Abstract
Following hair cell elimination in severely traumatized cochleae, differentiated supporting cells are often replaced by a simple epithelium with cuboidal or flat appearance. Atoh1 (previously Math1) is a basic helix-loop-helix transcription factor critical to hair cell differentiation during mammalian embryogenesis. Forced expression of Atoh1 in the differentiated supporting cell population can induce transdifferentiation leading to hair cell regeneration. Here we examined the outcome of adenovirus mediated over-expression of Atoh1 in the non-sensory cells of the flat epithelium. We determined that seven days after unilateral elimination of hair cells with neomycin, differentiated supporting cells are absent, replaced by a flat epithelium. Nerve processes were also missing from the auditory epithelium, with the exception of infrequent looping nerve processes above the habenula perforata. We then inoculated an adenovirus vector with Atoh1 insert into the scala media of the deafened cochlea. The inoculation resulted in upregulation of Atoh1 in the flat epithelium. However, two months after the inoculation, Atoh1-treated ears did not exhibit clear signs of hair cell regeneration. Combined with previous data on induction of supporting cell to hair cell transdifferentiation by forced expression of Atoh1, these results suggest that the presence of differentiated supporting cells in the organ of Corti is necessary for transdifferentiation to occur.
Keywords: Cochlea, deafness, aminoglycosides, neomycin, adenovirus, guinea pig, Atoh1, gene transfer
Introduction
The cochlear sensory epithelium contains two types of differentiated epithelial cells: hair cells and supporting cells. When hair cells degenerate, supporting cells expand and replace them to maintain a confluent layer of cells lining the scala media and separating endolymph from perilymph. In some cases, supporting cells in lesioned ears remain differentiated and the organ of Corti maintains its tall appearance despite the lack of hair cells. However, in many cases the supporting cells that remain after hair cell loss do not maintain their differentiated state. As a result, the area of the organ of Corti becomes a flat or cuboidal simple epithelium with no patterned organization (Forge et al., 1998; Kim et al., 2007). The condition of supporting cells in deaf ears will dictate the choice of therapy, once therapies such as hair cell regeneration or stem cell implantation become a reality.
The flat epithelium has been described after several types of trauma. For instance, ears that receive cochlear implants often exhibit a flat epithelium in both humans and animal models (Nadol et al., 1994). A variety of etiologies may lead to degeneration of the auditory epithelium to the flat state, including severe presbycusis (Bhatt et al., 2001), extremely severe ototoxic injury (Coco et al., 2007; Forge et al., 1998; Kim et al., 2007) or hereditary cochlear pathologies (Webster, 1992). In many cases, the loss of hair cells does not initially involve supporting cell degeneration, but over time the non-sensory auditory epithelium is replaced by a flat epithelium. Because of the prevalence of this pathology in humans, the flat epithelium constitutes the substrate for potential future therapy in many clinical cases. It is therefore important to characterize the flat epithelium and determine how it responds to therapeutic manipulations.
In the present study we have used the neomycin model to eliminate hair cells and induce transformation of supporting cells into the flat epithelium state. We tested the ability of the flat epithelium to be transduced with an adenovirus and whether forced expression of a developmental gene, Atoh1, in the flat epithelium can induce transdifferentiation of these cells into new hair cells. Atoh1 is the mouse homolog of the Drosophila gene atonal, a basic helix-loop-helix transcription factor that acts as a ‘pro-hair cell gene’ (Jones et al., 2006). Forced expression of Atoh1 in deaf ears with differentiated supporting cells can induce transdifferentiation of these supporting cells to new hair cells (Izumikawa et al., 2005; Shou et al., 2003).
We found that the adenovirus-mediated expression of a reporter gene in the flat epithelium was robust. However, forced expression of Atoh1 did not induce noticeable changes in the morphology of the flat epithelium. The results point to the importance of designing ways to prevent supporting cell degeneration and indicate that once the auditory epithelium is flat, therapies other than Atoh1 over-expression should be considered.
