/l camptothecin (Calibiochem, La Jolla, CA). NIT-1 cell apoptosis and secondary necrosis after treatment were confirmed using a TUNEL assay kit (Molecular Probes, Carlsbad, CA). Necrosis was induced by three cycles of freeze-thaw processes. Equal volumes of concentrated supernatants after each treatment were analyzed by Western blots for HMGB1 passive release (23). Similarly, primary islets were hand-picked under a dissection microscope after digestion and then dissociated into single cells for the same assay (24). For detection of HMGB1 active secretion, conditioned media from dendritic cells or macrophages were concentrated and analyzed as above.
ELISA for cytokine assay and intracellular Foxp3 or IFN-γ immunostaining.
The amount of TNF-α in the culture medium was determined using a sandwich ELISA kit (eBioscience, San Diego, CA). Intracellular Foxp3 or IFN-γ staining was carried out as reported (23). Flow cytometry data were analyzed using Quest v3.3 software (BD Bioscience, San Jose, CA).
Dendritic cell preparation and mixed lymphocyte reaction assay.
Bone marrow cells were flushed from femurs with RPMI 1640 supplemented with 10% FCS. Cells (5 × 105/ml) were plated in 150-mm Petri dishes supplemented with 5 ng/ml granulocyte macrophage–colony-stimulating factor and 1 ng/ml IL-4. Culture media were changed on days 4 and 7, stimulated with 0.5 μg/ml LPS (Sigma, St. Louis, MO) or 20 μg/ml rHMGB1 for 24 h, and then harvested on day 10 for flow cytometry. Splenic dendritic cells were purified using a mouse dendritic cell enrichment kit (StemCell, Seattle, WA). All antibodies used for flow cytometry were purchased from BD Biosciences (San Diego, CA). NOD dendritic cell allo-stimulatory capability was determined by mixed lymphocyte reaction as reported previously (25).
HMGB1 immunostaining.
NOD bone marrow–derived dendritic cells (BMDCs) or macrophages were fixed with formaldehyde and permeabilized with saponin. After blocking with 10% BSA, the cells were sequentially incubated with a monoclonal HMGB1 antibody (Abnova, Taiwan) and a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Nuclei were counterstained with propidium iodide. The cells were visualized on a LSM510 confocal microscope (Zeiss, Oberkochen, Germany). HMGB1 immunostaining in the pancreatic sections was carried out as previously described (23).
HMGB1 neutralizing antibody treatment of pre-diabetic NOD mice.
Eight- or 12-week-old NOD female mice were used for the study. The HMGB1 neutralizing antibody (600 μg/mouse) was intraperitoneally injected into each mouse once a week starting from the indicated age until 25 weeks of age. Mice that received the same amount of normal rabbit IgG (Sigma) that underwent the same purification process served as controls. For monitoring diabetes incidence, the mice were housed in the Medical College of Georgia SPF facility for up to 35 weeks of age. Urine glucose was checked every other day with Diastix strips (Bayer, Elkhart, IN) when the mice reached 14 weeks of age. Once urine glucose was detected, the mice were tested for blood glucose, and they were diagnosed with diabetes when two consecutive blood glucose readings were >250 mg/dl.
For monitoring insulitis, the selected mice were killed at 12, 15, and 18 weeks of age, respectively. Pancreatic sections were stained with hematoxylin-eosin using the established techniques (26). Insulitis severity was scored in a blinded fashion by two examiners using the following criteria: 0, intact islet, no cellular infiltrates; 1, peri-insulitis; 2, moderate insulitis, <50% of the islet was infiltrated; 3, severe insulitis, ≥50% of the islet was infiltrated. Eight mice (>200 islets) were examined at each time point for each study group.
Statistical analysis.
Diabetes incidence curves were generated by the Kaplan-Meier method. The differences of diabetes incidence between groups were determined using the log-rank (Mantel-Cox) test. χ2 test was used to determine the difference for insulitis severity at each time point. Comparisons between groups for flow cytometry, cytokine production, and intracellular cytokine expression were accomplished by one-way ANOVA using SPS 11.5 for Windows. Data are presented as means ± SD. P < 0.05 was considered statistically significant.
RESULTS
Purification of rHMGB1 and production of HMGB1 neutralizing antibodies.
rHMGB1 was first purified using the Ni-NTA affinity columns followed by weak cation exchange chromatography. The purified protein was further passed over polymyxin B columns to remove any contaminated endotoxin. The purity of rHMGB1 was high, as determined on SDS-PAGE (Supplemental Fig. S1A, which is detailed in the online appendix [available at http://dx.doi.org/10.2337/db07-1499]). Contamination of endotoxin was <60 pg/μg protein.
