Abstract
We previously identified the adaptor protein PACSIN2 as a negative regulator of ADAM13 proteolytic function. In Xenopus embryos, PACSIN2 is ubiquitously expressed, suggesting that PACSIN2 may control other proteins during development. To investigate this possibility, we studied PACSIN2 function during Xenopus gastrulation and in XTC cells. Our results show that PACSIN2 is localized to the plasma membrane via its Coiled-coil domain. We also show that increased levels of PACSIN2 in embryos inhibit gastrulation, fibronectin (FN) fibrillogenesis and the ability of ectodermal cells to spread on a FN substrate. These effects require PACSIN2 Coiled-coil domain and are not due to a reduction of FN or integrin expression and/or trafficking. The expression of a Mitochondria Anchored PACSIN2 (PACSIN2-MA) sequesters wild type PACSIN2 to mitochondria, and blocks gastrulation without interfering with cell spreading or FN fibrillogenesis but perturbs both epiboly and convergence/extension. In XTC cells, the over-expression of PACSIN2 but not PACSIN2-MA prevents the localization of integrin β1 to focal adhesions (FA) and filamin to stress fiber. PACSIN2-MA prevents filamin localization to membrane ruffles but not to stress fiber. We propose that PACSIN2 may regulate gastrulation by controlling the population of activated α5β1, integrin and cytoskeleton strength during cell movement.
Keywords: PACSIN2, Xenopus, gastrulation, integrin α5β1, filamin
Introduction
During development, cell movements are driving forces that shape the embryo. The earliest cell movements occur at gastrulation during which cells from the three primordial germ layers (ectoderm, mesoderm and endoderm) move with respect to one another. In the frog embryo, cells from the blastocoel roof (BCR) assemble a fibronectin-rich (FN) extracellular matrix (ECM) before gastrulation. During gastrulation, mesodermal cells involute, bind to, and migrate on the FN rich ECM toward the animal pole (Boucaut et al., 1984a; Winklbauer and Keller, 1996). The Central Cell Binding Domain (CCBD) of FN and the α5β1 integrin are involved in both the assembly of FN by ectodermal cells and the adhesion and migration of mesodermal cells (Darribere et al., 1992; Davidson et al., 2002; Hoffstrom, 2002; Na et al., 2003). The ability of mesodermal cells to spread and migrate on FN is regulated in both space (the involuted mesoderm) and time (at the onset of gastrulation) (Ramos et al., 1996). Previous studies have shown that ectodermal cells from the animal hemisphere can be induced to become mesodermal cells by Activin-A, a member of the TGF-β family of growth factors (Smith et al., 1988). While untreated ectoderm cells bind to FN, Activin-A treatment of these cells induces both spreading and migration on this substrate (Ramos and DeSimone, 1996; Smith et al., 1990). Interestingly, Activin-A treated ectoderm cells spread and migrate with the same timing as mesoderm cells in vivo.
The α5β1 integrin is expressed ubiquitously during gastrulation (Joos et al., 1995). There is no difference in cell surface expression of α5β1 integrin between the ectoderm and the mesoderm, suggesting that this integrin has different activation states in these two tissues (Ramos et al., 1996). The activation state achieved in the mesoderm is under the control of both mesoderm inducing factors and an “internal clock” (Dzamba et al., 2002). This control requires the cytoplasmic domains of both α and β subunits (Marsden and DeSimone, 2001; Na et al., 2003). However, little is known about the proteins involved in the control of the activation. Interestingly, the expression of the tyrosine phosphatase xPTP-PESTr induces the spreading of ectodermal cell before the onset of gastrulation. This suggests that either PTP-PEST or a binding partner could be part of the “internal clock” that controls the timing of α5β1 activation (Cousin and Alfandari, 2004).
The molecular events of cell adhesion and migration have been studied extensively using cell culture on various substrates including FN. During the early steps of cell adhesion to ECM, the integrins are activated through a conformational change (Hynes, 2002). Activated integrins then undergo a lateral assembly to form clusters, which subsequently recruit a large number of cytoplasmic proteins to form a structure known as the focal adhesion (FA)(Webb et al., 2002). These focal adhesions (FA) are sites of multiple signaling events from both outside and inside the cell. FA formation is tightly linked to the capacity of integrins to form clusters, but the exact mechanism of the clustering remains uncertain. In tissue culture cells, the interaction of β subunit with the cytoplasmic protein talin is required for integrin activation, cell spreading and migration (Calderwood, 2004; Ginsberg et al., 2005). The subsequent steps in cell adhesion and migration depend heavily on the capacity of cells to reorganize their actin cytoskeleton. The actin structures involved include (1) unipolar actin bundles within filopodia necessary for the cell to probe the environment, (2) a broad actin network in the lamellipodia whose polymerization drives cell migration and, (3) contractile actin stress fibers, containing acto-myosin bundles, which are essential for cell adhesion to the ECM and for the retraction of the trailing edge during migration (Ridley et al., 2003). Many proteins are involved in the regulation of these actin structures. One of these proteins, filamin, organizes the shape of the actin network (orthogonal or parallel bundles) and tethers it to the cortex (van der Flier and Sonnenberg, 2001). To achieve this function, filamin binds to many protein including integrin, actin and PACSIN family members.
PACSINs are members of the PCH (Pombe CDC15 Homology) family of proteins that play a role in remodeling the actin cytoskeleton (Chitu and Stanley, 2007). These adaptor proteins of 50 to 70 kDa contain (1) a Fer/CIP Homology (FCH) domain, (2) a CDC15 N-terminal homology (CDC15-NT) domain containing a Coiled coil (CC) domain involved in the binding of Filamin (Nikki et al., 2002a), (3) a linker domain containing NPF motifs or a Proline rich motif (PACSIN3), and (4) an SH3 domain. Many proteins are known to bind these different domains (Chitu and Stanley, 2007; Kessels and Qualmann, 2004). The PACSIN proteins homo and hetero-oligomerize through their FCH domain and possibly CDC15 NT domains (Halbach et al., 2007; Kessels and Qualmann, 2006; Nikki et al., 2002b) (Cousin and Alfandari, unpublished results). This oligomerization is thought to be important for the capacity of PACSINs to act as adaptor proteins. The over-expression of either the full length PACSINs or just the SH3 domains has been shown to inhibit endocytosis of the transferin receptor, suggesting a role for PACSINs in endocytosis (Modregger et al., 2000; Qualmann and Kelly, 2000; Simpson et al., 1999). Over-expression of PACSIN was also shown to induce actin-rich filopodia (Nikki et al., 2002a; Qualmann and Kelly, 2000; Sumoy et al., 2001), that could be rescued by co expressing the C-terminal region of N-WASP, an activator of the Arp2/3 complex (Qualmann and Kelly, 2000). Based on these results, it has been proposed that PACSINs are directly involved in endocytosis by linking the actin cytoskeleton to the endocytic machinery (Kessels and Qualmann, 2004; Qualmann and Kelly, 2000). Other groups have shown that PACSINs can also interact with proteins involved in signaling such as the death receptor ligand CD95L (Ghadimi et al., 2002) and the metalloprotease ADAM12 (Mori et al., 2003). In the case of ADAM12, PACSIN3 was shown to bind and activate the ADAM12-mediated shedding of HB-EGF in response to phorbol ester or angiotensin II treatment. We have reported that PACSIN2 is able to bind and inhibit the metalloprotease ADAM13, which is involved in the migration of cranial neural crest cell migration in Xenopus (Alfandari et al., 2001; Cousin et al., 2000). This inhibition is not linked to a decrease in cell surface expression of ADAM13 (Cousin et al., 2000). While the regulation of cell migration by a PACSIN family members is a new concept, the PCH family member CD2BP1/PST PIP1 have been implicated in the control of cell-cell adhesion (Badour et al., 2003; Li et al., 1998). Considering the ubiquitous expression of PACSIN2 in Xenopus embryos, we have used gastrulation, a stage where integrin function and regulation has been extensively studied, to further investigate the function of PACSIN2 in modulating cell migration.
In this report, we show that PACSIN2 over-expression inhibits gastrulation by down regulating the α5β1 integrin activity but not cell surface expression. We further show that PACSIN2 over-expression prevents the recruitment of integrin α5β1 to focal adhesions (FA) but not the recruitment of αv integrin. We also show that the membrane localization of PACSIN2, driven by a region near the Coiled-coil domain, is necessary for this function. Based on that observation and the fact that PACSIN2 forms multimers, we have used a mitochondrial anchored PACSIN2 (PACSIN2-MA) to remove endogenous PACSIN2 from the membrane. PACSIN2-MA blocks gastrulation but does not prevent α5β1 integrin activation or localization to FA. The over-expression of PACSIN2 and PACSIN2-MA has opposite effects on the cellular localization of filamin.
Materials and Methods
Eggs and embryos
Xenopus laevis eggs were obtained fertilized, cultured and staged as described previously (Cousin et al., 2000).
Constructs
The wild type PACSIN2 (wt) and ∂CC mutant were cloned into pCS2+ and pCS2-MT (Turner and Weintraub, 1994) between EcoRI and XbaI site (Cousin et al., 2000). The deletion of the residues 185 to 218 corresponding to the Coiled coil domain (CC) was performed by amplifying separately the PACSIN2 sequence in 5 ′ and 3 ′ of the CC domain. The junction of the two fragments was performed by the addition of a Bgl II site. The ∂CC construct was then cloned into pCS2+ vector and the open reading frame was verified by sequencing and by western blot. For the PACSIN2-MA construct, the PACSIN2 ORF was amplified by PCR (omitting the stop codon) and cloned in pCS2+ between the BamHI and EcoRI sites. The mitochondria anchor sequence was then amplified by PCSR and inserted at the c-terminus of PACSIN2 between the EcoRI and XbaI sites (Bubeck et al., 1997; Park et al., 2005; Pistor et al., 1994).
Microinjection experiments
Transcription reactions and injections were performed as previously described (Cousin and Alfandari, 2004; Cousin et al., 2000). The morpholinos (GeneTool) were designed by the manufacturer and injected similarly to transcripts (See Table 2).
Table 2.
List of the morpholinos
| PACSIN2 | 5′-ATATGTTCCCGACATGATCCTACGG- 3′ |
| PACSIN2′ | 5′-CGGAATCATCGTATGTGCCCGACAT- 3′ |
| Control | 5′-CCTCTTACCTCAGTTACAATTTATA-3′ |
Note: The ATG is underlined
Whole mount in situ hybridization
Whole mount in situ hybridization was carried out as previously described (Cousin et al., 2000). The following investigators generously provided the cDNA templates used to generate the probes: Xbra (J.Smith), Sox2 (R. Grainger), chordin (E.M. De Robertis) and N-tubulin (D.L.Shi).
Antibodies
The list and working dilutions of antibodies are presented in Table 1. The monoclonal antibodies were prepared and purified as described in (Alfandari et al., 2003). The biotinylation of 3D8 was performed using EZ-link-sulfo-NHS-LC-biotin (Pierce) at ratio of 1mg of biotin per mg of antibody during the purification process.
Table 1.
List of the antibodies
| Antibody | Antigen | IF | IP | WB | Reference |
|---|---|---|---|---|---|
| 8C8 | Integrin β1 | 10μg/ml | 10μg | 1μg/ml | (Gawantka et al., 1994) |
| P8D4 | Integrin α5β1 | 10μg | (Davidson et al., 2002) | ||
| 32F | fibronectin | 1:2000 | (Lee et al., 1984) | ||
| 4H2 | fibronectin | 1μg/ml | (Ramos and DeSimone, 1996) | ||
| P3C12 | Integrin αv | 10μg/ml | 10μg | (Cohen et al., 2000) | |
| 3D8 | PACSIN2 | 10μg/ml | 10μg | 1μg/ml | (Cousin et al., 2000) |
| 3D8 biotinylated | PACSIN2 | 10μg/ml | |||
| 3D8-Texas Red | PACSIN2 | 10μg/ml | |||
| filamin | Chicken filamin | 1:200 | 2μl | 1:5000 | AbCam cat# 11074 |
| MitoTraker Green FM | Mitochondrial marker | 200nM | Molecular Probes Cat#M7514 | ||
| 9E10 | myc | 30μg.ml | 10μg | 1μg/ml | Santa Cruz laboratory |
| 6615F | ADAM13 | 1:500 | (Alfandari et al., 1997) | ||
| DAPI | DNA | 0.5μg/ml | |||
| Phalloidin-Alexa594 | F-actin | 1:40 | Molecular Probes Cat#A12381 |
Protein extraction and analysis
The blastocoel content of 20 embryos was aspirated using a borosilicate needle and PLI-100 injection system and added to an equal volume of 2xLaemmli buffer containing 2% 2-mercaptoethanol. Cell surface biotinylation of embryonic cells was performed as described in (Alfandari et al., 1995) using EZ-link- sulfo-NHS-LC-biotin (Pierce). Embryonic proteins were extracted and analyzed by immunoprecipitation and/or western blot as previously described (Cousin et al., 2000). Co-immunoprecipitations were performed using about 1 to 2.106 XTC cells as described in (Cousin et al., 2000). For two dimensional gel electrophoresis analyses, the protein extracts were desalted on exclusion spin column (Pierce) and processed as described in (Jha et al., 2006).
