Abstract
OBJECTIVE—The worldwide prevalence of obesity is increasing at an alarming rate, along with the associated increased rates of type 2 diabetes, heart disease, and some cancers. While efforts to address environmental factors responsible for the recent epidemic must continue, investigation into the anorectic functions of potential molecules we present here, such as apolipoprotein (apo)E, offers exciting possibilities for future development of successful anti-obesity therapies.
RESEARCH DESIGN AND METHODS—Changes in feeding behavior after intracerebroventricular injection of apoE, the regulation of hypothalamic apoE gene expression by energy status, and the interaction of hypothalamic apoE with other neuropeptides were studied.
RESULTS—Intracerebroventricular apoE significantly decreased food intake without causing malaise, whereas intracerebroventricular infusion of apoE antiserum stimulated feeding, implying that endogenous apoE tonically inhibits food intake. Consistent with this, apoE was present in the hypothalamus, a brain site intimately involved in the integration of signals for energy homeostasis. Fasted rats exhibited significantly decreased apoE gene expression in the hypothalamus, and refeeding of these rats for 4 h evoked a significant increase of hypothalamic apoE mRNA levels. Both genetically obese (ob/ob) mice and rats with high-fat diet–induced obesity had significantly reduced hypothalamic apoE mRNA levels compared with their lean control counterparts, suggesting that decreased apoE may contribute to hyperphagia in these obese animals. Additionally, apoE-stimulated hypothalamic proopiomelanocortin gene expression and SHU9119, a melanocortin 3/4 receptor antagonist, attenuated the inhibitory function of apoE on feeding.
CONCLUSIONS—These data demonstrate that apoE suppresses food intake via a mechanism enhancing melanocortin signaling in the hypothalamus.
Elevated body weight resulting in obesity is a major health problem in the U.S. and is associated with significant morbidity and mortality from coronary heart disease, type 2 diabetes, and other disorders. One well-established risk factor for becoming obese is food consumption in excess of energy expenditure. Evidence links a complex circuitry in the mediobasal hypothalamus with the control of food intake and energy expenditure. Obese animals have a blunted response to satiation signals such as leptin and insulin, raising the possibility that defective satiation signaling within the central nervous system and/or peripheral tissues may contribute to the etiology of obesity.
Apolipoprotein (apo)E, a 34,000-kDa glycoprotein involved in lipid metabolism (1), is predominantly produced in the liver and brain (2). Recent data suggest that apoE plays additional roles in the brain, regulating both neuronal and astrocyte functions, including preventing excitotoxicity (3), promoting neuron survival and sprouting (4), protecting neurons against oxidative stress (5), and regulating innate and adaptive immune responses (6). We here describe a novel and critical role of brain apoE in the regulation of food intake.
Because targeted absence of apoE yields significantly elevated food intake and body weight in mice fed a diabetogenic diet (7), we hypothesized that apoE participates in regulating energy homeostasis. In support of our hypothesis, we found that 1) intracerebroventricular administration of apoE significantly reduces food intake and body weight without causing malaise, whereas intracerebroventricular infusion of apoE antiserum stimulates feeding; 2) apoE is present in the arcuate nucleus (ARC) and paraventricular nucleus (PVN) of the hypothalamus; 3) genetically obese (ob/ob) mice and high-fat (HF) diet–induced obese rats have significantly reduced hypothalamic apoE mRNA levels; 4) hypothalamic apoE gene expression is regulated by nutritional status; and 5) apoE exerts its anorectic action by interacting with the central melanocortin system.
RESEARCH DESIGN AND METHODS
Animals and surgical procedures.
Male adult Long-Evans rats (Harlan, Indianapolis, IN) and male ob/ob and wild-type C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were individually housed in a temperature-controlled vivarium on a 12/12-h light/dark cycle (lights on at 0200 h, except where noted). Laboratory chow (Purina 5001, Hudson, NH) and water were provided ad libitum except where noted. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
Rats anesthetized with ketamine (80 mg/kg)/xylazine (1.6 mg/kg) were implanted with 22-gauge stainless steel cannulas (Plastics One, Roanoke, VA) aimed at the third-cerebral ventricle. Coordinates were 2.2 mm posterior to bregma and 7.4 mm ventral to dura as described previously (8,9). Placement was confirmed 5 days later by administration of 10 ng angiotensin II in saline while the animals were water replete. Animals that did not drink at least 5 ml water within 30 min were considered to have failed cannula placement and were not used. Animals were allowed at least 7 days recovery before commencement of experimentation.
