Abstract
Previous research suggests that the frontal lobes are essential for temporal processing. We report a patient, MN, with probable Frontotemporal Dementia (FTD) who was tested on a battery of timing tasks with stimuli in the sub- and supra-second range. MN demonstrated a substantial over-estimation and under-production of target intervals on estimation and production tasks respectively but was as accurate as controls on a reproduction task. Furthermore, this deficit was markedly different for auditory and visual stimuli on production and estimation tasks; estimates of the duration of auditory stimuli were 3-4 times longer than for comparable visual stimuli. She performed normally on a task requiring her to judge whether a stimulus was longer or shorter than a standard duration with both sub- and supra-second stimuli. She performed well on control tasks involving estimation, production and reproduction of line lengths suggesting that her deficits were not attributable to a generalized cognitive impairment or an inability to make magnitude judgments. These data suggest that bifrontal pathology disrupts the “clock” or memory for time.
Keywords: Temporal processing, interval timing, Frontotemporal dementia, 0THERS?
Introduction
Humans are capable of regulating their behavior across time scales of several orders of magnitude, from hours to milliseconds (Buhusi & Meck; 2005). This capacity is suggestive of a relatively precise ability to process time that may involve both temporal and non-temporal processing modules (Lewis & Miall, 2006a; Harrington & Haaland, 1999). Disruption of timing operations may cause deficits in the ability to temporally adjust behavior that reveal the architecture of the systems involved in temporal processing (Rubia et al. 1997).
Traditional models have postulated the existence of an ‘internal clock’. One leading account, termed Scalar Expectancy Theory (SET), proposes that timing is mediated by a three-stage process, consisting of a clock, memory and decision stage mechanism for the measurement, storage and judgment of durations (Gibbon, Church & Meck, 1984). On this account, the clock stage includes a pacemaker that emits pulses at a constant rate. These pulses are then gated into an accumulator. Memory may be implicated in timing in several ways. For the purposes of comparing one interval to another, a representation of the outputs of the accumulator may be stored in memory; for purposes of judging duration or producing an interval, stored memory for durations (e.g., how long is a second) may be required. Finally, a decision stage module compares the information from the accumulator and memory representations to generate the appropriate response. In SET, the accuracy and variability of the clock mechanism are dependent upon both the level of attention being devoted to the task, and the ability of working memory to retain the pacemaker accumulation (Church, 1984). Studies investigating the relationship between operant responses and timed durations (e.g. reinforcement schedules) demonstrated that the variability of timed responses grows in proportion to the interval being timed in a manner that is consistent with Weber's law (Staddon, 1965; Dews, 1970). This consistent relationship between duration and variability was termed the ‘scalar property’ of time and is a hallmark of interval timing (Gibbon, 1977; Gibbon, Church & Meck, 1984).
The search for the physiological correlates to the internal clock has generated a substantial body of research in the past two decades (Matell & Meck, 2000; Buhusi & Meck 2005; Lewis & Miall, 2006a). One theory of neural timing, the Striatal Beat-Frequency (SBF) model, postulates that the coincident activity of oscillating cortical neurons, detected by converging input to the striatum, may serve as the signal for a to-be-timed stimulus (Matell & Meck, 2004). SBF proposes that cortico-striatal-thalamic feedback serves to maintain accurate temporal processing and motor responses. Support for this model comes from a variety of sources including neurophysiological recording and lesion studies in animals (Matell, Meck & Nicolelis, 2003; Meck, 2006) and neuroimaging data in humans (Hinton & Meck, 2004; Jahanshahi et al. 2006; Coull, 2004; Livesey, Wall & Smith, 2007; Pouthas et al., 2005; Stevens et al. 2007; but see Staddon & Higa, 2006).
Many investigations of timing suggest that the frontal cortex, particularly the Dorsolateral Prefrontral Cortex (DLPFC) is essential for temporal processing (Jones et al. 2004). In addition to contributing to the cortical time marker in SBF, the frontal cortex is also implicated in working memory models (D'Esposito, 2007; Postle, 2006; Frank, Loughry & O'Reilly, 2001). Indeed, it has been suggested that both working memory and temporal processing rely on the same neural networks (Lustig, Matell & Meck, 2005). Investigations in humans have repeatedly demonstrated the involvement of the frontal cortex in both systems. For example, numerous studies of patients with lesions to various parts of the frontal cortex have demonstrated disruptions in a variety of both temporal perception (Nichelli et al. 1995; Koch et al. 2002; Binkofski & Block 1996; Ivry & Keele 1989; Casini & Ivry 1999; Mangels, Ivry & Shimizu 1998; Mimura, Kinsborne & O'Conner, 2000; Kagerer et al. 2002) and working memory tasks (Markowitsch & Kessler, 2000; Müller & Knight, 2006; Thompson-Schill et al. 2002)
Neuroimaging data has also suggested that the frontal cortex is implicated in timing. However, the issue of laterality of function has yet to be resolved. Several neuroimaging studies utilizing functional Magnetic Resonance Imaging (fMRI), Positron Emission Tomography (PET), or Event-Related Potential (ERP) have suggested a preferential involvement of the right frontal cortex (Brunia et al. 2000; Maquet et al. 1996; Lewis & Miall, 2006b) while other studies suggest the involvement of the left frontal cortex (Jech et al. 2005; Hinton & Meck, 2004; Kawashima et al. 2000; Rubia et al. 1998; Harrington et al. 2004). Still other accounts suggest that both hemispheres are necessary for accurate temporal processing (Monfort, Pouthas & Ragot 2000; Livesey, et al. 2007; Coull et al. 2000; Macar et al. 2002; Schubotz et al. 2000; Rao et al. 2001).
Recently, studies utilizing repetitive Transcranial Magnetic Stimulation (rTMS) have explored the laterality of the frontal cortex in timing. Koch et al. (2003) demonstrated that rTMS over the right but not left DLPFC caused under-reproduction of both a sub-second and supra-second interval. Further work from these investigators revealed that rTMS over the right DLPFC improved time reproduction in Parkinson's disease (PD) patients (Koch et al. 2004). Jones et al. (2004) extended these findings by demonstrating that rTMS over the right DLPFC induced under-reproductions of a supra-second but not sub-second interval only when stimulation occurred during the reproduction phase and not the initial estimation phase. However, a recent study by Koch et al. (2007) found that rTMS on the right DLPFC led to over-reproduction of a supra-second interval, raising concerns regarding the consistency of findings in this area. Thus, although work to date has demonstrated that the frontal cortex is implicated in temporal processing and working memory, the specific contribution of this brain region to both systems remains uncertain (Andres, 2003; D'Esposito et al. 2006; Buhusi & Meck, 2005; Lewis & Miall, 2006a). We addressed this important issue by administering a variety of tasks assessing different aspects of timing at both sub- and supra-second intervals to a subject with bilateral frontal lobe dysfunction. The data are consistent with the claim that the frontal lobes are crucial for memory for time intervals or the clock mechanism itself.