Embryo and cell imaging
Picture of embryos and explants were taken using the Nikon SMZ stereomicroscope, the Nikon D50 digital camera and the Nikon Capture Software. Embryonic and XTC cells were observed using Zeiss Axiovert 200M inverted microscope equipped with a Ludl xyz-stage control and a Hamamatsu Orca camera. Images were taken using either the Openlab software (Improvision) (Figures 1C; 3; 5AB; 7C) or the AxioVision software (Zeiss) using the ApoTome structured light illumination system (Zeiss) (Figures 6BC; 7B; 10BC) or not (Figure 8).
Figure 1. A coiled coil domain is necessary to localize PACSIN2 near the cell membrane in embryos.
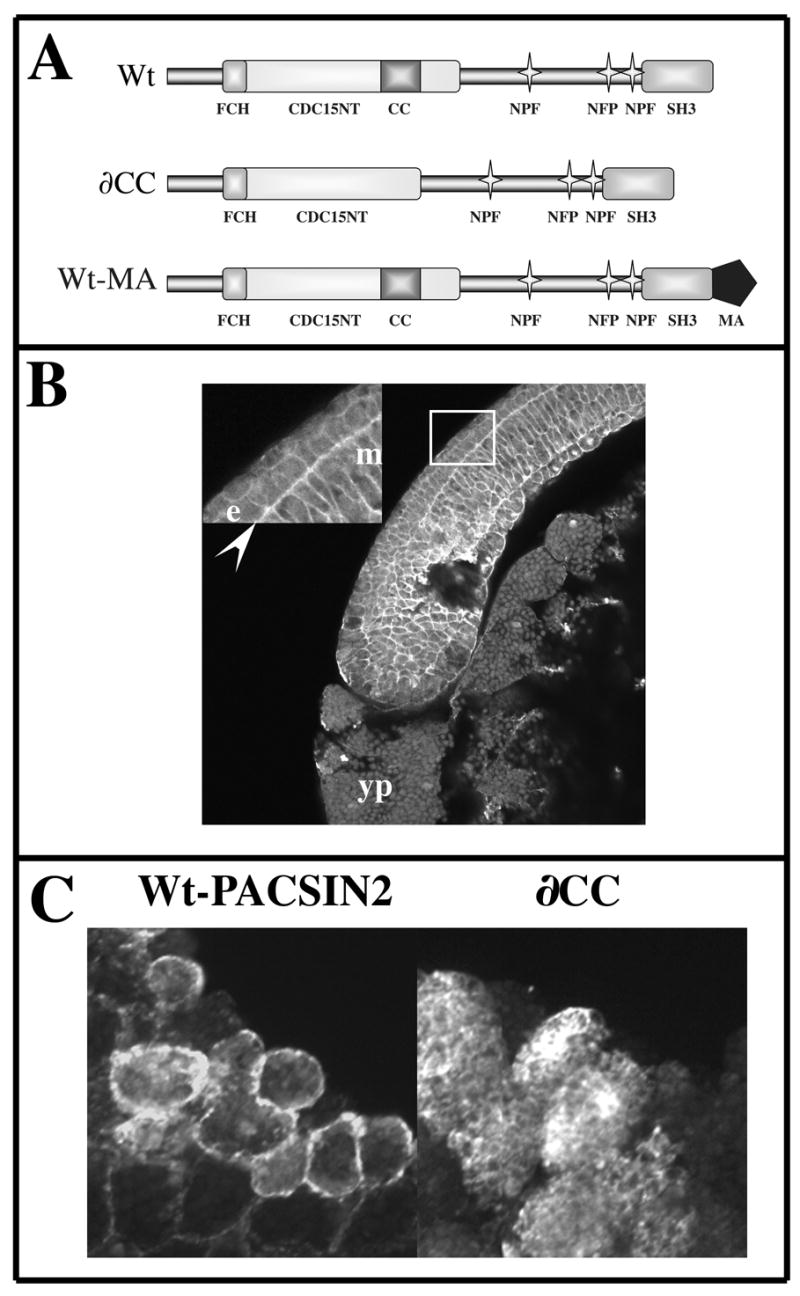
(A) Schematic representation of the wild type form of Xenopus laevis PACSIN2 (Wt), a mutant form where a putative Coiled Coil domain has been removed (∂CC) and a Wild type form of PACSIN2 with a mitochondria anchor sequence at the C-terminal end (Wt-MA). (B) Immunodetection of endogenous PACSIN2 on a sagital section of Xenopus embryo at stage 12 using the monoclonal antibody 3D8. PACSIN2 is expressed strongly near the membrane of ectoderm (e) and mesoderm cells (m). This membrane localization is more obvious in the mesoderm and at the interface between mesoderm and ectoderm (arrow head). (C) Embryos injected in 1 blastomere at the two-cell stage were grown to mid gastrula (12) and the over-expressed PACSIN2 detected by immunofluorescence on sagital section. The Wt-PACSIN2 is enriched at the vicinity of the cytoplasmic membrane while the mutant ∂CC remains cytoplasmic. yp: yolk plug; m: mesoderm; e: ectoderm.
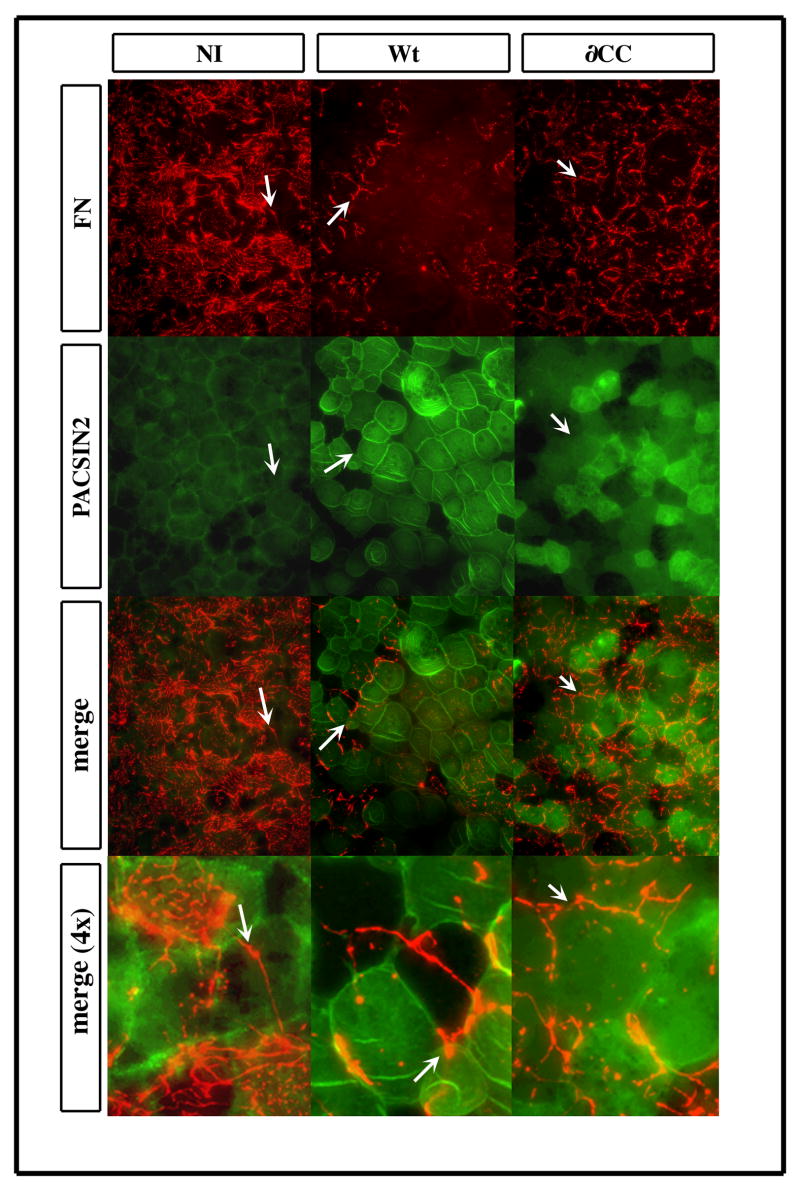
Figure 3. PACSIN2 over-expression inhibits fibronectin fibrillogenesis.
Embryos were injected in both blastomeres at the two-cell stage. The blastocoel roofs (BCR) were dissected at stage 12, fixed and processed for immunofluorescence. The FN fibrils of the BCR were detected using a rabbit polyclonal antibody 32F (red) while PACSIN2 was detected with the mAb 3D8 (green). The region indicated by white arrows is magnified in the merge (4x) inset. While all photographs were taken with the same settings, the endogenous PACSIN2 detection (4X, left) has been enhanced using Photoshop in the magnified image of NI cell to visualize the cell borders. Over-expression of Wt PACSIN2 in the entire BCR prevents FN fibrillogenesis. The expression of ∂CC PACSIN2 allows the BCR cells to assemble some FN fibrils.
Figure 5. Over-expression of PACSIN2 prevents cell spreading and perturbs convergence and extension.
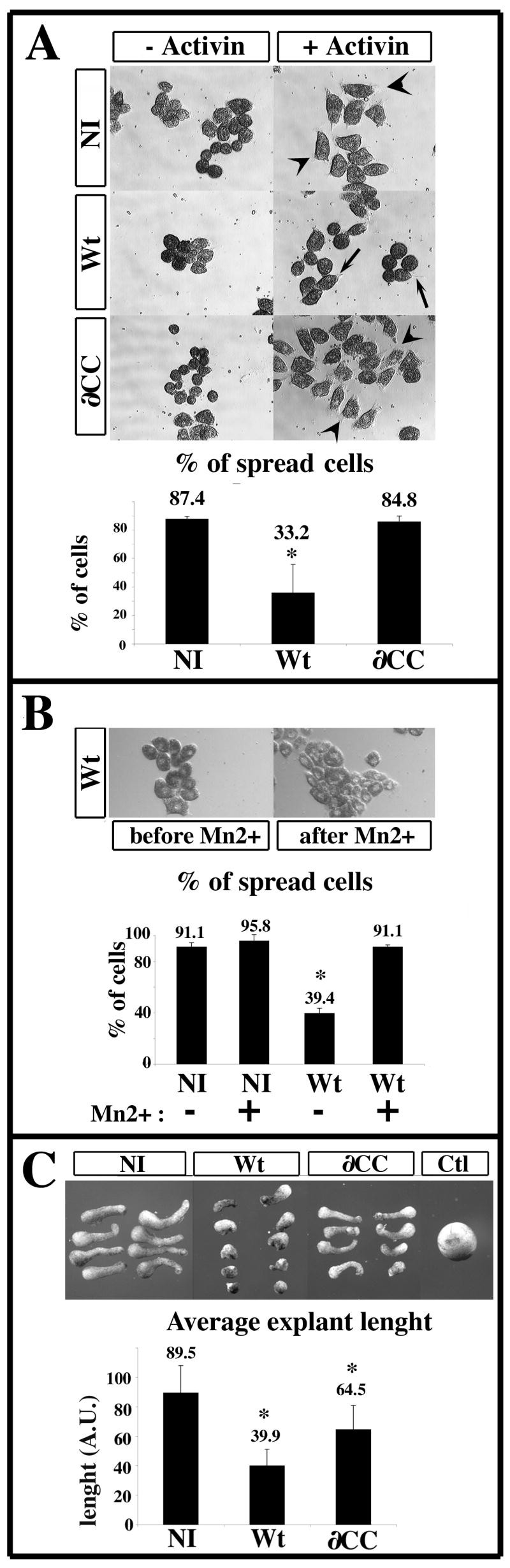
(A–B) Animal cap ectoderm of non injected embryos (NI) or embryos over-expressing the wild type (Wt) or the mutant form of PACSIN2 (∂CC) were dissociated at stage 8 and the cells seeded on GST fusion protein containing the CCBD domain of FN, in the presence (+ Activin) or absence of Activin (− Activin). Photographs were taken after sibling embryos initiated gastrulation movements. (A) Control cells (NI) or cells expressing ∂CC (∂CC) spread on the substrate in the presence of Activin-A. The cells over-expressing Wt-PACSIN2 (Wt) do not spread in those conditions. In the absence of Activin-A, the cells do not spread. The percentage of spread cells after Activin-A treatment was plotted on the histogram. The graph represent the mean of four independent experiments and error bars represent the standard deviation from the mean. The number of cells analyzed are as follow: NI=559; Wt=627; ∂CC=717. Arrows: filopodia; Arrowhead: lamelipodia. (B) Cells over-expressing Wt-PACSIN2 were treated as previously described. After 2h, cells were treated with 0.5 mM of MnCl2. Pictures were taken just before the addition of MnCl2 and 30 min after. The percentage of spread cells was plotted on the histogram. The graph represent the mean of 2 independent experiments and errors bars represent the standard deviation from the mean. The number of cells analyzed were as followed: NI= 269; NI+Mn2+= 332; Wt= 292; Wt+Mn2+= 206. The activation of integrins by Mn2+ is sufficient to rescue the spreading of the cells over-expressing Wt PACSIN2 (P<0.05). (C) Animal cap from non injected embryos (NI) or from embryos expressing Wt or ∂CC PACSIN2 were dissected at stage 8, induced with 5ng/ml of Activin-A and grown until the sibling embryos reached stage 20 (Ctl). While all animal caps from non-injected embryos or caps expressing ∂CC extend in response to Activin treatment, elongation is dramatically reduced in the animal caps over-expressing Wt- PACSIN2. The histogram represents the average length of explants in arbitrary units in a representative experiment. Three independent experiments were performed with similar results. The number of explants analyzed are as follow: NI =37; Wt =22; ∂CC =14. *: P<0.05
Figure 7. PACSIN2-MA blocks gastrulation but not fibrillogenesis or cell adhesion.