Materials and Methods
Animals
All animal experiments were approved by the University of Michigan Institutional Committee on Care and Use of Animals (UCUCA) and performed using accepted veterinary standards. We used 72 young adult guinea pigs (Elm Hills Breeding Laboratory). At the beginning of the experiments, animals weighed 250–400g and displayed normal Preyer’s reflex. All animals were deafened unilaterally with neomycin (see below) and received one of the following treatments: Ad.Atoh1 (n = 33), Ad.Atoh1-GFP (n = 21), Ad-GFP (n = 3), Ad.empty (adenovirus with no gene insert) (n = 9), artificial endolymph (NaCl 1 mM, KCl 126 mM, KHCO3 25 mM, MgCl2 0.025 mM, CaCl2 0.025 mM and K2HPO4 1.4 mM) (n = 2) and deafening alone (n = 4).
Deafening and inoculation surgery
All animals were deafened unilaterally (left ear), with a single bolus injection of 60 μl of 10% neomycin (Pharma-Tek, Huntington, NY) diluted in sterilized water. Neomycin was selected at this concentration because it leads not only to complete elimination of all hair cells in turns 1–3 of the guinea pig cochlea, but also to a drastic change in the morphology of supporting cells. The animals were anesthetized by the combination with Rompun (intramuscularly, xylazine, 10 mg/kg, Bayer, Shawnee Mission, KS, USA) and Ketalar (intramuscularly, ketamine HCl, 40 mg/kg, Parke Davis, Morris Plains, NJ, USA). We injected 1% lidocaine hydrochloride (subcutaneously, 0.5 ml) for local anesthesia in the postauricular region. The animals were placed at a prone position on a heated pad. An incision was made along the left postauricular region. The temporal bone was exposed, then opened by scalpel drilling and forceps to gain a view of the entire round window membrane. Using the bent tip of a 30 gauge needle and a 100 μl Hamilton syringe, 60 μl of 10% neomycin was injected into the scala tympani through the round window membrane, over 1 min. After the injection, the opening in the temporal bone was closed with carboxylate cement (Duleron) and the skin was sutured in two layers.
The vectors or artificial perilymph were inoculated into the scala media of left ear 7 days after the deafening surgery, using the procedure described previously (Ishimoto et al., 2002) except that inoculation was into second turn of the cochlea. Animals were sacrificed and prepared for morphological analysis (immunocytochemistry, plastic sections or SEM) 6 days after deafening, or 2 months after viral vector inoculation. In addition, a group of animals (N = 4) was used for immunocytochemical detection of Atoh1 gene expression at a time point 7 days after viral vector inoculation.
Adenoviral vectors
We used advanced generation replication-deficient recombinant adenoviral vectors with E1, E3 and partial E4 regions deleted (Brough et al., 1997). The vectors were Ad.Atoh1, Ad.Atoh1-GFP, Ad.GFP and Ad.empty. All vectors were provided by GenVec Inc. (Gaithersburg, MD, USA). The Atoh1 gene insert was driven by the human cytomegalovirus promoter and the GFP gene was driven by the chicken beta-actin promoter. We used undiluted vectors at a concentration of 1×1012 particles purified virus per ml. The viral suspensions were stored at −80 °C until thawed for use.
Immunocytochemistry
Animals were deeply anesthetized with xylazine and ketamine as above, decapitated, and the temporal bones were removed. The inner ears were perfused with 4% paraformaldehyde in phosphate buffered saline (PBS) for 2h. Further dissection was performed to remove the stria vascularis, Reissner’s membrane and the tectorial membrane. Then the tissue was permeabilized with 0.3% Triton-X-100 in PBS for 10 min. Non-specific binding of secondary antibody was blocked with 5% normal goat serum in PBS for 30 min. Immunocytochemistry was performed using primary antibodies: a mouse monoclonal anti-neurofilament 200 kDa antibody (Sigma, St. Louis, MO, diluted 1:200) or mouse monoclonal anti-Atoh1 (Hybridoma Core, Univ. of Iowa), followed by a secondary antibody, a goat monoclonal anti-mouse conjugated to rhodamine (Jackson ImmunoResearch, West Grove, PA) for 30 min. To double stain for actin, we used FITC-conjugated phalloidin (Molecular Probes, Junction City, OR, diluted 1:200). The specimens were further dissected to separate individual cochlear turns and mounted on glass slides using CrystalMount (Biomeda, Foster City, CA). The samples were examined and photographed using a Leica DMRB epifluorescence microscope (Leica, Eaton, PA) with a Cooled SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Scanning electron microscopy
Animals were deeply anesthetized (as described above) and perfused transcardially with 0.15M cacodylate buffer, followed by 2% glutaraldehyde in the same buffer. Cochleae were removed and the otic capsule opened to continue fixation for 2h. The tissues were postfixed using the osmium thiocarbohydrazide method (Osborne et al., 1991). The specimens were dehydrated with ethanol and dried by the critical point method with CO2 in a SamDri-790 (Tousimis, Rockville, MD). The samples were fixed to stubs with silver paste and photographed digitally using a Philips XL30 Field Emission Gun scanning electron microscope (FEI, Hillsboro, OR).