To implant intravenous cannulas, the skin was incised between the right front paw and the chin. The jugular vein was clamped ∼1 cm anterior to the external/internal branching, and a sterile silicon catheter containing heparinized saline was inserted with the tip positioned in the right atrium. The catheter was secured with sutures on both sides of the silicon ring. The catheter was passed subcutaneously to the top of the skull and connected to a piece of stainless steel tubing, which was secured by dental acrylic to the skull screws. To prevent clotting, the catheter was maintained with a heparin solution.
Materials.
Rat apoE was purified from plasma obtained from abdominal aorta blood of Sprague-Dawley rats. The plasma was centrifuged at a density of >1.065 g/ml to isolate apoB-containing lipoproteins. After dialysis to remove KBr, apolipoproteins in the HDL and greater were separated by SDS-PAGE and purified by gel extraction as described previously (10). We performed analytical PAGE on the isolated apoE and found only a single band consistent with the molecular weight of apoE protein (11). Goat polycolonal anti-apoE antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) (12,13) and SHU9119 (Phoenix Pharmaceuticals, Mountain View, CA) were used.
Food intake in ad libitum–fed and fasted rats.
Food intake of each rat was measured for at least 3 days to establish a baseline. On test days, ad libitum–fed rats were weighed and food was removed 2 h before the beginning of the dark cycle. Other rats were fasted for 24 h before lights off. Rats in both conditions received intracerebroventricular apoE in saline at doses of 0, 1, 2, 4, or 8 μg/rat just before dark. Infusions were delivered at a rate of 1 μl/min (total 4 μl). Food intake was recorded after 0.5, 1, 2, and 4 h.
Conditioned taste aversion.
Rats were adapted to 1-h daily access to water (available in two bottles) for 10 days. On day 11, rats were given 0.15% saccharin in water in each bottle for the 1-h period instead of water and then were injected with apoE (4 or 8 μg/rat i.c.v.). Other rats received LiCl as a positive control (0.15 mol/l; 127 mg/kg i.p.). On days 12–13, animals had water in both bottles during the 1-h session, and on the test day (day 14), rats were presented with one bottle containing saccharin and one containing water. Saccharin preference ratio was calculated as the amount of saccharin consumed divided by the total consumption of both liquids over the hour.
Intracerebroventricular injection of apoE antibody.
Rats with intracerebroventricular cannulas were subdivided into three groups of equal body weight. ApoE antibody (20 and 40 μg protein in 4 μl sterile saline) was administered intracerebroventricularly at a rate of 1 μl/min, starting at 1100 h, in the middle of the light period when rats normally eat very little. As a control, pre-absorbed apoE antiserum (40 μg protein) was infused intracerebroventricularly at the same rate. Preabsorbed apoE antiserum was generated using cyanogens-bromide–activated agarose matrices (Sigma) according to the manufacturer's instruction. Feeding and drinking behavior were observed for 60 min after the injections.
In a subsequent experiment, rats were divided into four groups. Groups 1 and 2 received intracerebroventricular saline (2 μl), and groups 3 and 4 received intracerebroventricular apoE antiserum (20 μg protein/rat) 30 min before dark. Thirty minutes later, groups 1 and 3 received 4 μl saline intracerebroventricularly, and groups 2 and 4 received intracerebroventricular apoE (4 μg/rat). Food intake was measured after 2 h.
Western blot analysis.
Hypothalamus samples were homogenized and centrifuged at 15,000 rpm for 20 min at 4°C. The supernatant (containing 40 μg protein per sample) was separated by 12% polyacrylamide gel electrophoresis, transferred to nitrocellulose sheets, and blotted with a polyclonal antibody against apoE (1:1,000). Immunocomplexes were quantitated using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were stripped and re-incubated with a monoclonal antibody against actin (1:10,000; Chemicon International). Membranes were exposed to X-ray film (Kodak Scientific Imaging Film; Kodak, Rochester, NY), and film density, measured as transmittance, was expressed as volume-adjusted optical density. The amount of apoE protein was normalized to the respective individual density values reflecting actin protein level and is expressed as a ratio.
Immunohistochemistry.
Rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and then perfused via the ascending aorta with 100 ml of a 0.9% NaCl solution followed by 500 ml of ice-cold 4% paraformaldehyde in 0.1 mol/l phosphate buffer (pH 7.4). Brains were removed and placed in perfusion buffer for 4 h at 4°C and then in buffered sucrose (30%) for at least 24 h, and 30-μm serial coronal sections were cut using a microtome. After washing, sections were blocked with 5% normal donkey serum in PBS containing 0.3% Triton X-100 for 2 h. Then, sections were incubated with goat anti-apoE antiserum (1:400) overnight at 4°C. The secondary antibody was donkey anti-goat immunoglobulin conjugated to fluorescein Alexa 594 (1:400; Molecular Probes). Imaging was performed on a Zeiss 510 microscope system. Omission of the primary antibody and substituting the primary antibody with apoE preabsorbed serum confirmed antibody specificity.
Quantitative PCR for apoE mRNA measurement.
Total RNA (100 ng), extracted from hypothalamus with Tri Reagent (Molecular Research Center, Cincinnati, OH), was reverse-transcribed to first-strand complementary cDNA (GE Healthcare Bio-Sciences, Piscataway, NJ). Quantitative real-time PCR (qPCR) was performed in a 25-μl final reaction volume with an iCycler iQ Detection System using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). qPCR conditions were as follows: 95°C for 3 min for one cycle, followed by 38 cycles of 95°C for 30 s and 58°C for 30 s. Melt curve analysis revealed one peak per sample. Threshold cycle (Ct) readings for unknown samples were calculated, and results were analyzed using the delta-delta CT method (Perkin-Elmer Applied Biosystems) (14). Other neuropeptide mRNA levels, including proopiomelanocortin (POMC), neuropeptide Y (NPY), and agouti-related protein (AgRP), were examined likewise. Cyclophilin was used as the internal control. Primer sequences, listed in Table 1, for rat and mouse apoE, rat POMC, NPY, AgRP, and cyclophilin were determined using primer design software (Integrated DNA Technologies, Coralville, IA).
TABLE 1.
Primer sequences and product size for qPCR
| Accession number | Forward primer | Reverse primer | Size (bp) | |
|---|---|---|---|---|
| Rat apoE | BC086581 | 5′-ttggtcccattgctgacag-3′ | 5′-accgtcagttcctgtgtgac-3′ | 189 |
| Mouse apoE | BC044785 | 5′-aacagacccagcaaatacgcc-3′ | 5′-ctcattgattctcctgggcc-3′ | 154 |
| POMC | NM_139326 | 5′-cgcccgtgtttcca-3′ | 5′-tgacccatgacgtacttcc-3′ | 69 |
| NPY | NM_012614 | 5′-ccttgcgacactacatcaa-3′ | 5′-ggggcattttctgtgcttt-3′ | 108 |
| AgRP | AF206017 | 5′-ttcccagagttctcaggtcta-3′ | 5′-atctagcacctctgccaaa-3′ | 98 |
| Cyclophilin | M19533 | 5′-attcatgtgccagggtggtgac-3′ | 5′-tcagtcttggcagtgcagat-3′ | 182 |
Rats with HF diet–induced obesity.
Two pelleted semi-purified nutritionally complete experimental diets prepared by Dyets (Bethlehem, PA) were used. As described previously (15), the HF diet contained 20 g fat (19 g butter oil and 1 g soybean oil)/100 g diet, and the low-fat (LF) diet contained 4 g fat (3 g butter oil and 1 g soybean oil)/100 g diet by weight. Rats were divided into three groups, with HF rats allowed ad libitum access to HF diet, LF rats allowed ad libitum access to LF diet, and a pair-fed HF group fed HF diet each day in an amount limited to the average daily caloric consumption of the rats fed the LF diet. Rats were maintained on their diet for 10 weeks, throughout which the mean energy intake of the LF and the pair-fed HF rats was 83% of that of the HF group (15). After 10 weeks, rats were fasted overnight and decapitated, and hypothalami were dissected and stored at −80°C until measurement of apoE mRNA.
Effects of apoE on the gene expression of neuropeptides in the hypothalamus.
We investigated the effects of apoE on gene expression of several neuropeptides in four groups of rats. Groups 1 and 2 were ad libitum–fed, and groups 3 and 4 were fasted for 46 h. Groups 2 and 4 received intracerebroventricular apoE (8 μg), and groups 1 and 3 received intracerebroventricular saline. Rats were killed 2 h after injections, and hypothalamic gene expression was characterized using qPCR measurement of POMC, NPY, and AgRP.
Effect of SHU9119 on apoE-elicited suppression of food intake and body weight.