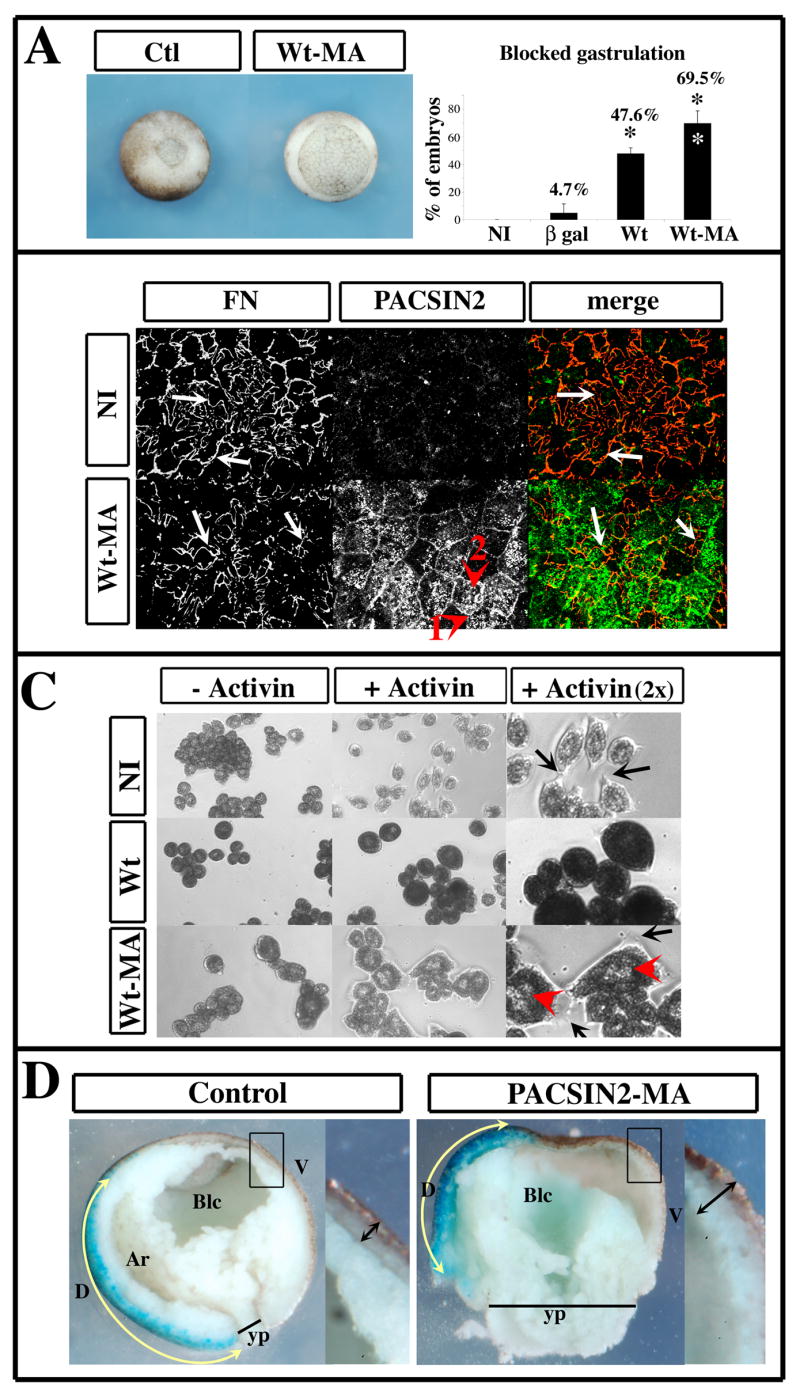
Embryos were injected in both blastomeres at the two-cell stage with transcripts encoding β-galactosidase (β gal), Wt-PACSIN2 (Wt) or the mitochondria-anchored PACSIN2 (Wt-MA). (A) When control embryos reached late gastrula stage, images of a representative control embryo (Ctl) and an embryo expressing PACSIN2-MA (Wt-MA) were taken. When embryos reached early neurula stage, they were scored for their failure to close the blastopore. The graph represents the percentage of embryos failing to close their blastopore and represents the average of 3 (β gal and Wt) or 4 experiments (Wt-MA and NI). Error bars represent the standard deviation to the mean. The number of embryos analyzed was as follow: NI=132; βgal=76; Wt=100; Wt-MA=225. While both PACSIN2 and PACSIN2-MA inhibits blastopore closure, PACSIN2-MA is significantly more efficient at blocking gastrulation compared to Wt-PACSIN2 (white asterisk). (B) Immunofluorescence performed on blastocoel roofs (BCR) dissected at stage 12. The FN fibrils of the BCR were detected using a rabbit polyclonal antibody 32F (red) and the PACSIN2 protein detected with mAb 3D8 (green). PACSIN2-MA is localized in dots in the middle of the cell (arrowhead 1) or at the periphery (arrowhead 2) likely to represent mitochondria. BCR from non-injected embryos and embryos expressing PACSIN2-MA display FN fibrils (arrows). (C) Animal cap ectoderm were dissociated at stage 8 and the cells seeded on the CCBD domain of FN, in the presence (+ Activin) or absence of Activin-A (− Activin). Photographs were taken after sibling embryos initiated gastrulation movements. A magnification of the activin treated cells is also included (+ Activin 2x). The control cells (NI) spread at the onset of gastrulation while cells over-expressing Wt-PACSIN2 failed to do so. The cells expressing PACSIN2-MA spread like control cells with large lamellipodia (arrow). However these cells are significantly larger, displaying multiple nuclei (red arrowhead) indicating a failure of the cytokinesis during cell division. Quantifications are described in the manuscript. (D) Transcripts encoding PACSIN2-MA were injected in 1-cell stage embryos. When embryos reached the 8-cell stage, β-galactosidase was injected in a dorsal vegetal blastomere as a lineage tracer. Control embryos only received β-galactosidase transcripts. When control embryos reached late gastrula stage, embryos were fixed, processed for β-galactosidase detection and bisected trough their dorso-ventral axis. The β-galactosidase expressing cells (dorsal mesoderm) show that PACSIN2-MA perturbed the convergence/extension movement (yellow double arrows). The magnification of the ventral ectoderm (black rectangles) shows that epiboly was also perturbed (black double arrows). Yp: yolk plug; Blc: blastocoel; Ar: archenteron; D: dorsal; V: ventral. *: P<0.05
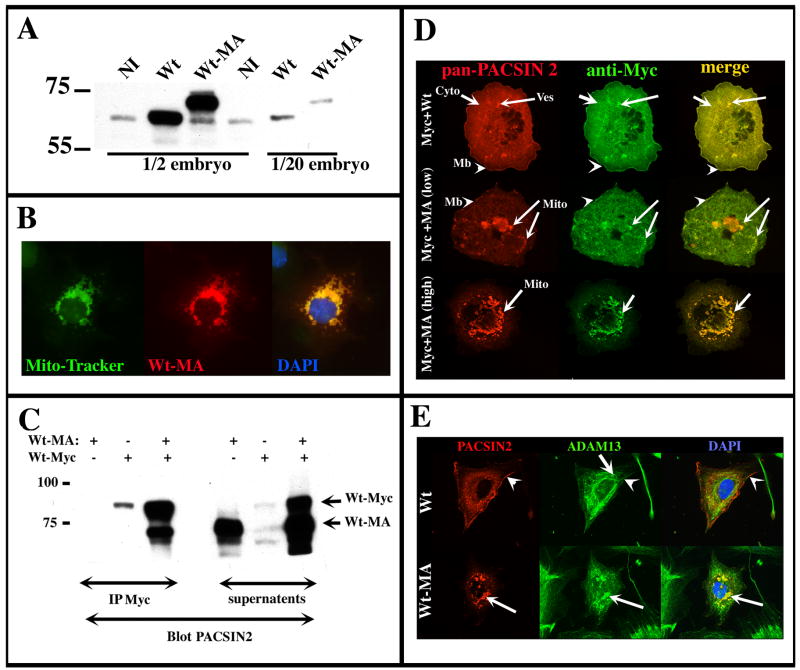
Figure 6. PACSIN2-MA recruits Wt-PACSIN2 to mitochondria.
(A) Western blot on embryo extracts. Embryos were injected in both blastomeres at the two-cell stage with 1ng of transcripts encoding Wt-PACSIN2 (Wt) or PACSIN2 containing the mitochondria anchor sequence of Act-A (Wt-MA). Proteins of non-injected (NI) and injected embryos were extracted at gastrula stage and the equivalent of 1/2 and 1/20 of an embryo was analyzed by western blot with the PACSIN2 mAb 3D8. The PACSIN2-MA protein is 4 kDa heavier than Wt-PACSIN2 and is expressed in a 10 fold excess compare to the endogenous PACSIN2 (NI). (B) Immunofluorescence on Cos-7 cells transfected with PACSIN2-MA. The mitochondria, the PACSIN2-MA protein and the nuclei were stained with the MitoTracker FM (green), mAb 3D8 (red) and DAPI (blue) respectively. The co localization of PACSIN2-MA (Wt-MA) with the mitochondria indicates that the construct is successfully targeted. (C) PACSIN2-MA co-immunoprecipitate with Wt-PACSIN2. Cos-7 cells were transfected with PACSIN2-MA (Wt-MA), PACSIN2-Myc tagged (Wt-Myc) or a mix of these two constructs. After 24h of expression, Wt-Myc was immunoprecipitated with the anti-Myc mAb (9E10). The immunoprecipitates were analyzed by western blot using the PACSIN2 mAb 3D8. An aliquot of protein extract after immunoprecipitation (supernatants) was analyzed in parallel to control protein expression. The 9E10 mAb was able to effectively precipitate PACSIN2-Myc. PACSIN2-MA was co-precipitated only when co-expressed with PACSIN2-Myc. (D) PACSIN2-MA depletes Wt-PACSIN2 localization in the cytoplasm and membrane. Cos7 cells were transfected with a 1:1 or 1:5 mixture of PACSIN2-Myc and PACSIN-MA (Wt-Myc + Wt-MA). Co-transfection with a 1:1 mixture of PACSIN2-Myc and wt-PACSIN2 was performed as a positive control. A sequential immunofluorescence was performed to detect the Myc antigen (mAb 9E10, green) and PACSIN2 protein (Texas red coupled mAb 3D8). When co-transfected with Wt-PACSIN2, PACSIN2-Myc localizes to the membrane (arrowhead; Mb), cytoplasm (Cyto) and some cytoplasmic vesicles (Ves). When Co-transfected with low levels of PACSIN2-MA, PACSIN2-Myc partially co-localizes with mitochondria clumps of various sizes (Mito). Some membrane localization was still observed. At high levels of PACSIN2-MA, PACSIN2-Myc is completely re-localized to the mitochondria by PACSIN2-MA.(E) XTC cells were transfected with Wt-PACSIN2 or PACSIN2-MA and processed for immunofluorescence with PACSIN2 antibody (3D8; red), ADAM13 antibody (6615F; green) and DAPI (blue). In Wt-PACSIN2 transfected cells, ADAM13 is found in the endoplasmic reticulum (arrow) and at the plasma membrane where it co-localizes with PACSIN2 (arrow head). In PACSIN2-MA expressing cells, some of the ADAM13 protein is re-localized to mitochondria (arrow).