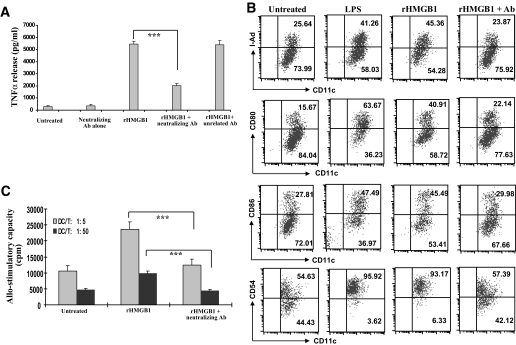
A proportion of the purified rHMGB1 was used to raise antibodies in five rabbits (Covance). Antibodies produced from each rabbit showed high specificity and titers against HMGB1, as shown on the Western blots (Supplemental Fig. S1B). RAW264.7 cells were then used to test the neutralizing effect. A small scale of each antibody was purified by protein-A columns and then passed through polymyxin B beads to remove potential contaminated endotoxin. rHMGB1 is potent in stimulating RAW264.7 cell activation in a dose-dependent manner. When 20 μg/ml rHMGB1 was added into the cultures, RAW264.7 cells secreted 18-fold higher TNF-α (Fig. 1A). Next, 25 μg/ml of each purified antibody was added into the cultures 1 h before rHMGB1 stimulation. Only one antibody showed a strong neutralizing effect, which reduced TNF-α production by twofold (P < 0.001), whereas the rest of antibodies showed either weak or undetectable neutralizing effect, and the control rabbit IgG failed to inhibit the stimulatory effect, indicating the specificity of the neutralizing effect (Fig. 1A).
FIG. 1.
rHMGB1 is potent to stimulate RAW264.7 cell and NOD dendritic cell activation. A: rHMGB1 stimulated RAW264.7 cells to secret high levels of TNF-α, whereas blockade of HMGB1 by a neutralizing antibody significantly inhibited TNF-α secretion. Of note, the neutralizing antibody alone did not show any stimulating effect on RAW264.7 cells, and addition of an unrelated antibody (Ab) failed to block HMGB1-induced RAW264.7 cell activation, indicating the specificity of the blocking effect. B: rHMGB1 is potent in stimulating NOD BMDC maturation. When 25 μg/ml HMGB1 neutralizing antibody was added into the cultures 1 h before the addition of 20 μg/ml rHMGB1, the stimulatory effect of rHMGB1 was almost completely blocked by the neutralizing antibody. Of note, 100 units polymyxin B was also added into the cultures to exclude the effect of endotoxin. Data shown in the figure are a representative of four independent experiments performed. C: HMGB1 neutralizing antibody pretreatment resulted in a reduced NOD BMDC allo-stimulatory capacity. Data shown in the figure are representative of four independent experiments carried out in each study group. ***P < 0.001.
rHMGB1 is potent in stimulating NOD dendritic cell maturation.
HMGB1 has been shown to be potent in stimulating dendritic cell activation in humans, C57BL/6, and BALB/c mice (27–29), but its effect on NOD dendritic cells remains unexplored. To this end, we first purified the antibody that showed the strongest neutralizing effect. To stimulate day-9 culture of NOD BMDCs for 24 h, 20 μg/ml rHMGB1 was used. It was found that rHMGB1 significantly stimulated NOD BMDC maturation, as manifested by increased surface marker expressions shown in Fig. 1B. To confirm this observation, 25 μg/ml neutralizing antibody was added into the cultures 1 h before rHMGB1 stimulation. As expected, addition of neutralizing antibody significantly inhibited rHMGB1-induced dendritic cell maturation, and as a result, they showed significantly lower surface marker expressions than the cells with rHMGB1 treatment alone (Fig. 1B). Consistently, these dendritic cells had significantly less allo-stimulatory capacity than those dendritic cells treated with rHMGB1 alone (Fig. 1C).
HMGB1 passive release from damaged pancreatic β-cells.
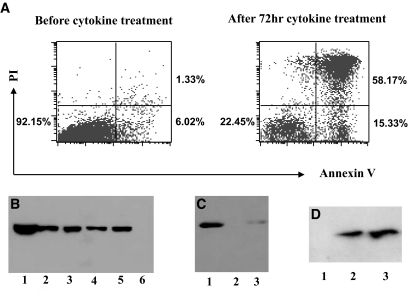
Because HMGB1 can be passively released from damaged cells, we sought to examine whether it can also be released from damaged β-cells. We first checked HMGB1 passive release in NIT-1 cells, a NOD-derived β-cell line. To induce apoptosis, 3 × 106 NIT-1 cells were treated with combination of IL-1β, TNF-α, and IFN-γ. In the normal condition, NIT-1 cells showed >92% viability, whereas >73% of the cells became apoptotic after cytokine treatment, of which >79% of the apoptotic cells were undergoing secondary necrosis (Fig. 2A). The culture supernatants were then collected and concentrated by the Centricon 10-kDa filter devices (Millipore, Billerica, MA). Equal volume of each supernatant was used for Western blot analysis of HMGB1 passive release. NIT-1 cell lysates and supernatant from necrotic NIT-1 cells were used as positive controls (Fig. 2B, lanes 1 and 5). HMGB1 was present in the supernatants of NIT-1 cells after cytokine (Fig. 2B, lane 2), UV light (Fig. 2B, lane 3), and camptothecin (Fig. 2B, lane 4) treatment, whereas it was undetectable in the control supernatant (Fig. 2B, lane 6).
FIG. 2.
