Abstract
Inoculation of BALB/c mice with rhesus rotavirus (RRV) in the newborn period results in biliary epithelial cell (cholangiocyte) infection and the murine model of biliary atresia. Rotavirus infection of a cell requires attachment, which is governed in part by cell-surface expression of integrins such as α2β1. We hypothesized that cholangiocytes were susceptible to RRV infection because they express α2β1. RRV attachment and replication was measured in cell lines derived from cholangiocytes and hepatocytes. Flow cytometry was performed on these cell lines to determine whether α2β1 was present. Cholangiocytes were blocked with natural ligands, a monoclonal antibody, or small interfering RNA against the α2-subunit and were infected with RRV. The extrahepatic biliary tract of newborn mice was screened for the expression of the α2β1-integrin. Newborn mice were pretreated with a monoclonal antibody against the α2-subunit and were inoculated with RRV. RRV attached and replicated significantly better in cholangiocytes than in hepatocytes. Cholangiocytes, but not hepatocytes, expressed α2β1 in vitro and in vivo. Blocking assays led to a significant reduction in attachment and yield of virus in RRV-infected cholangiocytes. Pretreatment of newborn pups with an anti-α2 monoclonal antibody reduced the ability of RRV to cause biliary atresia in mice. Cell-surface expression of the α2β1-integrin plays a role in the mechanism that confers cholangiocyte susceptibility to RRV infection.
Keywords: rhesus rotavirus, bile ducts, cholangiopathy
biliary atresia (BA) is a unique disease of infancy in which affected children develop fibroinflammatory obliteration of the biliary tract. It is the most common indication for pediatric liver transplantation (24). Because pathogenic viruses have been found in the livers of children afflicted with biliary atresia (10, 12, 18, 26, 29), a proposed etiology for biliary atresia is perinatal infection by a virus triggering inflammatory destruction of the biliary epithelium (3, 23, 32). A murine model of biliary atresia (30) supports a viral pathogenesis where newborn mice infected with rhesus rotavirus (RRV) develop inflammation within the portal tract (31) and extrahepatic bile duct obstruction (9, 27).
Recently, it has been shown that in the murine model of biliary atresia, RRV targeted the biliary epithelial cell (cholangiocyte) for infection (1, 31). To determine the basis for cholangiocyte susceptibility to RRV, an in vitro model of RRV infection of the two dominant cell types found within the liver (cholangiocytes and hepatocytes) was established. Consistent with the in vivo findings, RRV was better able to replicate in cholangiocytes than hepatocytes. Because the ability of rotavirus to infect cells appears to be regulated by cell-surface expression of the integrins α2β1, α4β1, αvβ3, and αxβ2, which serve as viral receptors (8, 13, 14, 16, 21), the cholangiocyte was surveyed for integrin expression and found to uniquely express the α2β1-integrin when compared with hepatocytes. Based on this information, we hypothesized that expression of the α2β1-integrin serves as a determinant governing cholangiocyte susceptibility to RRV infection contributing to the mechanism by which rotavirus initiates the experimental model of biliary atresia.
EXPERIMENTAL PROCEDURES
Murine Model of Biliary Atresia
All animal studies were performed in accordance with institutional animal welfare guidelines and with the use of an approved animal protocol. The experimental model of biliary atresia was induced in BALB/c mice (Harlan Labs, Indianapolis, IN) by previously described techniques (1).
To determine the role of the α2β1-integrin in vivo, newborn pups were injected intraperitoneally with monoclonal anti-α2 IgG (Ha1/29; Biogen Idec, Cambridge, MA) or anti-keyhole limpet hemocyanin (KLH) IgG (Ha4/8; Biogen Idec), an isotype control, at a dose of 100 μg per injection on days of life 0, 2, and 4. This dose was based on pharmacokinetic data derived from adult mice that showed that 100 μg injected intravenously yielded 20 μg /ml in blood after 24 h and nearly full clearance (0.1 μg/ml in blood) by 7 days (data not shown). On day of life 1, mice were inoculated with RRV. Pups were monitored for 30 days for clinical signs of hepatobiliary injury (i.e., jaundice in non-fur-covered skin, acholic stools, and bilirubinuria) and survival. Subsets of these mice were killed at 7 days after infection, and their extrahepatic biliary tract and liver were harvested. The tissues were analyzed for the presence of replication-competent virus by focus-forming assay (FFA).