Rats were fasted 4 h before lights-off (1400 h) and received two injections on the test day: saline (vehicle for SHU9119) + saline (vehicle for apoE), saline + apoE (2 μg), SHU9119 (0.1 nmol) + saline, and SHU9119 (0.1 nmol) + apoE (2 μg). The dose of SHU9119 has been reported to have no effect on food intake or body weight of rats (16). The first injection occurred at 1330 h and was followed 30 min later by the second injection. Food was then returned, and intake was measured after 4 h. Every rat was subjected to each treatment, with at least 5 days between tests.
Statistical analysis.
Data were analyzed using parametric statistics (Sigma Stat version 3.1). Two-way repeated-measures ANOVA followed by Tukey's test was used for analysis of food intake. For other comparisons, i.e., apoE or other gene mRNA levels, either a one-way ANOVA (for more than a two-group comparison) followed by Tukey's test or an unpaired Student's t test (for two-group comparison) was used. P values <0.05 were considered statistically significant.
RESULTS
Intracerebroventricular apoE suppressed food intake.
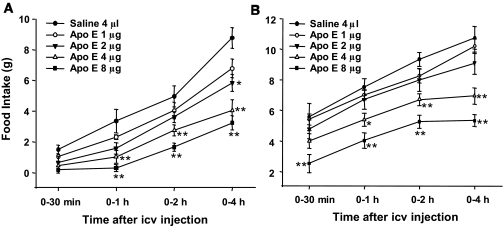
To examine the effect of apoE on food intake, we administered doses from 1 to 8 μg of apoE into the third-cerebral ventricle of rats. As depicted in Fig. 1, intracerebroventricular apoE at doses ≥2 μg produced a significant and dose-dependent inhibition of food intake in both ad libitum (Fig. 1A) and 24 h–fasted (Fig. 1B) conditions. To determine whether the central nervous system is the critical site of action for intracerebroventricular apoE, intake of rats administered apoE intravenously at doses that were effective centrally (4 and 8 μg) did not differ in food intake from those receiving vehicle at any time point, implying that intracerebroventricular apoE acts within the central nervous system to suppress food intake rather than leaking into the blood and acting at peripheral apoE receptors.
FIG. 1.
Central administration of apoE dose-dependently reduced food intake at the beginning of the dark period in ad libitum–fed (A) or 24 h–fasted (B) rats. Values are expressed as means ± SE, n = 7. icv, intracerebroventricular. *P < 0.05, **P < 0.01 vs. saline controls.
Reduction of food intake was not associated with illness.
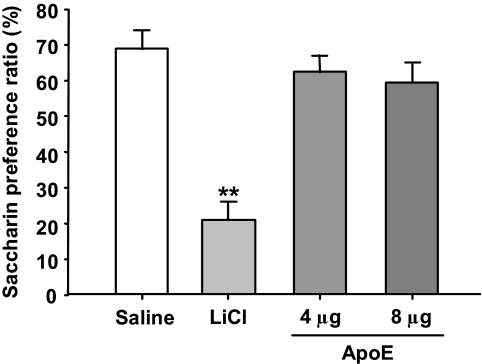
To determine whether apoE-induced reduction in food intake is secondary to illness, a conditioned taste aversion paradigm was conducted (8). Rats receiving saccharin paired with intracerebroventricular apoE (4 or 8 μg) had comparable preference for saccharin, as did the saline controls (Fig. 2). Animals receiving LiCl paired with saccharin consumed significantly less saccharin than the controls (P < 0.01), indicating that malaise is not a factor in the suppressive activity of apoE on food intake.
FIG. 2.
Intraperitoneal injection of lithium chloride (LiCl), but not intracerebroventricular apoE (4 or 8 μg), significantly reduced saccharin intake. Values are expressed as means ± SE, n = 8. **P < 0.01 vs. saline controls.
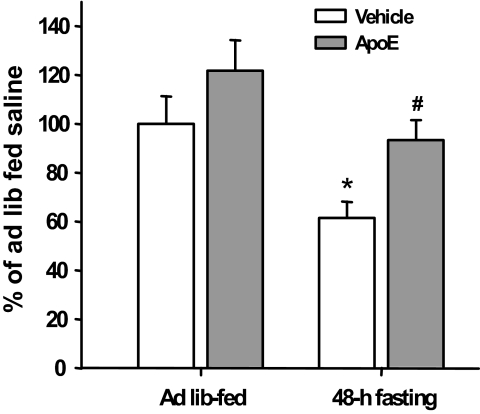
Intracerebroventricular apoE antiserum stimulated food intake and attenuated the anorectic effect of apoE.