Subject Description
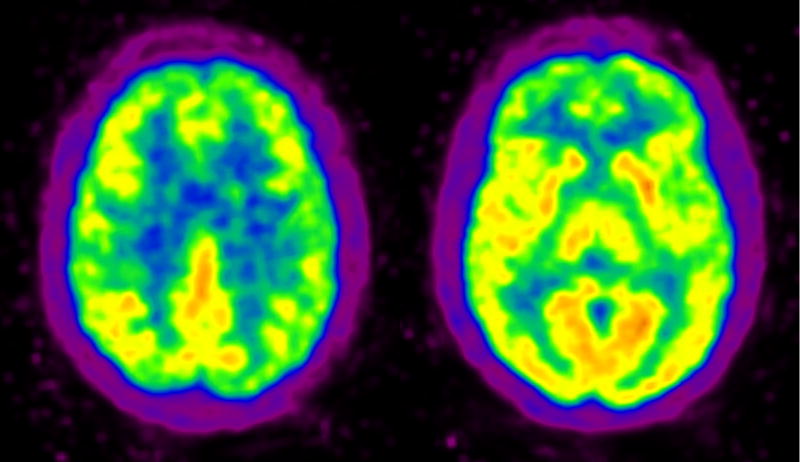
MN is a 52 year-old woman with a high school education who presented with a several year history of increasing forgetfulness, difficulty organizing her activities, and a reduced ability to perform more than one activity at a time (multi-tasking). For example, she claimed to have difficulty cooking because she was unable to time the preparation of elements of the meal so that some were getting cold as others were still cooking. Of greatest relevance to the present report, MN experienced substantial problems keeping track of time; on one occasion, after waiting several hours for a ride she wasn't sure whether she had waited for minutes or all day. After taking a nap, she often thought that it was the next day. Despite her problems, MN performed well in a variety of settings. She worked part-time at a daycare center, drove, took care of her house and provided care for ill family members. Neurological examination was normal except for the cognitive deficits described below. FDG-PET revealed decreased metabolic activity in the frontal lobes bilaterally, including the cingulate gyri. There was relative preservation of metabolic activity in the temporal, parietal and occipital regions as well as subcortical structures. (See Figure 1)
Figure 1.
Formal neuropsychological examination revealed poor performance on a variety of tasks sensitive to frontal lobe function. She performed poorly on the Wisconsin Card Sorting Test-64, solving only one category and failing to maintain set four times. She scored in the 2nd percentile on the Trails B task. She performed well on a number of tasks. Performance on the Controlled Oral Word Association Test was normal as was semantic fluency for animals (n=18). She also performed well on a number of tasks assessing visuo-spatial processing (e.g., clock drawing, Judgment of Line Orientation). Finally, she performed poorly on the Boston Naming Test (5th percentile) but did well on all other measures of language function.
Experiment 1: Estimation, Production and Reproduction of Supra-second Intervals
MN and 17 age-matched controls were tested with a battery of timing tasks, each designed to engage different aspects of temporal processing. MN was tested on the battery on two separate occasions, separated by a period of five months; the order of administration was reversed in the two sessions. As performance was quite similar in the two administrations, the data were combined. Stimuli were presented with a microcomputer.
Duration Estimation
This task was designed to assess temporal perception in both auditory and visual modalities in the supra-second range. At the onset of each trial, a fixation point was presented in the middle of the screen for one second. In the auditory version of the task, a tone was presented for 2, 4, 6, 8, 10 or 12 seconds. Auditory stimuli consisted of a free-field 250 Hz tone adjusted to a comfortable volume level for each subject. In the visual version of the task, a 4×4 cm red square was presented in the middle of the computer screen. At the offset of the stimulus, subjects were prompted by the word “respond” to indicate, in seconds, how long they believed the stimulus was present; subjects were told to respond with whatever precision they desired (that is, seconds, tenths of a second, etc.). Stimuli for each of the six durations were presented five times in random sequence for each modality. Thus, there were 60 trials in each block; as the battery was administered twice, each mean reported here for this and other tasks from this battery are based on 10 observations. Subjects were not told the range of stimulus durations and were not given feedback regarding accuracy.
Duration Production
This task was designed to assess temporal processing in both auditory and visual modalities in the supra-second range. Subjects initiated each trial with a keypress. At the onset of each trial, a fixation point was presented in the middle of the screen for one second. The fixation point was replaced by a number (2, 4, 6, 8, 10, or 12) that indicated the duration of the interval to be generated. The subjects initiated the stimulus onset by depressing the space bar on the keyboard. When the subjects believed the required interval had elapsed, they pressed the space bar a second time to terminate the trial. In the auditory task, depressing the space bar generated the same tone used in the duration estimation task; in the visual task, depressing the space bar generated the red square used in the estimation task. For both auditory and visual versions of the task, each duration was presented five times for a total of 60 trials in each administration of the task. Different durations were presented randomly. Subjects were not told the range of durations in the experiment and were not given feedback regarding accuracy.
Duration Reproduction
This task was designed to assess temporal memory in the supra-second range. Subjects initiated each trial with a keypress. At the onset of each trial, a fixation point was presented in the middle of the screen for one second. Following this, the fixation point extinguished and was replaced by a red square. The stimulus persisted for a fixed duration (2, 4, 6, 8, 10, or 12 seconds). After the prescribed duration, the stimulus extinguished and subjects initiated the reproduction stimulus by pressing the space bar causing the red square to appear; subjects pressed the space bar a second time when they believed the target interval had been reached. Five trials at each of the 6 durations were presented randomly, for a total of 30 trials. Subjects were not told the range of stimulus durations and were not given feedback regarding accuracy. There was no auditory version of this task.
Results
For all comparisons between patient and control scores we utilized the Crawford & Howell modified one-tailed t-test for significant differences in single-case studies (Crawford & Howell, 1998). Significance level was always set to P<0.05.
The mean response time for each task (estimation, production, and reproduction) was plotted against the stimulus interval. As a measure of overall accuracy, the mean response times were then fit with a linear regression (y = y0 + ax), and slope values were obtained. As a measure of variability, we utilized the standard deviation normalized by the trial target time (standard deviation/target time) and the coefficient of variation (CV; standard deviation/mean response time); the average standard deviation and CV across durations was then tested for differences between patient and controls. For the estimation and production tasks, differences between auditory and visual tasks were addressed by conducting a repeated measures ANOVA, in which modality (auditory × visual) and duration (2-12 seconds) served as factors.
Linear regressions fit to the mean response times for each duration are displayed in Figure 2. First, it should be noted that for MN the R2 for the four linear regressions ranged from .93 - .98, demonstrating that the variable of stimulus duration accounted for at least 93% of the variance in all conditions; thus, in all tasks, MN's responses were strongly correlated with stimulus duration. Analysis of the data from the Estimation task revealed that MN, but not controls, exhibited a significant effect of modality [F(1,11) = 3.32, p<0.05], as she overestimated auditory stimuli to a greater degree than visual stimuli. Because of this difference, data from these tasks were analyzed separately. MN exhibited a significantly steeper slope than controls on both auditory [t(16) = 36.72, p<0.0001] and visual [t(16) = 5.31, p<0.0001] tasks, indicating a strong tendency to overestimate durations.
Figure 2.

As MN's performance did not differ for the visual and auditory versions of the production task, data from these tasks were combined. MN differed from controls on the temporal production task in that she exhibited a significantly smaller slope value [t(16) = -1.98, p<0.04]. As shown in Figure 1, MN produced shorter durations than controls for all six stimulus durations. Finally, MN performed normally with respect to mean accuracy on the temporal reproduction task [t(16) = 0.34, p = 0.37].
