Abstract
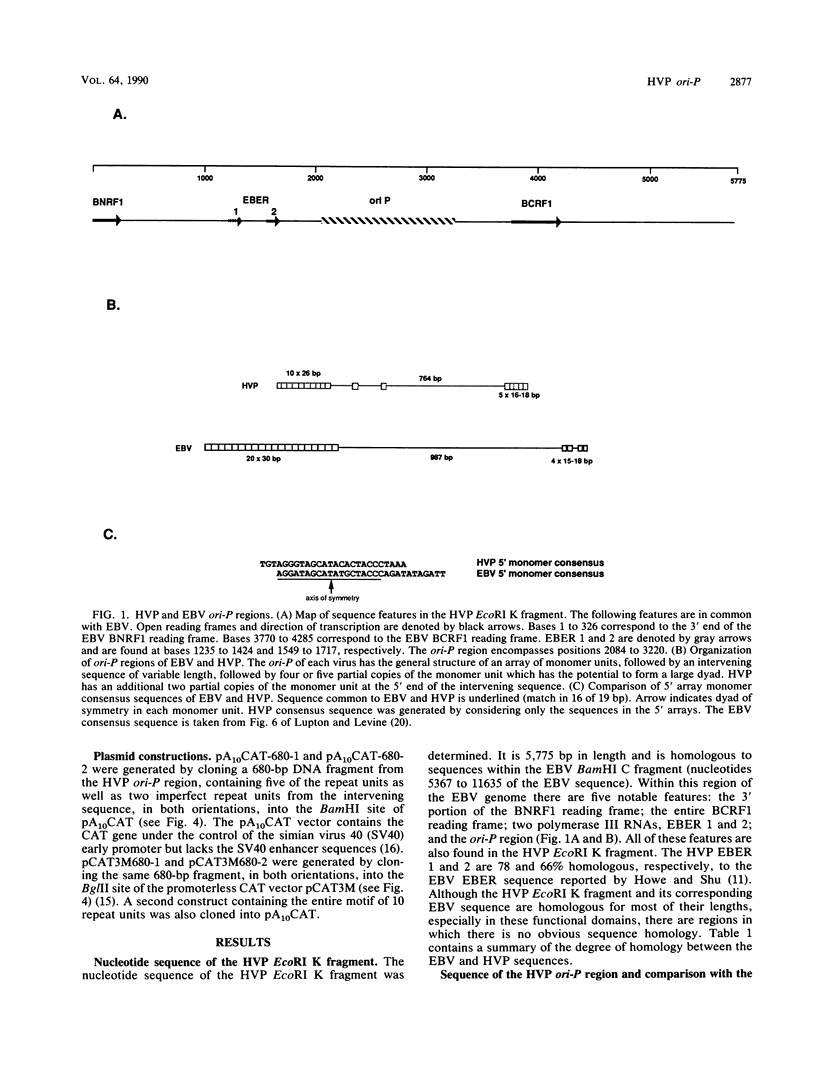
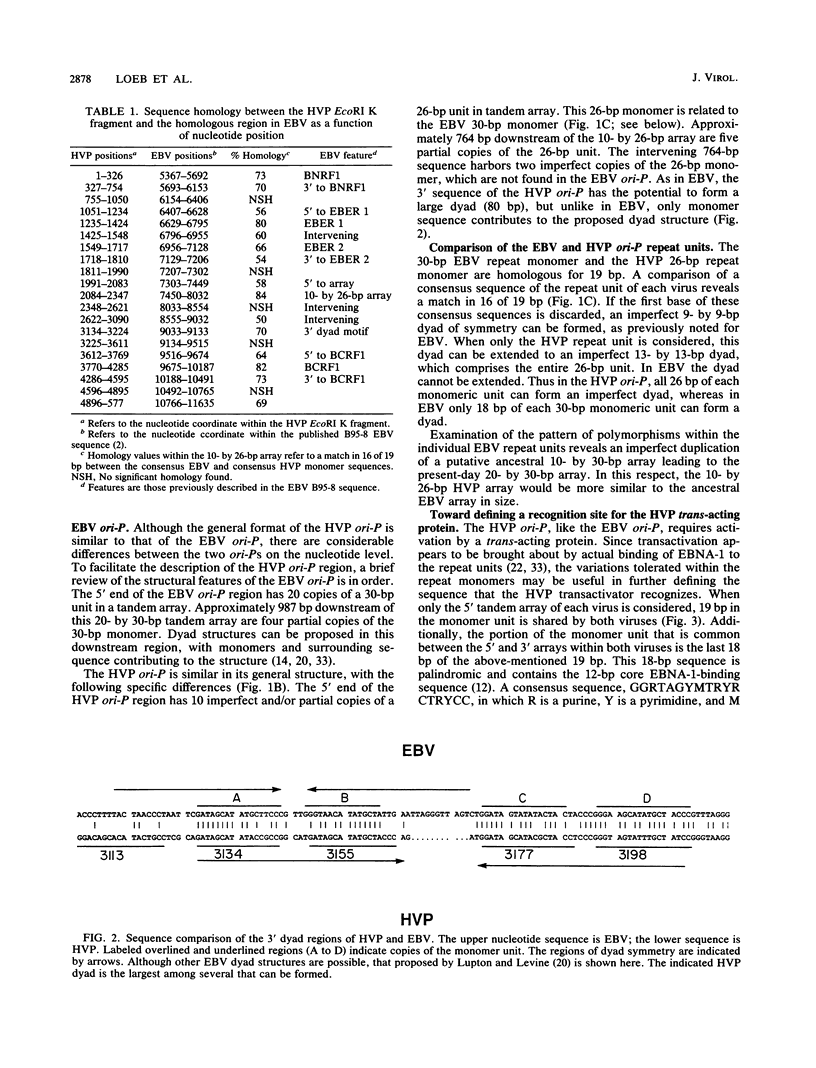
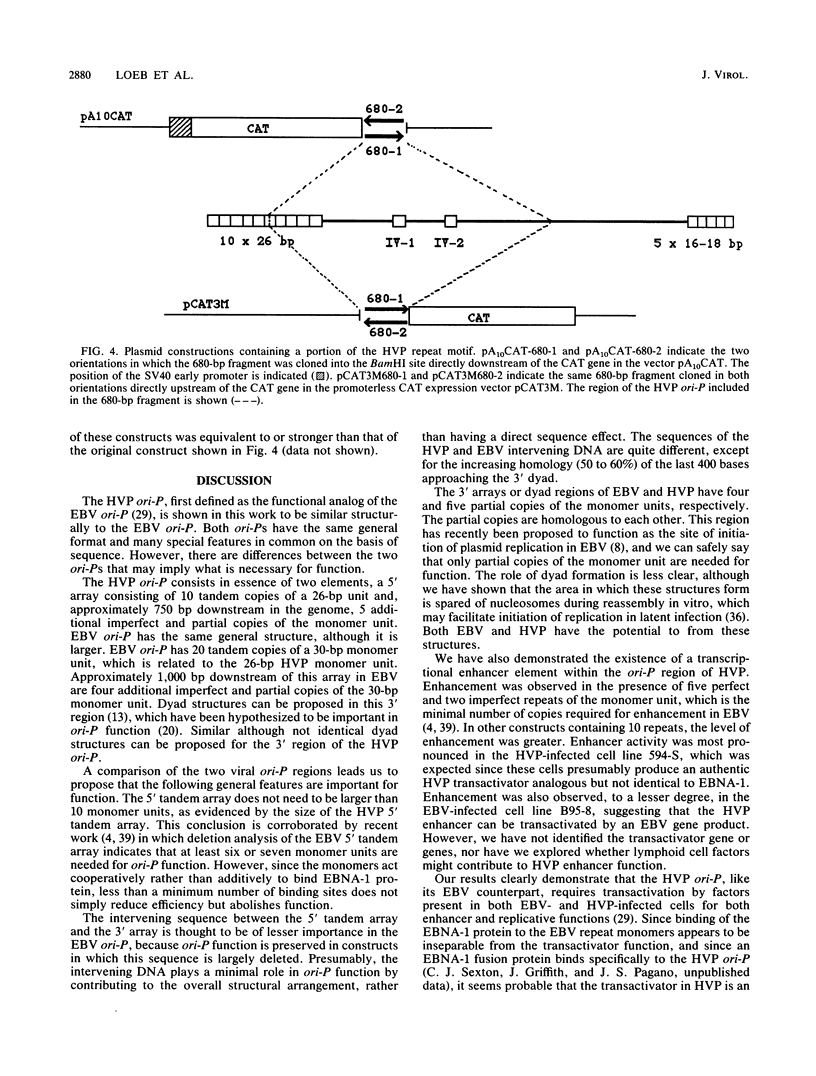
Herpesvirus papio (HVP) is a lymphotropic virus of baboons which is related to Epstein-Barr virus (EBV) and produces latent infection. The nucleotide sequence of the 5,775-base-pair (bp) EcoRI K fragment of HVP, which has previously been shown to confer the ability to replicate autonomously, has been determined. Within this DNA fragment is a region which bears structural and sequence similarity to the ori-P region of EBV. The HVP ori-P region has a 10- by 26-bp tandem array which is related to the 20- by 30-bp tandem array from the EBV ori-P region. In HVP there is an intervening region of 764 bp followed by five partial copies of the 26-bp monomer. Both the EBV and HVP 3' regions have the potential to form dyad structures which, however, differ in arrangement. We also demonstrate that a transcriptional enhancer which requires transactivation by a virus-encoded factor is present in the HVP ori-P.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T. Epstein-Barr virus genomes with properties of circular DNA molecules in carrier cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1477–1481. doi: 10.1073/pnas.72.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Lupton S., Levine A. J. Functional limits of oriP, the Epstein-Barr virus plasmid origin of replication. J Virol. 1989 Jul;63(7):3016–3025. doi: 10.1128/jvi.63.7.3016-3025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Rabin H., Letvin N., Henle W., Henle G., Klein G. Nuclear DNA-binding proteins determined by the Epstein-Barr virus-related simian lymphotropic herpesviruses H. gorilla, H. pan, H. pongo and H. papio. J Gen Virol. 1987 Jun;68(Pt 6):1587–1596. doi: 10.1099/0022-1317-68-6-1587. [DOI] [PubMed] [Google Scholar]
- Falk L., Deinhardt F., Nonoyama M., Wolfe L. G., Bergholz C. Properties of a baboon lymphotropic herpesvirus related to Epstein-Barr virus. Int J Cancer. 1976 Dec 15;18(6):798–807. doi: 10.1002/ijc.2910180611. [DOI] [PubMed] [Google Scholar]