Plastic sections
Animals were anesthetized and decapitated and the temporal bones removed and placed in 4 % paraformaldehyde in PBS for 2 hours. The otic capsule was dissected away and the modiolus, along with the organ of Corti, were decalcified for a 2–3 days in 3% EDTA with 0.25% glutaraldehyde. Once tissues appeared soft, specimens were postfixed with 1% osmium tetroxide in phosphate buffer, dehydrated in ethanol and embedded in Embed 812 epoxy resin. Sections were obtained with glass knives and photographed using a Leica DMRB microscope.
Results
Morphological analysis reported here is based on observations in the first three turns of the cochlea. Little variation was seen among individuals. In normal ears that were not deafened, the combined staining with phalloidin and neurofilament shows presence of hair cell and nerves extending in the direction of the hair cells (Fig. 1a). In contrast, in animals sacrificed at six days after the deafening procedure, whole-mounts of the organ of Corti stained with phalloidin show that hair cells are absent and the reticular lamina lacks orderly organization (Fig. 1b). The typical organization of supporting cells in the organ of Corti is not present and rows of cells cannot be distinguished. Instead, irregular intercellular borders reveal an epithelium in which cell contour is variable and distinctively different from the normal organ of Corti.
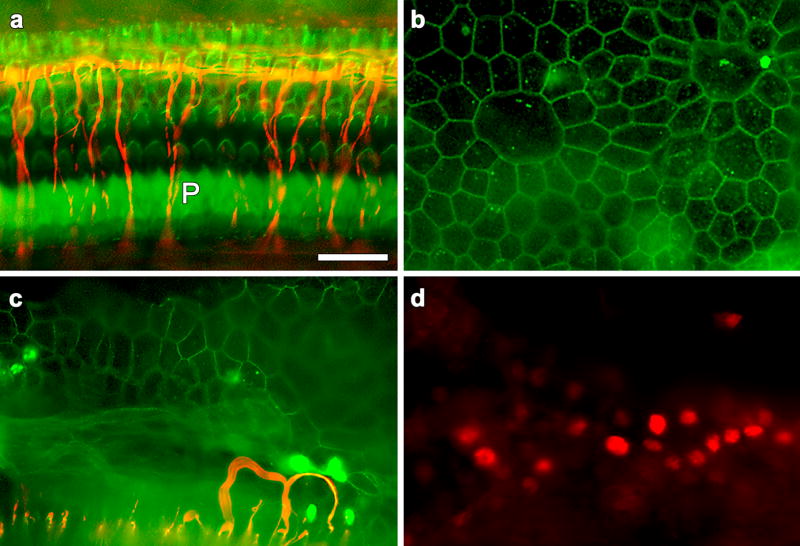
Figure 1.