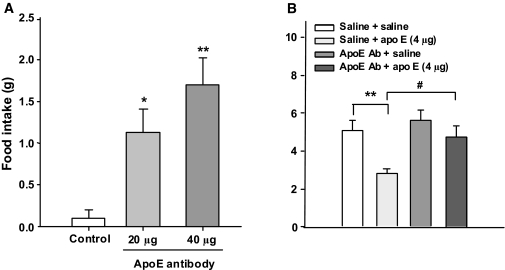
To determine whether endogenous apoE signaling influences food intake, we administered apoE antiserum intracerebroventricularly to ad libitum–fed rats in the middle of the light. ApoE antiserum elicited feeding and significantly increased mean food intake (Fig. 3A); prandial drinking also increased (data not shown). No adverse physiological reactions such as sedation or ataxia were observed. The intracerebroventricular infusion of preabsorbed apoE serum failed to elicit a feeding response. A subsequent experiment determined if apoE antiserum could specifically block apoE anorectic action. As depicted in Fig. 3B, intracerebroventricular apoE alone significantly reduced food intake, and this effect was attenuated by the antibody at 2 h.
FIG. 3.
A: Blocking the action of endogenous apoE in the brain with its antibody induces feeding even during the light phase. Data are means ± SE, n = 8, *P < 0.05, **P < 0.01 vs. control (apoE preabsorbed serum) group. B: The anorectic effect of apoE was attenuated by the infusion of its antibody in ad libitum–fed rats. n = 7, **P < 0.01 vs. saline group; #P < 0.05 vs. the apoE group. ApoE Ab, apoE antibody.
ApoE is present in the hypothalamus.
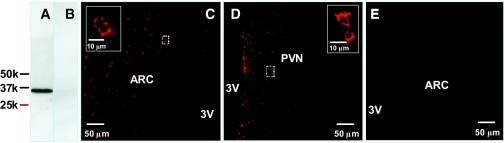
Using apoE antiserum, Western immunoblotting of hypothalamic proteins revealed an immunoreactive band of weight 34,000 kDa (Fig. 4A), corresponding to the molecular weight of rat apoE (11). Signal abolition by preabsorbing the antibody with purified rat apoE confirmed antibody specificity (Fig. 4B). To localize the distribution of apoE in the hypothalamus we used immunofluorescence labeling and observed a prominent presence of apoE protein in the ARC (Fig. 4C) and PVN (Fig. 4D). No immunoreactivity was observed in brain sections of apoE KO mice treated likewise, further confirming specificity of immunostaining. The distribution of apoE in the brain is consistent with a role in the regulation of energy homeostasis (17).
FIG. 4.
A and B: Western blot analysis of hypothalamic proteins using apoE antibody and apoE preabsorbed antibody, respectively. C and D: Immunofluorescence photomicrograph showing the presence of apoE in the ARC and PVN of rat hypothalamus. E: No apoE immunostaining was observed in the ARC of apoE KO mice using the same apoE antiserum. Solid boxes in each image are a high-magnified image of the area dotted boxes. 3V, third ventricle. (Please see http://dx.doi.org/10.2337/db08-0291 for a high-quality digital representation of this figure.)
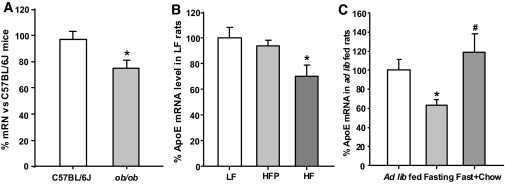
ApoE mRNA levels are reduced in the hypothalamus of genetically obese (ob/ob) mice and rats with HF-diet–induced obesity.
ob/ob mice are a well-known animal model for obesity (18). Using qPCR, we found that apoE mRNA levels are significantly reduced in the hypothalami of ob/ob mice compared with those in lean control animals (P < 0.05, Fig. 5A). To determine whether diet-induced obesity alters apoE gene expression in the hypothalamus, rats were fed an HF or LF diet for 10 weeks, after which the body weight of HF rats was significantly heavier than that of the other groups. As expected, pair-fed HF rats weighed less than HF rats (P < 0.05) and were not different from the LF rats (data not shown), consistent with what we have reported previously (15). Compared with LF rats, HF rats had significantly reduced hypothalamic apoE gene expression. Hypothalamic apoE levels of the LF and pair-fed HF groups were not statistically different (Fig. 5B).
FIG. 5.