- Gahn T. A., Schildkraut C. L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989 Aug 11;58(3):527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Gerber P., Kieff E. Herpesvirus papio DNA is similar in organization to Epstein-Barr virus DNA. J Virol. 1981 Feb;37(2):698–709. doi: 10.1128/jvi.37.2.698-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. G., Shu M. D. Isolation and characterization of the genes for two small RNAs of herpesvirus papio and their comparison with Epstein-Barr virus-encoded EBER RNAs. J Virol. 1988 Aug;62(8):2790–2798. doi: 10.1128/jvi.62.8.2790-2798.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Hayward S. D., Rawlins D. R. Interaction of the lymphocyte-derived Epstein-Barr virus nuclear antigen EBNA-1 with its DNA-binding sites. J Virol. 1989 Jan;63(1):101–110. doi: 10.1128/jvi.63.1.101-110.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S., Blaisdell B. E. A model for the development of the tandem repeat units in the EBV ori-P region and a discussion of their possible function. J Mol Evol. 1987;25(3):215–229. doi: 10.1007/BF02100015. [DOI] [PubMed] [Google Scholar]
- Karlin S. Significant potential secondary structures in the Epstein-Barr virus genome. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6915–6919. doi: 10.1073/pnas.83.18.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux G., Perricaudet M., Farrell P. J. A spliced Epstein-Barr virus gene expressed in immortalized lymphocytes is created by circularization of the linear viral genome. EMBO J. 1988 Mar;7(3):769–774. doi: 10.1002/j.1460-2075.1988.tb02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Nonoyama M., Rabin H. Collinear relationships of herpesvirus papio DNA to Epstein--Barr virus DNA. Virology. 1981 Apr 15;110(1):248–252. doi: 10.1016/0042-6822(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Lupton S., Levine A. J. Mapping genetic elements of Epstein-Barr virus that facilitate extrachromosomal persistence of Epstein-Barr virus-derived plasmids in human cells. Mol Cell Biol. 1985 Oct;5(10):2533–2542. doi: 10.1128/mcb.5.10.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Hwang E. S. Epstein-Barr virus nuclear antigen forms a complex that binds with high concentration dependence to a single DNA-binding site. J Virol. 