Abstract
OBJECTIVE— Acute activation of G protein–coupled receptor 40 (GPR40) by free fatty acids (FFAs) or synthetic GPR40 agonists enhances insulin secretion. However, it is still a matter of debate whether activation of GPR40 would be beneficial for the treatment of type 2 diabetes, since chronic exposure to FFAs impairs islet function. We sought to evaluate the specific role of GPR40 in islets and its potential as a therapeutic target using compounds that specifically activate GPR40.
RESEARCH DESIGN AND METHODS— We developed a series of GPR40-selective small-molecule agonists and studied their acute and chronic effects on glucose-dependent insulin secretion (GDIS) in isolated islets, as well as effects on blood glucose levels during intraperitoneal glucose tolerance tests in wild-type and GPR40 knockout mice (GPR40−/−).
RESULTS— Small-molecule GPR40 agonists significantly enhanced GDIS in isolated islets and improved glucose tolerance in wild-type mice but not in GPR40−/− mice. While a 72-h exposure to FFAs in tissue culture significantly impaired GDIS in islets from both wild-type and GPR40−/− mice, similar exposure to the GPR40 agonist did not impair GDIS in islets from wild-type mice. Furthermore, the GPR40 agonist enhanced insulin secretion in perfused pancreata from neonatal streptozotocin-induced diabetic rats and improved glucose levels in mice with high-fat diet–induced obesity acutely and chronically.
CONCLUSIONS— GPR40 does not mediate the chronic toxic effects of FFAs on islet function. Pharmacological activation of GPR40 may potentiate GDIS in humans and be beneficial for overall glucose control in patients with type 2 diabetes.
Loss of glucose-dependent insulin secretion (GDIS) from the pancreatic β-cell is responsible for the onset and progression of type 2 diabetes (1,2). Oral agents that stimulate insulin secretion, such as sulfonylureas and related ATP-sensitive K+ channel blockers, reduce blood glucose and have been used as a first-line type 2 diabetes therapy for nearly 30 years (3,4). However, these agents act to force the β-cell to secrete insulin continuously regardless of prevailing glucose levels, thereby promoting hypoglycemia and accelerating the loss of islet function and, eventually, diminished efficacy (5,6). Despite the availability of a range of agents for type 2 diabetes, many diabetic patients fail to achieve or to maintain glycemic targets (7–9). In addition, stricter glycemic guidelines have been proposed to help define a path toward diabetes prevention through identifying and treating the pre-diabetes state (10). Agents that induce GDIS have great potential to replace sulfonylureas as a first-line therapy for the treatment of type 2 diabetes. In particular, agents that have positive effects on arresting or even reversing β-cell demise would represent a major therapeutic advance toward addressing the lack of durability seen with current therapies and perhaps obviate the need for eventual insulin intervention (11–13). The recent emergence of glucagon-like peptide 1–based GDIS agents (14–16), including inhibitors of dipeptidyl peptidase-4 (17) and peptidase-stable analogs such as exendin-4 (18), is undoubtedly a major advance in such a direction. Nevertheless, it remains to be observed whether glucagon-like peptide 1–related agents truly exert durable beneficial effects on β-cell mass and function.
The molecular pharmacology of lipid and lipid-like mediators that signal through G protein–coupled receptors (GPCRs) has expanded significantly over the past few years. To date, several orphan GPCRs have been paired with lysophospholipids, bile acids, arachidonic acid metabolites, dioleoyl phosphatidic acid, and short-, medium-, and long-chain free fatty acids (FFAs) (19–21). From these discoveries, GPCR 40 (GPR40), GPR119, and GPR120 have been reported to play a role in regulating GDIS and therefore have potential as novel targets for the treatment of type 2 diabetes (22–26). GPR40 is a Gq-coupled, family A GPCR that is highly expressed in β-cells of human and rodent islets. Several naturally occurring medium- to long-chain FFAs and some thiazolidinedione peroxisome proliferator–activated receptor-γ agonists specifically activate GPR40 (27,28). Activation of GPR40 by FFAs (29–32) or synthetic compounds (23,33) enhances insulin secretion through the amplification of intracellular calcium signaling.
The pleiotropic effects of FFAs on the pancreatic β-cell are well known. The fact that FFAs are in vitro ligands for GPR40 is suggestive of the link to the wealth of existing literature data on the acute, stimulatory effects of FFAs on insulin release (34,35). However, FFAs also exert suppressive or detrimental effects on β-cells. Lipotoxicity of β-cells, a condition observed with chronic exposure to high FFA levels, results in impairment in their function and a resulting diminution in their insulin secretory capacity (36,37). Currently, there is an ongoing debate on whether GPR40 mediates the deleterious effects of FFAs on islet function (lipotoxicity) and whether an antagonist of GPR40 is preferable to an agonist for the treatment of type 2 diabetes (38,39). Since FFAs can both be metabolized within cells to act as intracellular signaling molecules (35) and activate more than one receptor (20), they cannot be used as specific and selective tools to unravel the role that GPR40 plays in the β-cell. It is therefore necessary to identify small molecules that specifically activate GPR40.
In the following discussion, we will detail the identification and in vitro pharmacology of a novel series of synthetic GPR40 agonists. Using isolated islets from wild-type and homozygous GPR40 knockout (GPR40−/−) mice (to confirm the on-target activity of small-molecule activators), we not only extended previous findings that acute activation of GPR40 enhances GDIS in pancreatic β-cells but also showed that long-term exposure to the GPR40 agonist, in contrast to FFAs, did not impair β-cell function, thus dissociating the activation of GPR40 from β-cell lipotoxicity. Finally, acute and subchronic dosing of the GPR40 agonist robustly reduced the blood glucose excursion during an intraperitoneal glucose tolerance test (IPGTT) in wild-type, but not GPR40−/−, mice.