A and B: Comparison of hypothalamic apoE mRNA levels determined by qPCR in ob/ob vs. C57BL/6J mice and 10-week HF–fed vs. LF-fed rats. *P < 0.05 vs. lean control mice and LF-fed rats, respectively. C: Fasting significantly reduces hypothalamic apoE mRNA levels, which was completely reversed by chow refeeding. Values are expressed as means ± SE, n = 7–8 per group, *P < 0.05, compared with ad libitum–fed rats; #P < 0.05 vs. fasted rats.
Hypothalamic apoE mRNA was regulated by fasting and chow refeeding.
To examine whether brain apoE is influenced by energy status, we measured hypothalamic apoE mRNA levels by qPCR in rats that were ad libitum–fed, 36 h–fasted, and 32 h–fasted followed by 4-h refeeding. Animals were killed in the dark phase (2200 h, lights off at 1800 h). Fasted rats lost 12% (from 273.4 ± 12.4 to 241.2 + 11.8 g) of their body weight and had significantly reduced hypothalamic apoE mRNA levels compared with ad libitum–fed controls (0.64 ± 0.05 vs. 1.0 ± 0.13; P < 0.05) (Fig. 5C). ApoE mRNA expression was completely reversed to levels of ad libitum–fed rats after 4 h of refeeding (1.18 ± 0.19) (Fig. 5C).
ApoE prevented the reduction of POMC mRNA levels induced by fasting.
The 48-h fasting significantly reduced POMC mRNA levels (P < 0.05), and intracerebroventricular apoE prevented this reduction, demonstrating the stimulatory effect of apoE on hypothalamic POMC gene expression (Fig. 6). Consistent with a previous report (19), 48-h fasting significantly increased both NPY and AgRP mRNA levels. After apoE administration, there was a trend toward decreased NPY and AgRP gene expression, but it was not statistically significant (data not shown).
FIG. 6.
Hypothalamic POMC mRNA levels measured by qPCR. The 48 h of fasting caused a significant reduction of POMC mRNA levels, which were reversed by intracerebroventricular administration of apoE (8 μg). Data are means ± SE, n = 7. *P < 0.05 vs. the ad libitum–fed saline group. #P < 0.05 vs. 48-h–fasted saline controls.
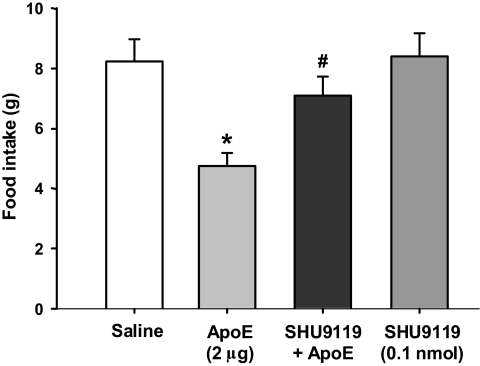
ApoE-induced reduction of food intake was attenuated by SHU9119.
The hypothalamic melanocortin system is important in the regulation of food intake and body weight (16,20). We hypothesized that apoE interacts with the central melanocortin system to control food intake. If so, then blockade of downstream melanocortin receptor(s) should either block or attenuate the influence of apoE on food intake. To evaluate this possibility, a subthreshold dose (0.1 nmol) of SHU9119, an antagonist specific for the melanocortin-3/4 receptors, was administered concomitantly with apoE in rats. As depicted in Fig. 7, intracerebroventricular SHU9119 alone did not produce a reliable effect on food intake, whereas 2 μg apoE significantly reduced food intake 4 h after injection. However, when the two injections were combined, the SHU9119 completely attenuated the anorectic effect of apoE, implying that an interaction occurs between apoE and the melanocortin system with regard to feeding regulation by the brain.
FIG. 7.
Cumulative food intake in rats at 4 h after apoE (2 μg), SHU9119 (0.1 nmol), or saline treatments. Data are means ± SE, n = 8–9. *P < 0.05 vs. saline ± saline group. #P < 0.05 vs. saline ± apoE group.
DISCUSSION
We have demonstrated for the first time that centrally administered apoE suppresses food intake dose-dependently and without malaise. Taken with the fact that comparable doses of apoE administered systemically have no effect on food intake, this anorectic action likely occurs within the brain. Moreover, when apoE antiserum was administered intracerebroventricularly to ad libitum–fed rats during the light cycle (when rats normally eat very little), increased food intake was observed, and intracerebroventricularly infusion of preabsorbed apoE antiserum failed to elicit feeding. This implies that apoE antiserum neutralizes endogenous apoE present in a brain area with access to the third ventricle and suggests that endogenous apoE might provide an important tonic inhibition of food intake. The observation that the anorectic effect of apoE was significantly attenuated by apoE antiserum further supports that the effect is specific to apoE.
