Abstract
When confronted with two identical stimuli in a very brief period of time subjects often fail to report the second stimulus, a phenomenon termed “repetition blindness”. The “type-token” account attributes the phenomenon to a failure to individuate the exemplars. We report a subject, KE, who developed simultanagnosia (the inability to see more than one item in an array) as a consequence of bilateral parietal lobe infarctions. With presentation of two words, pictures or letters for an unlimited time, KE typically reported both stimuli on less than half of trials. Performance was significantly influenced by the semantic relationship between items in the array. He reported both items significantly more frequently if they were semantically related; in contrast, when presented either identical or visually different depictions of the same item, he reported both items on only 2-4% of trials. Performance was not influenced by the visual similarity between the stimuli; he reported visually dissimilar objects less frequently than visually similar but different objects. We suggest that KE's bilateral parietal lesions prevent the binding of preserved object representations to a representation computed by the dorsal visual system. More generally, these data are consistent with the claim that the posterior parietal cortex is crucial for individuating a stimulus by computing its unique spatio-temporal characteristics.
Keywords: repetition blindness, parietal lobe, simultanagnosia, binding, visual attention
Introduction
Although the response to a word or object is often facilitated by the previous presentation of the same stimulus (“repetition priming”), there are circumstances under which the presentation of a stimulus not only fails to facilitate but interferes with the response to a previously presented item. This phenomenon, designated “repetition blindness” (Kanwisher, 1987; hereafter RB) is typically observed when stimuli are presented rapidly (e.g., 80-150 ms. between stimuli). For example, when asked to count the number of stars presented in a series of shapes presented serially at a rate of eight forms/second, subjects often fail to identify the second star in the string if there are few intervening stimuli. RB has also been observed with simultaneous displays (Bavelier, 1994; Chialant and Caramazza, 1997; Kanwisher, Driver and Machado, 1995) and in the auditory modality (“repetition deafness”; Soto-Faraco and Spence, 2001). The effect has also been reported for homonyms (“laze” and “lays”; Bevalier and Potter, 1992) and for words from different languages that have the same meaning (MacKay and Miller, 1994).
A number of explanations for RB have been proposed (see Park and Kanwisher, 1994; Kanwisher, Kim and Wickens, 1996, Parasuraman and Martin, 2001). Perhaps the most influential hypothesis is Kanwisher's (1987, 1991) “type-token” theory. On this account, recognition (here taken to assume awareness) of a stimulus entails linking of a “type”, or input representation of a stimulus, to a “token”, a representation that defines the unique spatio-temporal circumstances under which the item was encountered. The creation of a token is assumed to be a capacity-limited process that unfolds over time. On this account, RB occurs under conditions (e.g., brief stimulus presentation) that preclude the binding of both exemplars of the repeated stimulus (“types”) to “tokens” specifying the spatio-temporal characteristics of the stimulus. Thus, the failure to report the second star in a series of shapes would reflect a failure to “individuate” the repeated stimulus because the short interval between the stars as well as the need to process the other shapes in the string exceeds the capacity of the system to generate linkages between the percept and the episodic representation specifying the location and time at which the stimulus was presented.
The type-token account of RB is consistent with the widely accepted view that visual information is processed in two distinct but interacting pathways (Unglieder and Miskin, 1982). On this influential account and its subsequent elaborations (e.g., Milner and Goodale, 1995), the dorsal stream computes location information that is critical for establishing the location (and presumably, temporal characteristics) of an object in terms appropriate for action whereas the ventral system processes color, shape, texture and other types of information that permit the stimulus to be matched to stored information regarding object identity. Multiple lines of investigation demonstrate that the capacity for visual awareness is limited (Luck and Vogel, 1997; Mack and Rock, 1998; Kahneman, Treisman and Gibbs, 1992).
Simultanagnosia, initially described by Balint (1909) as a component of the syndrome that bears his name, is a disorder characterized by the inability to see more than one object at a time. Patients with this disorder typically exhibit a striking deficit in apprehending the visual world. In naturalistic settings such as a dinner table laden with food and utensils, these patients will often report seeing only a single item such as a fork.
Coslett and Saffran (1991) reported a simultanagnosic patient (BP) for whom the primary deficit was considered to be a failure to bind information computed in the dorsal and ventral stream. Her report of two item arrays (both line drawings and words) was enhanced on trials on which stimuli were semantically related. For example, she reported both items correctly significantly more frequently on trials on which the items were drawn from the same semantic category (e.g., tools, animals, etc) as compared to trials on which the items were drawn from different semantic categories (e.g, clothes and fruits). This observation suggested that BP's deficit was not attributable to “early” visual processing deficits: stimuli that were not processed to the level of identity could not influence performance.
The type-token hypothesis of RB as described above is, in many respects, similar to the account of the simultanagnosic patient BP as expressed in the following: “we suggest that the patient's simultanagnosia is attributable to an impairment in the process by which activated structural descriptions are linked to information coding the location of the object” (Coslett and Saffran, 1991). If simultanagnosia and RB are attributable to a failure to bind information computed in the ventral and dorsal visual processing streams, (at least some) simultanagnosic patients would be expected to exhibit RB; furthermore, as this hypothesis assumes that processing in the ventral stream is preserved, one might expect to find evidence that the stimulus attributes that are not reported are processed to the level of object identity.
We tested these predictions in another simultanagnosic patient, KE. After documenting that KE is profoundly simultanagnosic, we demonstrate that he reports both items in an array significantly more frequently if they are semantically related but almost never reports two instances of the same item; this effect is observed for both words (Experiment 1) and drawings (Experiment 2). In Experiment 3, we manipulated the variable of visual similarity and object identity. KE's report of both items in an array was influenced by object identity (that is, he exhibited RB) but not by visual similarity. His ability to report both instances of a cat, for example, was not influenced by the visual similarity of the drawings of the cat. The same effect of identity but not visual form was exhibited with letter stimuli in Experiment 4. Finally, in Experiment 5 we assessed the effect of sequential presentation on RB. Although he made more errors than would be expected for normal subjects, there was no evidence of RB with an inter-stimulus interval of either 500 or 1500 ms. We suggest that KE's bilateral parietal lesions prevent the binding of preserved object representations to a representation computed by the dorsal visual system. More generally, these data are consistent with the claim that the posterior parietal cortex is crucial for individuating a stimulus by computing its unique spatio-temporal characteristics.
Patient Description
KE was a 58 year-old factory worker with a high school education who noted an inability to “see” and difficulty in performing routine tasks after suffering a hemorrhagic infarction of the right posterior parietal cortex. KE had suffered an infarction of the left hemisphere causing minor language problems and clumsiness of the right hand three years prior to the investigations reported here. MRI at the time of the testing reported here (see Figures 1 and 2) revealed small areas of infarction involving the left middle temporal gyrus, the left middle frontal gyrus and a larger left posterior parietal infarction; additionally, residual effects of a right parietal hemorrhage were also noted. Increased signal in the subcortical white matter of both hemispheres was also noted on T-2 weighted images.
Figure 1.