RESEARCH DESIGN AND METHODS
Generation of GPR40 stable cell lines.
Human and mouse GPR40 stable cell lines were generated in either Chinese hamster ovary (CHO) cells, stably expressing nuclear factor of activated T-cells β-lactamase (NFAT BLA), or human embryonic kidney (HEK) 293 cells. The expression plasmids were transfected using lipofectamine (Invitrogen), following the manufacturer's instructions. Stable cell lines were generated following the appropriate drug selection.
Fluorometric imaging plate reader–based intracellular calcium assay.
GPR40/CHO NFAT BLA cells were seeded into black-wall clear-bottom 384-well plates (Costar) 1 day before the assay. The cells were incubated with 20 μl/well of Hanks’ buffered salt solution buffer with 0.1% BSA, 2.5 mmol/l probenecid, and 8 μmol/l Fluo-4-AM at room temperature for 100 min. Compounds were dissolved in DMSO and diluted to desired concentrations with assay buffer and added to the cells as 5× solution (13.3 μl/well). Fluorescence output was measured using a fluorometric imaging plate readerII (FLIPRII) (Molecular Devices) 10 s before compound addition.
Measurement of inositol 1,4,5-triphosphate production.
Human GPR40-HEK293 stable cells were plated at 16,000 cells/well on 96-well poly-d-lysine–coated plates and cultured for 72 h in Dulbecco's modified Eagle's medium (25 mmol/l glucose) with 10% fetal bovine serum, 25 mmol/l HEPES, and a selection of antibiotics. Cells were then washed with Hanks’ buffered salt solution buffer and further incubated for 18 h in 150 μl 3H-inositol labeling media (inositol- and serum-free Dulbecco's modified Eagle's medium), to which 3H-myo-inositol (NEN/PerkinElmer, Waltham, MA) was added to a final specific radioactivity of 1 μCi/150 μl. Agonist titrations have typically been performed by half-log dilutions run in duplicate in 11-point curves. The plates were counted in the MicroBeta instrument (PerkinElmer).
Isolation of pancreatic islets and the static GDIS assay.
Pancreatic islets of Langerhans were isolated from wild-type and GPR40−/− mice (littermates) by collagenase digestion and discontinous Ficoll gradient separation (40). The islets were cultured overnight in RPMI-1640 medium with 11 mmol/l glucose to facilitate recovery from the isolation process. Insulin secretion was determined by a 1-h static incubation in Krebs-Ringer bicarbonate (KRB) buffer in a 96-well format as previously described (41). Briefly, islets were first preincubated in KRB medium with 2 mmol/l glucose for 30 min and were then transferred to a 96-well plate (one islet/well) and incubated with 200 μl of the KRB medium with 2 or 16 mmol/l glucose in the presence or absence of oleate, palmitate, or testing compounds for 60 min. The buffer was removed from the wells at the end of the incubation and assayed for insulin levels using the Ultrasensitive Rat Insulin ELISA kit (ALPCO, Salem, NH).
Chronic treatment of islets and GDIS.
Islets, isolated from wild-type and GPR40−/− mice (littermates), were cultured in RPMI-1640 medium (11 mmol/l glucose and 10% FCS) with vehicle or 125 μmol/l FFAs (a 1:1 mixture of oleate:palmitate), as described previously (42), or a 5 μmol/l GPR40 small-molecule agonist for 3 days. The FFAs were added directly to the culture medium from 100× stock solutions in distilled water (for oleate) or 95% ethanol (for palmitate). After the 3-day exposure to oleate, palmitate, or GPR40 small-molecule agonist, insulin secretion was determined by the 1-h static incubation in KRB buffer with either 2 or 16 mmol/l glucose following a 30-min preincubation in the KRB buffer with 2 mmol/l glucose, as described above for the acute GDIS assay.
Islet perifusion.
For islet perifusion, batches of 25 islets each were perifused in parallel microchambers (Biovail International, Minneapolis, MN) with oxygenated KRB medium with 2 or 16 mmol/l glucose at a rate of 0.8 ml/min, and the fractions of the perfusate were collected once per minute for insulin measurement (43). Insulin concentration in aliquots of the incubation or perifusion buffers was measured by the Ultrasensitive Rat Insulin EIA kit from ALPCO Diagnostics (Windham, NH).
The neonatal streptozotocin-induced diabetes rat model and pancreas perfusion.
Timed pregnant Wistar rats were purchased from Charles River Laboratories. Pups were dosed with vehicle (0.5 mol/l citrate, pH 4.5) or 100 mg/kg i.p. streptozotocin (STZ) (Sigma-Aldrich) 48 h after birth. At 3 weeks of age, male pups were separated and housed, two per cage. Food and water were given ad libitum, and rats were maintained on a 12-h light-dark cycle. Perfusions were performed when rats were 8 weeks old, as described previously (44). For each surgery, rats were sedated with nembutal anesthesia (100 mg/kg i.p.). The peritoneal cavity was then opened, and the celiac artery was ligated dorsally. A 27-g cannula was inserted into the celiac artery for perfusant afflux, and another cannula was inserted in the portal vein for perfusant efflux. Immediately following surgery, rats were placed into a 37°C humidified whole-body perfusion chamber and perfused at 3 ml/min with a modified KRB buffer (O2 saturated; 37°C). Perfusant buffer contained 2 or 16 mmol/l glucose supplemented with vehicle (DMSO), 10 μmol/l compound B, or 30 mmol/l l-arginine. Perfusant (∼90% recovery) was collected in 1-min intervals and stored frozen at −70°C until analysis. Insulin was determined using a rat-specific insulin radioimmunoassay kit (Millipore, Billerica, MA). All procedures were approved by the Merck Rahway Institutional Animal Care and Use Committee.
IPGTT.