Statistical tests of the normalized standard deviation scores demonstrated significantly higher variability for MN on both auditory [t(16) = 80.94, p<0.0001] and visual [t(16) = 14.58, p<0.0001] estimation tasks, while MN's production task performance demonstrated a significantly lower variability [t(16) = -1.84, p<0.05]. Additionally, although accuracy was preserved, MN was significantly more variable than normal controls on the temporal reproduction task [t(16) = 4.73, p<0.001]. Non-normalized standard deviation scores for both MN and normal controls were additionally fit with linear regressions. Although slope values obtained for all fits were positive, R2 values for MN were substantially lower than control scores (0.49 – 0.59 vs. 0.84 – 0.99). MN's variability showed little consistency in size relation to the timed durations, demonstrating a violation of the scalar property.
Analysis of CVs revealed significantly higher variability on visual estimation [t(16) = 2.83, p<0.01] and auditory estimation [t(16) = 3.16, p<0.01], and lower variability on temporal production [t(16) = -2.07, p<0.03] tasks. Higher variability was also detected on temporal reproduction for CVs [t(16) = 2.00, p<0.04].
Discussion
On temporal estimation tasks MN dramatically overestimated target durations; for example, she estimated that an auditory 6-second stimulus lasted 46 seconds and that a 12 second stimulus lasted 142 seconds. Furthermore, this deficit was observed to a greater degree for auditory stimuli as opposed to visual stimuli; for example, she estimated that a visual 12 second stimulus lasted for 28 seconds. On temporal production tasks, she underestimated the target duration; although the difference between visual and auditory stimuli was not significant, perhaps because of floor effects, she underestimated to a greater extent with auditory as compared to visual stimuli. Also of note is the fact that her variability was significantly less than controls on this task. On a temporal reproduction task MN was accurate but significantly more variable than controls.
The pattern of results demonstrated by MN is, at least in some respects, consistent with the notion of a pacemaker or accumulator running at a faster rate (Nichelli, 1996). On the notion that supra-second interval judgments are made using a pacemaker that either produces “beats” too frequently or counts them at an accelerated rate, one would expect to observe overestimation of intervals because the total number of “beats” registered would be high. On the assumption that the metric for converting beats to time intervals (e.g., seconds) is preserved, one would therefore estimate the interval to be too long. Similarly, if production of a time interval entails matching the number of beats recorded by the clock to a number that reflects one's expectation of beats for the designated time interval, one would expect that the interval produced would be too short because of the pathologically rapid accumulation of beats. A fast clock should also yield values on a production task that are reciprocal to the values on an estimation task. For example, if MN estimated a 20 second duration as 40 seconds, her production for that duration should be 10 seconds. For the auditory estimation this is difficult to assess because of possible floor effects: the reciprocal of MN's auditory estimations are all <1000ms. For visual estimations, however, the reciprocals for each estimate were plotted and compared to the data for visual productions (see Figure 3). The data and linear regressions are approximate to one another, suggesting that a fast clock could have produced both sets of data. Finally, a fast pacemaker would not be expected to affect performance on the reproduction task because the same fast-running clock is used to both assess the duration of the stimulus and generate the response.
Figure 3.
There are, however, several aspects of the data that argue against this account. First, SET proposes that the output of the pacemaker conforms to a Poisson distribution. As such, the variability of a pacemaker should equal the square root of its mean output. On the assumption that the clock operates in this manner, one would expect a “fast clock”, characterized by a greater number of pulses for a given duration, to exhibit less variability on reproduction and production tasks; as previously noted, MN exhibited greater variability than normal controls on a reproduction task. Second, the “fast clock” hypothesis does not explain the striking differences in performance with auditory and visual stimuli on the estimation and production tasks. To explain this effect one would have to postulate not only the existence of multiple modality specific clocks but also that these putative clocks run at quite different speeds.
An alternative account is that MN exhibits an impairment in memory for time. Memory may be implicated in temporal processing in a number of ways. For example, temporal estimation tasks require that the subject compare the perceived duration to a reference memory store (how long is 5 seconds). Temporal production tasks also require access to reference memory, as the response duration must be defined with respect to stored knowledge of time intervals. Temporal reproduction tasks, in contrast, require the subject to retain the perceived interval in working memory after the encoding phase, then utilize that value during the reproduction phase; temporal reproduction tasks do not require access to reference memory. One possible explanation for MN's impaired performance is that reference memory is impaired. We return to this point in the General Discussion. MN's performance on the present experiment is not sufficient to dissociate a deficit between memory types.
Experiment 2: Temporal Discrimination with Sub- and Supra-second Intervals
A number of investigators distinguish between “automatic” and “cognitive” timing mechanisms. For example, Kagerer et al. (2002) demonstrated that subjects with right hemispheric brain damage performed worse on a temporal reproduction task only for intervals above 2-3 seconds. Numerous neuroimaging studies have supported the idea that different neural networks support temporal processing for sub-second and supra-second intervals (Lewis & Miall, 2003a). Interestingly, frontal cortex activation has been implicated in the function of both mechanisms (Lewis & Miall, 2003b). The following experiment was performed to assess temporal processing with both the sub- and supra-second intervals. To that end, MN and control subjects were asked to make judgments about the relative duration of stimuli lasting 300 ms, 600 ms, 2000 ms and 8000 ms. This task also provides an opportunity to seek converging evidence regarding supra-second intervals using a task that differs from those employed in Experiment 1 with respect to task demands.
A temporal discrimination task was administered to MN and ten age-matched controls. We utilized the Parameter Estimation by Sequential Testing (PEST) algorithm (Pentland, 1980) to estimate temporal perception for target intervals of 300, 600, 2000, and 8000 milliseconds. At the onset of each trial, subjects were presented with a fixation point for one second, followed by the presentation of a red square for one of the above target intervals (standard duration). When the target interval was reached, the red square extinguished for one second. A second red square was then presented for a variable duration of time (comparison duration) as determined by the adaptive staircase procedure of the PEST algorithm. The comparison duration boundaries were set not to exceed 150% or go below 50% of the standard interval for determining upper and lower thresholds respectively. For example, on trials with a 600ms standard, the shortest comparison would be 300ms and the longest comparison would be 900ms. Subjects were required to indicate on the keyboard whether they judged the second stimulus to be longer (by pressing the ‘L’ key) or shorter (by pressing the ‘S’ key) than the first stimulus. Subjects were not told the range of stimulus durations and were not given feedback regarding accuracy. The red square was the same as in Experiment 1.