HMGB1 passive release from damaged pancreatic β-cells. A: Flow cytometry analysis of NIT-1 cell apoptosis and secondary necrosis before/after cytokine (10 units/ml IL-1β, 100 units/ml TNF-α, and 100 units/ml IFN-γ) stimulation. More than 73% of the cells became apoptotic after 72 h of cytokine treatment, 79% of which were undergoing secondary necrosis as determined by propidium iodide (PI) and annexin V staining. B: Western blot analysis of culture supernatants for detection of HMGB1 passive release from damaged NIT-1 cells. NIT-1 cells were treated with cytokines (IL-1β, TNF-α, and IFN-γ), UV light (1 min), and 4 μmol/l camptothecin, respectively. Supernatant from each apoptotic culture was concentrated, and equal volume of elutes was then subjected to Western blot analysis. Lane 1, NIT-1 cell lysates (positive control); lanes 2–4, culture supernatants of NIT-1 cells treated with combination of cytokines, UV light, and camptothecin, respectively; lanes 5 and 6, supernatants from necrotic (positive control) and untreated NIT-1 cells (negative control). C: Z-VAD-FMK pretreatment of NIT-1 cells inhibited cytokine-induced HMGB1 passive release. Z-VAD-FMK (100 μmol/l) was added into the cultures 3 h before the addition of cytokines. Lanes 1–3, NIT-1 cell culture supernatants after cytokine, normal medium (control), and Z-VAD-FMK+cytokine treatment. D: HMGB1 passive release from damaged primary islet cells. Lanes 1–3, culture supernatants from untreated, cytokine-treated, and necrotic primary islet cells.
Next, we pretreated NIT-1 cells with 100 μmol/l Z-VAD-FMK (Calbiochem), a broad-spectrum caspase inhibitor, before cytokine treatment. Z-VAD-FMK significantly prevented cytokine-induced NIT-1 cell apoptosis (data not shown) and resulted in significantly lower HMGB1 passive release than the cells treated with cytokine alone (Fig. 2C, lane 3 vs. 1). We further investigated HMGB1 passive release from apoptotic primary islet cells, and similar results were obtained as shown in Fig. 2D.
HMGB1 active secretion by NOD autoreactive immune cells.
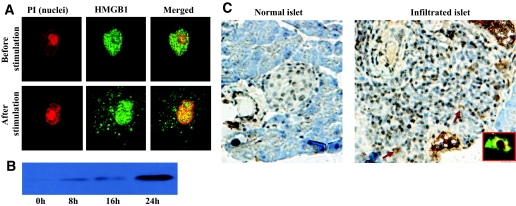
In addition to passive release, HMGB1 can also be secreted by activated immune cells, such as dendritic cells (28,30). To check the capacity of NOD dendritic cells secretion of HMGB1, we first examined HMGB1 subcellular localization in NOD BMDCs. HMGB1 was stained with a monoclonal antibody followed by a FITC-labeled secondary antibody. Nuclei were counterstained by propidium iodide. Before stimulation, HMGB1 was solely localized in the nucleus, while a large proportion of HMGB1 translocated into the cytoplasm after LPS or TNF-α/IFN-γ stimulation, suggesting HMGB1 active secretion (Fig. 3A). Western blot analysis of culture supernatants confirmed NOD BMDC secretion of HMGB1 in a time-dependent manner, with the highest levels observed after 24 h of stimulation (Fig. 3B). We further stained for BMDCs with propidium iodide and annexin V and failed to detect a significant difference for cell apoptosis and secondary necrosis before/after cytokine stimulation (data not shown). These results excluded the possibility that the detected HMGB1 in the BMDC culture supernatants originated from damaged BMDCs. To further confirm our results, we performed similar studies in macrophages, and consistent results were obtained (data not shown).
FIG. 3.
HMGB1 active secretion by NOD dendritic cells and islet infiltrated immune cells. A: Cytoplasmic translocation of HMGB1 after stimulation in NOD dendritic cells. HMGB1 was stained green, and nuclei were stained red. Before stimulation, HMGB1 was exclusively localized in the nuclei of NOD BMDCs. On 0.5 μg/ml LPS stimulation, a large proportion of HMGB1 had translocated into the cytoplasmic vesicles for active secretion. B: NOD dendritic cells secrete high levels of HMGB1 into the culture medium in a time-dependent manner. HMGB1 was present in the culture supernatant after 8 h of stimulation, with the highest levels observed after 24 h of stimulation. Of note, BMDCs showed similar viability before and after stimulation as determined by annexin V and propidium iodide (PI) staining, which excluded HMGB1 passive release from damaged BMDCs. C: HMGB1 active secretion by islet infiltrated immune cells. HMGB1 was visualized by DAB (brown). Nuclei were also counterstained blue by hematoxylin. In a normal islet, HMGB1 was solely localized within the nuclei of islet cells (left). In an islet with severe insulitis (right), HMGB1 was also exclusively localized in the nuclei in the majority of the cells. However, HMGB1 had translocated into the cytoplasm in some of the infiltrated immune cells (ring-like cells, indicated by arrows), indicating HMGB1 active secretion. Double staining for HMGB1 (green) and CD11c (red) confirmed that some of these ring-like cells were CD11c+ dendritic cells (inset). (Please see http://dx.doi.org/10.2337/db07-1499 for a high-quality digital representation of this figure.)