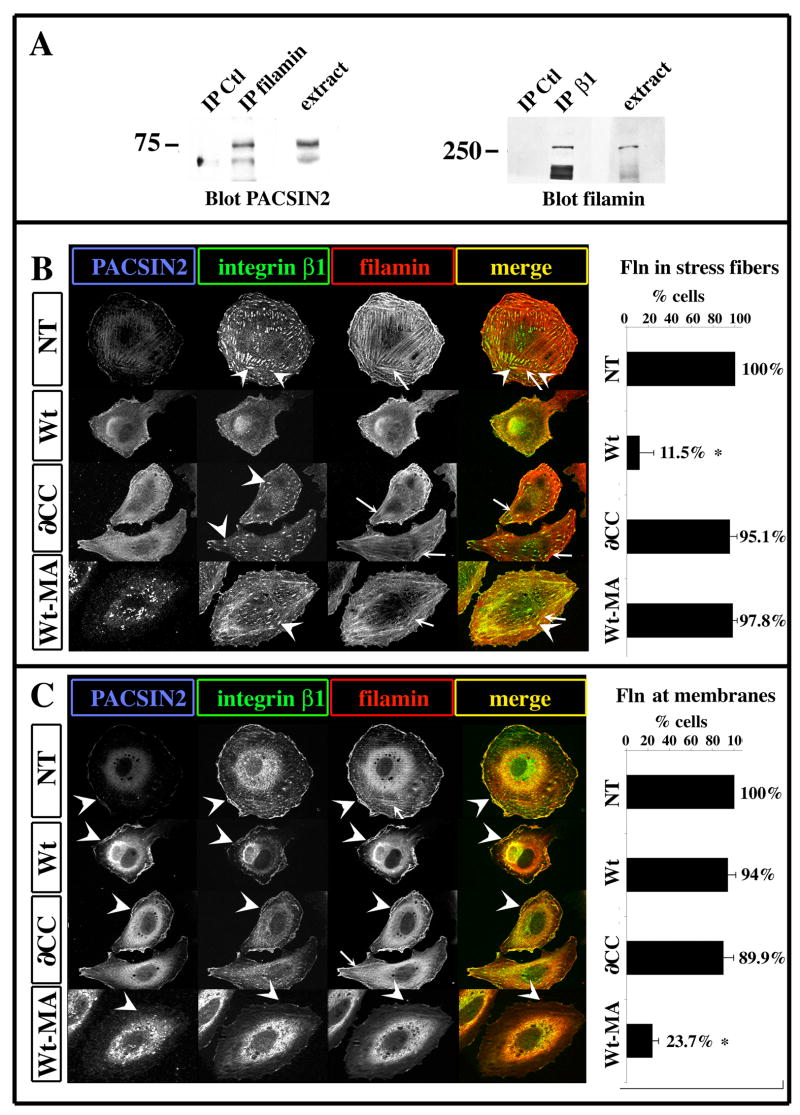
Figure 10. PACSIN2 alters the distribution of filamin in XTC cells.
(A) Co-immunoprecipitation of filamin with PACSIN2 and integrin β1. The proteins extracted from XTC cells were immunoprecipitated using antibodies to filamin (Fln) or integrin β1. Negative controls were performed by immunoprecipitation with non-immune mouse serum (IP Ctl). The immunoprecipitates as well as total extracts were analyzed by western blot for the presence of PACSIN2 and filamin respectively. In XTC cells, filamin co-precipitate with both integrin β1 and PACSIN2. (B-C) XTC cells were transfected with Wt-PACSIN2 (Wt), the ∂CC mutant (∂CC) or the PACSIN2-MA (Wt-MA) and processed by immunofluorescence with antibodies against filamin (red), integrin β1 (green) and PACSIN2 (blue). A merged image between the Fln and integrin β1 staining is presented (merge). Optical sections were performed using a structured light illumination system (ApoTome) at the level of the cell-substrate interaction (B) and 1μm above (C). (B) In non-transfected cells, filamin is mostly localized to stress fibers (arrow) and β1 integrin is localized to FA in which the stress fibers are anchored to (arrowhead). The over-expression of Wt-PACSIN2 inhibits both β1 integrin localization to FA and filamin localization to stress fibers while the ∂CC and the PACSIN2-MA mutant do not. (C) At 1μm above the substratum level, both β1 integrin and filamin are localized at the cell membrane of non-transfected cells (arrowhead). The over-expression of neither Wt-PACSIN2 nor the ∂CC mutant seems to perturb this localization. However, PACSIN2-MA appears to decrease filamin at the cell membrane. The graphs represent the mean of 4 (NT, Wt, Wt-MA) and 2 (∂CC) independent experiments. The error bar represents standard deviation from the mean. The number of cells scored was as followed: NT= 28, Wt PACSIN2=66, ∂CC=77, PACSIN2-MA= 70. * P<0.05. Arrowhead: focal adhesions (B) and membrane (C). Arrow: stress fiber.
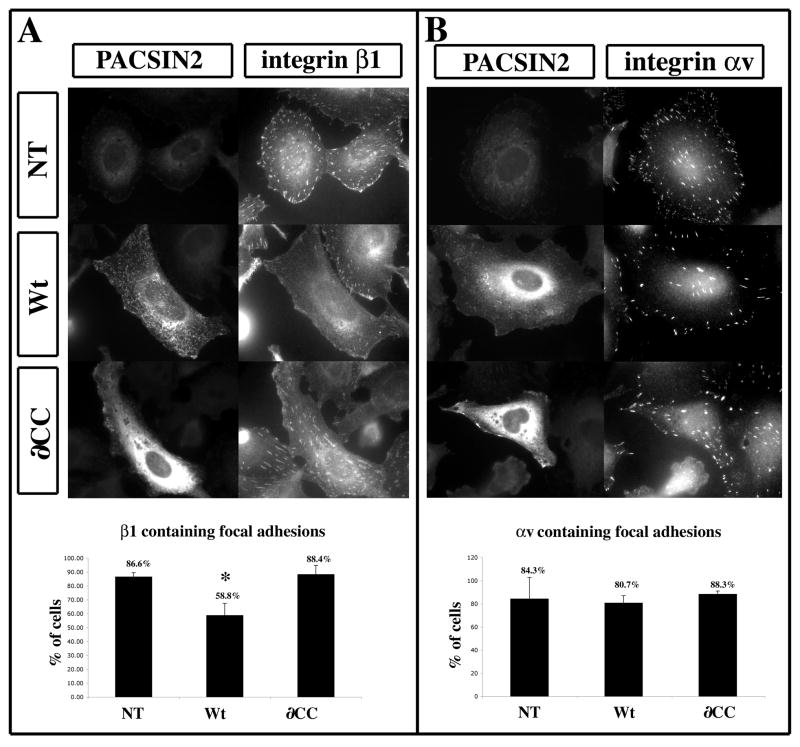
Figure 8. PACSIN2 over-expression perturbs the recruitment of integrinβ1 but not αv to focal adhesion in XTC cells.
XTC cells transfected with Wt-PACSIN2 or the ∂CC mutant were processed for immunofluorescence with a monoclonal antibody against integrin β1 (A) or αv (B) and biotinylated mAb 3D8 against PACSIN2. (A) In non-transfected cells (NT), β1 integrin localizes to focal adhesion (FA). The cells transfected with Wt-PACSIN2 (Wt) display an absence of integrin β1 localization to FA. The expression of the mutant ∂CC did not affect the localization of β1 integrin to FA (∂CC). The percentage of cells displaying integrin β1 positive focal adhesions is plotted on the histogram. The graph represents the mean of three independent experiments. The number of cells scored was as followed: NT=230, Wt PACSIN2=226, ∂CC=153. (B) Compared to the non-transfected cells (NT), the localization of integrin αv to FA is unchanged upon expression of Wt-PACSIN2 (Wt) or the ∂CC (∂CC). The histogram represents the percentage of cells displaying integrin αv positive focal adhesions and corresponds to the mean of two independent experiments. The number of cells scored was as followed: NT; n=148, Wt-PACSIN2; n=89, ∂CC; n=85. The error bar represents standard deviation from the mean. * P<0.05.
Embryo sectioning and immunostaining
Embryos were fixed, sectioned and processed for immunofluorescence as previously described (Cousin et al., 2000). Pictures were taken using a Zeiss Axiovert 35 inverted microscope equipped with a Hamamatsu Orca camera and were processed using Openlab software.
Immunostaining and confocal imaging
Immunofluorescence on bisected embryos was performed as described in (Marsden and DeSimone, 2001).
Immunodetection of fibronectin fibrils
Immunostaining of FN fibrils was performed as previously described (Cousin and Alfandari, 2004).
Cell adhesion and animal cap assay
Those assays were performed as previously described (Cousin and Alfandari, 2004). The length of animal cap was determined using the Openlab software (Improvision). The motility of animal cap cells was determined by averaging the mean speed of 10 cells using the Openlab software.
Cell transfection and staining
XTC and Cos7 cells were maintained and transfected as previously described (Cousin et al., 2000; Gaultier et al., 2002). After 48h of expression, the cells were trypsinized and seeded onto glass coverslips coated with 10μg/ml of fibronectin for 12h. Cells were fixed 20 min in 3.7% formaldehyde in MBS at room temperature, permeabilized in PBS 0.5% Triton X100 for 5 min at room temperature and blocked in 1% BSA in PBS 0.1% Tween (PBT) overnight at 4°C. Antibody incubations were performed sequentially (1h at room temperature for each antibody) in PBT 1% BSA as described in the figure legends. The list and use of primary antibody is presented in Table 1. All secondary antibodies were purchased from Jackson labs.
Results
The CC domain is critical for PACSIN2 cell membrane localization
We previously identified PACSIN2 as an adaptor protein capable of binding and inhibiting the function of ADAM13, a transmembrane metalloprotease specifically expressed in cranial neural crest and somitic mesoderm in Xenopus laevis embryos (Alfandari et al., 2001; Alfandari et al., 1997; Cousin et al., 2000). Immunodetection using the mAb 3D8 showed that PACSIN2 is expressed earlier and in a broader range of tissues than ADAM13 (Cousin et al., 2000). At gastrula stages, PACSIN2 is enriched in ectodermal and mesodermal derivatives (Cousin et al., 2000)(Fig. 1B). Confocal microscopy performed on a bisected gastrula shows that PACSIN2 is localized near the cell membrane particularly in the mesoderm (Fig. 1B, m) and at the junction of germ layers where the cell membrane contact the FN rich ECM (Fig. 1B, arrowhead). Upon over-expression, PACSIN2 (Wt) protein also localizes near cell membranes (Fig. 1C, Wt). However, a mutant form of PACSIN2 lacking one of the Coiled-coil domains (residues 185–218; Fig. 1A) does not display this membrane localization (Fig. 1C∂CC). These results show that the CC domain is critical for targeting PACSIN2 to the plasma membrane.
PACSIN2 over-expression perturbs gastrulation
To investigate the function of PACSIN2 during gastrulation, we over-expressed either the wild type (Wt) or mutant form (∂CC) of PACSIN2 in embryos. Typically, two-cell stage embryos were injected in both blastomeres with 1ng of mRNA encoding the wt-PACSIN2, the ∂CC mutant or GFP as a control, and embryonic development was carefully monitored. The expression of these proteins was followed by western blot (Fig. 2A). At the onset of gastrulation, the appearance of the blastopore on the dorsal-vegetal aspect of the embryo is observed in all embryos (Fig. 2B, arrowhead). At the end of gastrulation, while the blastopore of embryos expressing GFP is closed, the embryos injected with Wt PACSIN2 still have yolk plugs (Fig. 2C, double arrow). Interestingly, embryos expressing the mutant ∂CC close their blastopore and, like GFP-expressing embryos, develop normally until at least the tail bud stage (Fig. 2C; data not shown).
Figure 2. Over-expression of PACSIN2 inhibits gastrulation but does not perturb mesoderm induction.