Results
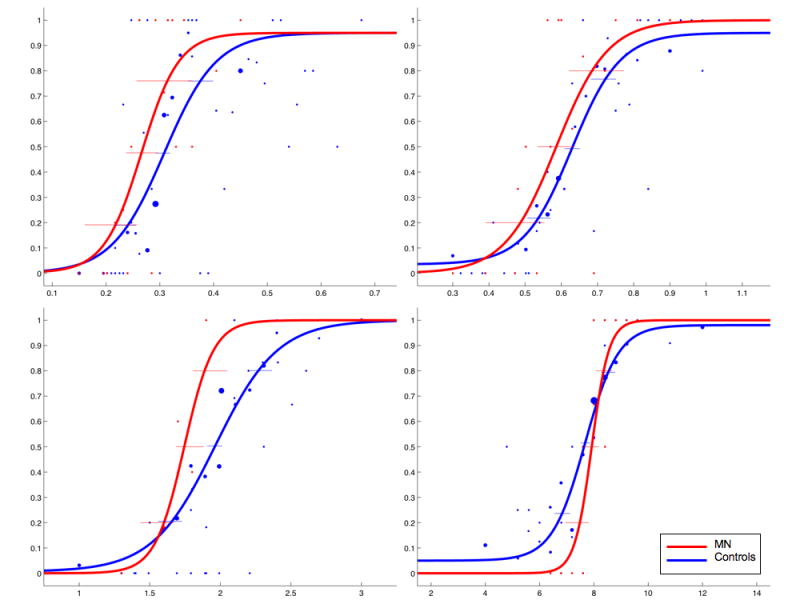
For each of the four standard intervals (300 ms, 600 ms, 2000 ms, 8000 ms), the probability of the subject making a “longer” response choice was plotted as a function of the comparison interval. This data was then fit with a sigmoidal, psychometric curve using the psignifit version 2.5.6 software package (see http://bootstrap-software.org/psignifit/) for Matlab, which implements the maximum-likelihood method described by Wichmann & Hill (2001a).
Upper and lower thresholds, the approximate points at which the subject is 25% or 75% likely to judge the stimulus as longer, were calculated by using the BC bootstrap method implemented by psignifit, based on 4999 simulations (Wichmann & Hill, 2001b). The results of this analysis yield the point of subjective equality (PSE; the time value when subjects were equally likely to judge the stimulus as longer or shorter), the difference limen (DL; upper – lower thresholds), and the coefficient of variation (CV; difference limen / PSE). Each value was averaged across normal controls and compared to the patient's scores.
Analyses for all variables obtained from the PEST temporal discrimination task exhibited no significant differences from normal controls at any of the durations tested. Mean control and patient scores, as well as their corresponding comparison p-values, are displayed in Table 1; psychometric functions are displayed in Figure 4.
Table 1.
| Duration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 300 | 600 | 2000 | 8000 | |||||||||
| Mean
MN |
Controls | P-value | MN | Controls | P-value | MN | Controls | P-value | MN | Controls | P-value | |
| PSE | 0.2659 | 0.3361 | 0.1473 | 0.6017 | 0.6187 | 0.3506 | 1.8099 | 1.9190 | 0.3246 | 7.9202 | 7.7404 | 0.3672 |
| DL | 0.0482 | 0.1183 | 0.1551 | 0.1003 | 0.1659 | 0.1078 | 0.2459 | 0.4353 | 0.4376 | 0.4390 | 0.6711 | 0.4736 |
| CV | 0.1606 | 0.3478 | 0.0908 | 0.1671 | 0.2696 | 0.1236 | 0.1230 | 0.2217 | 0.1313 | 0.0549 | 0.0971 | 0.4612 |
| Upper | 0.3141 | 0.3961 | 0.1972 | 0.7020 | 0.7016 | 0.4974 | 2.0558 | 2.1367 | 0.3986 | 8.3592 | 8.0760 | 0.4298 |
| Lower | 0.2177 | 0.2762 | 0.0950 | 0.5014 | 0.5357 | 0.2624 | 1.5639 | 1.7013 | 0.2267 | 7.4813 | 7.4049 | 0.4850 |
Figure 4.
Discussion
The data from this task corroborate and extend the results of the reproduction task employed in Experiment 1. Like the reproduction task, this task requires that the subject judge the duration of a target interval, maintain that duration in working memory while judging a second duration and make a comparison between the two durations. Consistent with her normal accuracy on the reproduction task, MN performed normally for each duration in this task.
There is one substantial difference between MN's performance on Experiments 1 and 2, however. In the first experiment, MN exhibited significantly greater variability than controls on the reproduction task. In Experiment 2, MN did not differ from controls at any interval with respect to variability as assessed by either the DL or the CV. Previous studies involving patients with unilateral prefrontal lesions have demonstrated increased variability on temporal discrimination tasks such as those employed in Experiment 2 (Mangels et al. 1998; Casini & Ivry 1999). One possible explanation for this discrepancy is that auditory stimuli were employed in these studies whereas visual stimuli were used in the present study. As MN exhibited greater deficits on estimation tasks for auditory as compared to visual stimuli, it is possible that she would have demonstrated greater variability than controls had auditory stimuli been employed.
An alternative explanation for MN's abnormal variability on the reproduction task in Experiment 1 but not in Experiment 2 is that in the former experiment the timing of the motor response was crucial as the motor response terminated the trial. In contrast, in Experiment 2, responses were generated after the trial ended with no time constraints. Thus, one possible explanation for the differences in variability exhibited by MN on the two tasks is that the variability may be, at least in part, a property of the motor system. Experiment 3, in which timing was assessed by a motor task (rhythmic finger tapping) addresses this possibility.
Experiment 3: Timing in a Finger-tapping task
The patient and ten normal controls were presented with a synchronization/continuation tapping task in which stimuli were presented in the auditory and visual modalities (Wing & Kristofferson, 1973). After initiating a trial with a key press, subjects were presented, in different blocks of trials, with a series of 440 Hz tones (auditory condition), or a series of blue squares (visual condition); both the tones and squares were 50ms in duration and appeared at 400 ms intervals. Subjects were instructed to observe the stimuli until they felt comfortable that they understood the pattern of occurrence, and then begin tapping a response key in time with the stimuli. After the first response was detected, stimuli were presented until 14 taps were recorded (synchronization phase). After 14 taps were detected, the stimuli were extinguished while subjects continued to tap at the same rate (continuation phase). After 31 taps had been recorded, the trial terminated and the subject was presented with feedback in the form of a normalized average response time, as measured by the Inter-Tap Interval (ITI) divided by the stimulus onset asynchrony (SOA); for example, if the mean tapping interval produced was 388 ms, the subject would see “.97” displayed on the screen. Subjects responded with the each hand for 12 series of taps with both the auditory and visual stimuli; right and left hand sequence was random. Subjects performed 12 trials with each hand in each modality, for a total of 48 trials. Auditory and visual conditions were administered in separate blocks on different days separated by several weeks, with MN performing the auditory task first and the visual task second.