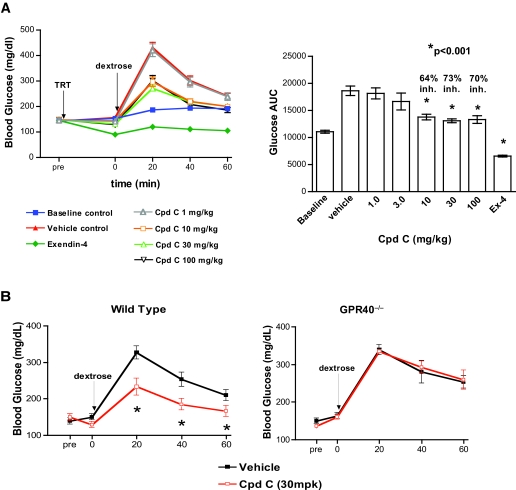
Male GPR40−/− and littermate wild-type C57BL/6N mice (7–11 weeks of age) from Taconic Farms (Germantown, NY) were housed 10 per cage and fed with rodent diet (Teklad 7012) and water ad libitum. On the morning of study, mice (n = 5–7 per group) were fasted for 5–6 h. Animals were then treated orally with vehicle (10 ml/kg 0.25% methylcellulose), Cpd-B, or Cpd-C 60 min before the IPGTT (2 g/kg i.p. dextrose). Blood glucose levels were determined from tail bleeds taken at −60, 0, 20, 40, and 60 min after dextrose challenge. The blood glucose excursion profile from t = 0–60 min was used to integrate an area under the curve (AUC) for each treatment. Percent inhibition values for each treatment were generated from the AUC data after the subtraction of the AUC of the vehicle and water group, which received vehicle at −60 min and water at 0 min. Concentrations of test compound in mouse plasma were determined by liquid chromatography/tandem mass spectrometry in blood samples collected at 60 min of the IPGTT (2 h after dosing).
Chronic treatment of established diet-induced obesity mice with GPR40 agonist.
C57BL/6N mice (Taconic Farms) were switched to a high-fat diet (60% kcal, R4129; Research Diet) at the age of 6 weeks, which was continued through out the study. Cpd-A was given to the established diet–induced obesity (eDIO) mice at age 20 weeks (14 weeks on the high-fat diet) at 10 mg/kg (oral gavage, once a day) for 10 days. On day 10 of the treatment, an IPGTT was performed as described above.
Calculations and statistics.
All data are expressed as means ± SE. Statistical analysis was conducted by using either single-factor ANOVA or Student's t test, as appropriate. Statistical significance was defined as P < 0.05.
RESULTS
Identification of small-molecule GPR40 agonists.
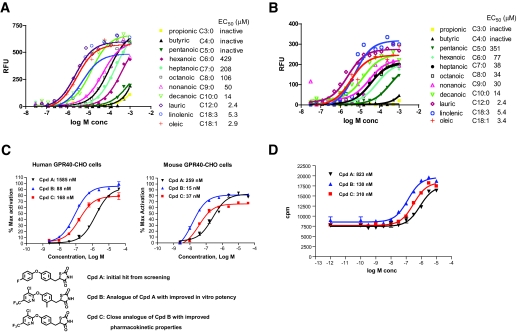
The intracellular signal transduction pathway of GPR40 proceeds through the activation of the Gq class of Gα proteins with subsequent phospholipase C activation, generation of inositol 1,4,5-triphosphate (IP3), and intracellular Ca2+ release. We confirmed that multiple medium- and long-chain FFAs activated human and mouse GPR40 expressed in CHO cells (Fig. 1A and B), whereas the short-chain FFAs (propionic, butyric, and pentanoic acid) had minimal activation against the mouse and human receptors. There also appeared to be a general increase in potency across the saturated fatty acids with increasing chain length from hexanoic acid (C6:0) to lauric acid (C12:0), as reported previously. A good correlation of potency was observed between human and mouse GPR40 by those fatty acids.
FIG. 1.
Activation of human and mouse GPR40 by fatty acids and small-molecule agonists. Representative dose responses of various fatty acids induced calcium mobilization in CHO cells stably expressing human (A) and mouse GRP40 (B) measured by the FLIPR assay. EC50 values for various fatty acids are means ± SE of three independent titration experiments. C: Chemical structures of the small-molecule GPR40 agonists and their dose responses (EC50) measured in the FLIPR-based calcium mobilization assay. D: IP3 accumulation assay for GPR40 agonists in human GPR40-HEK293 stable cells.
Kotarsky et al. (27) first showed that some thiazolidinedione ligands of the peroxisome proliferator–activated receptor-γ also activate GPR40. We thus screened ∼2,000 thiazolidinedione compounds from the Merck compound collection using the FLIPR assay in human GPR40-CHO stable cells and identified a partial agonist (relative to oleate) for GPR40 with an EC50 of 1,585 nmol/l (Fig. 1C, Cpd-A). This compound was inactive in binding assays (at concentrations up to 10 μmol/l) against human peroxisome proliferator–activated receptor-α, -δ, and -γ isoforms. Subsequent lead optimization (L. Yang, unpublished data) significantly improved the properties of Cpd-A and resulted in a series of specific and high-affinity GPR40 agonists exemplified by Cpd-B and Cpd-C. As shown in Fig. 1C and D, Cpd-B and Cpd-C elicited a dose-dependent increase in calcium mobilization (as detected by FLIPR assays) and IP3 accumulation in GPR40 stable cell lines. Cpd-B (and its des-methyl analog Cpd-C) is a full agonist at both the human and mouse GPR40 receptors, with EC50s ranging from 15 to 300 nmol/l. In general, a good correlation was observed between both assays with respect to the rank order of potency for a variety of analogs.