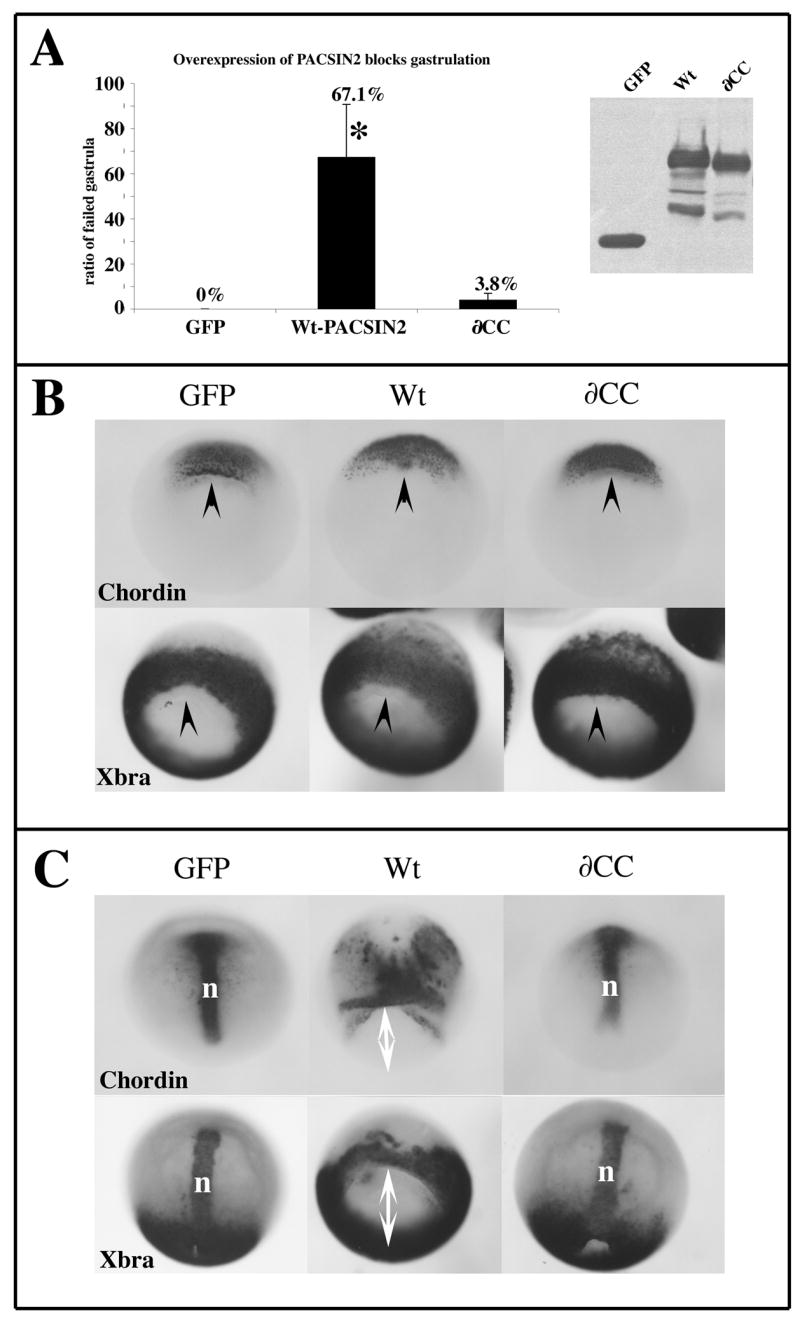
(A) Embryos were injected with 1ng of mRNA encoding GFP, Wt-PACSIN2 or the ∂CC in both blastomeres at the two-cell stage. The embryos were scored for gastrulation inhibition when control embryos reached stage 13. The average of 3 independent experiments is plotted on the graph. The error bars represent the standard deviation to the mean. The number of embryos analyzed are as follow GFP=74; Wt=143; ∂CC=104. The expression of proteins was monitored by western blot using both the anti-myc mAb 9E10 to detect GFP and the PACSIN2 mAb 3D8 to detect Wt and ∂CC. The equivalent of 0.5 embryos was loaded in each lane. (B–C) Injected embryos were fixed at early gastrula (B) or late gastrula stage (C) and treated by whole mount in situ hybridization with the markers chordin and brachyury (Xbra). At stage 10.5, all embryos started gastrulation at the same time as indicated by the presence of the blastopore lip (black arrow head). At stage 13, embryos over-expressing Wt-PACSIN2 fail to close their blastopore (white double arrow) and the notochord fails to elongate (n) even though chordin is expressed. This indicates that the mesoderm induction occurred but that the gastrulation movements did not. Embryos expressing the ∂CC mutant or GFP gastrulated properly. *: P<0.05
The inhibition of gastrulation could be caused by perturbation of mesoderm induction or defects in morphogenetic movements. To determine whether mesoderm induction was affected by PACSIN2 over-expression, we performed whole-mount in situ hybridization on injected embryos using the pan-mesodermal marker Xbra and the notochord marker Chordin at early gastrula (Fig. 2B) and late gastrula stage (Fig. 2C). Our results show that embryos with excess PACSIN2 expressed Xbra and chordin, indicating that mesoderm induction has occurred.
Together, these results indicate that over-expression of PACSIN2 induces no major changes in cell fate and suggest that the gastrulation defects may be caused by a direct interference with cell movements during gastrulation. This perturbation requires the localization of PACSIN2 near the cell membranes, since the ∂CC mutant expression does not interfere with gastrulation. To investigate the impact of PACSIN2 over-expression on morphogenesis, we looked at the two main components of the adhesive machinery: the extracellular matrix (ECM) underlying the blastocoel roof (BCR) and the adhesive property of the cells.
PACSIN2 over-expression prevents fibronectin matrix assembly
During amphibian gastrulation, mesodermal cells attach to and migrate on the FN matrix assembled by the blastocoel roof (BCR) cells. Inhibiting either FN matrix assembly or mesoderm cell adhesion to FN results in a failure of gastrulation movements (Boucaut et al., 1984b; Darribere et al., 1990; Marsden and DeSimone, 2001; Ramos et al., 1996). We first addressed the effect of PACSIN2 over-expression on FN fibrillogenesis. BCRs from gastrula-stage embryos (stage12) expressing the various constructs, were dissected and processed by whole mount immunofluorescence using a polyclonal antibody directed against Xenopus FN (32F) and the monoclonal antibody against PACSIN2 (3D8) (Fig. 3). While the control BCR displays the typical intricate network of FN fibrils (Fig. 3, NI), explants over-expressing PACSIN2 fail to assemble fibrils (Fig. 3, Wt). However, in these explants, FN staining is still observed at sites of cell-cell contact where cells are not over-expressing PACSIN2 (Fig. 3, arrow in Wt-merge). This suggests that the absence of fibrils is not due to the absence of FN and that the effect of PACSIN2 over-expression is cell autonomous. BCR expressing the ∂CC mutant assembled FN fibrils (Fig. 3A, ∂CC).
Together, these results show that the over-expression of PACSIN2 inhibits FN fibrillogenesis and this phenotype requires a functional CC domain.
PACSIN2 does not alter fibronectin secretion and integrin cell surface expression
The formation of fibronectin fibrils depends mainly on the presence of two proteins: soluble fibronectin secreted into the blastocoel fluid and integrin α5β1 expressed at the surface of BCR cells. Because PACSIN2 is thought to be involved in protein trafficking (Kessels and Qualmann, 2004), we investigated whether PACSIN2 over-expression perturbs the secretion of fibronectin into the blastocoel and/or the cell surface expression of integrins in the embryo.
To address the issue of FN secretion, the blastocoel contents of 20 embryos and the cell extracts of half an embryo were analyzed by western blot using a monoclonal antibody directed against fibronectin (4H2). The western blot of cellular extracts using the mAb 3D8 was performed in parallel to monitor the levels of PACSIN2 expressed in these embryos (Fig. 4A). The results demonstrate that non-injected and PACSIN2 over-expressing embryos both express and secrete FN. Therefore the absence of fibrils on the blastocoel roof of PACSIN2 over-expressing embryos is not due to an absence of FN in the blastocoel fluid. The cell surface expression of integrins was analyzed by biotinylating the extracellular proteins of dissociated embryos. Integrins β1, α5β1 and αv were immunoprecipitated with mAb 8C8, P8D4 and P3C12 respectively, and then probed with streptavidin-HRP (Fig. 4B). The results show that embryos over-expressing either Wt or the ∂CC PACSIN2 display no significant change in the expression levels of the integrins tested including integrin α5β1, which is directly responsible for FN fibrillogenesis.
Figure 4. Over-expression of PACSIN2 does not prevent integrin or fibronectin expression.
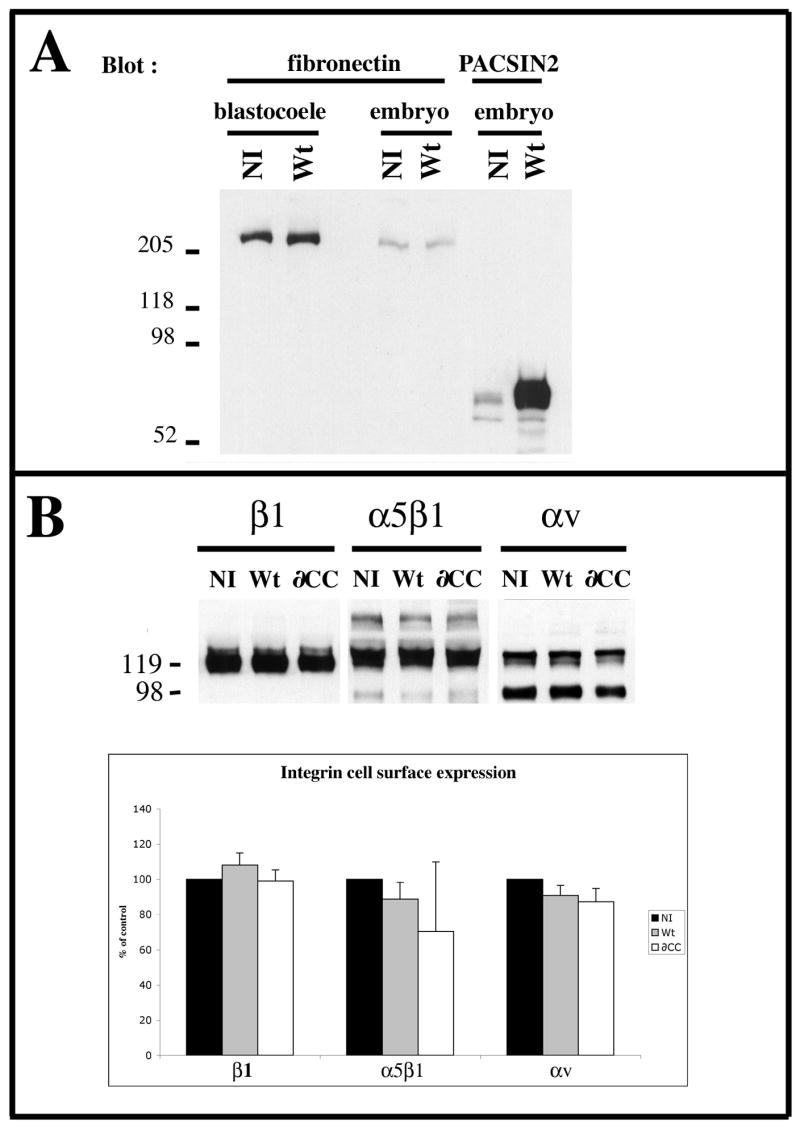
Embryos injected in the both blastomeres at the two cells stage were grown to early gastrula. (A) The protein content of either whole embryos (0.5 embryo equivalent) or their blastocoel fluid (20 blastocoels) was analyzed by western blot using a monoclonal antibody to FN (4H2) and a monoclonal antibody to PACSIN2 (3D8). Non-injected embryos (NI) and the embryos over-expressing PACSIN2 (Wt) express and secrete fibronectin. (B) The embryos were dissociated and cell surface proteins were biotinylated. Immunoprecipitations were performed using monoclonal antibodies against integrin β1 (8C8), α5β1 (P8D4) and αv (P3C12) and the precipitates were analyzed by western blot using streptavidin-peroxidase. The graph represents the quantification of 3 (integrins α5β1 and αv) and 4 (β1-integrin) independent experiments using the Histograms of the Photoshop software. Signal intensities have been normalized to the non-injected control embryos (100%). The error bars correspond to the standard deviation from the mean. None of the differences between injected and non-injected embryos were statistically significant (P>0.05).
Altogether, these results show that over-expression of PACSIN2 does not impair the steady state levels of either the integrins at the cell surface or the soluble FN in the blastocoel.
PACSIN2 over-expression inhibits cell spreading
The effects of PACSIN2 over-expression on matrix assembly are not due to changes in the levels of either FN or the integrins involved in assembling it into fibrils. A possible explanation is that PACSIN2 interferes with the functional activation of α5β1 integrin. To investigate this hypothesis, we tested the capacity of ectodermal cells to spread on the central cell binding domain (CCBD) of fibronectin containing the RGD and synergy sites following Activin-A induction (Ramos and DeSimone, 1996). BCR cells from non-injected embryos or embryos injected with Wt or ∂CC PACSIN2 were dissociated at the mid blastula stage. The dissociated cells were put on the CCBD domain of FN in the presence or absence of Activin-A. Images were obtained after the sibling embryos initiated gastrulation movements (Fig. 5A). When treated with Activin-A, the percentage of spread cells were calculated and plotted on the histogram. In the absence of Activin-A, none of the cells are able to spread. In contrast, following Activin-A induction, the control cells and cells expressing ∂CC spread, displaying polygonal shapes and sending multiple lamelipodial protrusions (Fig. 5A, arrowhead). This morphology is associated with actively motile cells, which was confirmed by time-lapse microscopy (data not shown). Conversely, the cells over-expressing PACSIN2 display a rounded morphology with very few filopodial extensions typical of non-spread cells (Fig. 5A, arrows). The artificial activation of integrins (including α5β1) by the addition of 0.5mM of Mn2+ is sufficient to restore a cell spreading phenotype indicating that the non-spread cells are still alive but unable to spread on the CCBD domain (Fig. 5B)(Mould et al., 1995). Not surprisingly, the absence of spreading was associated with low motility (data not shown).