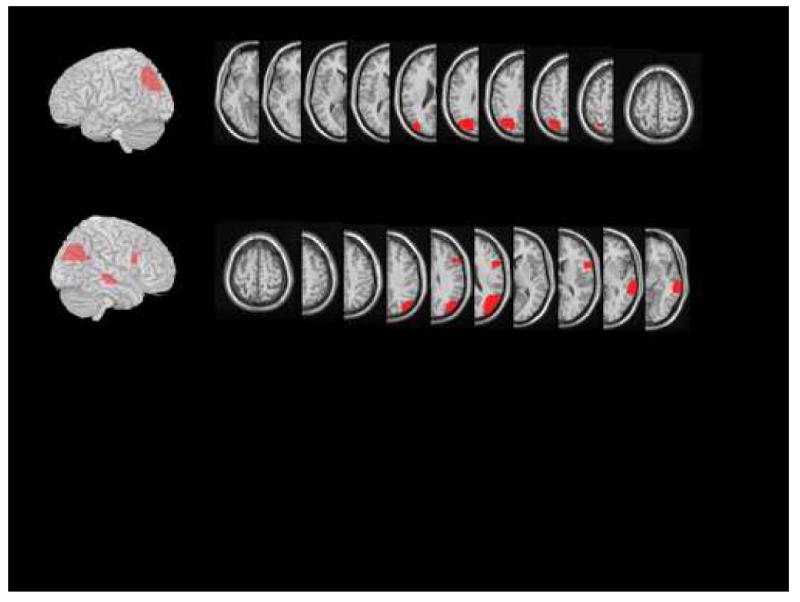
Figure 2.
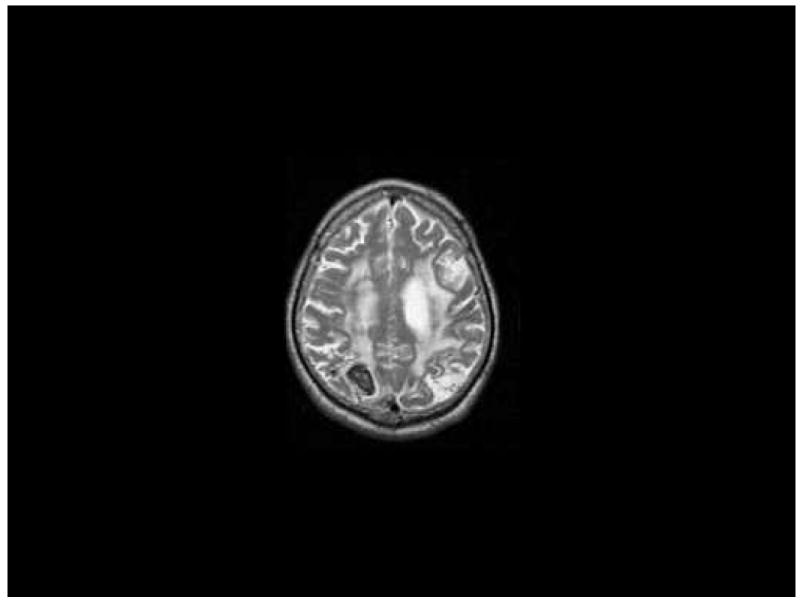
Neurologic examination revealed him to be fully oriented. He manifested pyramidal clumsiness with both hands but no significant weakness. KE exhibited prominent misreaching to visualized targets (optic ataxia) with both hands. Gait was slow with short steps. There was no tactile or auditory extinction.
Assessment of visual processing revealed a visual acuity of 20/30 OU. When instructed to look at the examiner's face without focusing attention, visual fields were full to confrontation; when asked to focus attention on the examiner's nose, visual fields were restricted to approximately 30° concentrically with no evidence of a scotoma or asymmetry. He exhibited inconsistent extinction to double simultaneous stimulation in the left visual field. Ocular movements to command (e.g., look to the door) as well as smooth and saccadic pursuit were normal in all directions.
Neuropsychological Assessment
The neuropsychological assessment was limited by KE's optic ataxia as well as his inability to consciously process more than one object at a time; the former prevented him from responding reliably on tasks requiring a pointing response. Spoken language was relatively preserved. He was fluent with an appropriate range of syntactic structure in spontaneous speech. Repetition of words and sentences was normal. He exhibited mild word finding problems in conversation and was slightly impaired in naming single objects. He named 48/60 words on the Boston Naming Test. There were three semantic errors, four circumlocutions and five instances in which he named a part of the object. He description of the Cookie Theft picture was slow and labored; after approximately ten minutes of scrutiny, he reported six people in the picture, apparently counting the girl 3 times and the boy twice. He never reported most of the objects in the array and did not achieve a coherent understanding of the scene.
His report of arrays of objects was markedly deficient. When shown two or more real objects, pictures or words he usually reported only one object (see below for details). Furthermore, when he reported a single stimulus, he was unaware of the presence of a second stimulus. For example, KE was shown a randomized sequence of 25 cards on which a single line drawing was presented and 25 cards on which two line drawings were presented. He was asked to name what he saw; on trials on which he reported only one item, he was asked to indicate how many line drawings were present. Cards were presented for five seconds. On the 25 trials on which two items were presented, he reported both items on 7 trials. On the remaining 18 trials, he reported that only one item was present. Thus, KE did not appear to detect stimuli that he was unable to report.
He correctly read 58% (46/80) of the words from Subtest 31 of the Psycholinguistic Assessment of Language Processing in Aphasia (Kay, Lesser and Coltheart, 1992), often in a slow, laborious fashion. Twenty-five errors (74% of errors) were visually based (church read as “couch”), 5 (15%) were reports of one or more letters and 4 (12%) were omissions. On the latter trials his attention appeared to be captured by irrelevant aspect of the stimulus (e.g., the corner of the card) and he was unable to find the word. Non-word reading was markedly impaired; he correctly pronounced only 3 of 50 four-letter non-words. As has been reported in previous investigations of simultanagnosic subjects, many errors (66%) involved the report of one or more letters (Coslett and Saffran, 1991; Baylis et al, 1994). Despite being told that stimuli contained by a “big” and “small” letters, he reported only the small letter on 19/20 trials when shown hierarchical stimuli in which a single large letter was composed of a number of smaller, different letters (16) c.f., Navon, 1977).
Finally, KE also demonstrated a finding that has not to our knowledge been reported: he was unable to report more than one attribute of a single object. For example, he was unable to name the color of the ink in which words were written despite naming the word correctly. Several experiments demonstrated, however, that perceptual attributes that he was unable to report influenced his performance. These data are reported in detail in another manuscript (Coslett and Lie, in press).
Because of his profound deficits, stimuli for the tasks described below were presented on 5″ × 7″ (12.7 × 17.8 cm) white cards or with a monitor. Words and line drawings were either horizontally or vertically aligned and were separated by approximately 7 cm. Targets subtended approximately 2° of visual angle. Stimuli were presented in KE's midline for an unlimited period of time. We note that in preliminary testing, KE's performance was quite similar with stimuli presented on cards or a computer monitor. He was more likely to report the right stimulus with horizontal (side by side) arrays and the top stimulus with vertical arrays. Collapsing across all tasks, on those trials on which he reported one stimulus correctly it was on the right or the top in approximately 62% of trials. Overall performance with vertical and horizontal arrays was quite similar. Thus, his directional biases, while consistent, were not severe.
It should be emphasized that efforts were made to maximize performance on all tasks. KE was told that two stimuli were present on each card. On those trials on which only one target was reported, he was reminded that a second target was present and was encouraged to continue searching.
