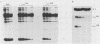Abstract
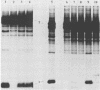
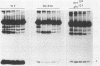
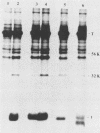
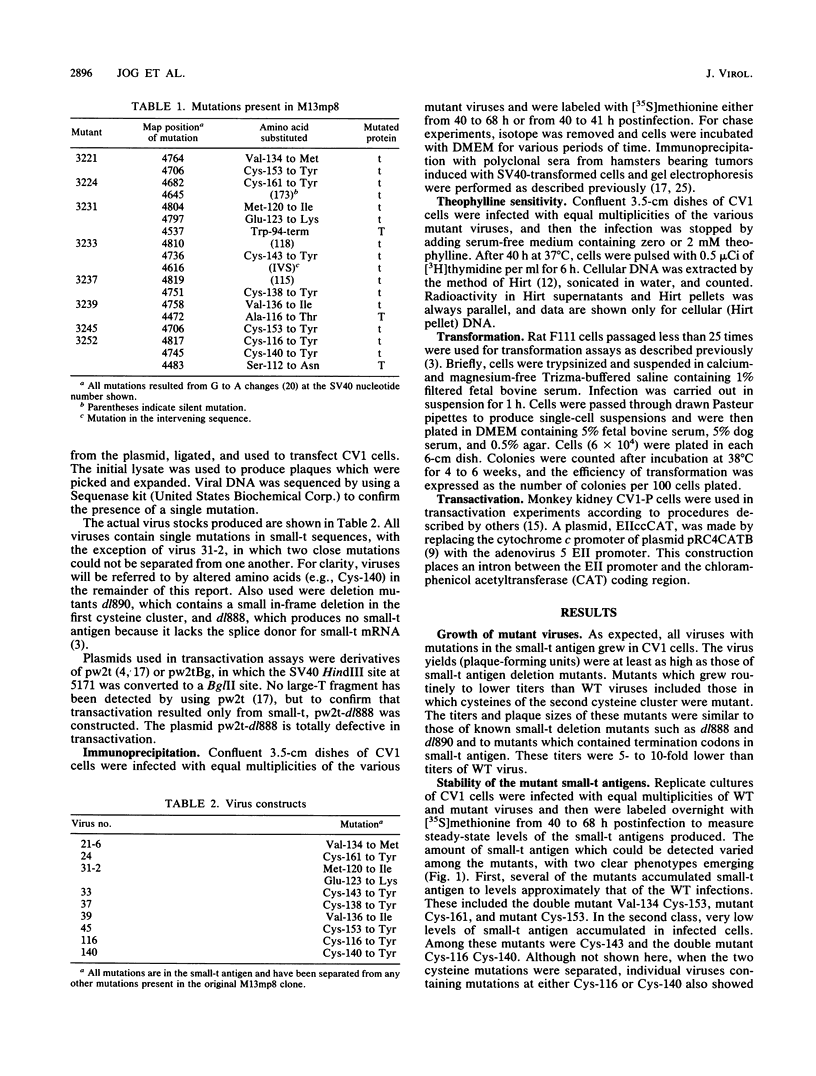
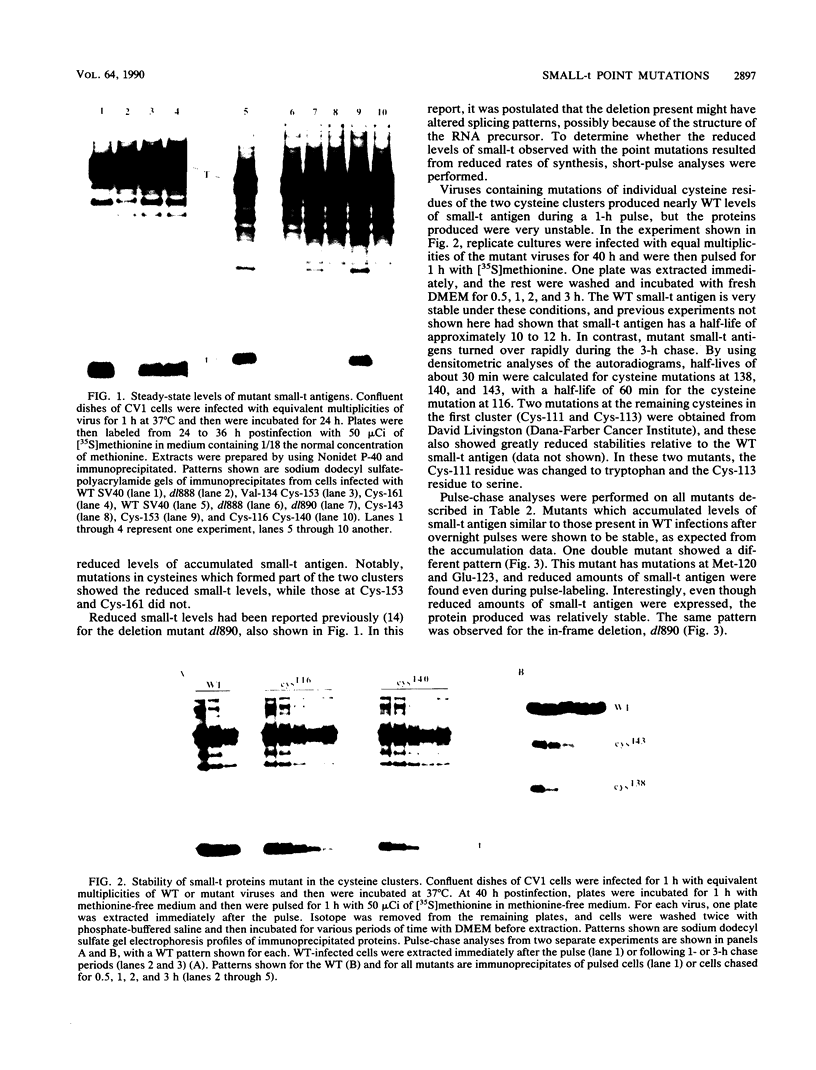
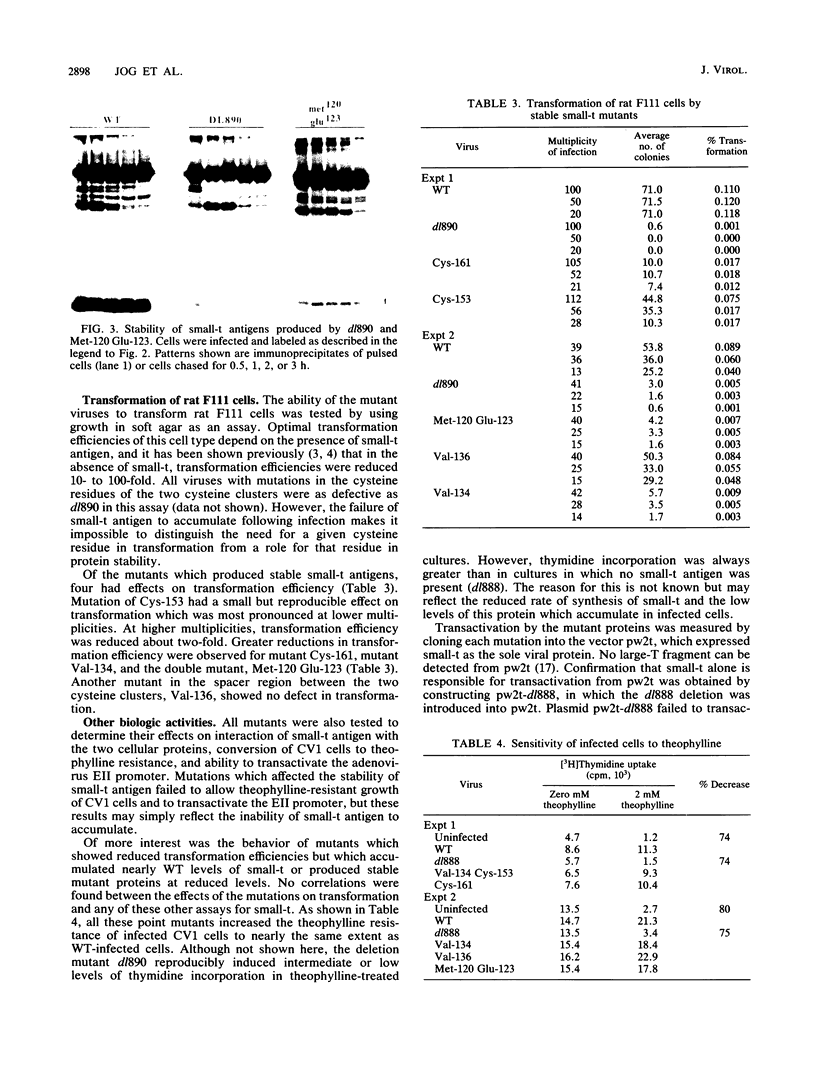
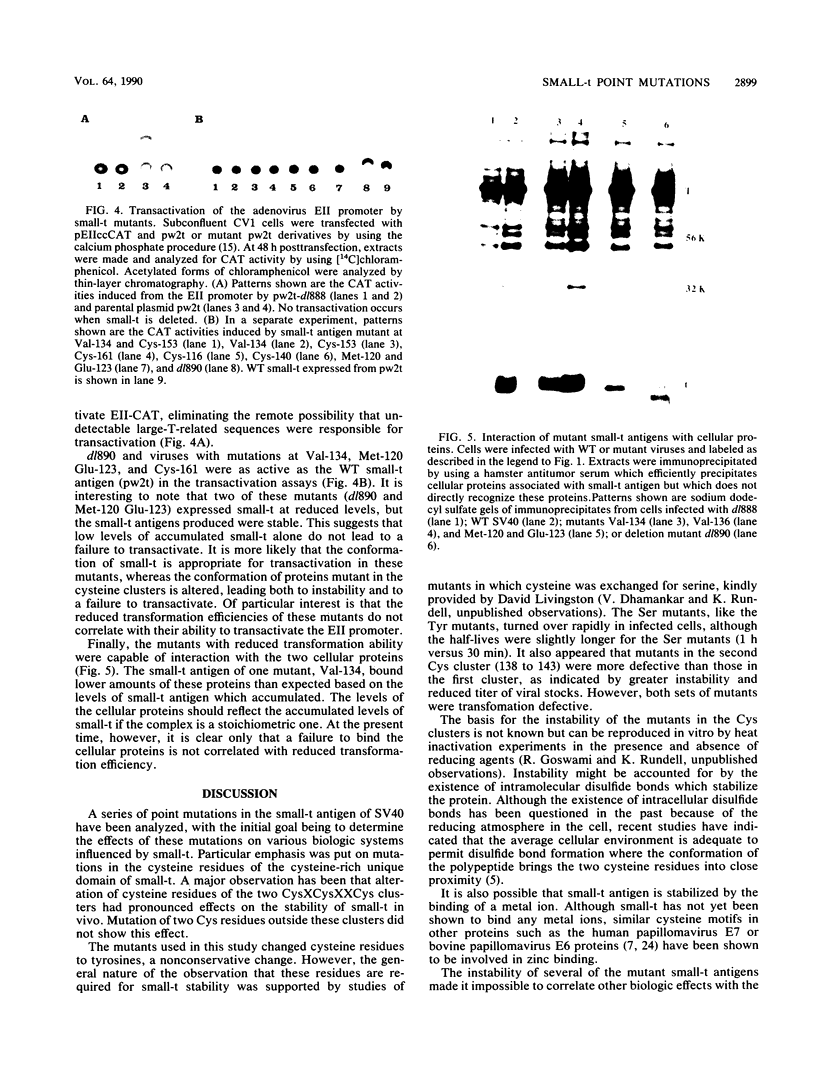
Several point mutations in the simian virus 40 (SV40) small-t antigen have been analyzed for their effects on protein stability, transformation, transactivation, and binding of two cellular proteins. All mutations which affected cysteine residues in two cysteine clusters produced highly unstable small-t antigens. Four point mutations outside these clusters and one in-frame deletion mutant, dl890, produced stable proteins but reduced transformation efficiency. These were able to transactivate the EII promoter and bind the cellular proteins, suggesting that these activities are not sufficient for small-t-mediated enhancement of transformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikel I., Mamon H., Brown E. L., Boltax J., Agha M., Livingston D. M. The t-unique coding domain is important to the transformation maintenance function of the simian virus 40 small t antigen. Mol Cell Biol. 1986 Apr;6(4):1172–1178. doi: 10.1128/mcb.6.