Whole-mounts of the auditory epithelium stained with markers for neurofilament, actin or Atoh1 and photographed with epi-fluorescence. a. Fluorescent phalloidin (green) shows the distribution of actin in the normal auditory epithelium, depicting the outer pillar cells (P) and a normal array of hair cells and supporting cells. Co-localization with neurofilament (red) shows radial fibers extending towards the outer hair cell area and longitudinal fibers near the 3rd row outer hair cells. b. Phalloidin staining 6 days after neomycin administration shows that hair cells are absent. The auditory epithelium consists of non-sensory cells with irregular apical junctional contours. c. Double-staining with phalloidin and neurofilament antibody reveals absence of hair cells and lack of radial nerve fibers 6 days after neomycin. A small number of fibers extend and loop into the auditory epithelium. d. A cochlea deafened with neomycin and inoculated with Ad.Atoh1 7 days later. High efficiency of gene expression is detected by nuclear staining of Atoh1-specific antibody. Bar=30μm.
Double-stained tissues in which phalloidin was applied in conjunction with the neurofilament antibody reveal that hair cells are absent, the remaining cells do not maintain the typical organization of the organ of Corti, and neuronal processes are not extending in the direction of the area where hair cells usually reside (Fig. 1c). Neurites are present medially of where the organ of Corti would have been, close to the habenula perforata. Occasionally, 1–2 neural processes are found looping into the sensory epithelium.
In animals that were deafened with neomycin, inoculated with Ad.Atoh1 and sacrificed 7 days later, high efficiency of Atoh1 transgene expression is observed in non-sensory cells that remain in the auditory epithelium, as determined by use of Atoh1 specific antibodies (Fig. 1d). The density of Atoh1-positive nuclei was highest in the area adjacent to the inoculation site where it exceeded 50% efficiency. The efficiency decreased with distance from the inoculation site. Control animals that were deafened and then inoculated with Ad.empty or not inoculated at all were negative for the Atoh1 antibody (not shown).
Plastic sections of ears obtained 6 days after the deafening procedure show that no hair cells survive in the tissue, and also that supporting cells in a differentiated state are not found either (Fig. 2). The epithelium on the basilar membrane is simple, and cells appear cuboidal or flat. The sites of the habenula perforata and the vas spiral are visible. Supporting cells that are usually found in and around the organ of Corti are not identifiable.
Figure 2.
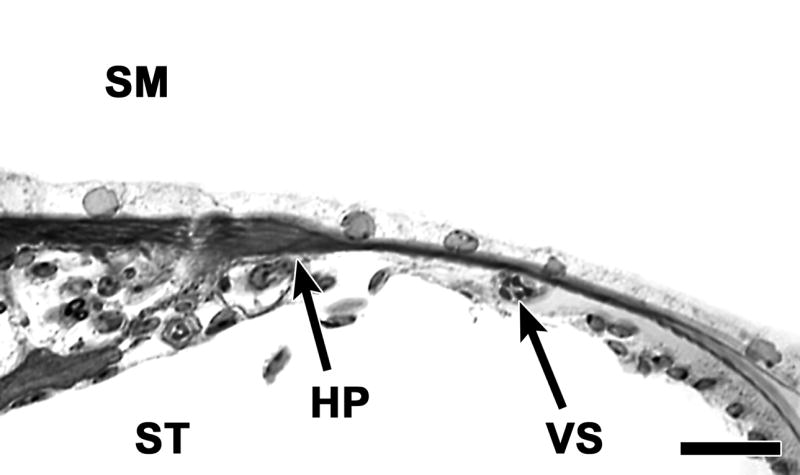
A plastic cross section of the auditory epithelium 6 days after neomycin showing absence of hair cells and of differentiated supporting cells. The auditory epithelium consists of a monolayer of flat epithelial cells. The habenula perforate (HP) and vas spiral (VS) provide landmarks for localization in the tissue. The flat epithelium extends to areas flanking the original site of the organ of Corti. SM: scala media, ST: scala tympani. Bar=20μm.
SEM analysis of the cochlea at 6 days after the deafening showed complete absence of hair cells in the epithelium (Fig. 3a). In ears deafened with neomycin, inoculated with artificial endolymph a week later, and sacrificed 2 months after the inoculation (Fig. 3b), the morphology was similar to that seen in ears that were deafened with no further treatment. Hair cells were not seen and the apical contour of the auditory epithelium lacked an organized pattern. In deafened ears inoculated with Ad.Atoh1 and sacrificed 2 months (Fig. 3c) or 10 weeks later (Fig. 3d) no hair cells could be identified and the morphology was similar to that seen in the deafened ears that received no further treatment (Fig. 3a). In all ears presented in Figure 3, the non-sensory cells lacked regular organization and hair cells could not be found.