To explore HMGB1 active secretion by NOD islet infiltrated immune cells, we performed in situ HMGB1 immunostaining using pancreatic sections originated from NOD mice with insulitis (14 weeks old). HMGB1 (visualized by diaminobenzidine) was exclusively localized in the nuclei of cells in the normal islets (Fig. 3C, left). HMGB1 was also localized in the nuclei in the majority of cells of islets with insulitis. However, a large proportion of HMGB1 had translocated into the cytoplasm in some of the infiltrated cells (Fig. 3C, ring-like cells indicated by arrows), suggesting HMGB1 active secretion by infiltrated immune cells. To confirm this notion, we double-stained pancreatic islets for HMGB1 (green) and CD11c (red) and found that some of these ring-like cells were CD11c+ dendritic cells (Fig. 3C, inset). Taken together, our data suggest that during autoimmunity, HMGB1 can be either passively released from damaged β-cells or actively secreted by islet infiltrated immune cells, such as dendritic cells, which could then enhance autoimmune progression.
Administration of HMGB1 neutralizing antibody prevents diabetes in NOD mice.
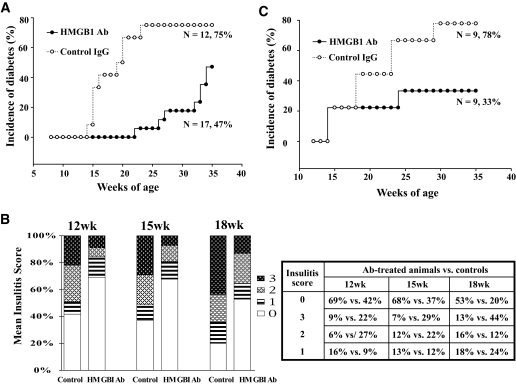
To determine the implication of HMGB1 in autoimmune progression, we first performed studies in the early stage of autoimmunity in 8-week-old NOD mice. Forty-one mice were selected for HMGB1 neutralizing antibody treatment, 17 of which were housed for monitoring diabetes incidence, and the rest were used for monitoring insulitis. For controls, 36 mice were treated with normal IgG, among which 12 were housed for monitoring diabetes incidence, and 24 were used for examining insulitis progression. The mice were treated once a week starting from 8 to 25 weeks of age and housed up to 35 weeks of age for diabetes onset. Diabetes incidence was plotted as Kaplan-Meier survival curves. Of important note, HMGB1 antibody treatment significantly reduced diabetes incidence (Fig. 4A, 47 vs. 75%, P < 0.01). Furthermore, the treatment significantly delayed the onset of diabetes. In average, the age for onset of diabetes in HMGB1 antibody–treated mice was 28.7 ± 3.4 weeks, whereas the control IgG–treated mice was only 18.4 ± 3.1 weeks (P < 0.0001).
FIG. 4.
Blockade of extracellular HMGB1 prevents insulitis progression and diabetes onset in NOD mice. A: HMGB1 neutralizing antibody (Ab) treatment prevented diabetes onset in 8-week-old NOD mice. Blockade of HMGB1 in 8-week-old NOD mice not only decreased diabetes incidence (75 vs. 47%, P < 0.01) but also delayed the onset of diabetes (28.7 ± 3.4 vs. 18.4 ± 3.1 weeks, P < 0.0001). B: A bar graph showing insulitis severity examined at 12, 15, and 18 weeks after HMGB1 neutralizing antibody treatment (left). A table showing the distribution and parallel comparison of insulitis score at each time point examined (right). Insulitis scores were estimated in a blinded fashion by two examiners. The differences for insulitis severity between antibody- and control IgG–treated animals at each time point were analyzed by χ2 test with corrections. HMGB1 antibody treatment significantly inhibited insulitis severity at 12-week (P < 0.0005), 15-week (P < 0.0006), and 18-week (P < 0.0001) time points. C: HMGB1 neutralizing antibody treatment prevented diabetes onset in 12-week-old NOD mice. The mice were treated with either 600 μg HMGB1 blocking antibody or same amount of control IgG once a week, starting from the indicated age (8 or 12 weeks) until 25 weeks and were housed up to 35 weeks of age for monitoring diabetes onset.
Next, we checked insulitis severity. Eight mice were killed for each group at each time point, and a total of three time points (12, 15, and 18 weeks of age) were examined. Sections of each pancreas were stained with hematoxylin-eosin, and insulitis was scored as described above. As can be seen in Fig. 4B, the extent of insulitis in HMGB1 antibody–treated mice was significantly less severe than the control IgG–treated mice examined at 12 (P < 0.0005), 15 (P < 0.0006), and 18 (P < 0.0001) weeks old. These data demonstrate that blockade of HMGB1 prevented insulitis progression.
To examine the implication of HMGB1 in the late stage of autoimmunity, we performed a prevention study in 12-week-old NOD mice, because previous reports suggest that autoimmune infiltration at this stage has already progressed for at least 5 weeks (31). As above, the mice were treated with HMGB1 antibody once a week starting from 12 to 25 weeks of age. Consistently, HMGB1 antibody treatment significantly decreased diabetes incidence (Fig. 4C, 33 vs. 78%, P < 0.01). However, there was no significant delay for diabetes onset (18.3 ± 5.8 vs. 20.9 ± 5.5 weeks) as observed in 8-week-old NOD mice. All together, blockade of HMGB1 significantly inhibited insulitis progression and, as a result, decreased diabetes incidence in NOD mice.