Effects of Stimulus Identity on Performance: Semantic Priming and Repetition Blindness
When presented a visual array, patients with simultanagnosia typically explicitly deny seeing more than one item; in several instances, however, the performance of these subjects may be influenced by the semantic relationship between the items in an array, strongly suggesting that the items in the array have been processed to the level of identity (e.g, Coslett and Saffran, 1991; Egly and Robertson, 1993; Robertson et al, 1997). Similar findings have been reported in patients with neglect (Berti and Rizzolatti, 1992; Marzi et al, 1996; Vuillemier and Rafal, 1999; McGlinchey-Berroth et al, 2000; Rees et al 2000; Driver and Vuillemier, 2001). In the following series of experiments we sought evidence of semantic priming as well as RB by presenting stimuli that were unrelated, semantically related or identical.
Experiment 1: Words
Methods
To assess the effect of semantic relatedness and identity on recognition two words were displayed vertically on a monitor until he either reported both words or until 15 seconds elapsed. Stimuli for this experiment were generated from a pool of words that included 14 animals, 14 tools, 12 items of clothing and 12 fruits/vegetables. All stimuli were 2-5 letters in length. At the viewing distance of approximately 40 cm, words subtended approximately 2-3° of visual angle. There were a total of 228 trials; on 76 trials the same word appeared twice, on 76 trials different words drawn from the same semantic category were displayed and on 76 trials different words from different semantic categories were included. Each word appeared in all three conditions and in an equal number of times in the upper and lower positions in the array. The sequence of trials was randomized. Because of KE's reduced (and variable) stamina, testing was performed over 4 sessions. The same patterns of performance were observed in all sessions.
KE was told that there would be two words on all trials and that he should report both words. He was explicitly told that the same word would be repeated on some trials and was instructed to name the word twice on those trials.
Results
KE reported both items correctly on 30% (23/76) of Within Class trials, 14% (11/76) of Across Class trials but only 4% (3/76) of Same Item trials. KE was significantly worse on the Same Item trials as compared to Within or Across Category trials (Fisher's Exact P=.0151 and .0003 respectively). He was also significantly more likely to identify both items correctly on Within as compared to Across Class trials (Fisher's Exact = .032).
Across all types of trials, the most common error was the correct report of one item but a failure to report a second item; this occurred on 152 (67%) of trials. (See Table 2) He reported neither word correctly on 30 trials (13% of trials); 22 of these were visual errors involving one word (e.g., dog read as “dig”) and 8 were failures to report any word. Finally, KE read one word correctly but produced a visual error in response to the second word on 9 trials (4%). There were no unambiguous semantic errors on trials on which he reported one word correctly.
Table 2. Error Analysis for Experiment 1.
| Unrelated | Semantically Related | Identical | Total | |
|---|---|---|---|---|
| Correct | 11 | 23 | 3 | 37 |
| One Correct | ||||
| Plus Omission | 51 | 41 | 60 | 152 |
| Plus Visual Error | 3 | 4 | 2 | 9 |
| Neither Correct | ||||
| 1 Visual Error | 8 | 5 | 9 | 22 |
| 2 Omissions | 3 | 3 | 2 | 8 |
Discussion
The results are discussed after Experiment 2.
Experiment 2: Drawings
Methods
This experiment is quite similar to Experiment 1 except that line drawings served as stimuli. Two item arrays were generated from a corpus of line drawings that included 14 animals, 14 tools, 11 items of clothing and 11 fruits/vegetables. Most drawings came from the Snodgrass and Vanderwart (1980) corpus but other stimuli were taken from additional corpora available in the lab. On each trial, two line drawings were presented side by side on a card. Each drawing was approximately 3 × 3 cms in size, corresponding to approximately 4.3° of visual angle.
Three different types of trials were included. There were 50 Across Category trials on which the items were drawn from different categories, 50 Within Category Trials on which stimuli were drawn from the same category and 50 Same Item trials on which the same drawings were presented twice. Each item appeared 6 times, twice in each of the three conditions. Each item appeared once on the left and once on the right.
KE was given an unlimited amount of time to respond. He was told that there were always two drawings present and was encouraged to keep looking if he reported only one item. As before, he was told that the same item would be repeated on some trials and that he should report the item twice on those trials. Trial types were randomized.
Results
KE correctly identified both stimuli on 32/50 trials in the Semantically Related condition, 19/50 trials in the Unrelated condition and 2/50 trials in the Same Object condition. (See Table 1) He performed significantly worse in the Same Object as compared to the Within Category (Fisher's Exact P<.0001) and Across Category (Fisher's Exact P<.0001) conditions. He also performed significantly better on Semantically Related as compared to the Across Category condition (Fisher's Exact P=.0149).
Table 1. Report of 2 item arrays.
| Unrelated | Semantically Related | Same | |
|---|---|---|---|
| Exp. 1: Words | 14% | 30% | 4% |
| 11/76 | 23/76 | 3/76 | |
| Exp. 2: Drawings | 38% | 64% | 4% |
| 19/50 | 32/50 | 2/50 |
As indicated in Table 3, errors were similar to those observed in the previous experiment; 74 errors (76%) involved the correct report of one item. There were no major differences in errors as a function of condition. There were no unambiguous semantic errors in any condition.
Table 3. Error Analysis for Experiment 2.
| Unrelated | Semantically Related | Identical | Total | |
|---|---|---|---|---|
| Correct | 19 | 32 | 2 | 53 |
| One Correct | ||||
| Plus Omission | 20 | 9 | 37 | 66 |
| Plus Visual Error | 2 | 1 | 5 | 8 |
| Neither Correct | ||||
| 1 Visual Error | 8 | 6 | 5 | 19 |
| 2 Omissions | 1 | 2 | 1 | 4 |
Discussion
For both pictures and words, KE exhibited facilitation with semantically related stimuli as well as a marked decrement in performance with identical stimuli. The effect of object identity demonstrated by both semantic facilitation as well as RB exclude the possibility that KE's deficit reflects solely a “low-level” perceptual problem: one would not expect a stimulus that was not registered by the visual system to influence processing (cf, Kanwisher et al, 1995; Ptak and Schnider, 2005). It should also be noted that the better performance on within category trials is not likely to be related to guessing. If, for example, KE's recognition of one item prompted him to respond with the name of another item from that category one might expect to observe errors in which the incorrectly named item was semantically but not visually related to the identified item. Errors of this type were not observed.
We argue that the type/token account of RB (Kanwisher 1987, 1991) can be elaborated to accommodate KE's performance. Like many previous investigators, we contend that the processing of attributes such as form and color that are critical for object recognition is mediated by the temporal lobe; object recognition, that is the process by which a “token” is generated, requires that this perceptual information contact stored object and word representations for which the temporal lobe is also crucial (Milner and Goodale, 1995; Farah, 2004).
As suggested by Kanwisher (2001), the explicit recognition that two exemplars of a stimulus are present in an array or series of stimuli may require that the object specific information - or type - be linked to location and time computed in the dorsal visual stream. We assume that this binding process is a limited capacity operation. Support for this claim comes from a variety of investigations in normal subjects demonstrating that the number of object-location pairings that can be computed and maintained is limited (Pylyshyn and Storm, 1988; Kahneman et al, 1992; Luck and Vogel, 1997). Thus, we suggest that KE's RB is attributable to a pathologic restriction in the linking of information computed in the ventral visual system to a location specified in a spatial or spatio-motor system that is computed in the parietal lobe. If this account is correct, one would expect KE to be unable to “see” more than one exemplar of the same object because the individuation of a stimulus requires the limited capacity process that is deficient in KE, the binding of what and where systems.