Results
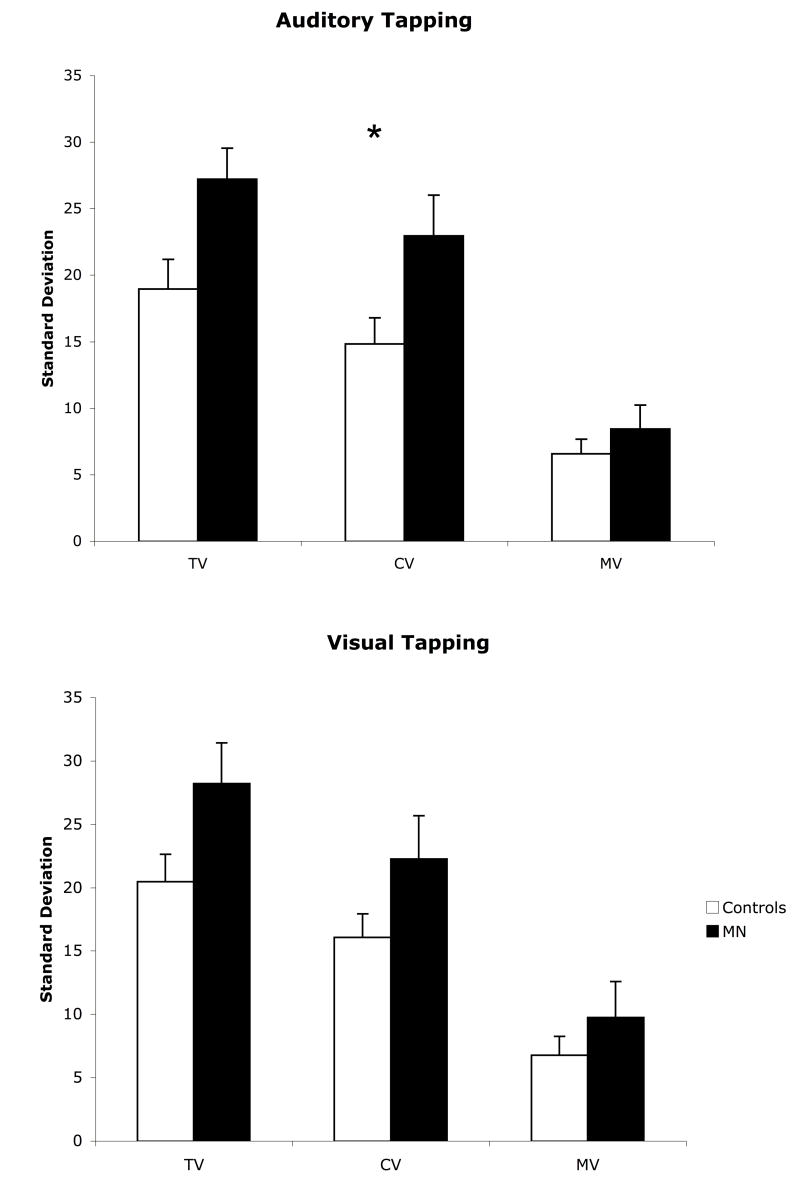
Only tapping from the continuation phase was analyzed, and the first tap from each trial was removed from the analysis. Drift was accounted for by fitting the tap times within each trial with a regression line; the residuals from each regression were then used to calculate the lag 1 autocovariance in order to calculate central and implementation variance scores (for a further discussion of methods and theory see Vorberg & Wing, 1998). The method of Wing & Kristofferson (1973) was used to generate an estimate of central and motor implementation variability. Average variance scores for each modality were then compared between the patient and normal controls.
MN demonstrated relatively normal accuracy on the synchronization/continuation tapping task for both auditory [Mean Control Accuracy = 394.1± 25, Mean MN Accuracy = 389.9±28; t(9) = -0.16, p = 0.4384] and visual [Mean Control Accuracy = 392.2±31, Mean MN Accuracy = 350.3±31; t(9) = -1.27, p = 0.1183] tasks. Variance scores are displayed in Figure 5. Decomposition of the variance into central and implementation variance scores revealed a significantly higher central variability for auditory stimuli [t(9) = 1.97, p<0.04]; there was a trend for greater central variability for visual stimuli as well [t(9) = 1.59, p = 0.0732]. There were no significant differences with respect to total or motor implementation variability.
Figure 5.
Discussion
MN performed normally with respect to accuracy but demonstrated increased central variability with both auditory and visual stimuli. This pattern of performance, disruption in central variability with preserved accuracy, has been demonstrated previously in patient populations (Harrington et al. 2004; Harrington, Haaland & Hermanowicz, 1998; Casini & Ivry, 1999). However, there has been some debate as to the source of central variability (Harrington et al. 1998), as increases in working memory and attentional demands have been shown to increase central variability scores (Sergent, Hellige & Cherry, 1993; Casini & Ivry, 1999). In light of these findings, the cause of MN's increase in central variability cannot be stated with certainty. The fact that variability was increased on this task as well as the reproduction task of Experiment 1 but not in Experiment 2 is consistent with the possibility that the increased variability is associated with the requirement to produce a timed response to terminate the trial. Thus, this finding suggests that the abnormal variability reflects processing deficits after the clock mechanism has registered interval duration.
Experiment 4: Estimation, Production and Reproduction of Line Length
MN appears to be suffering from a degenerative process primarily involving the prefrontal cortex. Furthermore, she exhibited deficits on a number of tasks on the neuropsychological assessment described previously. In light of these findings, a number of control tasks were administered to demonstrate that her deficits on temporal tasks were not attributable to generalized cognitive deficits or a failure to understand the task demands. To this end, a battery of tasks modeled on the temporal estimation, production and reproduction tasks employed in Experiment 1 was administered; in this experiment, MN was asked to make judgments about line length rather than temporal duration.
MN and five normal controls participated. The Experiment included line estimation, line production and line reproduction tasks. For line estimation, a random series of lines of differing lengths (5, 10, 15, 20, 25, and 30 cm) were presented and the subject was asked to estimate the length of the line. Each line was printed in black ink on a 20×36 cm sheet of white paper. The line was presented until the subject responded (typically within 2 seconds). Subjects were not permitted to touch the paper.
In the line production task, subjects were asked to draw a line of a specified length (5, 10, 15, 20, 25, 30 cm) on a blank 20×36 cm white sheet of paper. Each line was drawn on a separate sheet of paper with a black marker. In the line reproduction task, subjects were shown a line of 5, 10, 15, 20, 25, or 30 cm in length and asked to draw a line of the same length on a blank piece of 20×36 cm paper. The paper on which the target line was printed was placed on a table in the midline at a distance of approximately 50 cm, and displayed for no longer than two seconds. The blank paper on which the line was drawn was placed in the midline and centered at a distance of approximately 25 cm from the subject. For all three tasks, stimuli of each length were presented five times in random sequence for a total of 30 trials for each task. Data were analyzed as described in Experiment 1.
Results
Linear regressions fit to the line task are displayed in Figure 6. MN performed normally with respect to both accuracy and variability on line estimation, production, or reproduction tasks.
Figure 6.

Discussion
One might perform poorly on time production, reproduction and estimation tasks for a variety of reasons including an inability to maintain set, impaired working memory, inattentiveness, a failure to understand the task, etc. As these tasks are at least broadly comparable with respect to these dimensions, MN's normal performance with line length judgments suggests that her impaired performance on Experiments 1-3 is attributable to a deficit in temporal processing rather than a more general or pervasive cognitive or behavioral impairment.
General Discussion
The data from the first three experiments reveal a consistent deficit in the perception of temporal information. Furthermore, MN's performance on the tasks described in Experiment 4 suggests that her impairment is not attributable to a general cognitive or behavioral deficit. There are several potential accounts of these data. One possibility is that MN exhibits a “fast clock”. According to information processing models of timing (e.g., Gibbon, Church & Meck, 1984), an increase in the rate at which the pacemaker beats or an impairment in the accumulator characterized by accelerated registration of beats would lead one to estimate intervals to be longer (Harrington & Haaland, 1999). For example, if experience indicates that 100 beats represents 8 seconds but the clock is running faster such that 100 beats accumulate in 4 seconds, one would be expected to judge a stimulus of 12 seconds to be 24 seconds. This is because during that 12 second interval a total of 300 beats accumulate. On the same logic, a fast clock would be expected to lead to the production of shorter intervals. In contrast, reproduction of intervals should not be influenced by clock speed because the same clock is used to measure the length of intervals to be estimated or produced; thus, if one is asked to reproduce an interval, it doesn't matter if the interval is 100 beats or 400 beats.