We found that apoE is highly expressed in the ARC and PVN of the hypothalamus, key areas in the regulation of energy homeostasis (17). Obesity is a complex metabolic problem, and evidence indicates that obese animals have blunted satiation, raising the possibility that defective satiation signaling in the brain may contribute to the etiology of obesity (21). Our data implicate apoE as a physiological regulator of food intake, such that impairment in apoE production could lead to chronic increases in meal size resulting in obesity. Consistent with this, we observed significantly lower levels of hypothalamic apoE mRNA in genetically obese mice (ob/ob) compared with lean controls, which also suggests that reduced apoE levels contribute to hyperphagia of these obese animals.
Epidemiological studies yield a significant positive correlation between average dietary fat intake and the incidence of obesity (22,23). Using an HF-induced obese rat model (24), we confirmed that hypothalamic apoE mRNA levels are significantly lower than in LF-fed rats, which directed us to assess if altered apoE gene expression results from HF diet exposure or the resultant obesity. Rats with restricted caloric intake of HF diet neither became obese nor had decreased hypothalamic apoE gene expression, suggesting that reduced apoE gene expression after HF diet consumption is secondary to consequent obesity and not due to mere exposure to HF diet itself.
Leptin acts in the hypothalamus to decrease food intake and increase energy expenditure (25) through its long form of the receptor (Ob-Rb) (26,27), which is highly expressed in the rat hypothalamus, especially in the ARC (28). Our data from ob/ob mice (leptin-deficient) and HF-induced obese rats (leptin-resistant) suggest that a deficiency in leptin signaling may be related in part to the altered apoE gene expression in the hypothalamus. Further support comes from the observation that fasting-induced reduced leptin levels are associated with reduced hypothalamic apoE mRNA levels. Leptin's facilitation of the gene expression of apoE and anorectic effects will be explored in future experiments.
Caloric deprivation invokes a complex array of neural and metabolic responses to reduce energy expenditure, one of which is an increased drive to eat related to the alteration of neuropeptide gene expression in the hypothalamus (29). Food deprivation increases hypothalamic AgRP and NPY gene expression, but decreases POMC mRNA levels in the hypothalamus (30). Consistent with the change in POMC, we demonstrated that hypothalamic apoE mRNA levels also decreased when the animals were fasted for 36 h compared with those in ad libitum–fed rats. This diminished gene expression was completely restored by refeeding, indicating that hypothalamic apoE mRNA is indeed regulated by energy status. These observations are potentially critical and strongly suggest a physiological role of apoE in the regulation of food intake.
Compelling evidence suggests that food intake and body weight are controlled by neuropeptides acting within the central nervous system, and this circuitry can be conceptually divided into anabolic and catabolic systems. NPY, AgRP, and POMC are key peptides in these systems, respectively (29), and the balance between these systems ultimately determines the animal's ingestive behavior and energy expenditure (17,29). We hypothesized that apoE exerts its anorectic action by interacting with these neuropeptides and therefore assessed the effects of intracerebroventricular apoE administration on hypothalamic POMC, NPY, and AgRP gene expression in 48 h–fasted rats. ApoE treatment prevented the reduction of POMC mRNA observed in fasted rats (Fig. 6), implying that apoE exerts its anorectic action by enhancing activity of the melanocortin system.
Given this finding, it could be further hypothesized that blockade of downstream melanocortin receptor(s) will block or attenuate the anorectic action of apoE. In a subsequent experiment, a subthreshold dose of SHU9119 (0.1 nmol), an antagonist specific for the melanocortin-3 and melanocortin-4 (MC3/4) receptors, was administered concomitantly with apoE. This resulted in complete abolition of apoE-induced satiety (Fig. 7), indicating that an interaction indeed occurs between apoE and the melanocortin system with regard to the control of feeding by the brain.
Molecular mechanisms mediating the anorectic action of apoE currently remain unclear. Because apoE exerts its physiological function through its receptors, most of which are in neurons (31), the secreted apoE likely binds to its receptors, perhaps located on POMC neurons. Activation of apoE receptors might stimulate POMC gene expression, from which the α-melanocyte–stimulating hormone is cleaved and released. The liberated α-melanocyte–stimulating hormone could bind melanocortin-3/4 receptors to reduce food intake and body weight. Detailed molecular mechanisms of how apoE increases POMC gene expression requires further study.