Many subjects with simultanagnosia have profound spatial deficits. For example, Robertson et al (1997), Humphreys et al () and McCrae et al (2006) have reported simultanagnosic subjects who exhibit frequent illusory conjunctions on a visual search task, a pattern of performance consistent with a spatial impairment. Could KE's impairments reflect a deficit in spatial processing rather than a disorder of binding? Although KE may indeed have some impairment in spatial processing, we think that this is an unlikely explanation for his RP for reasons that are discussed more fully in Coslett and Lie (in press). First, unlike many (but not all; see Coslett and Chatterjee, 2003) simultanagnosics, KE does not make illusory conjunction errors. Second, KE not only fails to report more than one item in an array, he exhibits a phenomenon not to our knowledge previously reported: he is unable to report more than one attribute of the same object. For example, when shown the word table written in red ink, he reports the word correctly but is unable to report the color and even denies that a color is present. As the colored ink constitutes the stimulus that he reports, the failure to report the color of the ink cannot be attributed to a spatial deficit. For these and other reasons we believe that KE's RB is not attributable to an inability to mark the location of a visual stimulus.
There is another aspect of KE's performance, however, that requires an explanation: KE was significantly more likely to identify both items if they were semantically related. We argue that KE was more likely to report both words or pictures when confronted with an array containing “cow” and “deer” as compared to “cow” and “hammer” because the former two entities share more semantic features than the latter two. For example, cow and deer are both living, have four legs, have eyes, and are covered with short hair; a cow and hammer share none of these features. The activation of a structural description for one stimulus is assumed to result in the activation of the relevant semantic features; on an interactive model of visual processing (e.g., Heinke and Humphreys, 2003), activation of semantic features would be expected to boost the activation of structural descriptions that share these same features. Similarly, on recent connectionist models of semantic processing, activation of semantic “features” would be expected to facilitate object recognition (Rogers and McClelland, 2004). If, as suggested above, the binding of ventral and dorsal stream information is a limited capacity operation that is pathologically reduced in KE, the enhanced activation of a structural description may facilitate the linking of the structural description and location information.
Performance with two exemplars of the same entity may be disadvantaged because the second exemplar does not provide any additional semantic information that serves to boost the activation of the object representation. Consider, for example, a trial on which two drawings of a “cat” are presented. The identification of one exemplar of “cat” would be expected to activate all of the semantic feature information relevant to that entity. Thus, although activated semantic features would feed back to the structural description system to activate related entities such as dog, cow, pet food, etc, this top-down activation would be irrelevant for the second exemplar of “cat” as the structural description for this entity would be maximally activated by the first exemplar. Note that on this account, performance on “unrelated” items is likely to be better than performance with two exemplars of the same object because even “unrelated” objects are likely to share some semantic features (e.g., mode of manipulation, color, function) that serves to boost activation of the structural description and thereby facilitate the binding of the structural descriptions to a representation of location.
Thus, on our account, both semantic facilitation and RB emerge from the same processing architecture. Semantic similarity facilitates recognition of both items because recognition of one item is associated with activation of the object's semantic information; to the extent that the semantic features are shared by other objects, activation of the shared semantic features would be expected to boost the activation of structural descriptions, thereby facilitating recognition. In the limiting case of object identity, however, the requirement that objects be individuated over-rides the effects of semantic activation.
We note that performance consistent with RB has been reported in brain-lesion subjects. Baylis et al (1993) reported data from five patients with extinction in a task in which they were shown colored letters and asked to report either the letter or color of the stimuli. Extinction was more severe when the stimuli were identical on the dimension to be reported; that is, when shown both a red “S” and a blue “S” subjects were less likely to report both stimuli when asked to name the letters as compared to a condition in which they were asked to report the colors. We return to this issue after below.
Repetition Blindness and Perceptual Similarity
The next two experiments were performed to explore the effects of visual similarity on KE's performance. The account of KE's RB discussed above attributes the phenomenon to interactions between the ventral and dorsal streams occurring after the visual form of the stimulus has been computed. One might speculate, however, that priming of visual features or the object description contributed to KE's semantic priming and RB. The possibility that similarities in visual form contributed to KE's performance was addressed in two additional experiments in which the perceptual similarity between the same and different stimuli was manipulated.
We recognize that several lines of evidence suggest that visual priming does not entirely account for KE' performance. First, the fact that both facilitation and RB were observed with words, for which no principled relationship between form and semantic category would be expected, suggests the effects are unlikely to reflect priming of visual form exclusively. Second, investigations in normal subjects have demonstrated that RB is observed even when the same objects are less visually similar than different objects (Kanwisher et al, 1999).
Experiment 3: Semantic Priming and Repetition Blindness with Perceptual Similarity Control
Methods
This experiment was modeled on a task reported by Kanwisher, Yin and Wojciulik (Experiment 7, 1999). KE was asked to name two line drawings presented on cards. Stimuli were generated using twenty basic-level objects (e.g., belt). For each object, two different line drawings were selected from the corpus of Snodgrass and Vanderwart (1980) or were generated by the experimenters. There were 20 stimuli in each of the four categories for a total of 80 trials. There were four different types of trials generated by crossing the variables of object identity and visual similarity. In the “Same/High Similarity” (Figure 3a) stimuli included two identical line drawings; in the “Same/Low Similarity” condition stimuli included two visually dissimilar exemplars of the same object (Figure 3b). In the “Different/High Similarity” condition two visually similar but different objects were presented (Figure 3c). Finally, in the “Different/Low Similarity” condition two visually dissimilar different objects were presented (Figure 3d). An example of these stimuli is shown in Figure 3. In this stimulus set, open and closed umbrellas (Figure 3b) were rated by eight normal controls to be significantly less visually similar than the closed umbrella and the carrot (Figure 3c). The mean visual similarity ratings for the 20 visually dissimilar same pairs of objects was significantly lower than the mean ratings for the 20 visually similar but different pairs of objects (p<.01).
Figure 3.