The convergence and extension of axial mesoderm is a tissue rearrangement process occurring during gastrulation that results in the extension of the antero-posterior axis (Keller et al., 2000). This process is the driving force of gastrulation in Xenopus embryos and requires both the presence of FN fibrils and the activation of integrin α5β1 function (Davidson et al., 2006; Marsden and DeSimone, 2001; Marsden and DeSimone, 2003). Because PACSIN2 over-expression inhibits these two key components of gastrulation, we tested the effect of over-expression on convergence and extension using the animal cap assay. The animal caps of blastulae (stage 8) were dissected and incubated in the presence of mesoderm inducer Activin-A until sibling embryos reached early tailbud stage (Fig. 5C, Ctl). Animal caps from non-injected or ∂CC-PACSIN2 injected embryos elongate in response to Activin-A (Fig. 5C; NI ∂CC). This elongation is typical of the induction of dorsal mesoderm and the formation of a notochord. As expected, the explants expressing Wt-PACSIN2 show a dramatically reduced elongation (Fig. 5C, Wt). Interestingly, the ∂CC expressing explants also display a small reduction in elongation (Fig. 5C, ∂CC).
Altogether, these results show that PACSIN2 over-expression inhibits FN fibrillogenesis and cell spreading by interfering with α5β1 integrin function. This ultimately inhibits the morphogenetic movement necessary for the completion of gastrulation in vivo.
Loss of PACSIN2 function: Knock Down and dominant negative approaches
The PACSIN2 over-expression induces dramatic morphogenetic defects during gastrulation and suggests that PACSIN2 may be involved in the gastrulation process. To investigate this hypothesis, the loss of function of PACSIN2 was performed. Using morpholino oligonucleotides to both sequences of PACSIN2 (Genebank accession no BC128665 and AJ277159), we saw only a modest reduction in protein expression, no effect on gastrulation and only a minor delay in neurulation (see Supplemental Figure S1 and S2). This failure could be attributed to the large pool of maternal PACSIN2, or the presence of other PACSINs like PACSIN1 and PACSIN3 that could compensate for the loss of PACSIN2. We therefore designed a dominant negative construct based on PACSIN2 ability to form multimeres (Kessels and Qualmann, 2004), and our previous observation that PACSIN2 localization at specific site of the plasma membrane was essential for its activity (see Fig. 1 and 2). Thus, a mitochondrial-anchored form of PACSIN2 was engineered by adding to the C-terminus of PACSIN2 a 25 amino acid stretch of Act-A protein, which is responsible for targeting the protein to mitochondrial membranes (Fig. 1A)(Bubeck et al., 1997; Park et al., 2005). The goal was to promote multimerization of this construct with endogenous PACSIN2 to sequester the complex at the mitochondria thus competing with its normal localization at the membrane ruffles of the cells. The expression of this construct was assessed by injecting embryos at two-cell stage with 1 μg of mRNA encoding either the Wt PACSIN2 (Wt) or the mitochondria-anchored PACSIN2 (Wt-MA). At gastrula stage, the proteins were extracted and analyzed by western blot using the PACSIN2 mAb 3D8. The result shows that PACSIN2-MA is 4kDa heavier than Wt-PACSIN2, and both forms are expressed at 10-fold greater levels than the endogenous protein (Fig. 6A). To ascertain that the PACSIN2-MA is effectively targeted to the mitochondria, Cos cells were transfected and processed by immunofluorescence using a mitochondrial marker (MitoTracker FM), the PACSIN2 mAb and the nuclei marker DAPI. The results show co-localization of the PACSIN2-MA protein and the mitochondria (Fig. 6B). The same experiment was performed in XTC cells and while the PACSIN2-MA displayed a similar localization, the MitoTraker marker was ineffective at staining Xenopus mitochondria (data not shown). We investigated the capacity of PACSIN2-MA to displace wild-type PACSIN2 to mitochondria in two ways. First, we confirmed the direct interaction between PACSIN2 and PACSIN2-MA by co-immunoprecipitation (Fig. 6C). Second, a Myc tag version of PACSIN2 (Wt-Myc) was co-transfected with PACSIN2-MA at 1:1 and 1:5 ratios in Cos-7 cells (Fig. 6D). As a control, Wt-Myc was co-transfected with wt-PACSIN2 (Wt). When co-transfected with Wt-PACSIN2 (Wt-Myc + Wt), PACSIN2-Myc is localized to the cytoplasm (cyto), membrane (arrow) and some vesicle (ves). When co-transfected with PACSIN2-MA, PACSIN2-Myc has two different kind of localization depending on how strong the PACSIN2-MA is expressed (low or high). At low levels, PACSIN2-MA is localized to a big clump of mitochondria around the nucleus and smaller clumps or strings of mitochondria in the cytoplasm and cell membrane. The clumping of mitochondria is likely caused by PACSIN2 dimerization. At these low levels of Wt-MA, PACSIN2-Myc partially co-localizes with PACSIN2-MA to mitochondria but some cytoplasm and membrane staining are also present. This indicates that, at these levels, PACSIN2-Myc is incompletely removed from its original localization. At high levels, PACSIN2-MA is localized only in a very large size clump of mitochondria around the nucleus. At these high levels, PACSIN2-Myc strictly co-localizes with PACSIN2-MA and, as far as we can tell, could no longer be detected in the cytoplasm or the membrane. These “high level” phenotype were seen in 18% of cells transfected with the 1:1 DNA ratio (n=60) and 71% of cells transfected with the 1:5 DNA ratio (n=66).
Lastly, we investigated the effect of PACSIN2-MA on the localization of previously identified PACSIN2 partners, like ADAM13 (Cousin et al., 2000). In wt-PACSIN2 transfected XTC cells, endogenous ADAM13 is localized to the endoplasmic reticulum (Fig. 6E; arrow) and at the plasma membrane where it co-localizes with PACSIN2 (Fig. 6E, arrow head). In PACSIN2-MA expressing cells, endogenous ADAM13 is partially re-localized to mitochondria.
These data demonstrate that PACSIN2-MA binds PACSIN2 and re-localizes it to mitochondria indicating that it likely acts as a dominant negative.
PACSIN2-MA blocks gastrulation
To study the effect of PACSIN2-MA on development, 1μg of mRNA encoding β-galactosidase (β gal), Wt-PACSIN2 (Wt) or PACSIN2-MA (Wt-MA) was injected in both blastomeres of two-cell stage embryos and the development carefully monitored. All embryos developed synchronously until early gastrula stage (data not shown). At mid gastrula stage, embryos expressing Wt-PACSIN2 and PACSIN2-MA display a delay (Fig. 7A). When embryos reached the neurula stage, the majority of Wt and Wt-MA injected embryos fail to close their blastopores (Fig. 7A, histogram). In order to investigate the cause of gastrulation failure, we studied the presence of FN fibrils on the blastocoel roof by performing immunofluorescence on animal caps from stage 12 embryos as described earlier. Non-injected embryos display an extensive FN matrix on the blastocoel roof (Fig. 7B, NI). As expected, PACSIN2-MA localizes in dot-like structures likely to represent mitochondria (Fig. 7B, arrowhead). Surprisingly, most of these clusters localize near the cell membranes. In Wt-MA expressing embryos, the FN fibrils are present. We also investigated the effect of PACSIN2-MA expression on the spreading and motility of Activin-A treated animal cap cells on FN substrate. In the absence of Activin-A, none of the cells spread (Fig. 7 C;-Activin). As previously described, 89.7% of the control cells (NI) spread in the presence of Activin-A (± 4.6; n=314; 3 independent experiments) while only 9.2% of the cells over-expressing wt-PACSIN2 do (± 4.4; n=273; P<0.01)(Fig. 7C). The cells expressing PACSIN2-MA spread like control cells (93.1% ± 3.2; n=385; P>0.05) (Fig. 7C). The cells from NI, ∂CC and Wt-MA expressing embryos have comparable motilities with an average speed of 6.2 μm/h (±1.9), 5.0 μm/h (±0.85) and 4.8 μm/h (±0.87) respectively (P>0.05). Not surprisingly, the cells over-expressing Wt-PACSIN2 display a significant decrease in motility (3.6 μm/h ± 0.81, P<0.05). We noticed that these cells are bigger and display multiple nuclei (arrowhead) and large lamelipodia (arrow), which is typical of cells that have failed cytokinesis. These multinucleated cells are commonly seen when cells fail to spread (Cousin and Alfandari, unpublished observation; Fig. 7C, Wt). However PACSIN2-MA expressing cells are able to spread, pointing to a different reason for cytokinesis failure. This cytokinesis failure is unlikely to play a role in the overall phenotype of the embryos since we did not observe a significant number of enlarged cells or abnormal cell division in vivo (data not shown). In order to understand the cause of gastrulation failure, we investigated the effect of PACSIN2-MA on the various cell rearrangements necessary for gastrulation, namely the epiboly of the ectoderm and the convergence and extension of the mesoderm. After injecting PACSIN2-MA transcripts at 1 cell stage, β-galactosidase was injected as a lineage tracer in the dorsal-vegetal blastomere in 8-cell stage embryos. Embryos expressing PACSIN2-MA display an average thickness of 5 cells instead of 2-cells apparent in control embryos indicating that the epiboly was inhibited (Fig. 7D, black double arrow). Furthermore, the dorsal mesoderm stained for β-galactosidase appears shorter and thicker in PACSIN2-MA expressing embryos indicating that convergence and extension movements were also perturbed (Fig. 7D, yellow double arrow).
The results show that PACSIN2-MA inhibits cell intercalation movements during gastrulation most likely by preventing the normal localization of endogenous PACSIN2 within the cell. However, PACSIN2-MA expression inhibits neither FN fibrillogenesis nor mesoderm cell spreading and migration on FN substrate, suggesting that these functions can be achieved in the absence of PACSIN2. To better understand the function of PACSIN2, we used Xenopus XTC cells to investigate the behavior of integrin and cytoskeletal components in PACSIN2 gain and loss of function experiments.
PACSIN2 over-expression inhibits the localization of β1 integrin but not αv integrin to focal adhesion
Our data show that embryos over-expressing PACSIN2 have normal levels of integrin α5β1 expressed at the cell surface while its activity appears to be inhibited. To further investigate the mechanism by which PACSIN2 may control integrin function, we first tested whether localization of β1 integrin within the membrane was perturbed. Immunodetection of β1 integrins was performed on Xenopus XTC fibroblasts transfected with either Wt-PACSIN2 or the ∂CC mutant (Fig. 8A).
The majority of non-transfected cells display focal adhesions (FA) containing β1 integrins (Fig. 8A and 9, NT). When over-expressing Wt-PACSIN2, this proportion significantly decreases (Fig. 8A and 9, Wt). Cells over-expressing the ∂CC mutant display a profile statistically indistinguishable from non-transfected cells (Fig. 8A ∂CC). The reduction of β1-integrin containing focal adhesions could be due to a general failure of FA formation or a specific lack the recruitment of β1-integrin to FA. To test this hypothesis, we performed the same immunofluorescence assay with a αv integrin-specific mAb (P3C12; Fig. 8B). The αv-integrin distribution in FAs of non-transfected cells is similar to β1-integrin, with the same percentage of cells showing FAs containing αv-integrin (Fig. 8B). However, the over-expression of neither Wt nor ∂CC forms of PACSIN2 changes this distribution. Other results show that the localization of paxillin to FA is also unchanged when Wt or ∂CC-PACSIN2 are expressed (data not shown). Together, these data demonstrate that the over-expression of PACSIN2 perturbs the localization of integrin β1 to FA but not the formation of focal adhesions per se. Moreover, αv-integrins are not affected by PACSIN2 suggesting that the mechanism of regulation by PACSIN2 may be specific to individual integrins.
Figure 9. PACSIN2-MA does not prevent the recruitment of integrinβ1 to focal adhesion.