HMGB1 regulates dendritic cell subpopulation and functionality.
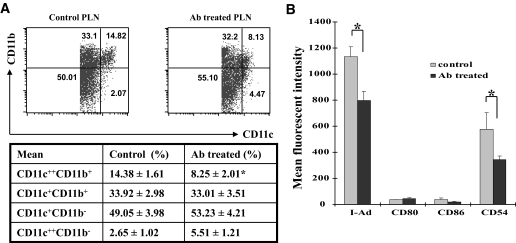
To dissect the mechanisms underlying the protective effects, we first examined the impact on dendritic cells. Dendritic cells pooled from pancreatic lymph nodes (PLNs), inguinal lymph nodes (ILNs), and spleens of four mice from each study group were used for the study, and three pools were analyzed. PLN- or ILN-derived dendritic cells were analyzed without pre-enrichment to prevent any loss of potentially important cells. We gated CD11c+ cells and analyzed dendritic cell subpopulations by CD11c and CD11b expression levels. Interestingly, HMGB1 antibody treatment significantly reduced PLN CD11c++CD11b+ dendritic cells (Fig. 5A, 14.38 ± 1.61 vs. 8.25 ± 2.01%, P < 0.01). Because CD11c++CD11b+ dendritic cells are probably implicated in islet antigen presentation to autoreactive T-cells (32), we then examined their maturation status by analyzing surface marker expressions. Surprisingly, HMGB1 antibody treatment not only reduced PLN CD11c++CD11b+ dendritic cells but also suppressed their maturation, as shown in Fig. 5B. We next did similar study for PLN and ILN dendritic cell subpopulations in HMGB1 antibody–treated animals. Only very few CD11c++CD11b+ dendritic cells were present in the ILN; however, the number and maturation status for ILN CD11c+CD11b+ dendritic cells were significantly lower than that in the PLN (15.33 ± 1.49 vs. 22.09 ± 2.22%, P < 0.05; Supplemental Fig. S2). We finally compared ILN dendritic cell subpopulations between HMGB1 antibody–and control IgG–treated animals and failed to detect significant differences in terms of subpopulations and maturation status (data not shown).
FIG. 5.
Blockade of extracellular HMGB1 regulates PLN dendritic cell subpopulation and maturation. A: HMGB1 antibody (Ab) treatment reduced the number of PLN CD11c++CD11b+ dendritic cells. PLN cells pooled from four mice were gated for CD11c+ cells and then analyzed for subpopulations based on CD11c and CD11b expression levels. A representative of flow cytometry data are shown in the top. The data are presented in the table (bottom) as means ± SD of three pools studied in each group. B: A bar graph showing the effect of HMGB1 antibody treatment on PLN CD11c++CD11b+ dendritic cell maturation. The treatment significantly inhibited I-Ad and CD54 expression compared with the control IgG–treated mice. The data shown here are means ± SD of three pools studied. *P < 0.01.
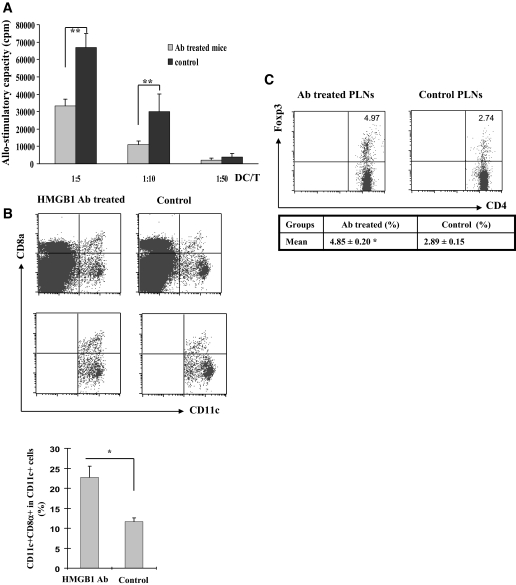
Unlike PLN dendritic cells, HMGB1 antibody treatment did not show significant effect on splenic dendritic cell maturation (Supplemental Fig. S3). However, the treatment significantly inhibited splenic dendritic cell allo-stimulatory capability (Fig. 6A). To dissect its possible mechanism, we analyzed splenic dendritic cell subpopulations based on CD11c and CD8α expressions. Interestingly, antibody treatment significantly increased the number of splenic CD11c+CD8α+ dendritic cells (23.4 ± 2.8 vs. 11.6 ± 1.0%, P < 0.001; Fig. 6B). Because previous studies suggest that they are probably a subset of tolergenic dendritic cells (33), the reduced allo-stimulatory capability of splenic dendritic cells could be caused by the increase of CD11c+CD8α+ dendritic cells.
FIG. 6.