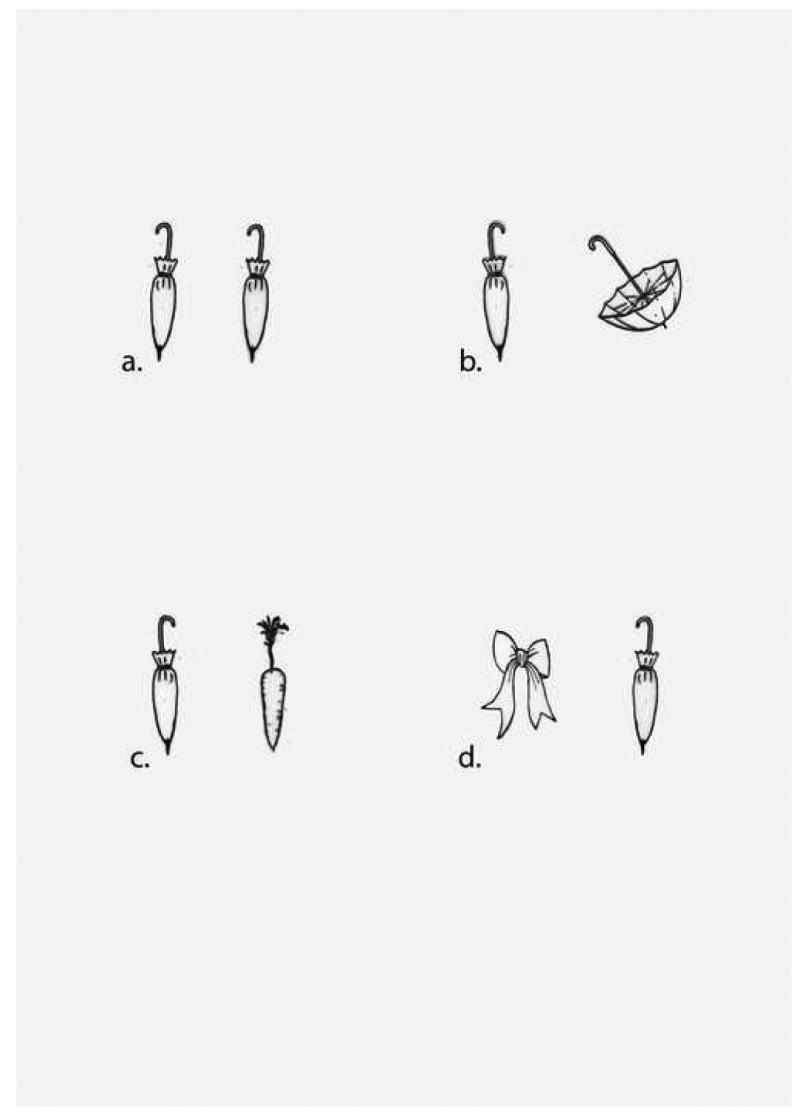
Stimuli were presented in random order for an unlimited time. KE was specifically told that on some trials the two pictures depicted the same object and that he should name the object twice. Individual stimuli were approximately 3 × 3 cm in size, separated by approximately 5 cm and presented side by side on white cards. The task was administered on 3 occasions. There was a 2-week interval between the first and second sessions, and a 3-month interval between the second and third sessions. As performance was similar across the three administrations, data from the three administrations are summed in Table 4.
Table 4. Repetition Blindness as a Function of Visual Similarity.
| Same/High Sim. | Same/Low Sim. | Diff./High Sim. | Diff./Low Sim. |
|---|---|---|---|
| 7% | 5% | 25% | 33% |
| 4/60 | 3/60 | 15/60 | 20/60 |
| 6% | 29% | ||
| 7/120 | 35/120 | ||
Results
There are several noteworthy results. First, there was no effect of visual similarity. That is, when two exemplars of the same object were presented, there was no difference in performance between the visually similar and visually dissimilar items (3/60 vs. 4/60; Fisher's Exact P=.50); similarly, on trials with two different items, there was no difference in performance on trials with visually similar and visually dissimilar items (15/60 vs. 20/60, Fisher's Exact P=.21). In contrast, there was a significant effect of stimulus identity. KE reported both items more frequently on trials when the items differed with respect to identity whether the items were visually similar (15/60 vs. 4/60, Fisher's Exact P=.0055) or visually dissimilar (20/60 vs. 3/60, Fisher's Exact P=.00006). As there was no effect of visual similarity, we collapsed across this variable; KE responded correctly on 35/120 (29%) trials with different objects but only 7/120 (6%) trials with identical objects (Fisher's Exact P<.00001). Finally, KE performed significantly better on visually similar but not identical objects (15/60) as compared to visually dissimilar exemplars of the same object (3/60; Fisher's Exact P=.0019).
Experiment 4: Letter Identify and Perceptual Similarity
This experiment was designed to exploit the fact that the letters differ with respect to visual appearance both as a function of case (upper, lower) as well as identity (X, E). We reasoned that if KE's impaired ability to report more than one exemplar of an object was attributable to perceptual similarity he would be more accurate in the mixed case condition as compared to the same case trials and more accurate with visually similar different letters (e.g., “s z”). If letter identity is the relevant dimension, KE would be expected to perform better with different letters (regardless of case) and would not be expected to exhibit an influence of case in the same letter trials; additionally, the visual similarity between the letters would not be expected to influence performance.
Methods
Six types of letter pairs were generated by crossing the factors of letter identity (same, different) and case (Upper, Lower, Mixed). There were 77 trials with Same Letter pairs (see Table 5). The different letter pairs included 27 pairs of upper case, 25 of lower case and 25 mixed case pairs. For the Different Letter Pairs, twenty five trials consisted of pairs of visually similar letters (13 capital and 12 lower case), and 25 trials consisted of pairs of visually less similar letters (13 capital and 12 lower case; for example, visually similar pairs included “A H” and “s z” whereas visually less similar pairs included “P U” and “j n”. Pairs were generated using data provided in Gilmore et al (1979) and Dunn-Rankin et al (1968). Trials were randomized.
Table 5. Repetition Blindness in Letter Naming.
| Same Letter | Different Letter | |||||
|---|---|---|---|---|---|---|
| AA | aa | Aa | AB | ab | Ab | |
| 0% | 4% | 4% | 44% | 48% | 48% | |
| 0/27 | 1/25 | 1/25 | 12/27 | 12/25 | 12/25 | |
| Total: | 4% | 47% | ||||
| 2/77 | 36/77 | |||||
Letters were presented in 48 point Times font. Stimuli were presented side by side on 3 × 5 index cards. Stimuli subtended approximately 3° of visual angle and were separated by 3° of visual angle. KE was asked to name both letters in a display. He was told that on some trials, the same letter would be shown twice. The test was completed in 2 sessions 2-months apart. As performance was quite similar across the two administrations, the data were collapsed.
Results
As indicated in Table 5, KE again exhibited profound RB, naming both letters on only 2/77 trials with same name letters but on 36/77 trials with different letters (Fisher Exact P<.0001). In contrast, there was no effect of visual similarity; he named both letters on only 1/52 trials when they were visually identical (both upper or lower case letters) and 1/25 trials when they were visually different (Fisher's Exact=.5468). Furthermore, there was no effect of the visually similarity of the letters in the different letter conditions. Finally, he named both letters on 12/25 trials for both visually similar and dissimilar pairs.
Discussion of Experiments 3-4
These findings replicate and extend the findings of Experiments 1 and 2. KE was significantly less likely to report two exemplars of the same picture or letter as compared to two different stimuli. Whereas there was a robust effect of object identity, there was no effect of visual similarity. These data strongly suggest that, at least in the context of a task requiring object identification, the semantic priming and RB exhibited by KE did not arise by virtue of priming of visual form or feature but arises after the point at which object identify has been computed. In this sense, the data from KE are consistent with studies in normal subjects (Kanwisher et al, 1999).
The fact that RB was determined by object identity rather than visual form demonstrates that RB may arise at a relatively late stage in processing, that is after object identity has been computed. It does not indicate, however, that the effects of RB only occur at this stage of processing. Kanwisher (1987) demonstrated that RB occurs only for the attended stimulus dimension. That is, when presented stimuli that differ on two dimensions (color and letter), RB is exhibited only for the stimulus attribute that is to be reported. For example, when asked to attend to color, the second “S” in a series of letters is not omitted if it differs in color from the preceding “S”; using the same stimuli, RB will be observed if subjects are asked to attend to letter identity. Consistent with the fact that RB is influenced by task demands, the phenomenon has been observed in patients with extinction with respect to color and form (Baylis et al, 1993; see also Danckert et al, 1999), phonology (Rafal et al, 2002), and meaning (Ptak and Schnider, 2005).