Data from MN are consistent with the fast clock hypothesis in a number of respects. She over-produced and under-estimated intervals but performed normally with respect to accuracy on tasks involving the reproduction (Experiment 1) or discrimination (Experiment 2) of both sub- and supra-second intervals. It is noteworthy that MN also performed well on a task in which the timing measure is generated by the motor system (Experiment 3). Another finding consistent with the “fast clock” hypothesis is that at least for visual input, MN exhibited a reciprocal relationship between the severity of the over-estimation and under-production. This effect was not observed for auditory stimuli, perhaps because of floor effects on the production tasks.
An alternative explanation of the data is that MN exhibits an impairment in the memory processes involved in timing. SET proposes that memory is necessary for temporal processing in a variety of ways. First, as the clock-stage emits pacemaker pulses, the collection of these pulses is stored in an accumulator module, from which it is then encoded into working memory. Second, estimating the length of an interval or producing an interval of a designated duration (e.g., 10 seconds) requires access to a representation of time duration (e.g., second, minute, hour, etc). Reproduction of a time interval may be performed without accessing a stored representation of temporal duration; rather, storing the output of the accumulator in a working memory system may complete this task.
MN's impairment on production and estimation tasks is consistent with the hypothesis that her memory for temporal intervals is disrupted. On this account, if her stored representation of “one second” is, in fact, a duration of 250 ms., her production of 10 seconds would last 2.5 seconds whereas her estimation of 10 seconds would be 40 seconds. Additionally, as reproduction of intervals of the duration included in our investigations does not require reference memory, this account predicts good performance on this task. As previously noted, MN's performance was consistent with all of these prediction.
The performance of amnesic subjects is consistent with the view that memory processes are important for temporal memory (Mimura et al. 2000; Kinsbourne & Hicks, 1990). Pouthas and Perbal (2004) recently reported an amnesic subject who is relevant in this context. This subject was impaired on reproduction tasks with long durations but performed well on production tasks with both long and short durations. As reference memory would be expected to be crucial for production of any time interval, the authors interpreted this as reflecting a deficit in short-term memory with preserved reference memory for time. If MN suffers from an impairment in reference memory with preserved working and short-term memory, she might be expected to exhibit the opposite pattern of performance. Consistent with this account, MN is substantially impaired on production tasks but performs well on reproduction tasks.
There are several aspects of the data, however, that are not readily accommodated by either the fast clock or memory deficit accounts. The first is the striking difference in performance as a function of stimulus modality. Although small differences in timing have been reported for visual as compared to auditory stimuli by some investigators (Penney, Gibbon & Meck, 2000; Meck, 1984), modality differences of the magnitude exhibited by MN would not be predicted by either a fast clock or a memory impairment. A second issue is MN's increased variability on the reproduction tasks in Experiment 1 and Experiment 3. This finding suggests that there may be additional sources of variance, perhaps including such factors as impulsivity or inattentiveness.
Finally, we are unable to exclude the possibility that MN's impairment reflects a disruption when setting or defining response thresholds.
Timing and the Frontal Lobes
A number of lines of evidence reviewed above suggest that the prefrontal cortex is implicated in temporal processing (e.g., Harrington et al. 2004; Rao et al. 2001; see Lewis and Miall, 2006a). Data from MN are consistent with this claim. On models of timing incorporating a clock mechanism, one might suggest that either the pacemaker mechanism or the accumulator, or both are supported by the frontal cortex. Data from MN do not permit one to distinguish between impairments of the rate at which pulses are generated or the accuracy/efficiency with which they are counted. Second, the frontal cortex may subserve the encoding of temporal intervals into reference memory, perhaps from working memory. Indeed, recent studies utilizing a variety of working memory tasks have suggested that the frontal cortex does not store encoded information (Cohen et al. 1997; Rao et al. 2001; Campo et al. 2005; D'Esposito et al. 2006; Postle, 2006; Postle et al. 2006, Lamar et al. 2007). Rather, the frontal cortex may serve to manipulate this information in memory, while the value is stored in other cortical or subcortical areas (D'Esposito, 2007; Frank, Loughry & O'Reilly, 2001). Finally, the frontal lobes may be crucial to the representation of reference memory (e.g., the duration of 5 seconds).
A physiological model of timing, Matell and Meck's (2004) SBF model, proposes that fronto-striatal interactions constitute a critical phase in the encoding and detection of duration length, as oscillating firing rates from cortical neurons are detected by striatal medium spiny neurons where they are transformed into a temporal code. If frontal efferents are altered such that their output no longer corresponds to the same temporal code, a new coincident pattern would become associated with a previously learned temporal duration. As such, the striatum would have to contend with two coincident patterns for the same physical duration, leading to impairment in interval judgments.
Modality Differences
MN demonstrated a striking dissociation in the estimation of auditory and visual stimuli. Her estimates of the duration of auditory stimuli were as much as five times greater than her estimates for comparable visual stimuli. While not significantly different, perhaps because of floor effects, she produced intervals to auditory stimuli that are approximately one-half of the duration of intervals produced to visual stimuli.
Differences in the perception of temporal intervals as a function of stimulus modality, albeit of a far lesser magnitude, have been demonstrated previously in both humans and animals (Meck, 1984; Penney, Gibbon & Meck, 2000). Auditory stimuli have been suggested to drive the clock at faster rates, thereby leading to estimates that are longer for auditory stimuli. On the assumption that attending to visual stimuli requires greater resources than attending to auditory stimuli, this has been attributed to an effect of attention on gating or accumulating beats. Such an account seems unlikely for MN as there was no evidence of discrepancies to visual as opposed to auditory stimuli on neuropsychological examination that would explain the striking dissociation in performance on production and estimation tasks as a function of stimulus modality.
Furthermore, it is highly unlikely that MN's deficit has generated a five-fold difference in the speed at which auditory as compared to visual “clocks” operate. The alternative explanation that reference memory is modality specific and that MN exhibits differential deficits at this level also appears unlikely.
Timing Deficits in Degenerative Diseases
Disruption of temporal processing has been demonstrated in patients with Parkinson's and Huntington's Diseases (Malapani, 1998; Woodruff-Pak & Papka, 1996; O'Boyle et al. 1996). These degenerative diseases affect dopamine projections and are often characterized as disorders of the basal ganglia; although temporal processing deficits in these conditions have been taken as evidence for the role of the basal ganglia in timing, pathology has been identified in a wide range of neural structures (Carbon & Marie, 2003; Taylor, Saint-Cyr & Lang, 1986). Investigations of subjects with Alzheimer's disease have also demonstrated increased variability and impaired accuracy in timing tasks, with a tendency to overestimate larger durations (Papagano, Allegra & Cardaci 2004; Nichelli et al., 1993). Additionally, impairments have been demonstrated in subjects with cerebellar degeneration, most notably for sub-second intervals (Nichelli, Always & Grafman, 1996).