Blockade of extracellular HMGB1 regulates splenic dendritic cell function and PLN Tregs. A: Splenic dendritic cells originated from antibody (Ab)-treated mice showed a reduced allo-stimulatory capacity. There was no surface marker expression difference observed between the experimental and control animals, but the allo-stimulatory capability was significantly lower in antibody-treated mice. **P < 0.001. B: HMGB1 antibody treatment increased the number of splenic CD11c+CD8α+ dendritic cells. Top: Splenocytes pooled from four mice were gated for B220− cells and then analyzed for CD11c and CD8α expression. Bottom: The data are presented as bar graphs of means ± SD of three pools studied. *P < 0.01. C: Blockade of extracellular HMGB1 increased the number for PLN CD4+Foxp3+ Tregs. PLN CD4 T-cells pooled from four mice were analyzed, and the results are presented in a table as means ± SD of three pools studied. *P < 0.05.
Blockade of HMGB1 upregulates PLN Tregs.
We next examined the effect of antibody treatment on Treg production. CD4+ T-cells pooled from PLNs and spleens were further analyzed for Foxp3 expressions. It was found that the proportion of CD4+Foxp3+ T-cells in the PLN CD4+ T-cells examined was significantly higher in HMGB1 antibody–treated mice than the control mice (4.85 ± 0.20 vs. 2.89 ± 0.15%, P < 0.05; Fig. 6C). In contrast, there was no difference for the splenic Tregs between the two groups of mice (data not shown).
Blockade of HMGB1 affects CD8+ T-cell subpopulations.
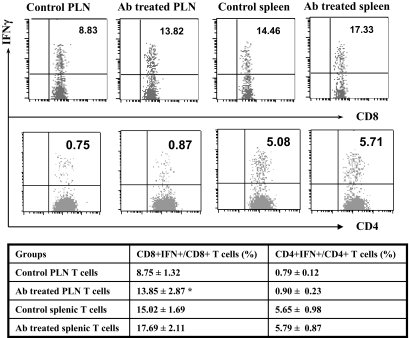
To dissect the impact of HMGB1 antibody treatment on T-cell activation, PLN or splenic CD4+ and CD8+ T-cells were analyzed by flow cytomety for activation markers, such as CD69, CD62Llow, and CD44. To our surprise, antibody treatment showed no effect on the maturation status for CD4+ or CD8+ T-cells (data not shown). We then checked CD4+ and CD8+ T-cell subpopulations. Unexpectedly, antibody treatment significantly increased PLN CD8+ T-cells (16 ± 1.9 vs. 11.96 ± 2.5%, P < 0.05). Next, we examined the proportion of CD4+IFN-γ+ (Th1) and CD8+IFN-γ+ (Tc1) cells. PLN or splenic CD4 or CD8 T-cells were intracellularly stained for IFN-γ expression as described above. As above, a significantly higher proportion of PLN CD8+IFN-γ+ (Tc1) cells was observed in antibody-treated animals (13.85 ± 2.87 vs. 8.75 ± 1.32%, P < 0.01; Fig. 7). A similar trend was also observed in the splenic T-cells examined but did not reach statistical significance (17.69 ± 2.11 vs. 15.02 ± 1.69%). On the contrary, there was no significant difference detected for the proportion of PLN or splenic CD4+IFN-γ+ (Th1) cells between the two groups of mice.
FIG. 7.
HMGB1 antibody (Ab) treatment regulates T-cell subpopulations. PLN and splenic T-cells originated from HMGB1 antibody–treated mice and control mice were harvested for intracellular IFN-γ staining. The percentages of CD4+ and CD8+ T-cells that are also positive for IFN-γ were determined by flow cytometric analysis. The data are presented in the table as means ± SD of three independent experiments performed. The number of CD8+IFN-γ+ T-cells (Tc1) in the PLN of antibody-treated animals was significantly higher than that of control animals (P < 0.01). A similar trend was also observed in the splenic T-cells. *P < 0.01.
DISCUSSION
Hmgb1 probably originated >500 million years ago before the split between the animal and plant kingdoms. It is among the most evolutionarily conserved proteins in the eukaryotic kingdom and shares 99% amino acid identity between rodents and humans. Recently, we have demonstrated a pivotal role for HMGB1 in the initiation and progression of allograft rejection by acting as an innate alarmin. Because allograft rejection and type 1 diabetes–associated autoimmune response share many similar features, we reasoned that HMGB1 could also be implicated in type 1 diabetes pathogenesis. Additionally, the nature of HMGB1 acting as an innate “danger signal” made it a perfect molecule for us to dissect the role of innate immunity in type 1 diabetes development.
We first demonstrated the potency for HMGB1 in inducing NOD dendritic cell and macrophage activation, and we investigated the origin of extracellular HMGB1 during autoimmunity in NOD mice. HMGB1 passive release from necrotic cells has been demonstrated in the previous studies (34). Recent studies also support that HMGB1 passive release is a feature of apoptosis at least in some cell types and likely occurs during late apoptosis (35,36). β-Cells do not seem to be exceptional in this regard, as shown in our study. Given that apoptosis and secondary necrosis may be the dominant form of β-cell death during insulitis course (37), we induced β-cell death by cytokines, UV, and camptothecin and then checked HMGB1 release. More than 79% of apoptotic β-cells were undergoing secondary necrosis after cytokine treatment, which resulted in HMGB1 passive release (Fig. 2). We also demonstrated NOD BMDC and macrophage secretion of HMGB1 after stimulation (Fig. 3A), which resembled the observations obtained from human dendritic cells. In situ immunostaining further indicated HMGB1 secretion by islet- infiltrated immune cells, such as dendritic cells (Fig. 3C). These findings suggest that extracellular HMGB1 in the pancreatic islets during autoimmunity could originate from both damaged β-cells and infiltrated immune cells, such as dendritic cells.