1987 Feb;61(2):465–471. doi: 10.1128/jvi.61.2.465-471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G., Henle W., Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971 Nov 15;8(3):443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Detection of Epstein-Barr viral genome in nonproductive cells. Nat New Biol. 1971 Sep 22;233(38):103–106. doi: 10.1038/newbio233103a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Luka J., Falk L., Klein G. Detection of a nuclear, EBNA-type antigen in apparently EBNA-negative Herpesvirus papio (HVP)-transformed lymphoid lines by the acid-fixed nuclear binding technique. Int J Cancer. 1977 Dec 15;20(6):941–946. doi: 10.1002/ijc.2910200618. [DOI] [PubMed] [Google Scholar]
- Pesano R. L., Pagano J. S. Herpesvirus papio contains a plasmid origin of replication that acts in cis interspecies with an Epstein-Barr virus trans-acting function. J Virol. 1986 Dec;60(3):1159–1162. doi: 10.1128/jvi.60.3.1159-1162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Neubauer R. H., Hopkins R. F., 3rd, Dzhikidze E. K., Shevtsova Z. V., Lapin B. A. Transforming activity and antigenicity of an Epstein-Barr-like virus from lymphoblastoid cell lines of baboons with lymphoid disease. Intervirology. 1977;8(4):240–249. doi: 10.1159/000148899. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Reisman D., Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986 Nov;6(11):3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C. J., Pagano J. S. Analysis of the Epstein-Barr virus origin of plasmid replication (oriP) reveals an area of nucleosome sparing that spans the 3' dyad. J Virol. 1989 Dec;63(12):5505–5508. doi: 10.1128/jvi.63.12.5505-5508.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E., Levinger L. F., Carter C. W., Jr Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J Virol. 1979 Feb;29(2):657–665. doi: 10.1128/jvi.29.2.657-665.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysokenski D. A., Yates J. L. Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J Virol. 1989 Jun;63(6):2657–2666. doi: 10.1128/jvi.63.6.2657-2666.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Warren N., Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. 1985 Feb 28-Mar 6Nature. 313(6005):812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]