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Montano X., Agha M. E., Brown M., McCormack M., Boltax J., Livingston D. M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987 Jan 30;48(2):321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. S., Pan S., Pater M. M., Di Mayorca G. Differential requirement for SV40 early genes in immortalization and transformation of primary rat and human embryonic cells. Virology. 1985 Oct 30;146(2):246–261. doi: 10.1016/0042-6822(85)90008-x. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Dhar R., Seif I., Khoury G. Nucleotide sequence of the BK virus DNA segment encoding small t antigen. Proc Natl Acad Sci U S A. 1979 Feb;76(2):565–569. doi: 10.1073/pnas.76.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C., Vousden K. H. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989 Jun;63(6):2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman M., Bikel I., Figge J., Roberts T., Schlossman R., Livingston D. M. Localization of the simian virus 40 small t antigen in the nucleus and cytoplasm of monkey and mouse cells. J Virol. 1984 May;50(2):623–628. doi: 10.1128/jvi.50.2.623-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. Both upstream and intron sequence elements are required for elevated expression of the rat somatic cytochrome c gene in COS-1 cells. Mol Cell Biol. 1988 Jan;8(1):35–41. doi: 10.1128/mcb.8.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Doolittle R. F., Walter G. Amino acid sequence homology between polyoma and SV40 tumour antigens deduced from nucleotide sequences. Nature. 1978 Jul 20;274(5668):291–293. doi: 10.1038/274291a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981 Feb;37(2):802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Phillips B., Rundell K. Failure of simian virus 40 small t antigen to disorganize actin cables in nonpermissive cell lines. J Virol. 1988 Mar;62(3):768–775. doi: 10.1128/jvi.62.3.768-775.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J Virol. 1987 Apr;61(4):1240–1243. doi: 10.1128/jvi.61.4.1240-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Cox J. Simian virus 40 t antigen affects the sensitivity of cellular DNA synthesis to theophylline. J Virol. 1979 Apr;30(1):394–396. doi: 10.1128/jvi.30.1.394-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutila J. E., Christensen J. B., Imperiale M. J. Viral growth, origin binding, and p53 binding properties of simian virus 40 large T antigen transformation and replication mutants. Oncogene Res. 1989;4(4):303–310. [PubMed] [Google Scholar]
- Rutila J. E., Imperiale M. J., Brockman W. W. Replication and transformation functions of in vitro-generated simian virus 40 large T antigen mutants. J Virol. 1986 May;58(2):526–535. doi: 10.1128/jvi.58.2.526-535.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Androphy E. J., Schiller J. T., Lowy D. R. Mutational analysis of bovine papillomavirus E6 gene. J Virol. 1989 May;63(5):2340–2342. doi: 10.1128/jvi.63.5.2340-2342.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Hearing P., Rundell K. Cellular proteins associated with simian virus 40 early gene products in newly infected cells. J Virol. 1979 Oct;32(1):147–154. doi: 10.1128/jvi.32.1.147-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]