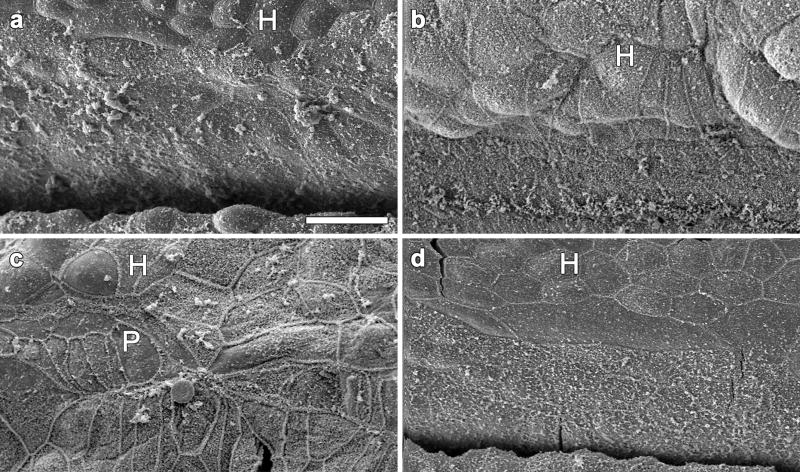
Figure 3.
SEM images of the surface of the auditory epithelium in ears treated with neomycin. a. In a cochlea obtained 6 days after neomycin administration, the epithelium is completely devoid of hair cells. The apical contour of cells is disorganized and irregular but the border area with Hensen cell region (H) is visible. b. A cochlea treated with neomycin, inoculated with artificial endolymph a week later and sacrificed 2 months after the inoculation showing similar surface morphology to that seen in (a). c–d. In a deafened cochlea inoculated with Ad.Atoh1 and obtained 2 months (c) or 10 weeks later (d), no hair cells are seen. A few cells with apical morphology resembling pillar cells (P) are sometimes seen (c). The surface morphology is similar to deafened ears that received no Ad.Atoh1 (a–b). Bar=20μm.
Discussion
The data show that 6 days after placing neomycin in the perilymph, neither hair cells nor differentiated supporting cells can be found. The rapid and devastating effect of this ototoxic regimen on hair cells has been described in the past (Jyung et al., 1989; Zappia et al., 1989). In this study, we show an equally devastating effect on supporting cells, such that the epithelium replacing the organ of Corti is flat and neuronal fibers are absent. We also show that this transformation has negative implications for adenovirus mediated therapeutic treatments. Adenovirus transduction of the tissue is substantial but less robust than in differentiated supporting cells. Forced expression of Atoh1 in the flat epithelium does not lead to appearance of new hair cells.
The mechanism inducing transformation of differentiated supporting cells to an undifferentiated flat epithelium after hair cell removal is not clear. If kanamycin and ethacrynic acid are used to eliminate hair cells, supporting cells can remain differentiated for up to 10 weeks in the guinea pig model (Izumikawa et al., 2005) and perhaps longer. Presence of differentiated supporting cells in deaf ears with no hair cells was shown using other models (Sugawara et al., 2005). Together, these data suggest that the loss of hair cells is not sufficient to cause the loss of differentiated supporting cells. It may be that the supporting cells, themselves, are sensitive to extremely ototoxic aminoglycosides such as neomycin. Furthermore, this might not be the only reason for flattening of the auditory epithelium. In some experimental conditions, supporting cells become flat a long time after the ototoxic insult is stopped, suggesting that secondary degeneration may take place (Forge et al., 1998), which might be attributed to a mechanism that is distinct from the direct effects of the ototoxic drugs. Thus, several factors may determine the survival or degeneration of non-sensory cells in the auditory epithelium. Our results suggest that future reparative approaches will be more successful when differentiated supporting cells remain in the tissue, and therefore it will be important to understand the transition to the flat epithelium and design ways to prevent it.