The present study is the first of which we are aware to explore temporal processing deficits in FTD. Although, the pathology in this condition is typically more severe in the frontal and, in some instances, the temporal lobes, the full extent of the pathology cannot be stated with certainty in any individual. The fact that the PET scan revealed an abnormality that is restricted to the prefrontal cortex suggests that, at least for MN, the pathology is greatest in that location.
One potentially relevant aspect of MN's disorder is that the pathology involves both hemispheres. Although strongly lateralized cognitive faculties may be identified (e.g., language), most faculties are supported by both hemispheres. As a consequence, bihemispheric lesions and degenerative conditions typically produce the most striking behavioral disorders. Thus, one possible explanation for the fact that MN exhibits a deficit in producing and estimating temporal intervals that appears to be more substantial than that exhibited by subjects reported to date is that most previously reported subjects have suffered unilateral lesions whereas MN's disorder involves both hemispheres.
It is interesting to note that, despite her striking impairment in estimating and producing time MN lives a relatively normal life. For example, although she estimated 12 seconds to be as long as 120 seconds, she drives, cooks and performs relatively well on a wide range of daily tasks. One possible explanation for this is that many activities for which timing may be crucial are embedded in an action stream in which time intervals are judged relative to one another rather than in an absolute fashion. Consider for example, the temporal computations that underlie the ability to turn across a lane of oncoming traffic. This may require that one generate an estimate of the time interval between oncoming cars. One may then, on the basis of previous experience or a mental simulation of the act of accelerating across the lane of traffic, determine whether there is sufficient time to make the turn. It is important to note that this process requires that two intervals be compared – the interval between oncoming cars and the time required to cross the lane of traffic. Both intervals are generated by the same timing mechanism; thus, deficits in clock speed or reference memory are unlikely to produce profound alterations in routine tasks. In this respect then, we suggest that most activities for which timing is crucial are, from a computational perspective, most similar to temporal reproduction tasks. As MN performed well on this and the related tasks described in Experiments 2 and 3, it may not be surprising that she performs well on everyday tasks for which temporal computations are critical.
Acknowledgments
Supported by RO1 MH76227 to HBC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres P. Frontal cortex as the central executive of working memory: time to revise our view. Cortex. 2003;39(45):871–895. doi: 10.1016/s0010-9452(08)70868-2. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Block RA. Accelerated time experience after left frontal cortex lesion. Neurocase. 1996;2:485–493. [Google Scholar]
- Brunia CH, de Jong BM, van den Berg-Lenssen MM, Paans AM. Visual feedback about time estimation is related to a right hemisphere activation measured by PET. Experimental Brain Research. 2000;130(3):328–337. doi: 10.1007/s002219900293. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):766–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Carbon M, Marie RM. Functional imaging of cognition in Parkinson's disease. Current Opinion in Neurobiology. 2003;16(4):475–480. doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- Casini L, Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13(1):10–21. doi: 10.1037//0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- Church RM. Properties of the internal clock. Annals of the New York Academy of Sciences. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- Campo P, Maestu F, Ortiz T, Capilla A, Santiuste M, Fernandez A, Amo C. Time modulated prefrontal and parietal activity during the maintenance of integrated information as revealed by magnetoencephalography. Cerebral Cortex. 2005;15(2):123–130. doi: 10.1093/cercor/bhh115. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Coull JT. fMRI studies of temporal attention: allocating attention within or towards time. Cognitive Brain Research. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38(6):808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual's test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society of London. 2007;362(1481):761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Cooney JW, Gazzaley A, Gibbs SEB, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and fMRI data. Journal of the International Neuropsychological Society. 2006;12:248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- Dews PB. The theory of fixed-interval responding. In: Schoenfeld WN, editor. The Theory of Reinforcement Schedules. New York: Appleton-Century-Crofts; 1970. pp. 43–61. [Google Scholar]
- Elvevåg B, Brown GD, McCormack T, Vousden JI, Goldberg TE. Identification of tone duration, line length, and letter position: an experimental approach to timing and working memory deficits in schizophrenia. Journal of Abnormal Psychology. 2004;113(4):509–521. doi: 10.1037/0021-843X.113.4.509. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O'Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: A computational model. Cognitive, Affective, and Behavioral Neuroscience. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and weber's law in animal timing. Psychological Review. 84(3):279–325. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research. Reviews Neurosciences. 1999;10(2):91–116. doi: 10.1515/revneuro.1999.10.2.91. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12(1):3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Handy TC, Gazzaniga MS, Ivry RB. Cortical and subcortical contributions to the representation of temporal information. Neuropsychologia. 2003;41:1461–1473. doi: 10.1016/s0028-3932(03)00093-9. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, Rao SM. Neural representation of interval encoding and decision making. Cognitive Brain Research. 2004;21(2):193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. Frontal-striatal circuity activated by human peak-interval timing in the supra-seconds range. Cognitive Brain Research. 2004;21(2):171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Dirnberger G, Frith CD. The substantia nigra pars compacts and temporal processing. The Journal of Neuroscience. 2006;26(47):12266–12273. doi: 10.1523/JNEUROSCI.2540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jech R, Dusek P, Wackermann J, Vymazal J. Cumulative blood oxygenation-level-dependent signal changes support the ‘time accumulator’ hypothesis. Neuroreport. 2005;16(13):1467–1471. doi: 10.1097/01.wnr.0000175616.00936.1c. [DOI] [PubMed] [Google Scholar]
- Jones CR, Rosenkranz K, Rothwell JC, Jahanshahi M. The right dorsolateral prefrontal cortex is essential in time reproduction: an investigation with repetitive transcranial magnetic stimulation. Experimental Brain Research. 2004;158(3):366–372. doi: 10.1007/s00221-004-1912-3. [DOI] [PubMed] [Google Scholar]
- Kagerer FA, Wittmann M, Szelag E, Steinbuchel N. Cortical involvement in temporal reproduction: evidence for differential roles of the hemispheres. Neuropsychologia. 2002;40(3):357–366. doi: 10.1016/s0028-3932(01)00111-7. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Okuda J, Umetsu A, Sugiura M, Inoue K, Suzuki K, Tabuchi M, Tsukiura T, Narayan SL, Nagasaka T, Yanagawa I, Fujii T, Takahashi S, Fukuda H, Yamadori A. Human cerebellum plays an important role in memory-timed finger movement: an fMRI study. Journal of Neurophysiology. 2000;83(2):1079–1087. doi: 10.1152/jn.2000.83.2.1079. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M, Hicks RE. The extended present: evidence from time estimation by amnesics and normals. In: Shallice T, editor. Neuropsychological Impairments of short-term Memory. Cambridge University Press; Cambridge UK: 1990. pp. 319–329. [Google Scholar]