Cell Lines and Virus
A mouse cholangiocyte cell line, mCl, derived from primary cholangiocytes, harvested from BALB/c, mice and immortalized with SV40 large T-cell antigen was provided by the laboratory of Dr. James Boyer (Yale Liver Care Center, Hartford, CT). These cells express gamma-glutamyl transpeptidase (GGT) and cytokeratin-7, consistent with their biliary epithelium origin (data not shown). H2.35 cells, a hepatocyte cell line derived from BALB/c mice, were purchased from the American Type Culture Collection (Manassas, VA). The cell lines were maintained in DMEM (Cellgro, Herndon, VA) with 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA), penicillin (10,000 U/ml)-streptomycin (10,000 μg/ml) (Invitrogen), 1% l-glutamine (Invitrogen), and amphotericin B (250 μg/ml; Cellgro).
The rotavirus strain RRV, obtained from Dr. Harry Greenberg (Stanford University, Palo Alto, CA), was maintained in MA104 cells. Triple-layered RRV particles were purified by cesium chloride centrifugation and were used in all in vitro assays.
Viral Assays
Measurement of replication-competent virus by using FFA.
Virus within samples was enumerated by the formation of foci in MA104 cells as previously described (1, 25).
Measurement of the ability of rotavirus to infect cell lines in vitro.
Cells were seeded in culture tubes at a quantity of 5 × 105 in DMEM and were incubated at 37°C for 3 days. Tubes were washed with Earle's balanced salt solution and were infected with RRV at varying multiplicities of infection (MOI) at 37°C for 1 h. At an MOI of 1, the amount of virus equals the number of cells. The cultures were washed and incubated with serum-free DMEM + 4 μg trypsin/ml at 37°C for 24 h. Cultures were monitored for the development of cell lysis [cytopathic effect (CPE)]. CPE was determined by visual inspection of the cell layer and graded based on a previously described scale (17), and viral yield was assessed by FFA.
Measurement of viral binding by using attachment and blocking assays.
Cells were grown to confluence in 24-well plates. The cells, medium, and inoculating virus were cooled to 4°C. Viral binding was assessed by using previously described attachment assays (17). For each assay, the sample size consisted of at least three wells of cells per experimental condition, and the experiment was repeated between three and six times.
For blocking assays, cells were pretreated with different concentrations of human collagen I, mouse collagen IV (BD Discovery Labs, Bedford, MA), mouse laminin (Invitrogen), the short peptides aspartic acid-glycine-glutamic acid-alanine [(DGEA) Anaspec, San Jose, CA], arginine-glycine-aspartic acid-alanine (RGDA), glycine-proline-arginine-proline (GPRP), glycine-histidine-arginine-proline (GHRP), or the monoclonal antibodies Ha1/29, Ha4/8, and Ha31/8 (Biogen Idec) for 1 h at 4°C. The peptide sequence aspartic acid-glycine-glutamic acid (DGE) is the collagen-binding domain to the α2β1-integrin (33). The peptide sequence arginine-glycine-aspartic acid (RGD) is the laminin-binding domain to the α2β1-integrin (28). The peptide sequence glycine-proline-arginine (GPR) is the fibrinogen-binding domain to the αvβ3-integrin (13). The peptide sequence GHRP served as a negative control. Ha31/8 is a function-blocking monoclonal antibody directed against the murine α1-integrin subunit.
The blocked cells then underwent attachment assay. Other blocked cells were inoculated with RRV for 1 h at 4°C, washed (to remove any unbound virus), warmed to 37°C, and incubated for 24 h. Viral yield was determined by FFA. For each blocking assay, the sample size consisted of between three and five wells of cells per experimental condition, and each experiment was repeated at least three times.
Flow Cytometry
Direct immunofluorescent staining for the individual subunits of the integrins α1β1, α2β1, α4β1, αvβ3, and αxβ2 was performed by using FITC-conjugated or R-phycoerythrin-conjugated monoclonal antibodies (BD Biosciences, San Jose, CA). Confluent monolayers were washed with PBS and were detached by using trypsin-0.75 mM EDTA for 10 min at 37°C. When testing for the α4- and αx-integrin subunits, cells were detached using PBS + 0.75 mM EDTA without trypsin because trypsin can degrade these integrins (15). H2.35 and mCl cells were resuspended to a concentration of 1 × 106 cells/ml in FACS buffer (PBS + 0.1% sodium azide) containing 10 μg/ml of FITC-conjugated antibody and were incubated for 30 min at 4°C. Cells were washed with PBS + 0.1% sodium azide, pelleted by centrifugation, and resuspended in PBS + 1% formaldehyde. Background fluorescence was evaluated with isotype-control antibodies. Cells were analyzed (10,000 events per sample) by using a FACSCalibur dual-laser flow cytometer (BD Biosciences) and CellQuest software (BD Biosciences).