Acute effects of FFAs and GPR40 agonist on GDIS in islets from wild-type and GPR40−/− mice.
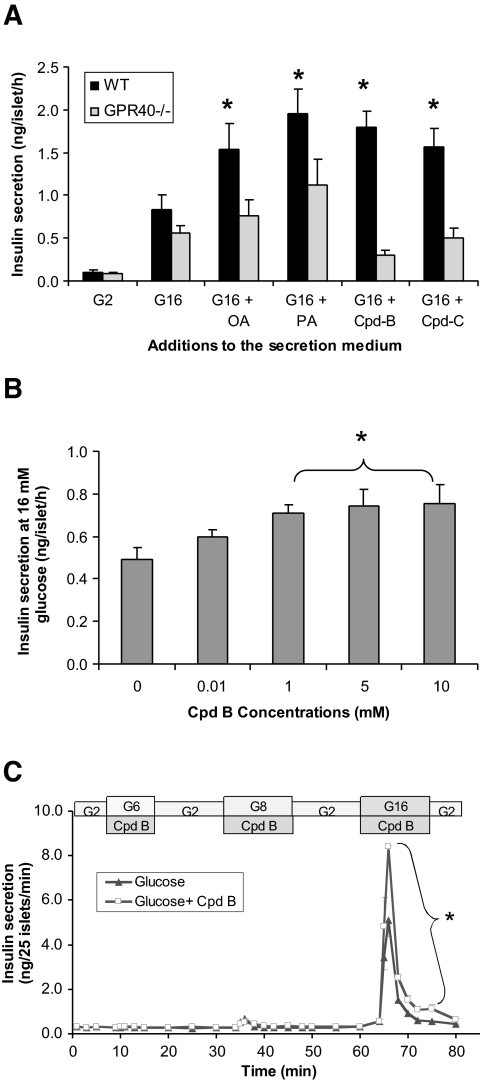
Small-molecule agonists of GPR40 have been shown by others (23,33) to enhance GDIS in insulinoma cell lines, but it has yet to be established that activation of GPR40 with synthetic agonists would enhance GDIS in primary islets. We thus examined the acute effects of FFAs and GPR40 agonists (Cpd-B and Cpd-C) on GDIS in islets from GPR40−/− and wild-type mice in both 1-h static incubations (Fig. 2A and B) and in islet perifusion experiments (Fig. 2C). Insulin secretory responses to glucose were comparable in wild-type and GPR40−/− islets in the static incubation assay. Fatty acid treatment (200 μmol/l oleate or palmitate) significantly promoted glucose-dependent insulin secretion in wild-type islets (oleate 1.9 ± 0.4-fold, palmitate 2.4 ± 0.3-fold; n = 6; P < 0.01 for both) but not in the GPR40−/− islets (oleate 1.2 ± 0.3-fold, palmitate 1.8 ± 0.5-fold; n = 6; P > 0.05 for both). Likewise, the two small-molecule GPR40 agonists, when tested at 10 μmol/l, significantly augmented GDIS in wild-type islets but were totally inactive in the GPR40−/− islets (Fig. 2A). The effects of Cpd-B on GDIS in wild-type islets were concentration dependent, and a maximal effect was reached at 5 μmol/l (Fig. 2B).
FIG. 2.
The acute effect of fatty acids and GPR40 agonists on GDIS in islets isolated from wild-type and GPR40−/− mice. A: Effects of oleate, palmitate, and the GPR40 agonists (Cpd-B and -C) on insulin secretion measured by the static incubation assay in islets from wild-type (WT) and GPR40−/− mice. Following a 30-min preincubation in KRB medium (containing 0.2% BSA) with 2 mmol/l glucose, islets were incubated with either 2 or 16 mmol/l glucose or 16 mmol/l glucose with oleic acid (OA; 200 μmol/l), palmitic acid (PA; 200 μmol/l), Cpd-B (10 μmol/l), or Cpd-C (10 μmol/l). Data are means ± SE of two independent experiments with six replicates in total. *P < 0.05 compared with G16 of wild-type islets (with DMSO added as vehicle). B: Effects of increasing concentrations of Cpd-B on insulin response at 16 mmol/l glucose in wild-type islets measured by the static incubation assay. Data are means ± SE of three experiments. *P < 0.05 compared with vehicle (DMSO) control. C: Glucose dependency of GPR40-mediated insulin secretion in mice islets. Batches of islets from C57BL/6 mice were perifused with KRB medium containing 2, 6, and 8 mmol/l glucose for 10 min each sequentially (with 10 min washout by 2 mmol/l glucose in between). Insulin released during those stimulation was measured once per minute. Data are means ± SE of three independent experiments. *P < 0.05 compared with 16 mmol/l glucose alone.
The glucose dependency of GPR40-mediated insulin secretion was also examined in the islet perifusion system. Islets from wild-type mice were sequentially stimulated by 2, 6, 8, and 16 mmol/l glucose together with Cpd-B, and insulin responses to those conditions were monitored at 1-min intervals. As shown in Fig. 2C, Cpd-B significantly enhanced insulin secretion triggered by 16 mmol/l glucose but not 6 and 8 mmol/l glucose. The AUC of insulin secretion in the presence of Cpd-B was significantly greater than that stimulated by glucose alone (32 ± 0.1 vs. 19 ± 1.2 ng/25 islet × 10 min; n = 3; P < 0.01).
Chronic effects of FFAs and GPR40 agonist in isolated islets from wild-type and GPR40−/− mice.