- Koch G, Oliveri M, Carlesimo GA, Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002;59(10):1658–1659. doi: 10.1212/01.wnl.0000032504.45792.8f. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60(11):1844–1846. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Brusa L, Stanzione P, Torriero S, Caltagirone C. High-frequency rTMS improves time perception in Parkinson disease. Neurology. 2004;63:2405–2406. doi: 10.1212/01.wnl.0000147336.19972.82. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Gerfo EL, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Experimental Brain Research. 2007;179(2):291–299. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- Lamar M, Price CC, Libon DJ, Penney DL, Kaplan E, Grossman M, HeiMNan KM. Alterations in working memory as a function of leukoaraiosis in dementia. Neuropsychologia. 2007;45(2):245–254. doi: 10.1016/j.neuropsychologia.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003a;41(12):1583–1592. doi: 10.1016/s0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Current Opinion in Neurobiology. 2003b;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Remembering the time: a continuous clock. Trends in Cognitive Sciences. 2006a;10(9):401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. A right hemispheric prefrontal system for cognitive time measurement. Behavioral Processes. 2006b;71(23):226–234. doi: 10.1016/j.beproc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Livesey AC, Wall MB, Smith AT. Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia. 2007;45(2):321–331. doi: 10.1016/j.neuropsychologia.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: frontal-striatal synchronization in working memory and timing. Memory. 2005;13:441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, Maquet P. Activation of the supplementary motor area and of attentional networks during temporal processing. Experimental Brain Research. 2002;142(4):475–485. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson's disease: a dopamine-related dysfunction. Journal of Cognitive Neuroscience. 1998;10(3):316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Cognitive Brain Research. 1998;7(1):15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Kessler J. Massive impairment in executive functions with partial preservation of other cognitive functions: the case of a young patient with severe degeneration of the prefrontal cortex. Experimental Brain Research. 2000;133(1):94–102. doi: 10.1007/s002210000404. [DOI] [PubMed] [Google Scholar]
- Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit-Berthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D. Brain activation induced by estimation of duration: a PET study. Neuroimage. 3(2):119–126. doi: 10.1006/nimg.1996.0014. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22(1):94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Research: Cognitive Brain Research. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MA. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behavioral Neuroscience. 2003;117(4):760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- Meck WH. Attentional bias between modalities: effect on the internal clock, memory, and decision stages used in animal time discrimination. Annals of the New York Academy of Sciences. 1984;423:528–541. doi: 10.1111/j.1749-6632.1984.tb23457.x. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research. 2006;1109(1):93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Mimura M, Kinsbourne M, O'Conner M. Time estimation by patients with frontal lesions and by Korsakoff amnesics. Journal of the International Neuropsychological Society. 2000;6:517–528. doi: 10.1017/s1355617700655017. [DOI] [PubMed] [Google Scholar]
- Monfort V, Pouthas V, Ragot R. Role of the frontal cortex in memory for duration: an event-related potential study in humans. Neuroscience Letters. 2000;286(2):91–94. doi: 10.1016/s0304-3940(00)01097-1. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139(1):51–58. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Venneri A, Molinari M, Tavani F, Grafman J. Precision and accuracy of subjective time estimation in different memory disorders. Cognitive Brain Research. 1993;1(2):87–93. doi: 10.1016/0926-6410(93)90014-v. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Clark K, Hollnagel C, Grafman J. Duration processing after frontal lobe lesions. Annals of the New York Academy of Sciences. 1995;769:183–190. doi: 10.1111/j.1749-6632.1995.tb38139.x. [DOI] [PubMed] [Google Scholar]
- Nichelli P. Time perception measurements in neuropsychology. In: Paster MA, Artieda J, editors. Time, internal clocks and movement. Elsevier; 1996. pp. 187–204. [Google Scholar]
- Nichelli P, Always D, Grafman J. Perceptual timing in cerebellar degeneration. Neuropsychologia. 1996;34(9):863–871. doi: 10.1016/0028-3932(96)00001-2. [DOI] [PubMed] [Google Scholar]
- O'boyle DJ, Freeman JS, Cody FW. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson's disease. Brain. 1996;119(1):51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- Papagano C, Allega A, Cardaci M. Time estimation in Alzheimer's disease and the role of the central executive. Brain and Cognition. 2004;54:18–23. doi: 10.1016/s0278-2626(03)00237-9. [DOI] [PubMed] [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Differential effects of auditory and visual signals on clock speed and temporal memory. Journal of Experimental Psychology: Human Perception & Performance. 2000;26(6):1770–1787. doi: 10.1037//0096-1523.26.6.1770. [DOI] [PubMed] [Google Scholar]
- Pentland A. Maximum likelihood estimation: the best PEST. Perception & Psychophysics. 1980;28(4):377–379. doi: 10.3758/bf03204398. [DOI] [PubMed] [Google Scholar]
- Pouthas V, Perbal S. Time perception depends on accurate clock mechanisms as well as unimpaired attention and memory processes. Acta Neurobiologiae Experimentalis. 2004;64(3):367–385. doi: 10.55782/ane-2004-1520. [DOI] [PubMed] [Google Scholar]
- Pouthas V, George N, Poline JB, Pfeuty M, Vandemoorteele PF, Hugueville L, Ferrandez AM, Lehericy S, Lebihan D, Renault B. Neural network involved in time perception: an fMRI study comparing long and short interval estimation. Human Brain Mapping. 2005;25(4):433–441. doi: 10.1002/hbm.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139(1):23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. Journal of Cognitive Neuroscience. 18(10):1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nature Neuroscience. 2001;4(3):317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rubia K, Schuri U, v Cramon DY, Poeppel E. Time estimation as a neuronal network property: a lesion study. Neuroreport. 1997;8:1273–1276. doi: 10.1097/00001756-199703240-00043. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, BulMNore E. Prefrontal involvement in “temporal bridging” and timing movement. Neuropsychologia. 1998;36(12):1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Friederici AD, von Cramon DY. Time perception and motor timing: a common cortical and subcortical basis revealed by fMRI. Neuroimage. 2000;11(1):1–12. doi: 10.1006/nimg.1999.0514. [DOI] [PubMed] [Google Scholar]
- Sergent V, Hellige JB, Cherry B. Effects of responding hand and concurrent verbal processing on time-keeping and motor-implementation processes. Brain and Cognition. 1993;23(2):243–262. doi: 10.1006/brcg.1993.1058. [DOI] [PubMed] [Google Scholar]
- Staddon JE. Some properties of spaced responding in pigeons. Journal of the Experimental Analysis of Behavior. 1965;8(1):19–27. doi: 10.1901/jeab.1965.8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon JE, Higa JJ. Interval timing. Correspondence. Nature Reviews Neuroscience. 2006;7 [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Human Brain Mapping. 2007;28(5):394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson's disease. Brain. 1986;109(5):845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D'Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cognitive, Affective, Behavioral Neuroscience. 2002;2(2):109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Vorberg D, Wing AM. Handbook of perception and action. New York, NY: Academic Press; 1996. Modeling variability and dependence in timing; pp. 181–262. [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling and goodness of fit. Perception & Psychophysics. 2001a;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Perception & Psychophysics. 2001b;63(8):1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Wing AM, Kristofferson A. Response delays and the timing of discerete motor responses. Perception & Psychophysics. 1973;14:5–12. [Google Scholar]
- Woodruff-Pak DS, Papka M. Huntington's disease and eyeblink classical conditioning: normal learning but abnormal timing. Journal of the International Neuropsychological Society. 1996;2(4):323–334. doi: 10.1017/s135561770000134x. [DOI] [PubMed] [Google Scholar]