The presence of HMGB1 in the insulitis lesion suggests its importance in type 1 diabetes pathogenesis. To address this question, we first treated 8-week-old NOD mice with an HMGB1 blocking antibody. As expected, blockade of HMGB1 not only significantly prevented insulitis progression and decreased diabetes incidence but also delayed the onset of diabetes (Fig. 4). Similar results were also obtained for the studies in 12-week-old NOD mice. However, blockade of HMGB1 in 12-week-old NOD mice failed to delay the onset of diabetes, indicating that it is more difficult to block autoimmunity once it progressed to the late stage. However, this phenomenon may also be associated with shorter blocking antibody treatment, because blocking antibody administration did not start before 12 weeks of age and was stopped once the mice reached 25 weeks of age.
Given the critical role of dendritic cells in type 1 diabetes–associated autoimmunity, we first checked the impact for blockade of HMGB1 on dendritic cells, the most potent APCs known today. It was found that antibody treatment reduced the number and maturation of CD11c++CD11b+ dendritic cells in the PLN (Fig. 5A and B). This observation is consistent with the functionality of these dendritic cells because they are probably associated with autoantigen presentation to naïve T-cells in the PLN (32). We further noticed higher number and more matured CD11c+CD11b+ dendritic cells in the PLN than that in the ILN in HMGB1 antibody-treated animals (Supplemental Fig. S2), suggesting that they might also be implicated in autoantigen presentation. Of note, HMGB1 antibody treatment apparently did not have an effect on these dendritic cells, because both HMGB1 antibody–and control IgG–treated animals showed similar characteristics for these dendritic cells. Our studies also suggested a role for HMGB1 regulation of splenic dendritic cells. After blockade of HMGB1, the mice showed a skewed population of CD11c+CD8a+ dendritic cells (Fig. 6B), which have been suggested to be responsible for inducing peripheral tolerance to tissue-associated antigens (33). Consistently, splenic dendritic cells originated from antibody-treated mice had a decreased allo-stimulatory capability (Fig. 6A).
We also noticed that HMGB1 antibody treatment resulted in increased CD4+Foxp3+ Tregs in the PLN (Fig. 6C). The basis for this observation is not clear from our studies. Previous studies in other model systems have shown that a subset of dendritic cells can induce Treg production (38–40). Therefore, blockade of HMGB1 may modulate dendritic cells to an appropriate maturation state that can induce Treg development. Also, blockade of HMGB1 may have an effect on Treg migration, which leads to Treg accumulation in the PLN.
Of note, blockade of HGMB1 did not show an observable effect on T-cell activation. However, blockade of HMGB1 unexpectedly increased the number of total CD8+ T-cells in the PLN. Consistent with this observation, the proportion of CD8+IFN-γ+ cells (Tc1) in the PLN and spleen was higher in antibody-treated mice compared with the control mice (Fig. 7). The exact mechanism responsible for this observation remains unclear. Based on the fact that HMGB1 antibody treatment significantly inhibited insulitis progression characterized by the reduction of islet-infiltrated immune cells (Fig. 4B), this phenomenon could be associated with retarded migration of activated autoreactive T-cells from PLN into the pancreatic islets after blockade of HMGB1. In fact, a similar result was observed in the experimental autoimmune encephalomyelitis model. Yan et al. (41) noticed that after blockade of receptors for advanced glycation end products, a co-receptor for HMGB1 prevented the entry of encephalitogenic T-cells into the central nervous system. Taken together, these results indicate that HMGB1 could affect the migration of activated autoreactive T-cells into the target organ.
In summary, we demonstrated strong evidence indicating the involvement of HMGB1 in type 1 diabetes pathogenesis. Blockade of extracellular HMGB1 significantly inhibited insulitis progression and diabetes development in NOD mice. The mechanisms underlying this protective effect are associated with the inhibition of inflammation and the regulation of autoimmune response.
Supplementary Material
Acknowledgments
C.-Y.W. has received grants from the American Diabetes Association and the Juvenile Diabetes Research Foundation International. J.Z. is an international graduate exchange student from the Tongji Medical College, Huazhong University of Science and Technology, PR China.