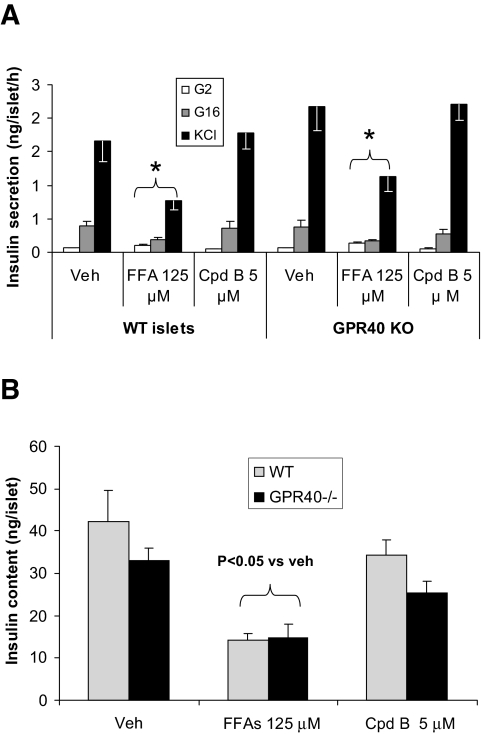
To investigate the effects of long-term activation of GPR40 by FFAs or GPR40-selective agonists on islet function, islets were isolated from the GPR40−/− and wild-type littermates and cultured for 72 h with or without FFAs (a 1:1 mixture of palmitate and oleate at a total final concentration of 125 μmol/l) or Cpd-B (5 μmol/l). Insulin secretion was measured in 1-h static incubation assays after the 3-day culture period. There was no difference in glucose- or KCl-stimulated insulin secretion between GPR40−/− and wild-type islets cultured in normal medium. As previously reported (38), the 3-day exposure to FFAs equally and significantly inhibited GDIS in wild-type and GPR40−/− islets (Fig. 3A). In addition, insulin secretion in response to membrane depolarization caused by 30 mmol/l KCl and islet insulin content were also diminished identically by the 3-day FFA treatment in islets from wild-type and GPR40−/− animals (Fig. 3A and B). In contrast, chronic treatment of islets (wild-type and knockout) with Cpd-B did not have any effect on GDIS, indicating that the GPR40 agonism, whether evoked with FFAs or structurally distinct small molecules, is not involved in the impairment of insulin secretion seen with chronic fatty acid treatment. The 3-day continuous exposure to Cpd-B apparently did not cause desensitization of the β-cells to GPR40 activation, as GDIS could be enhanced equally well when fresh compound was added to the islets that had been treated for 3 days by the compounds (data not shown).
FIG. 3.
Effects of a 3-day exposure to FFAs or Cpd-B on insulin secretion and insulin content in islets from wild-type (WT) and GPR40−/− mice. Islets from the wild-type and GPR40−/− mice were cultured for 3 days with or without fatty acids (65 μmol/l of palmitate + 65 μmol/l of oleate) or Cpd-B (5 μmol/l). Insulin secretion (A) was measured by the static insulin secretion assay in KRB medium with no FFAs or Cpd-B present. Similarly treated islets were also used for islet insulin measurement following acid ethanol extraction (B). Data are means ± SE of three separate experiments. *P < 0.05 when compared with vehicle (veh) control.
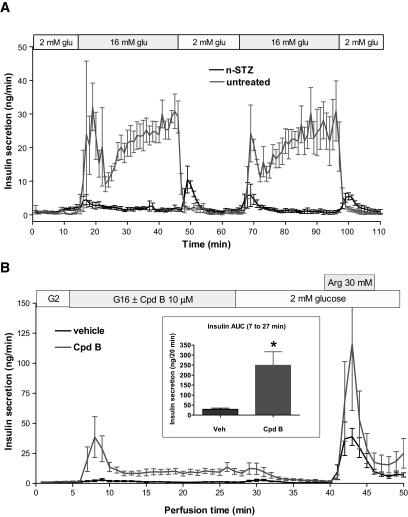
Effects of a GPR40 agonist on insulin secretion from the in situ pancreas perifusion of the neonatal STZ (nSTZ)-induced diabetic rat.
To begin to explore the potential of GPR40 agonism for the treatment of type 2 diabetes, we tested the efficacy of GPR40 agonists on ex vivo GDIS from the perfused pancreata of nSTZ-induced diabetic rats (44,45). Compared with isolated islets, this model is an attractive way to study insulin secretion dynamics in situ, as it provides improved resolution and fidelity that approaches the native setting. As shown in Fig. 4A, the pancreata from vehicle (sham) rats exhibited identical biphasic insulin secretory responses to both glucose (16 mmol/l) pulses, which were totally lost in pancreata from the nSTZ-induced diabetic rats. When present in the perfusate at 10 μmol/l during the glucose (16 mmol/l) stimulation phase, Cpd-B induced a pronounced enhancement of insulin secretion when compared with vehicle-treated pancreata (AUCinsulin: 249 ± 67 vs. 29 ± 6 ng/20 min with glucose alone; n = 4; P < 0.01). The restoration of insulin secretion by Cpd-B mainly occurred in the first phase of β-cell responses. Cpd-B also enhanced insulin secretion stimulated by 30 mmol/l arginine at the end of the perifusion experiment.
FIG. 4.
A: Insulin responses to two pulses of 16 mmol/l glucose stimulation in pancreata perfused in situ from normal and nSTZ-induced diabetic rats. Data are means ± SE of three preparations for each group. The pancreata from the normal rats exhibited identical biphasic insulin secretory responses to both glucose pulses, which were totally lost in pancreata from the nSTZ-induced diabetic rats. B: Insulin responses to glucose stimulation in the presence or absence of Cpd-B in perfused pancreata from the nSTZ-induced diabetic rats. Pancreata from the nSTZ-induced diabetic rats were challenged first by 16 mmol/l glucose and then by 30 mmol/l arginine (with 15 min washout in between) with or without 10 μmol/l Cpd-B. Data are means ± SE of five pancreatic preparations for both groups.
Effect of GPR40 agonist on IPGTT glucose levels in wild-type and GPR40−/− mice.