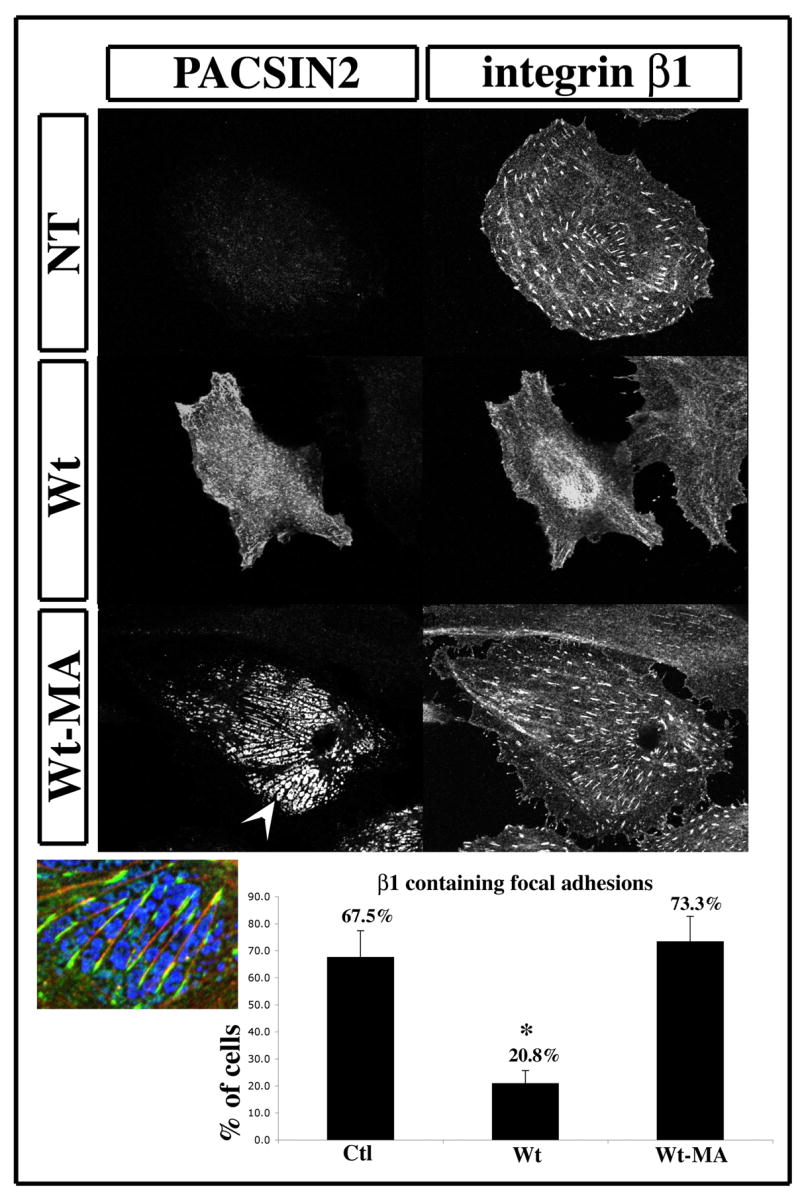
XTC cells were transfected with Wt-PACSIN2 (Wt) or PACSIN2-MA (Wt-MA) and processed as described in Figure 8A. The percentage of cells displaying integrin β1 positive focal adhesions is plotted on the histogram. Non-transfected cells (NT) and cells expressing the mitochondria-anchored PACSIN2 displayed integrin β1 in focal adhesions. PACSIN2 over-expression inhibits integrin β1 localization to focal adhesion as described in Figure 8. The colored inset represents a magnification of the region of the Wt-MA expressing cell indicated by the arrowhead (PACSIN2-MA in blue, β1 integrin in green and F-actin detected with phalloidin in red). The graph represents the mean of two independent experiments. The error bar represents standard deviation from the mean. The number of cells scored was as followed: NT= 80, Wt PACSIN2=79, PACSIN2-MA= 80. * P<0.05.
The immunofluorescence performed with the β1-integrin antibody were repeated on XTC cells transfected with PACSIN2 –MA and analyzed with a structured light imaging system. The cells expressing PACSIN2-MA displayed the same proportion of FA containing integrin β1 as the control cells (Fig. 9, NT and Wt-MA). We also noticed that, while PACSIN2-MA localizes to what seems to be mitochondria, most of these are in the same focal plan as the focal adhesion (Fig. 9 Wt-MA, arrowhead). This result concurs with the observation made on animal caps and suggests that, while the mitochondrial anchor of PACSIN2-MA is targeted to mitochondria, the membrane localizing feature of PACSIN2 brings mitochondria near the cell membrane but never at the ruffle membrane. The detection of actin filaments with Phalloidin-Texas red shows that FA and stress fibers do not co-localize with the mitochondria but rather are apposed to one another (Fig. 9, inset).
Filamin is mis-localized upon PACSIN2 mis-expression
We showed that PACSIN2 over-expression inhibits integrin α5β1 function and localization to FA. Because we could not detect any direct interactions between the integrin β1 cytoplasmic tail and PACSIN2 (Supplemental Fig. S3), we hypothesize that the effect is indirect. The members of the PACSIN family binds to several proteins involved in actin polymerization and/or organization (Kessels and Qualmann, 2004). Among these, filamin is of particular interest because it binds the CC domain of the PACSIN family member FAP52 (Nikki et al., 2002a) and the cytoplasmic domain of integrin β1 (van der Flier et al., 2002). Therefore, we investigated if filamin could be the target protein responsible for the phenotypes induced by PACSIN2 gain and loss of function.
First, we investigated the interaction of filamin with PACSIN2 and integrin β1 in Xenopus cell lines. An immunoprecipitation was performed using an XTC cell protein extract and either a filamin or integrin β1 antibody (pAb Fln and mAb 8C8 respectively). The immunoprecipitates were analyzed by Western blot using the PACSIN2 mAb or the filamin pAb respectively (Fig. 10A, IP). In parallel, crude protein extracts of XTC cells were analyzed for the presence of filamin and PACSIN2 (Fig. 10A; extract). The filamin antibody detects a single band of 280 kDa in both the β1-integrin immunoprecipitate and the XTC cell extract, which is consistent with the expected size for filamin. The PACSIN2 antibody recognizes a doublet of 72 and 76 kDa in both the extract and the filamin immunoprecipitate. However, the control immunoprecipitation also shows the 72kDa band indicating only the 76 kDa species specifically co-precipitates with filamin. Together, these data indicate that filamin can interact with both PACSIN2 and integrin β1 in XTC cells.
Secondly, we investigated the effect on filamin localization upon over-expression of Wt-PACSIN2, the ∂CC mutant or PACSIN2-MA in XTC cells. We performed triple-label-immunofluorescence on XTC cells using antibodies against PACSIN2, integrin β1 and filamin and analyzed the localization of each protein at the level of cell-substrate interface (Fig. 10B) and 1 μm into the cell (Fig. 10C) using structured light microscopy. In non-transfected cells, we detect filamin along bundles of fibers likely to represent the actin stress fibers (Fig. 10B, C; arrow). At each end of these fibers, we observed a co-localization with β1 integrins (Fig. 10B, arrowhead) representing the anchoring of stress fibers to FA. At 1 μm above the plane of the ECM substrate, filamin is localized near the cell membrane where it co-localizes with integrin β1 and PACSIN2 most likely at the cell cortex (Fig. 10C, arrowheads). In the PACSIN2 over-expression cells, filamin does not localize with any filamentous structure (Fig. 10B, Wt). In contrast, cells expressing ∂CC have filamin localized to fibers (Fig. 10B, ∂CC; arrows). However, neither the Wt-PACSIN2 nor the ∂CC mutant perturbs the co-localization of filamin with integrin β1 and PACSIN2 at membrane ruffles (Fig. 10C, arrowheads). PACSIN2-MA expression has the opposite effect of PACSIN2 over-expression on filamin localization: filamin is associated with fibers at the substratum level but is greatly reduced at the membranes ruffles (Fig. 10C, arrowhead).
Together, these results show that a gain of PACSIN2 function perturbs the localization of filamin to actin stress fiber but not to the membrane ruffles. This effect depends on the Coiled-coil domain. In contrast, the loss of PACSIN2 function using the dominant negative PACSIN2-MA has the opposite effect, decreasing filamin localization from ruffle membranes but not from actin stress fiber. This suggest that PACSIN2 function is to bring filamin to the membrane ruffles.
Discussion
PACSIN2 was previously shown to bind to and control the function of ADAM13 in cephalic neural crest cells (Cousin et al., 2000). However, PACSIN2 is expressed earlier than ADAM13 and in a broad range of tissues. Therefore, we analyzed PACSIN2 function during early development using both gain and loss of function approaches. The main findings of this work are summarized in Table 3. Here we present evidences that PACSIN2 is involved in the regulation of gastrulation, possibly through regulating cell adhesion.
Table 3.
Summary of data
| Gastrulation | FN fibrils | Cell spreading | C/E | Fln in S.F. | Fln in R.M. | β1 in F.A. | |
|---|---|---|---|---|---|---|---|
| Control | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wt | No | No | No | No | No | Yes | No |
| ∂CC | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wt-MA | No | Yes | Yes | No | Yes | No | Yes |
Note: FN=fibronectin; C/E=convergence and extension; Fln=filamin; S.F.=stress fiber; R.M.=ruffle membrane; F.A.= focal adhesion
PACSIN2 may control the level of α5β1 integrin activation in the embryo
Using both cell culture and embryo experiments, we have shown that an excess of PACSIN2 prevents integrin α5β1 localization to the focal adhesion (in XTC cells) and its function in FN matrix assembly and mesodermal cell spreading (in embryos). These experiments suggest that an excess of PACSIN2 prevents integrin α5β1 activation both in cell culture and the embryo while having no effect on αv containing integrins or the formation of focal adhesions. Surprisingly, the dominant negative experiments show no effects on integrin α5β1 localization in XTC cells or on the function of this integrin in embryos. These results demonstrate that in the absence of PACSIN2 activity, α5β1 integrin becomes activated in both tissue culture and embryonic cells. While these results may appear contradictory at first, they are in fact compatible with a model of PACSIN2 function in which PACSIN2 maintain a population of integrin α5β1 in a “lower activation” state at the membrane ruffles. In this case, removing PACSIN2 would not prevent the integrin activation necessary for either FN assembly or cell spreading while over-expression of PACSIN2 would maintain most of α5β1 integrin to the ruffle membrane and prevents its activation. Two pieces of evidence support this hypothesis. First, PACSIN2 does not appear to bind directly to the integrin (Supplemental Figure S3) but both PACSIN2 and the α5β1 integrin bind to filamin (Fig. 10A). This may explain why preventing PACSIN2 from going to the ruffle membrane does not necessarily affect integrin localization. Second, PACSIN2 appears to shuttle from the cytoplasm to the ruffle membrane. The dominant negative form of PACSIN2 decreases filamin localization to the membrane ruffle while the over-expressed PACSIN2 prevent filamin localization to stress fibers (which are present) without affecting its membrane localization (Fig. 10B, C; Fig. 9, insert). This suggests that the complex of PACSIN2/α5β1 integrin/filamin is present at the ruffle membranes under normal conditions. During cell spreading and migration, stable integrin-ECM contacts are formed at these ruffle membranes. Within these contacts, integrins switch to a higher affinity state for the substrate (the so called integrin activation event) and re-group into focal adhesions. The cytoplasmic protein talin plays a critical role during integrin activation by binding to the β1 integrin cytoplasmic domain at the NPxY motif (Tadokoro et al., 2003). Interestingly, the members of the filamin family are also known to bind the integrin β1 subunit at a region overlapping the NPxY motif (Kiema et al., 2006). The binding of talin and filamin are mutually exclusive and the affinity for one or the other can be modulated (Kiema et al., 2006). Therefore, it is possible that the PACSIN2 over-expression results in the inhibition of α5β1 integrin by promoting integrin interactions with filamin at the expense of talin. This could be achieved by stabilizing filamin at ruffled membranes as suggested by the decrease of filamin at the ruffle membrane in cells expressing the dominant negative PACSIN2. With the dominant negative PACSIN2-MA, the PACSIN2/filamin/α5β1 integrin complex is not formed and the integrin is free to move to the focal adhesion. Using this model, we can predict how the dominant negative PACSIN2-MA affects embryo development. First, PACSIN2-MA should have no effect on α5β1 integrin activation and therefore should not interfere with mesoderm cell spreading and fibronectin matrix assembly. This is exactly what is observed in Figure 7 B and C. Secondly, the decrease of PACSIN2 function may result in the unregulated activation of α5β1 integrin in all cells of the gastrula, which is likely to prevent cell/cell intercalation essential for the gastrulation movements. This would result in a perturbation of blastopore closure despite the presence of the ECM, and the ability of the mesodermal cell to spread and migrate. Again, this is what is observed (Fig. 7D).
Could PACSIN2 function during gastrulation independently of the α5β1 integrin?
The first model relies on the gain of function results to link PACSIN2 function to α5β1 activation. Another possible model that does not rely on the gain of function results is based on the observation that the PACSIN2-MA prevents filamin from localizing to the ruffle membrane of XTC cells (Fig. 10C). Filamin has been shown to be essential for the 3 dimensional organization of the actin cytoskeleton in the cell cortex. Flanagan and colleagues have found that filamin depletion leads to a thinner actin filament network at the cortex (Flanagan et al., 2001). Because, the cell cortex needs to be quite stiff in order to support cell movement, these cells have impaired motility (Glogauer et al., 1998; Tseng et al., 2004). In fact, cells depleted of filamin were found to be more easily deformed and unable to migrate in a Boyden chamber assay (Cunningham et al., 1992). In Xenopus, Davidson et al. have shown that actin-rich cell protrusions are essential for the ability of cells to rearrange during gastrulation (Davidson et al., 2006). Moreover, the cytokinesis failure induced by PACSIN2-MA is reminiscent of the cytokinesis defect induced by the loss of function of CDC15p, a S.pombe PCH family member, which was linked to a defect in the actin ring formation (Fankhauser et al., 1995). Therefore, it is likely that the dominant negative PACSIN2 compromises the cortical actin strength so that it can no longer support cell rearrangement leading to gastrulation failure.
Why are the morpholino ineffective at preventing PACSIN2 translation?