Experiment 5: Report of Stimuli with Sequential Presentation
Although associated with profound deficits in the processing of stimuli presented at the same time but at different locations, simultanagnosia associated with bilateral posterior hemisphere lesions is not typically associated with symptoms suggestive of impaired processing of information over time. Experimental support for this point comes from the investigation of BP (Coslett and Saffran, 1991). This patient performed normally in determining if an exemplar of a semantic category was present in a four-word sequence using a rapid serial visual display paradigm in which each word was presented for 50 ms. A similar finding was reported by Robertson et al (1997) in their investigations of RM; this subject was far less likely to produce illusory conjunction errors with sequential as compared to simultaneous presentation.
RB in normal subjects is observed not only for location (e.g, Epstein and Kanwisher, 1999) but also in time (Kanwisher, 1987, 1991); indeed, most investigations of the phenomenon have involved serial stimulus presentation. In Experiment 5, we sought to determine if KE exhibited RB in serial presentation.
Methods
Single capital letters were presented sequentially using a computer. In all instances, the first letter was presented in 56 point Geneva font and the second in 56 point Helvetica font. Prior to each trial, a 0.75 × 0.75 inch blank square was presented for 500 ms at the center of the monitor. Each letter was presented for 500 ms in the first block and for 1500 ms in the second block of trials. There was a 250 ms. inter-stimulus interval between letters in both administrations. There were 26 Same Letter and 26 Different Letter trials in each administration. In Different Letter trials, two letters were randomly selected. All letters appeared twice, once as a first stimulus and once as a second stimulus. There were two blocks of 26 trials for both the 500 ms and 1500 ms. stimulus presentation for a total of 104 trials. Testing was performed in four blocks of trials on the same day using an ABBA design.
Results
The number of trials on which KE identified both letters is indicated in Table 6. Data from two trials were omitted because of lapses of attention. There are several important findings. First, KE was correct on 76% of trials. Second, there was no evidence of RB; collapsing across both same and different letter trials, KE's performance with same and different trials did not differ. Finally, there was no effect of stimulus duration (500 vs. 1500 ms.).
Table 6. Letter Report with Serial Presentation.
| Same Letter | Different Letter | |||
|---|---|---|---|---|
| 500 ms. ISI | 1500 ms. ISI | 500 ms. ISI | 1500 ms. ISI | |
| 21/25 | 18/25 | 17/26 | 22/26 | |
| Total | 39/50 | 39/52 | ||
| 78% | 75% | |||
Discussion
KE performed abnormally on this task, exhibiting a 24% error rate on a task on which normal subjects perform at ceiling. Several lines of evidence, however, suggest that deficits contributing to his impaired performance on this task (e.g., impaired ability to maintain fixation at the site of stimulus presentation across trials) do not fully explain his RB. First, we note that his performance on this task was far superior to that observed on any task involving report of two objects presented at a different location. Second, KE did not exhibit RB on this task; at both ISIs there was no difference between same and different trials.
It is relevant to note that Robertson et al (1997) reported that the simultanagnosic subject RM exhibited illusory conjunctions on a visual search task but not with serially presented stimuli. In conjunction with the findings from KE, these data suggest that simultanagnosia is primarily a disorder of binding in space as opposed to time.
Finally, the fact that he reported both letters on a large majority of trials suggests that he understood the instructions to report both occurrences of a repeated stimulus and that his failure to do so in the previous experiments cannot be attributed to impaired comprehension of task demands or a bias against repeating the name of the stimulus (see Kanwisher et al, 1995 for a similar conclusion regarding normal subjects).
General Discussion
The present data confirm and extend our previous findings in a simultanagnosic patient, BP (Coslett and Saffran, 1993). Like BP, KE reports both items in an array significantly more frequently on trials in which there is a clear semantic relationship between the stimuli. Additionally, KE exhibits RB for pictures, letters and drawings, a finding not previously reported in simultanagnosia. The fact that performance is not influenced by visual similarity between items suggests that on a task requiring object identification, RB as well as semantic facilitation are attributable to a disruption after object identity has been computed.
We have argued that KE's deficit is best characterized as a failure to bind information computed in the occipito-temporal visual processing stream to information regarding object location computed in egocentric spatial coordinates in the parietal lobe. The binding account of simultanagnosia we have offered is not without precedent. Similar arguments have been advanced to explain data from normal subjects as well as patients with brain lesions. In a recent review of behavioral and imaging studies of the neural basis of awareness, Kanwisher (2001) concludes that “the binding of activated perceptual attributes with a representation that specifies the time and place that the word appeared” is a necessary prerequisite for awareness. Kanwisher's account appears to differ somewhat from the hypothesis developed here, however, in that she regards visual attention or “the same process that is necessary for the conjoining of visual feature information” to be critical for binding. We propose, in contrast, that binding of what and where information arises at a later stage of processing than visual feature integration (c.f., Humphreys et al, 2000).
Data from studies of disorders of perceptual awareness in patients with brain injury are also relevant to the issue of binding. Driver and Vuilleumier (2001) reviewed numerous studies of patients demonstrating that neglected or extinguished stimuli may be processed not only at a perceptual but also at a semantic level (Audet, Bub and Lecours, 1991; Baylis et al, 1993; McGlinchey-Berroth et al, 1993; Rees et al, 2001). Additionally, several fMRI studies of patients with neglect have demonstrated that even extinguished faces may be associated with activation in the “fusiform face area” (Rees et al, 1997; Vuilleumier et al, 2000). These studies are consistent with the view although objects and scenes may be processed to a the point of identity, awareness of the objects derives from linking the item to a marker specifying its spatial and temporal coordinates.
We (Coslett, 1999) reported data from subjects with focal brain injury whose performance is also relevant in this context; we found that the performance of subjects with parietal lobe lesions on motor and language tasks was influenced by the location to which they attended or acted. For example, subjects with a left hemisphere lesion performed better when attending to stimuli on the left side of the environment whereas subjects with right hemisphere lesions performed better on language and motor tasks when directing attention to right hemispace. These effects were only observed in subjects whose lesions involved the parietal lobe. The findings were taken as evidence that the parietal lobes support a spatial system that serves to link or bind representations generated in other brain regions. On this account, binding of processing systems – including those with no inherent “spatial” dimension such as speech – to a spatial representations serves to facilitate processing (see Marcel, 1983, Allport, 1987, Rizzolatti and Berti, 1992).
Recent studies of anatomic connections in non-human primates are also relevant in this context as they demonstrate anatomic structures that could be relevant to the binding of type and token representations. Using the anterograde tracer BDA, Zhong and Rockland (2003), for example, reported direct connections from much of the inferior parietal lobule to regions of TE, a region of temporal cortex that is thought to be crucial for object recognition. These data supplement previous reports demonstrating multiple indirect pathways by which TE and parietal cortex are connected (e.g, Cavada and Goldman-Rakic, 1989; Baizer et al, 1991; Seltzer et al, 1996).
Finally, KE's lesion loci are of interest. KE's bilateral inferior parietal lobe lesions are likely to the major cause of his simultanagnosia; this assertion is supported by studies of the anatomic underpinnings of the disorder (Rafal, 1999; Coslett and Chatterjee, 2003) as well as the fact that the disorder was first evident in the context of an acute right posterior parietal hemorrhage that followed an earlier left posterior parietal infarction. Several authors have argued that the posterior parietal lobe including the inferior parietal lobule may be crucial for linking representations computed in the dorsal and ventral stream. Watson et al (1994), for example, demonstrated that ablation of the cortex surrounding the superior temporal sulcus in monkeys is associated with contralesional neglect; on the basis of these and other data, they suggested that this region is the analogue of the human inferior parietal lobule. Furthermore, they reviewed the extensive anatomic and electrophysiologic literature demonstrating that the cortex surrounding the superior temporal sulcus receives extensive inputs from regions subserving the ventral and dorsal visual processing streams. Driver (1996) has also speculated that the inferior parietal lobe in man serves as the interface between the ventral and dorsal visual streams.