Published ahead of print at http://diabetes.diabetesjournals.org on 13 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Wang CY, Podolsky R, She JX: Genetic and functional evidence supporting SUMO4 as a type 1 diabetes susceptibility gene. Ann N Y Acad Sci 1079: 257–267, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Wang CY, She JX: SUMO4 and its role in type 1 diabetes pathogenesis. Diabete Metab Res Rev 24: 93–102, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Li M, Guo D, Isales CM, Eizirik DL, Atkinson M, She JX, Wang CY: SUMO wrestling with type 1 diabetes. J Mol Med 83: 504–513, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bach JF, Bendelac A, Brenner MB, Cantor H, De LG, Kronenberg M, Lanier LL, Raulet DH, Shlomchik MJ, von Herrath MG: The role of innate immunity in autoimmunity. J Exp Med 200: 1527–1531, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann MF, Kopf M: On the role of the innate immunity in autoimmune disease. J Exp Med 193: F47–F50, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyan H, Buckley LR, Yousaf N, Londei M, Leslie RD: A role for innate immunity in type 1 diabetes? Diabete Metab Res Rev 19: 89–100, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Carroll M: Innate immunity in the etiopathology of autoimmunity. Nat Immunol 2: 1089–1090, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, Zinkernagel R, Pircher H: Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J Exp Med 187: 763–774, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maxwell JR, Campbell JD, Kim CH, Vella AT: CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. J Immunol 162: 2024–2034, 1999 [PubMed] [Google Scholar]
- 10.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P: Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity 2: 261–270, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Fasciano S, Li L: Intervention of Toll-like receptor-mediated human innate immunity and inflammation by synthetic compounds and naturally occurring products. Curr Med Chem 13: 1389–1395, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DR: Toll-like receptors and other links between innate and acquired alloimmunity. Curr Opin Immunol 16: 538–544, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DR, Palmer SM: Role of Toll-like receptor-driven innate immunity in thoracic organ transplantation. J Heart Lung Transplant 24: 1721–1729, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Musette P, Auquit Auckbar I, Begon E: [Innate immunity: cutaneous expression of Toll-like receptors]. Med Sci (Paris) 22: 149–152, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ruse M, Knaus UG: New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol Res 34: 33–48, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Ulloa L, Messmer D: High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 17: 189–201, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bianchi ME, Beltrame M, Paonessa G: Specific recognition of cruciform DNA by nuclear protein HMG1. Science 243: 1056–1059, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ: HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Andersson U, Tracey KJ: HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheum Dis Clin North Am 30: 627–637, xi, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Izuishi K, Tsung A, Jeyabalan G, Critchlow ND, Li J, Tracey KJ, DeMarco RA, Lotze MT, Fink MP, Geller DA, Billiar TR: Cutting edge: high-mobility group box 1 preconditioning protects against liver ischemia-reperfusion injury. J Immunol 176: 7154–7158, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Barkauskaite V, Ek M, Popovic K, Harris HE, Wahren-Herlenius M, Nyberg F: Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus 16: 794–802, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Popovic K, Ek M, Espinosa A, Padyukov L, Harris HE, Wahren-Herlenius M, Nyberg F: Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum 52: 3639–3645, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, Wang S, Zheng X, Wang CY, Gong F: Extracellular hmgb1 functions as an innate immune-mediator implicated in murine cardiac allograft acute rejection. Am J Transplant 7: 799–808, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mathews CE, Graser RT, Savinov A, Serreze DV, Leiter EH: Unusual resistance of ALR/Lt mouse beta cells to autoimmune destruction: role for beta cell-expressed resistance determinants. Proc Natl Acad Sci U S A 98: 235–240, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G, Fang M, Gong M, Liu L, Zhong J, Feng W, Xiong P, Wang CY, Gong F: Steady state dendritic cells with forced IDO expression induce skin allograft tolerance by upregulation of regulatory T cells. Transpl Immunol 18: 208–219, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Xu JF, Huang BJ, Yin H, Xiong P, Feng W, Xu Y, Fang M, Zheng F, Wang CY, Gong FL: A limited course of soluble CD83 delays acute cellular rejection of MHC-mismatched mouse skin allografts. Transpl Int 20: 266–276, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N: High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 173: 307–313, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P: Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol 174: 7506–7515, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Telusma G, Datta S, Mihajlov I, Ma W, Li J, Yang H, Newman W, Messmer BT, Minev B, Schmidt-Wolf IG, Tracey KJ, Chiorazzi N, Messmer D: Dendritic cell activating peptides induce distinct cytokine profiles. Int Immunol 18: 1563–1573, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P: The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol 81: 84–91, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Andre I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D: Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A 93: 2260–2263, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turley S, Poirot L, Hattori M, Benoist C, Mathis D: Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 198: 1527–1537, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasquez AC, Feili-Hariri M, Tan RJ, Morel PA: Qualitative and quantitative abnormalities in splenic dendritic cell populations in NOD mice. Clin Exp Immunol 135: 209–218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scaffidi P, Misteli T, Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Bell CW, Jiang W, Reich CF, Pisetsky DS: The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291: C1318–C1325, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Jiang W, Bell CW, Pisetsky DS: The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol 178: 6495–6503, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Saldeen J: Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology 141: 2003–2010, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Steinman RM: Some interfaces of dendritic cell biology. APMIS 111: 675–697, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM: Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med 198: 235–247, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM: Dendritic cells are specialized accessory cells along with TGF-{beta} for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3- precursors. Blood 110: 4293–4302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan SS, Wu ZY, Zhang HP, Furtado G, Chen X, Yan SF, Schmidt AM, Brown C, Stern A, LaFaille J, Chess L, Stern DM, Jiang H: Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med 9: 287–293, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.