To extend the above results to an in vivo setting, we tested the effects of our small-molecule GPR40 agonists on glucose excursion during IPGTT in normal lean mice. Cpd-C was selected for this experiment based on pharmacokinetic considerations. Cpd-C possesses excellent oral bioavailability (∼100%), a plasma half-life of 8 h, time to reach maximum concentration (Tmax) of 3 h, and maximum concentration (Cmax) of 1.4 μmol/l, following a 2 mg/kg oral dose (data not shown). The oral administration of Cpd-C, 1 h before the dextrose challenge in the IPGTT, significantly reduced blood glucose excursion in a dose-dependent manner from 3 to 100 mg/kg, with maximum efficacy (73% inhibition of AUCglucose) achieved at ∼30 mg/kg and a corresponding plasma concentration of 37 μmol/l measured 2 h postdose (Fig. 5A). The glucagon-like peptide 1 mimetic exendin-4 was included as a positive control, and it completely prevented any glucose excursion at a concentration of 0.0025 mg/kg.
FIG. 5.
Glucose-lowering efficacy of Cpd-C in wild-type C57BL/6 and GPR40−/− mice. A: Effects of increasing doses of Cpd-C on blood glucose levels during the IPGTT in wild-type mice. C57BL/6 mice were dosed with vehicle (0.25% methylcellulose) or Cpd-C (1 ∼100 mg/kg) by oral gavage at −60 min, followed by intraperitoneal glucose challenge (2 g dextrose per kg body wt; same volume of H2O only for the vehicle-water group) at 0 min. Blood glucose levels were measured in whole-blood samples obtained by tail sniping at the intervals indicated in the graphs. The percent inhibition of glucose levels was calculated based on the glucose AUC during the 60-min IPGTT for each group after subtracting the values from those of the baseline (no drug and no glucose) control group. Data are means ± SE of 7–10 mice per group. *P < 0.01 compared with vehicle-treated animals. B: The GPR40−/− mice and the littermate wild-type mice were dosed with vehicle or 30 mg/kg Cpd-C 60 min before the IPGTT as described above for A. Data are means ± SE of 7–10 mice per group. *P < 0.05 compared with vehicle treated animals.
To demonstrate that the observed Cpd-C–induced glucose lowering was GPR40 dependent, the effects of the ligand on blood glucose excursion during an IPGTT were investigated again in a cohort of GPR40−/− mice and littermate wild-type mice. The administration of 30 mg/kg Cpd-C again resulted in a significant suppression of AUCglucose during IPGTT in the wild-type mice. In contrast, the same dose of the compound exerted no inhibition of blood glucose excursion in the GPR40−/− mice (Fig. 5B). The above findings demonstrated that robust glucose lowering in normal wild-type mice by Cpd-C is mediated by GPR40.
Effect of acute and chronic treatment with GPR40 agonist on IPGTT glucose levels in eDIO mice.
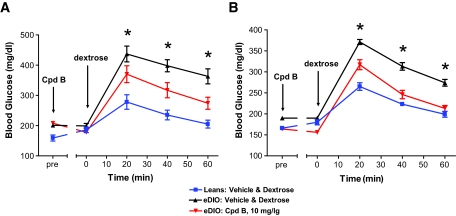
To further evaluate the potential of GPR40 activation for treatment of type 2 diabetes, we studied the effect of GPR40 agonist (Cpd-B) on IPGTT glucose levels in high-fat diet–induced obese (eDIO) mice both acutely and subchronically. We induced eDIO in C57BL/6 mice with a 60% high-fat diet for 14 weeks (started at age 6 weeks). The effects of Cpd-B (10 mg/kg, oral gavage) on IPGTT glucose were tested before and after 10 days of continuous dosing (10 mg/kg, daily). The eDIO mice weighed significantly heavier (42 ± 0.6 vs. 28 ± 0.4 g; n = 8; P < 0.001) and manifested impaired glucose tolerance compared with mice on regular diet (AUCglucose: 18,311 ± 272 vs. 13,540 ± 326 mg · dl−1 · 60 min−1; n = 8; P < 0.001). Acute treatment of the eDIO mice with Cpd-B (10 mg/kg) significantly reduced IPGTT glucose levels by ∼50% (Fig. 6A). The glucose-lowering efficacy of Cpd-B was well maintained after a subchronic dosing. As measured by the IPGTT performed on the last day of compound treatment (Fig. 6B), 10 mg/kg Cpd-B reduced glucose excursion by 70%, similar to the acute efficacy achieved in these mice before the initiation of the 10-day dosing period. Although we did not measure food intake in this study, the chronic efficacy does not appear to be attributable to any changes in food intake and body weight of the mice. There were no differences in body weight between vehicle- and Cpd-B–treated mice either before (42 ± 0.6 g for the vehicle vs. 41 ± 0.7 g for the Cpd-B group; n = 8; P > 0.05) or after (44 ± 0.8 g for the vehicle vs. 45 ± 0.8 g for the Cpd-B group; n = 8; P > 0.05) the chronic treatment.
FIG. 6.
Effects of chronic treatment with GPR40 agonist (Cpd-B) on IPGTT glucose levels in high-fat diet–induced obese (eDIO) mice. C57BL/6 mice were switched to a 60% high-fat diet (D12492i) at the age of 6 weeks and kept on the same diet throughout the study. The treatment with the GPR40 agonist (10 mg/kg Cpd-B, oral gavage, once a day) was started 14 weeks after the initiation of high-fat–diet feeding. An IPGTT (1 g dextrose/kg body wt) was performed on day 0 (A) and day 10 (B) of the 10-day-long treatment with Cpd-B to compare the glucose-lowering efficacy of Cpd-B before (A) and after (B) the chronic treatment. The final dose on day 10 was given 1 h before glucose challenge. Blood glucose levels were measured in whole-blood samples obtained by tail snipping at the intervals indicated in the graphs. There were eight mice per group. Single-factor ANOVA was used to compare the difference in glucose AUC among the groups. *P < 0.01 compared with vehicle-treated DIO mice.