The pathologic basis of KE's RB is somewhat less clear. To our knowledge, RB has not been reported previously in patients with simultanagnosia. It is not clear, however, that appropriate tests have been administered to patients with this disorder. A re-analysis of the data from BP (Coslett and Saffran, 1991), revealed data suggestive of RB but the phenomenon was not systematically explored. As BP suffered from lesions of the bilateral posterior parietal lobes, one possibility is that RB is associated with parietal lesions. Reports of RB in patients with extinction secondary to parietal lobe lesions (Baylis et al, 1993; Rafal et al, 2002, Danckert et al, 1999) are consistent with this proposal.
We cannot exclude the possibility that KE's left middle temporal and middle frontal gyrus infarctions contributed to his RB. Frith and Dolan (1997), for example, argued that that the top-down effects on attentional selection may be exerted by neural structures distant from the site at which selection occurs such as the prefrontal cortex. Mennemeier et al (1994) also reported a patient of interest in this regard. These investigators tested habituation to visual stimuli in a subject with left parietal and right frontal lesions. They found abnormally rapid habituation in the visual field contralateral to the parietal lesion but delayed habituation in the visual field contralateral to the opposite the frontal lesion. Based on these considerations, one might suggest that the left middle frontal gyrus lesion or the generalized white matter increased signal may be relevant to KE's RB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport DA. Selection for action: Some behavioural and neurophysiological considerations of attention and action. In: Heuer H, Sanders AF, editors. Perspectives on perception and action. 1987. pp. 395–419. [Google Scholar]
- Audet T, Bub D, Lecours AR. Visual neglect and left-sided context effects. Brain Cognition. 1991;16(1):11–28. doi: 10.1016/0278-2626(91)90082-j. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaque. J Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint R. Seelenlahhmung des “Schauens,” optische Ataxie, raumliche Storung der Aufmerksamkeit. Monatschr Psychiatrie Neurol. 1901;25:51–81. [Google Scholar]
- Bavelier D. Repetition blindness between visually different items: the case of pictures and words. Cognition. 1994;51:199–236. doi: 10.1016/0010-0277(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Driver J, Rafal RD. Visual extinction and stimulus repetition. Journal of Cognitive Neuroscience. 1993;5:453–466. doi: 10.1162/jocn.1993.5.4.453. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Driver J, Baylis L, Rafal R. Perception of letters and words in Balint's syndrome: Evidence for the unity of words. Neuropsychologia. 1994;32:1273–1286. doi: 10.1016/0028-3932(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Berti A, Rizzolatti G. Visual processing without awareness: evidence from unilateral neglect. Journal of Cognitive Neuroscience. 1992;4:345–351. doi: 10.1162/jocn.1992.4.4.345. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1980;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chialant D, Carmazza A. Identity and similarity factors in repetition blindness: implications for lexical processing. Cognition. 1997;63:79–119. doi: 10.1016/s0010-0277(96)00789-5. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–05. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Spatial Influences on Motor and Language Function. Neuropsychologia. 1999;37:695–706. doi: 10.1016/s0028-3932(98)00116-x. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Schwartz MF, Goldberg G, Haas D, Perkins J. Multi-Modal Hemispatial Deficits after Left Hemisphere Stroke: A Deficit in Attention? Brain. 1993;116:527–554. doi: 10.1093/brain/116.3.527. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Saffran EM. Simultanagnosia: To see but not two see. Brain. 1991;114:1523–1545. doi: 10.1093/brain/114.4.1523. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Chatterjee A. Balint's Syndrome. In: Feinberg T, Farah M, editors. Behavioral Neurology and Neuropsychology. 2nd. McGraw-Hill; 2003. [Google Scholar]
- Coslett HB, Lie E. Simultanagnosia: When a Rose is not Red. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.20002. In press. [DOI] [PubMed] [Google Scholar]
- Danckert J, Maruff P, Kinsella G, de Graaff S, Currie J. Attentional modulation of implicit processing of information in spatial neglect. Neuroreport. 1999;10:1077–1083. doi: 10.1097/00001756-199904060-00032. [DOI] [PubMed] [Google Scholar]
- Driver J, Mattingley JB, Rorden C, Davis G. Extinction as a paradigm measure of attentional bias and restricted capacity following brain injury. In: Thier P, Karnath HO, editors. Parietal lobe contributions to orientation in 3D space. Berlin: Springer-Verlag; 1997. pp. 401–29. [Google Scholar]
- Driver J, Vuillemier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Dunn-Rankin P, Leton DA, Shelton VF. Congruency factors related tovisual confusion of English letters. Perception Motor Skills. 1968;26:659–66. doi: 10.2466/pms.1968.26.2.659. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. Repetition Blindness for Locations. Journal of Experimental Psychology: Human Perception & Performance. 1999;25:1855–1866. doi: 10.1037//0096-1523.25.6.1855. [DOI] [PubMed] [Google Scholar]
- Esterman M, McGlinchey-Berroth R, Milberg W. Parallel and Serial Search in hemispatial neglect: Evidence for preserved preattentive but impaired attentive processing. Neuropsychology. 2000;14:599–611. doi: 10.1037//0894-4105.14.4.599. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions tovisual feature binding: Evidence from a patient with bilateral lesions. Science. 1995;269:853–855. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- Frith C, Dolan RJ. Brain mechanisms associated with top-down processes in perception. Phil Trans R Society London B. 1997;352:1221–1230. doi: 10.1098/rstb.1997.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghent L. Some effects of deprivation of eating and drinking behavior. Journal of Comparative Physiology and Psychology. 1957;50:172–6. doi: 10.1037/h0042629. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Hersh H, Caramazza A, Griffin J. Multidimensional letter similarity derived from recognition errors. Perception Psychophysics. 1979;25:425–31. doi: 10.3758/bf03199852. [DOI] [PubMed] [Google Scholar]
- Heinke D, Humphreys GW. Attention, Spatial Representation, and Visual Neglect: Simulating Emergent Attention and Spatial Memory in the Selective Attention for Identification Model (SAIM) Psychological Review. 2003;110:29–87. doi: 10.1037/0033-295x.110.1.29. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Livingstone MS. Segregation of form, color, and stereopsis in primate area 18. Journal of Neuroscience. 1987;7:3378–415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GW, Cinel C, Wolfe J, Olson A, Klempen N. Fractionating the binding process: Neuropsychological evidence distinguishing binding of form from binding of surface features. Vision Research. 2000;40:1569–1596. doi: 10.1016/s0042-6989(00)00042-0. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Treisman A, Gibbs BJ. The reviewing of object files: Object-specific integration of information. Cognitive Psychology. 1992;24:175–219. doi: 10.1016/0010-0285(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Treismen A, Gibbs BJ. The reviewing of object files: object specific integration of information. Cognitive Psychology. 1992;24:175–219. doi: 10.1016/0010-0285(92)90007-o. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Repitition blindness: type recognition without token individuation. Cognition. 