Phenotypic (immunocytochemical) characterization of the flat epithelium will help to determine the identity of these cells and facilitate attempts to manipulate these cells for therapeutic purposes. Several proteins have already been detected in this epithelium. For instance, these cells connect to each other with tight junctions, as determined by the presence of ZO-1 immunoreactivity, and express the supporting cell marker S-100 (Kim et al., 2007). More complete molecular characterization will help determine whether these cells are de-differentiated supporting cells of the organ of Corti (such as Deiters, pillar or Hensen cells), and/or cells that have migrated from the flanking areas such as the inner or outer sulcus.
In birds, ototoxic lesions that deplete hair cells, leave behind differentiated supporting cells and regeneration occurs spontaneously (Cotanche, 1999; Stone et al., 2000; Stone et al., 1998). A more severe lesion that also influences the supporting cells has been accomplished with noise (Cotanche et al., 1995). In such cases, non-sensory cells that flank the basilar papilla can migrate into the sensory epithelium, as may be the case in the flat epithelium. These cells then become the therapeutic target for regenerative attempts or insertion of stem cells. Previous data show that cell migration is indeed possible in the auditory epithelium in mammals (Forge et al., 1998).
Better understanding of the biology of the flat epithelium will assist in advancing tasks such as integration of stem cells, enhancement of neuronal survival or induction of transdifferentiation to the hair cell phenotype. In mammals, the flat epithelium can undergo a robust proliferative phase (Kim et al., 2007). This may be of help for designing therapies and inserting genes or stem cells. More work is necessary to characterize the origin of these cells, their general biology and their amenability to taking up external molecules or vectors for gene delivery. It will be important to identify surface receptors on these cells that will allow the design of gene transfer vectors that will have specificity to these cells. It will also be necessary to determine if these cells are heterogenous in their origin and characteristics, as tentatively suggested by their pattern of transduction with adenovirus, with only a sub-population showing positive transgene expression.
The inability of Atoh1 to induce transdifferentiation of non-sensory cells to new hair cells may be related to the state of differentiation of the epithelium. Specifically, the fate of developing cells that express Atoh1 (in the inner ear and elsewhere) depends on the context determined by previous developmental gene expression in each of these tissues. The expression of Atoh1 is usually a final step in differentiation. Thus, forced expression of Atoh1 would be expected to exert different developmental outcomes depending on the developmental history of the cell. The current findings show that the flat epithelium fails to undergo transdifferentiation following forced expression of Atoh1 and suggest that the flat epithelium has regressed to a very early state of differentiation and no longer present the commitment to the hair cell or supporting cell phenotype. Based on the lack of response to Atoh1, it can be assumed that the flat epithelium is less differentiated than cells in the vicinity of the organ of Corti, such as interdental cells, where forced Atoh1 expression induces ectopic hair cell formation (Kawamoto et al., 2003; Minoda et al., 2007).
These findings are important for conceptual and practical therapeutic approaches for hair cell regeneration. When considering transdifferentiation therapy, rebuilding the auditory epithelium may need to begin with inducing forced expression of early developmental genes, perhaps as early as otocyst specific genes that induce formation of the sensory areas, the epithelial ridges of the developing cochlear epithelium. Once expression of these early developmental genes recreates that epithelial ridge, Atoh1 over-expression may be used as a final stage for inducing generation of new hair cells.
Better understanding and ability to manipulate the flat epithelium may also be of help for enhancing cochlear implant procedures. The present data show a complete lack of hair cells along with a near complete loss of nerve fibers in the auditory epithelium. However, we found that some nerves continued to meander in the epithelium despite the lack of hair cells, possibly looking for a target. Similar finding were reported before in other models (Bohne et al., 1992; Strominger et al., 1995). The maintained ability of neurons to meander in the deafened epithelium, which appears to occur after several etiologies for hair cells loss, is important for the feasibility of innervating therapeutically-placed new hair cells or stem cells. However, the degree of neural survival may depend on the state of the supporting cells in the auditory epithelium (Sugawara et al., 2005). It would be important to characterize this correlation in human temporal bones, where deafferented spiral ganglion neurons tend to survive to a larger extent than in lab animals.