Our first attempt at obtaining a loss of function phenotype was made by injecting morpholinos against the two paralogs of PACSIN2. However, no obvious defects of gastrulation or FN fibrillogenesis could be seen in morphant embryos. Western blot carried out on embryonic protein extracts resolved on a 2D SDS-PAGE, showed a modest decrease of one out of four protein species recognized by the PACSIN2 mAb. These data suggest that the other forms of PACSIN2 recognized by our mAb are not simply phosphorylation variants as previously thought but may be other closely related PACSIN2 proteins expressed in embryos that may compensate for the loss of PACSIN2. This hypothesis is supported by the fact that at least two other PACSINs have been identified in Xenopus EST databases (Kessels and Qualmann, 2004). This redundancy could explain why only one group has, so far reported results with the knock down of a single PACSIN gene (Mori et al., 2003). The cloning of all the remaining members of the PACSIN family and their paralogs will be needed in order to accomplish a significant loss of function in early embryo development. In order to circumvent the possible PACSIN redundancies, we designed the mitochondria anchored form of PACSIN2. This design was based on the fact that PACSIN can form homo and heterodimers (Kessels and Qualmann, 2004) and the observation that PACSIN2 need to be localized to the membrane to exert its effect. As a proof of principle, we showed that PACSIN2-MA is capable of re-localizing a Myc tagged PACSIN2 to mitochondria (Fig. 6C and D). In addition, in mammals, a similar construct of PACSIN1 (Syndapin I) was shown to bring both N-WASP and Dynamin to the mitochondria (Kessels and Qualmann, 2006). While PACSIN heterodimer have been reported between PACSIN1, 2 and 3, we can only speculate that this is also the case in Xenopus since neither PACSIN1 nor 3 have been fully characterized in this species. Therefore we can’t rule out that the PACSIN2-MA protein may affect other members of the PACSIN family to contribute to the phenotype.
Supplementary Material
Acknowledgments
We thank Dr. Mungo Marsden and Dr.Bette Dzamba for their valuable discussion and advice, and Dr. Alban Gaultier and Dr. Ann Sutherland for their helpful comments and support. We thank Dr. Donna Webb and Dr. John Wallingford for the gift of the paxillin-EGFP and Act-A constructs respectively. This work is supported by the Association pour la Recherche contre le Cancer (ARC) awarded Dr. Hélène Cousin and the National Institute of Health R-01 grants HD26402 awarded to Dr. Douglas DeSimone and DE016289 to Dr. Dominique Alfandari and DE14365 to Drs. DeSimone and Alfandari.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev Biol. 2003;260:449–64. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Cousin H, Gaultier A, Smith K, White JM, Darribere T, DeSimone DW. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest- cell migration. Curr Biol. 2001;11:918–30. doi: 10.1016/s0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Whittaker CA, DeSimone DW, Darribere T. Integrin alpha v subunit is expressed on mesodermal cell surfaces during amphibian gastrulation. Dev Biol. 1995;170:249–261. doi: 10.1006/dbio.1995.1212. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Wolfsberg TG, White JM, DeSimone DW. ADAM 13: a novel ADAM expressed in somitic mesoderm and neural crest cells during Xenopus laevis development. Dev Biol. 1997;182:314–330. doi: 10.1006/dbio.1996.8458. [DOI] [PubMed] [Google Scholar]
- Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, Field D, Siminovitch KA. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18:141–54. doi: 10.1016/s1074-7613(02)00516-2. [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Boulekbache H, Thiery JP. Prevention of gastrulation but not neurulation by antibodies to fibronectin in amphibian embryos. Nature. 1984a;307:364–7. doi: 10.1038/307364a0. [DOI] [PubMed] [Google Scholar]
- Boucaut JC, Darribere T, Poole TJ, Aoyama H, Yamada KM, Thiery JP. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984b;99:1822–30. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck P, Pistor S, Wehland J, Jockusch BM. Ligand recruitment by vinculin domains in transfected cells. J Cell Sci. 1997;110(Pt 12):1361–71. doi: 10.1242/jcs.110.12.1361. [DOI] [PubMed] [Google Scholar]
- Calderwood DA. Talin controls integrin activation. Biochem Soc Trans. 2004;32:434–7. doi: 10.1042/BST0320434. [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley ER. Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol. 2007;17:145–56. doi: 10.1016/j.tcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Cohen MW, Hoffstrom BG, DeSimone DW. Active zones on motor nerve terminals contain alpha 3beta 1 integrin. J Neurosci. 2000;20:4912–21. doi: 10.1523/JNEUROSCI.20-13-04912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin H, Alfandari D. A PTP-PEST-like protein affects alpha5beta1-integrin-dependent matrix assembly, cell adhesion, and migration in Xenopus gastrula. Dev Biol. 2004;265:416–32. doi: 10.1016/j.ydbio.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Cousin H, Gaultier A, Bleux C, Darribere T, Alfandari D. PACSIN2 is a regulator of the metalloprotease/disintegrin ADAM13. Dev Biol. 2000;227:197–210. doi: 10.1006/dbio.2000.9871. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–7. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Darribere T, Guida K, Larjava H, Johnson KE, Yamada KM, Thiery JP, Boucaut JC. In vivo analyses of integrin beta 1 subunit function in fibronectin matrix assembly. J Cell Biol. 1990;110:1813–23. doi: 10.1083/jcb.110.5.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darribere T, Koteliansky VE, Chernousov MA, Akiyama SK, Yamada KM, Thiery JP, Boucaut JC. Distinct regions of human fibronectin are essential for fibril assembly in an in vivo developing system. Dev Dyn. 1992;194:63–70. doi: 10.1002/aja.1001940108. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev Biol. 2002;242:109–29. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Marsden M, Keller R, Desimone DW. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–44. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Bolton MA, DeSimone DW. Cell Adhesion: The integrin family of cell adhesion molecules. Oxford University Press; Oxford: 2002. [Google Scholar]
- Fankhauser C, Reymond A, Cerutti L, Utzig S, Hofmann K, Simanis V. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–44. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155:511–7. doi: 10.1083/jcb.200105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier A, Cousin H, Darribere T, Alfandari D. ADAM 13 disintegrin and cysteine-rich domains bind to the Hep II domain of fibronectin. J Biol Chem. 2002;19:19. doi: 10.1074/jbc.M201792200. [DOI] [PubMed] [Google Scholar]
- Gawantka V, Joos TO, Hausen P. A beta 1-integrin associated alpha-chain is differentially expressed during Xenopus embryogenesis. Mech Dev. 1994;47:199–211. doi: 10.1016/0925-4773(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Ghadimi MP, Sanzenbacher R, Thiede B, Wenzel J, Jing Q, Plomann M, Borkhardt A, Kabelitz D, Janssen O. Identification of interaction partners of the cytosolic polyproline region of CD95 ligand (CD178) FEBS Lett. 2002;519:50–8. doi: 10.1016/s0014-5793(02)02709-6. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr Opin Cell Biol. 2005;17:509–16. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Glogauer M, Arora P, Chou D, Janmey PA, Downey GP, McCulloch CA. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J Biol Chem. 1998;273:1689–98. doi: 10.1074/jbc.273.3.1689. [DOI] [PubMed] [Google Scholar]
- Halbach A, Morgelin M, Baumgarten M, Milbrandt M, Paulsson M, Plomann M. PACSIN 1 forms tetramers via its N-terminal F-BAR domain. Febs J. 2007;274:773–82. doi: 10.1111/j.1742-4658.2006.05622.x. [DOI] [PubMed] [Google Scholar]
- Hoffstrom BG. Thesis dissertation. Department of Biology, University of Virginia; 2002. Integrin function during Xenopus Laevis gastrulation. [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jha KN, Salicioni AM, Arcelay E, Chertihin O, Kumari S, Herr JC, Visconti PE. Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol Hum Reprod. 2006;12:781–9. doi: 10.1093/molehr/gal085. [DOI] [PubMed] [Google Scholar]
- Joos TO, Whittaker CA, Meng F, DeSimone DW, Gnau V, Hausen P. Integrin alpha 5 during early development of Xenopus laevis. Mech Dev. 1995;50:187–199. doi: 10.1016/0925-4773(94)00335-k. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels MM, Qualmann B. The syndapin protein family: linking membrane trafficking with the cytoskeleton. J Cell Sci. 2004;117:3077–86. doi: 10.1242/jcs.01290. [DOI] [PubMed] [Google Scholar]
- Kessels MM, Qualmann B. Syndapin oligomers interconnect the machineries for endocytic vesicle formation and actin polymerization. J Biol Chem. 2006 doi: 10.1074/jbc.M510226200. [DOI] [PubMed] [Google Scholar]
- Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, Campbell ID, Ylanne J, Calderwood DA. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–47. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Lee G, Hynes R, Kirschner M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell. 1984;36:729–40. doi: 10.1016/0092-8674(84)90353-2. [DOI] [PubMed] [Google Scholar]
- Li J, Nishizawa K, An W, Hussey RE, Lialios FE, Salgia R, Sunder-Plassmann R, Reinherz EL. A cdc15-like adaptor protein (CD2BP1) interacts with the CD2 cytoplasmic domain and regulates CD2-triggered adhesion. EMBO J. 1998;17:7320–36. doi: 10.1093/emboj/17.24.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–47. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol. 2003;13:1182–91. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- Modregger J, Ritter B, Witter B, Paulsson M, Plomann M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J Cell Sci. 2000;113:4511–4521. doi: 10.1242/jcs.113.24.4511. [DOI] [PubMed] [Google Scholar]
- Mori S, Tanaka M, Nanba D, Nishiwaki E, Ishiguro H, Higashiyama S, Matsuura N. PACSIN3 binds ADAM12/meltrin alpha and up-regulates ectodomain shedding of heparin-binding epidermal growth factor-like growth factor. J Biol Chem. 2003;278:46029–34. doi: 10.1074/jbc.M306393200. [DOI] [PubMed] [Google Scholar]
- Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin alpha 5 beta 1-fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ J Biol Chem. 1995;270:26270–7. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- Na J, Marsden M, DeSimone DW. Differential regulation of cell adhesive functions by integrin {alpha} subunit cytoplasmic tails in vivo. J Cell Sci. 2003:23. doi: 10.1242/jcs.00445. [DOI] [PubMed] [Google Scholar]
- Nikki M, Merilainen J, Lehto VP. FAP52 regulates actin organization via binding to filamin. J Biol Chem. 2002a;277:11432–40. doi: 10.1074/jbc.M111753200. [DOI] [PubMed] [Google Scholar]
- Nikki M, Merilainen J, Lehto VP. Focal adhesion protein FAP52 self-associates through a sequence conserved among the members of the PCH family proteins. Biochemistry. 2002b;41:6320–9. doi: 10.1021/bi015991n. [DOI] [PubMed] [Google Scholar]
- Park TJ, Gray RS, Sato A, Habas R, Wallingford JB. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr Biol. 2005;15:1039–44. doi: 10.1016/j.cub.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Pistor S, Chakraborty T, Niebuhr K, Domann E, Wehland J. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. Embo J. 1994;13:758–63. doi: 10.1002/j.1460-2075.1994.tb06318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B, Kelly RB. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J Cell Biol. 2000;148:1047–62. doi: 10.1083/jcb.148.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:227–40. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JW, Whittaker CA, DeSimone DW. Integrin-dependent adhesive activity is spatially controlled by inductive signals at gastrulation. Development. 1996;122:2873–2883. doi: 10.1242/dev.122.9.2873. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL. SH3-domain-containing proteins function at distinct steps in clathrin- coated vesicle formation. Nat Cell Biol. 1999;1:119–124. doi: 10.1038/10091. [DOI] [PubMed] [Google Scholar]
- Smith JC, Symes K, Hynes RO, DeSimone D. Mesoderm induction and the control of gastrulation in Xenopus laevis: the roles of fibronectin and integrins. Development. 1990;108:229–38. doi: 10.1242/dev.108.2.229. [DOI] [PubMed] [Google Scholar]
- Smith JC, Yaqoob M, Symes K. Purification, partial characterization and biological effects of the XTC mesoderm-inducing factor. Development. 1988;103:591–600. doi: 10.1242/dev.103.3.591. [DOI] [PubMed] [Google Scholar]
- Sumoy L, Pluvinet R, Andreu N, Estivill X, Escarceller M. PACSIN 3 is a novel SH3 domain cytoplasmic adapter protein of the pacsin-syndapin-FAP52 gene family. Gene. 2001;262:199–205. doi: 10.1016/s0378-1119(00)00531-x. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Tseng Y, An KM, Esue O, Wirtz D. The bimodal role of filamin in controlling the architecture and mechanics of F-actin networks. J Biol Chem. 2004;279:1819–26. doi: 10.1074/jbc.M306090200. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Kuikman I, Kramer D, Geerts D, Kreft M, Takafuta T, Shapiro SS, Sonnenberg A. Different splice variants of filamin-B affect myogenesis, subcellular distribution, and determine binding to integrin [beta] subunits. J Cell Biol. 2002;156:361–76. doi: 10.1083/jcb.200103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells -- over and over and over again. Nat Cell Biol. 2002;4:E97–100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Winklbauer R, Keller RE. Fibronectin, mesoderm migration, and gastrulation in Xenopus. Dev Biol. 1996;177:413–26. doi: 10.1006/dbio.1996.0174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.