1987;27:117–43. doi: 10.1016/0010-0277(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Repetition Blindness and Illusory Conjunctions: Errors in Binding Visual Types with Visual Tokens. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:404–421. doi: 10.1037//0096-1523.17.2.404. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Neural Events and Perceptual Awareness. Cognition. 2001;79:89–113. doi: 10.1016/s0010-0277(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Chun M, McDermott J, Ledden P. Functional Imaging of Human Visual Recognition. Cognitive Brain Research. 1996;5:55–67. doi: 10.1016/s0926-6410(96)00041-9. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Driver J, Machado L. Spatial repetition blindness is modulatedby selective attention to color and shape. Cognitive Psychology. 1995;29:303–37. doi: 10.1006/cogp.1995.1017. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Potter M. Memory and Cognition. Journal of Experimental Psychology. 1989;17:117–24. doi: 10.3758/bf03197061. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yin C, Wojciulik E. Repetition Blindness for Pictures: Evidence forthe Rapid Computation of Abstract Visual Descriptions. In: Coltheart V, editor. Fleeting Memories. Boston, MA: MIT Press; 1999. [Google Scholar]
- Kanwisher Nancy, McDermott J, Chun MM. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex: Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Luck S, Vogel E. The capacity of visual working memory for features andconjunctions. Nature. 1997;390:279–81. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Mack A, Rock I. Inattentional blindness. Cambridge, MA: MIT press; 1998. [Google Scholar]
- MacKay DG, Miller MD, Schuster SP. Repetition blindness and aging: evidence for a binding deficit involving single, theoretically specified connection. Psychology of Aging. 1994;2:251–8. doi: 10.1037//0882-7974.9.2.251. [DOI] [PubMed] [Google Scholar]
- Marcel AJ. Conscious and unconscious perception: an approach to the relations between phenomenal experience and perceptual processes. Cognitive Psychology. 1983;2:238–300. doi: 10.1016/0010-0285(83)90010-5. [DOI] [PubMed] [Google Scholar]
- Marzi CC, Smania N, Martini MC, Gambina G, Tomelleri G, Palamara A, Alessandrini F, Prior M. Implicit redundant-targets effect in visual extinction. Neuropsychologia. 1996;34:9–22. doi: 10.1016/0028-3932(95)00059-3. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Milberg WP, Verfaellie M, Alexander M, Kilduff PT. Semantic processing in the neglected visual field: evidence from a lexical decision task. Cognitive Neuropsychology. 1993;10:79–108. [Google Scholar]
- Mennemeier MS, Chatterjee A, Watson RT, Wertman E, Carter LP, Heilman KM. Contributions of the parietal and frontal lobes to sustained attention and habituation. Neuropsychologia. 1994;32:703–716. doi: 10.1016/0028-3932(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The visual brain in action. MIT Press; Cambridge: 1995. pp. 549–586. [Google Scholar]
- Kanwisher N, McDermott J, Marvin M, Navon CD. Irrelevance of figural identity for resolving ambiguities in apparent motion. Journal of Experimental Psychology. 1976;2:130–138. doi: 10.1037//0096-1523.2.1.130. [DOI] [PubMed] [Google Scholar]
- Park J, Kanwisher N. Determinants of repetition blindness. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:500–519. doi: 10.1037//0096-1523.20.3.500. [DOI] [PubMed] [Google Scholar]
- Perenin MT, Vighetto A. Optic Ataxia: A specific disruption in visual motor mechanisms. Brain. 1988;111:643–674. doi: 10.1093/brain/111.3.643. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–74. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R, Schnider A. Visual extinction of similar and dissimilar stimuli: Evidence for level-dependent attentional competition. Cognitive Neuropsychology. 2005;22:111–127. doi: 10.1080/02643290342000654. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A. Reflexive orienting in spatial neglect is biased towards behaviorally salient stimuli. Cerebral Cortex. 2006;16:337–45. doi: 10.1093/cercor/bhi111. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm R. Tracking multiple independent targets: Evidence for both serial and parallel stages. Spatial Vision. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Rafal R, Danziger S, Grossi G, Machad L, Ward R. Visual detection is gated by attending for action: Evidence from hemispatial neglect. PNAS. 2002;99:6371–6375. doi: 10.1073/pnas.252309099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000;123:1624–1633. doi: 10.1093/brain/123.8.1624. [DOI] [PubMed] [Google Scholar]
- Robertson L, Treisman A, Friedman-Hill S, Grabowecky M. The Interaction of Spatial and Object Pathways: Evidence from Balint's Syndrome. Journal of Cognitive Neuroscience. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Egly R, Lamb MR, Kerth L. Spatial attention and cuing to global and local levels of hierarchical structure. Journal of Experimental Psychology: Human. 1993 doi: 10.1037//0096-1523.19.3.471. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Semantic cognition: A parallel distributed processing approach. Cambridge, MA: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- Sagiv N, Vuilleumier P, Swick D. The neural fate of extinguished faces: electrophysiological correlates of conscious and unconscious perception in unilateral spatial neglect. J Cogn Neuroscience. 2001;98:3495–3500. [Google Scholar]
- Seltzer B, Cola MG, Gutierrez C, Massee M, Weldon C, Cusick CG. Overlapping and nonoverlapping cortical projections to cortex of the superior temporal sulcus in the rhesus monkey: double anterograde tracer studies. J Comp Neurol. 1996;370:173–190. doi: 10.1002/(SICI)1096-9861(19960624)370:2<173::AID-CNE4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology. 1980;2:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Soto-Faraco S, Spencer S. Spatial modulation of repetition blindness and repetition deafness. Quarterly Journal of Experimental Psychology. 2001;54:1181–202. doi: 10.1080/713756015. [DOI] [PubMed] [Google Scholar]
- Spatial and Object Pathways: Evidence from Balint's Syndrome. Journal of Cognitive Neuroscience. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Treisman A, Schmidt H. Illusory conjunctions in the perception of objects. Cognitive Psychology. 1982;14:107–141. doi: 10.1016/0010-0285(82)90006-8. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Miskin M. Two visual pathways. In Analysis of Visual Behavior. Perception and Performance. 1982;19:471–487. [Google Scholar]
- Vuilleumier P, Schwartz S, Husain M, Clarke K, Driver J. Implicit processing and learning of visual stimuli in parietal extinction and neglect. Cortex. 2001;37:741–4. doi: 10.1016/s0010-9452(08)70629-4. [DOI] [PubMed] [Google Scholar]
- Vuillemier P, Sagiv N, Hazeltine E, Poldrack R, Rafal R, Gabrielli J. The neural fate of seen and unseen faces in visuo-spatial neglect: A combined event-related fMRI and ERP study of visual extinction. Proceedings of the National Academy of Science, USA. 2001;98:3495–3500. doi: 10.1073/pnas.051436898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Valenstein E, Day A, Heilman KM. Posterior Neocortical Systems Subserving Awareness and Neglect. Archives of Neurology. 1994;51:1014–1021. doi: 10.1001/archneur.1994.00540220060015. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N. Implicit but not Explicit Feature Binding in a Balint's. Visual Cognition. 1998;5:157–181. [Google Scholar]
- Zeki S, Marini L. Three cortical stages of color processing in the human brain. Brain. 1998;121:1668–1685. doi: 10.1093/brain/121.9.1669. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Rockland KS. Inferior Parietal Lobule Projections to Anterior Interotemporal Cortex (Area TE) in Macaque Monkey. Cerebral Cortex. 2003;13:527–540. doi: 10.1093/cercor/13.5.527. [DOI] [PubMed] [Google Scholar]