To determine whether acute dosing of GPR40 agonist promotes in vivo insulin secretion in mice, we performed a slightly modified IPGTT experiment using mice that had been fed with the 60% high-fat diet for 4 weeks. In that cohort of high-fat diet–fed mice, Cpd-B (30 mg/kg, administrated orally 60 min before glucose challenge) did not affect the basal insulin levels at 0 min (1.72 ± 0.5 vs. 1.26 ± 0.2 ng/ml) but significantly enhanced insulin responses at 5 min during an IPGTT relative to vehicle control (3.8 ± 0.4 vs. 2.4 ± 0.4 ng/ml; n = 8; P < 0.02).
DISCUSSION
GPR40 is a Gq-coupled family A GPCR specifically expressed in the pancreatic β-cell (46,47). The discovery of its activation by medium- and long-chain FFAs has sparked considerable interest in and experimentation on this receptor, from basic research to potential drug discovery efforts. Nevertheless, there are still several important questions that remain to be answered. How much does GPR40 contribute to the acute (stimulatory) and chronic (inhibitory) effects of FFAs on islet function? What will be the consequences of acute and chronic activation of GRP40 with a pharmacophore on islet function and beyond? Can agonists of GPR40 stimulate sufficient GDIS to result in the reduction of blood glucose in normal and diabetic animals? We set out to address some of these questions in this study using potent selective agonists of the receptor in conjunction with GPR40 knockout mice.
GPR40 has been previously shown to mediate part of the enhancement of GDIS by FFAs (i.e., the acute effect of FFAs on insulin secretion) (22,23,29–33,38) but not the chronic toxic effects of FFAs in islets (38). The results from this study are largely consistent with those findings. Our data indicate that both oleate and palmitate lost the majority of their actions on GDIS in GPR40-depleted islets, thus suggesting that GPR40 is a major, if not the sole, mediator of the acute stimulatory action of FFAs on GDIS (no attempt was made to calibrate precisely their EC50 and maximal activity on insulin secretion). The residual effects of FFAs observed in the GPR40−/− islets could be mediated by the intracellular metabolism/oxidation of FFAs (35) or by additional cell surface receptors such as GPR120 (23).
The potential role of GPR40 in mediating the chronic inhibitory effects of FFAs on islet function is also a matter of debate. Overexpression of GPR40 selectively in pancreatic β-cells caused dramatic disintegration of the islets and severe hyperglycemia in an insulin promoter factor 1–GPR40 transgenic line (39). On the other hand, islets from the GPR40 knockout mice appear to be as vulnerable as wild-type islets to the detrimental effects from FFAs in vitro (38). Our study has provided additional support for the latter observation, namely, that GPR40 does not mediate β-cell lipotoxicity. Similar to what was shown by Latour et al. (38), we found that 3 days of exposure to FFAs caused comparable inhibition of GDIS in wild-type and GPR40−/− islets. In addition, we observed significant and identical reductions in islet insulin content in wild-type and GPR40−/− islets, an important feature of the chronic inhibitory effects exerted by FFAs in β-cells (42). We thus conclude that GPR40 is not responsible for lipotoxicity in β-cells exposed to elevated FFAs in vitro or in vivo.
Small-molecule agonists of GPR40 have recently been reported by at least two groups (23,33). Yet, to the best of our knowledge, the utility of such agents for potentiating GDIS as a novel therapy of type 2 diabetes has yet to be disclosed. Accordingly, we studied the effects of a novel GPR40 agonist discovered in our laboratories upon GDIS in several experimental paradigms. First, we showed that the GPR40 agonist enhanced GDIS in mouse islets using both static incubation and islet perifusion methods; the effects were dependent on the presence of GPR40. Second, we demonstrated that the action of our GPR40 agonist was strictly glucose dependent. Third, we found that the GPR40 agonist restored GDIS (at least the first phase) in pancreatic β-cells from the nSTZ rat, a diabetes model that possesses three key traits similar to those seen in the human disease: sustained hyperglycemia, a substantial reduction in β-cell mass (>90% reduction in pancreatic insulin content), and impaired GDIS in the residual β-cell population (44,45). The significant restoration of GDIS (particularly the first phase of insulin secretion) in this model by Cpd-B strongly suggests that such ligands will be efficacious in treating type 2 diabetic patients with compromised β-cell function and mass. Finally, our compound exerted robust glucose-lowering efficacy during an IPGTT in mice. This is the first report of a GPR40 agonist demonstrating glucose control efficacy in preclinical animals. Taken together, our findings support the proposition that GPR40 agonists may be beneficial for restoring GDIS and glucose control in type 2 diabetes and, therefore, merit further evaluation in clinical studies. Results from the present study do not support the assertion that chronic activation of GPR40 may harm β-cell function (the “lipotoxicity” hypothesis). In contrast, our study has provided strong evidence that activation of GPR40 can enhance GDIS in islet β-cells from normal and diabetic rodents and thereby improve glucose tolerance.
Published ahead of print at http://diabetes.diabetesjournals.org on 13 May 2008.
C.P.T. and Y.F. contributed equally to this article.