In conclusion, our data confirm that the neomycin model is an efficient method for creating a flat epithelium in the cochlea. We demonstrate that adenovirus is a useful gene carrier into the flat epithelium and Atoh1 does not induce transdifferentiation of the flat epithelium into new hair cells. As such, it is necessary to design ways to prevent degeneration of supporting cells in ears depleted of hair cells, and to direct specific therapies in cases where the flat epithelium does occur.
Acknowledgments
We thank Lisa Beyer for technical assistance. Viral vectors were kindly provided by Doug Brough (GenVec). Work was supported by the Taubman Institute, a gift from Berte and Alan Hirschfield, the R. Jamison and Betty Williams Professorship, a Research Grant from Kansai Medical University, and NIH/NIDCD Grants R01-DC01634, R01-DC05401, R01-DC03685, T32-DC00011 and P30-DC05188.
List of abbreviations
- GFP
Green fluorescent protein
- SEM
scanning electron microscopy
- PBS
phosphate buffered solution
- Fig
Figure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Bhatt KA, Liberman MC, Nadol JB., Jr Morphometric analysis of age-related changes in the human basilar membrane. Ann Otol Rhinol Laryngol. 2001;110:1147–53. doi: 10.1177/000348940111001212. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Neural regeneration in the noise-damaged chinchilla cochlea. Laryngoscope. 1992;102:693–703. doi: 10.1288/00005537-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Brough DE, Hsu C, Kulesa VA, Lee GM, Cantolupo LJ, Lizonova A, Kovesdi I. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J Virol. 1997;71:9206–13. doi: 10.1128/jvi.71.12.9206-9213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res. 2007;225:60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA. Structural recovery from sound and aminoglycoside damage in the avian cochlea. Audiol Neurootol. 1999;4:271–85. doi: 10.1159/000013852. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Messana EP, Ofsie MS. Migration of hyaline cells into the chick basilar papilla during severe noise damage. Hear Res. 1995;91:148–59. doi: 10.1016/0378-5955(95)00185-9. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–97. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–8. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyung RW, Miller JM, Cannon SC. Evaluation of eighth nerve integrity by the electrically evoked middle latency response. Otolaryngology - Head & Neck Surgery. 1989;101:670–82. doi: 10.1177/019459988910100610. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6:612–9. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- Minoda R, Izumikawa M, Kawamoto K, Zhang H, Raphael Y. Manipulating cell cycle regulation in the mature cochlea. Hear Res. 2007;232:44–51. doi: 10.1016/j.heares.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Ketten DR, Burgess BJ. Otopathology in a case of multichannel cochlear implantation. Laryngoscope. 1994;104:299–303. doi: 10.1288/00005537-199403000-00010. [DOI] [PubMed] [Google Scholar]
- Osborne MP, Comis SD. Preparation of inner ear sensory hair bundles for high resolution scanning electron microscopy. Scanning Microsc. 1991;5:555–64. [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–79. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Cellular studies of auditory hair cell regeneration in birds. Proc Natl Acad Sci U S A. 2000;97:11714–21. doi: 10.1073/pnas.97.22.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Oesterle EC, Rubel EW. Recent insights into regeneration of auditory and vestibular hair cells. Curr Opin Neurol. 1998;11:17–24. doi: 10.1097/00019052-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Strominger RN, Bohne BA, Harding GW. Regenerated nerve fibers in the noise-damaged chinchilla cochlea are not efferent. Hear Res. 1995;92:52–62. doi: 10.1016/0378-5955(95)00196-4. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB. Degeneration followed by partial regeneration of the organ of Corti in deafness (dn/dn) mice. Exp Neurol. 1992;115:27–31. doi: 10.1016/0014-4886(92)90216-d. [DOI] [PubMed] [Google Scholar]
- Zappia JJ, Altschuler RA. Evaluation of the effect of ototopical neomycin on spiral ganglion cell density in the guinea pig. Hear Res. 1989;40:29–37. doi: 10.1016/0378-5955(89)90096-8. [DOI] [PubMed] [Google Scholar]