As the prevalence of obesity is rapidly increasing worldwide and efforts to address the environmental factors responsible for this epidemic are slowly gaining ground, a deeper molecular and physiologic understanding of obesity must be pursued to combat obesity from multiple angles with various therapeutic approaches. We have revealed apoE as an important regulator of food intake and body weight. Characterization of the central effect of apoE on feeding should stimulate further research into the physiological functions of this protein, and mechanistic understanding mediating the anorectic function of apoE may lead to the development of effective new treatments for human obesity and related disorders.
Acknowledgments
This work was supported by National Institutes of Health grants DK70992, DK63907, DK56863, DK54504, DK17844, and DK56910.
Published ahead of print at http://diabetes.diabetesjournals.org on 16 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mahley RW: Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240: 622–630, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM: Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest 76: 1501–1513, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y, Aono M, Laskowitz D, Warner DS, Pearlstein RD: Apolipoprotein E protects against oxidative stress in mixed neuronal-glial cell cultures by reducing glutamate toxicity. Neurochem Int 44: 107–118, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Gutman CR, Strittmatter WJ, Weisgraber KH, Matthew WD: Apolipoprotein E binds to and potentiates the biological activity of ciliary neurotrophic factor. J Neurosci 17: 6114–6121, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cedazo-Minguez A, Cowburn RF: Apolipoprotein E: a major piece in the Alzheimer's disease puzzle. J Cell Mol Med 5: 254–266, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtiss LK, Boisvert WA: Apolipoprotein E and atherosclerosis. Curr Opin Lipidol 11: 243–251, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC: LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab 282: E207–E214, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, Woods SC, Seeley RJ, Davidson WS, Tso P: Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav 78: 149–155, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Chavez M, Seeley RJ, Woods SC: A comparison between effects of intraventricular insulin and intraperitoneal lithium chloride on three measures sensitive to emetic agents. Behav Neurosci 109: 547–550, 1995 [PubMed] [Google Scholar]
- 10.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P: Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res 31: 1613–1625, 1990 [PubMed] [Google Scholar]
- 11.Utermann G: Isolation and partial characterization of an arginine-rich apolipoprotein from human plasma very-low-density lipoproteins: apolipoprotein E. Hoppe Seylers Z Physiol Chem 356: 1113–1121, 1975 [DOI] [PubMed] [Google Scholar]
- 12.Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T: Blocking the apolipoprotein E/amyloid-beta interaction as a potential therapeutic approach for Alzheimer's disease. Proc Natl Acad Sci U S A 103: 18787–18792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore ZW, Hui DY: Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric oxide synthase. J Lipid Res 46: 2083–2090, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M, Shen L, Liu Y, Tajima D, Sakai R, Woods SC, Tso P: Diurnal rhythm of apolipoprotein A-IV in rat hypothalamus and its relation to food intake and corticosterone. Endocrinology 145: 3232–3238, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Cone RD: The central melanocortin system and energy homeostasis. Trends Endocrinol Metab 10: 211–216, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D'Alessio D, Seeley RJ, Tso P, Woods SC: Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol 290: R202–R207, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Woods SC, Seeley RJ, Porte D Jr, Schwartz MW: Signals that regulate food intake and energy homeostasis. Science 280: 1378–1383, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Tschop M, Heiman ML: Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109: 307–319, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Savontaus E, Conwell IM, Wardlaw SL: Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res 958: 130–138, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F: Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88: 131–141, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Woods SC: Gastrointestinal satiety signals. I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol 286: G7–G13, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kuller LH: Dietary fat and chronic diseases: epidemiologic overview. J Am Diet Assoc 97: S9–S15, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein AH, Kennedy E, Barrier P, Danford D, Ernst ND, Grundy SM, Leveille GA, Van Horn L, Williams CL, Booth SL: Dietary fat consumption and health. Nutr Rev 56: S3–S19, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P: A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133: 1081–1087, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Friedman JM, Halaas JL: Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM: Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632–635, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP: Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB: Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535–547, 1998 [PubMed] [Google Scholar]
- 29.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG: Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV: Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140: 4551–4557, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Beffert U, Stolt PC, Herz J: Functions of lipoprotein receptors in neurons. J Lipid Res 45: 403–409, 2004 [DOI] [PubMed] [Google Scholar]