R.M. is currently affiliated with Pharmasset, Princeton, New Jersey.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Leahy JL: Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36: 197–209, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Wajchenberg BL: Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28: 187–218, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Doyle ME, Egan JM: Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev 55: 105–131, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Rendell M: The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs 64: 1339–1358, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY: Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 90: 501–506, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Del Guerra S, Marselli L, Lupi R, Boggi U, Mosca F, Benzi L, Del Prato S, Marchetti P: Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J Diabetes Complications 19: 60–64, 2005 [DOI] [PubMed] [Google Scholar]
- 7.U.K. Prospective Diabetes Study Group: U.K. Prospective Diabetes Study 16: overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 44: 1249–1258, 1995 [PubMed] [Google Scholar]
- 8.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355: 2427–2443, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Grant RW, Wexler DJ, Watson AJ, Lester WT, Cagliero E, Campbell EG, Nathan DM: How doctors choose medications to treat type 2 diabetes: a national survey of specialists and academic generalists. Diabetes Care 30: 1448–1453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet P, Shaw J: International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 24: 451–463, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Donath MY, Halban PA: Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 47: 581–589, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Rhodes CJ: Type 2 diabetes: a matter of beta-cell life and death? Science 307: 380–384, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Prentki M, Nolan CJ: Islet beta cell failure in type 2 diabetes. J Clin Invest 116: 1802–1812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drucker DJ: The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Drucker DJ: Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 30: 1335–1343, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Brubaker PL: Incretin-based therapies: mimetics versus protease inhibitors. Trends Endocrinol Metab 18: 240–245, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Herman GA, Stein PP, Thornberry NA, Wagner JA: Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther 81: 761–767, 2007 [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28: 1092–1100, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Im DS: Discovery of new G protein-coupled receptors for lipid mediators. J Lipid Res 45: 410–418, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kostenis E: A glance at G-protein-coupled receptors for lipid mediators: a growing receptor family with remarkably diverse ligands. Pharmacol Ther 102: 243–257, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wu X, Simonavicius N, Tian H, Ling L: Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem 281: 34457–34464, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M: Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S: Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148: 619–628, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G: Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Chu Z-L, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J: A role for {beta}-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 148: 2601–2609, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Rayasam GV, Tulasi VK, Davis JA, Bansal VS: Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin Ther Targets 11: 661–671, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B: A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 301: 406–410, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI: The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Itoh Y, Hinuma S: GPR40, a free fatty acid receptor on pancreatic beta cells, regulates insulin secretion. Hepatol Res 33: 171–173, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Feng DD, Luo Z, Roh SG, Hernandez M, Tawadros N, Keating DJ, Chen C: Reduction in voltage-gated K+ currents in primary cultured rat pancreatic beta-cells by linoleic acids. Endocrinology 147: 674–682, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara K, Maekawa F, Yada T: Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab 289: E670–E677, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Schnell S, Schaefer M, Schofl C: Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from beta-cells through activation of GPR40. Mol Cell Endocrinol 263: 173–180, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Song F, Lu S, Gunnet J, Xu JZ, Wines P, Proost J, Liang Y, Baumann C, Lenhard J, Murray WV, Demarest KT, Kuo GH: Synthesis and biological evaluation of 3-aryl-3-(4-phenoxy)-propionic acid as a novel series of G protein-coupled receptor 40 agonists. J Med Chem 50: 2807–2817, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Prentki M, Vischer S, Glennon MC, Regazzi R, Deeney JT, Corkey BE: Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J Biol Chem 267: 5802–5810, 1992 [PubMed] [Google Scholar]
- 35.Warnotte C, Gilon P, Nenquin M, Henquin JC: Mechanisms of the stimulation of insulin release by saturated fatty acids: a study of palmitate effects in mouse β-cells. Diabetes 43: 703–711, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Zhou YP, Grill VE.: Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 93: 870–876, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poitout V, Robertson RP: Glucolipotoxicity: fuel excess and {beta}-cell dysfunction. Endocr Rev 29: 351–366, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V: GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 56: 1087–1094, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H: The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab 1: 245–258, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Lacy PE, Kostianovsky M: Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16: 35–39, 1967 [DOI] [PubMed] [Google Scholar]
- 41.Herrington J, Zhou YP, Bugianesi RM, Dulski PM, Feng Y, Warren VA, Smith MM, Kohler MG, Garsky VM, Sanchez M, Wagner M, Raphaelli K, Banerjee P, Ahaghotu C, Wunderler D, Priest BT, Mehl JT, Garcia ML, McManus OB, Kaczorowski GJ, Slaughter RS: Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 55: 1034–1042, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Zhou YP, Marlen K, Palma JF, Schweitzer A, Reilly L, Gregoire FM, Xu GG, Blume JE, Johnson JD: Overexpression of repressive cAMP response element modulators in high glucose and fatty acid-treated rat islets: a common mechanism for glucose toxicity and lipotoxicity? J Biol Chem 278: 51316–51323, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Zhou YP, Cockburn BN, Pugh W, Polonsky KS: Basal insulin hypersecretion in insulin-resistant Zucker diabetic and Zucker fatty rats: role of enhanced fuel metabolism. Metabolism 48: 857–864, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Portha B, Blondel O, Serradas P, McEvoy R, Giroix MH, Kergoat M, Bailbe D: The rat models of non-insulin dependent diabetes induced by neonatal streptozotocin. Diabetes Metab 15: 61–75, 1989 [PubMed] [Google Scholar]
- 45.Thyssen S, Arany E, Hill DJ: Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology 147: 2346–2356, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Tomita T, Masuzaki H, Iwakura H, Fujikura J, Noguchi M, Tanaka T, Ebihara K, Kawamura J, Komoto I, Kawaguchi Y, Fujimoto K, Doi R, Shimada Y, Hosoda K, Imamura M, Nakao K: Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic beta cells and implications for insulin secretion. Diabetologia 49: 962–968, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Bartoov-Shifman R, Ridner G, Bahar K, Rubins N, Walker MD: Regulation of the gene encoding GPR40, a fatty acid receptor expressed selectively in pancreatic beta cells. J Biol Chem 282: 23561–23571, 2007 [DOI] [PubMed] [Google Scholar]