To demonstrate that the α2β1 heterodimer was present, indirect immunofluorescence staining by FACS analysis of the cells was performed by using 10 μg/ml of rat anti-α2β1 primary IgG monoclonal antibody (BMA2.1; Chemicon International, Temecula, CA). A FITC-conjugated goat anti-rat IgG antibody (10 μg/ml) was added, and the cells were analyzed as above.
Transfection of Small Interfering RNA
mCl cells were seeded at a density of 5 × 104 cells per well in 24-well plates and were incubated overnight at 37°C. Cells that were 40% confluent were transfected with small interfering RNA (siRNA) or non-targeting siRNA (negative siRNA) according to the manufacturer's protocol in 100 μl of serum-free media with 1% l-glutamine and 3.35 μl/well of X-tremeGENE (Roche, Basel, Switzerland). A mixture of four siRNA sequences targeting the α2-subunit (Dharmacon, Lafayette, CO) and four siRNA sequences targeting the αv-subunit (Dharmacon) was used at a total concentration of 0.625 μg/sequence.
The α2 siRNA sequences were as follows: sequence 1, sense UGAAUUGUCUGGCGUAUAAUU, antisense UUAUACGCCAGACAAUUCAUU; sequence 2, sense CAACUGGGAUCUGUUCUGAUU, antisense PUCAGAACAGAUCCCAGUUGUU; sequence 3, sense GCCAAUGAGCCGAGAAUUAUU, antisense PUAAUUCUCGGCUCAUUGGCUU; sequence 4, sense GAUUGUCGGUUCACCUGUAUU, antisense PUACAGGUGAACCGACAAUCUU.
The αv siRNA sequences were as follows: sequence 1, sense GCGCAAUCCUGUACGUGAAUU, antisense PUUCACGUACAGGAUUGCGCUU; sequence 2, sense CCAAUUAGCAACACGGACUUU, antisense PAGUCCGUGUUGCUAAUUGGUU; sequence 3, sense GUGAGGAACUGGUCGCCUAUU, antisense PUAGGCGACCAGUUCCUCACUU; sequence 4, sense GUGAACAGAUGGCUGCGUAUU, antisense PUACGCAGCCAUCUGUUCACUU.
Nontargeting siRNA was purchased from Dharmacon (siCONTROL#1).
Cells were overlaid with 450 μl of DMEM containing 10% FBS and 1% l-glutamine. After 48 h, cells were trypsinized, pooled, re-plated at 1 × 106 cells/well, and incubated at 37°C for 24 h. Flow cytometry and Western blot analysis for the α2- and αV-subunits were performed to confirm RNA suppression. Attachment and infectivity assays were performed on confluent monolayers. The sample size consisted of between three and five wells of cells, and the experiment was repeated three times.
Immunohistochemistry
In vitro, 100 μl of 1 × 106 mCl and H2.35 cells were centrifuged onto slides by using a cytospin. Slides were fixed in methanol and were blocked with 10% normal goat serum. Slides were incubated with FITC-conjugated rat anti-mouse α2-antibody (Emfret Analytics, Wurzburg, Germany) diluted 1:40 in goat serum and analyzed.
Confocal microscopic analysis.
Liver and extrahepatic biliary sections harvested from mice were embedded in Histo Prep (Fisher Scientific, Pittsburgh, PA) over dry ice. Frozen samples were cut in 7-μm sections and were fixed in acetone for 10 min. Slides were blocked with 10% normal goat serum followed by subsequent incubations with FITC-conjugated rat anti-mouse α2-antibody diluted 1:40 in 10% goat serum, 10 ug/ml of Cy2-conjugated IgG fraction of monoclonal mouse anti-FITC (Jackson ImmunoResearch, West Grove, PA) in 10% goat serum, and a mixture of 0.25 μM TO-PRO-3 (Invitrogren) and 1 μg/ml rhodamine-conjugated wheat germ agglutinin (Invitrogen) diluted in 1× PBS. Slides were visualized by using a Zeiss LSM-510 laser-scanning microscope through a C-apochromat ×40 water objective with a 1.2 numerical aperture using a multitrack configuration. The Cy2 was excited by using a 488-line argon laser with a 488 diacroic and a 500–530 band-pass filter. The rhodamine-conjugated wheat germ agglutinin was excited using a 543 helium-neon laser with a 543 diacroic and a 565–615 band-pass filter. The TO-PRO-3 was excited by using a 633 helium-neon laser with a 633 diacroic and 650 long-pass filter. The images were compiled and analyzed by using Zeiss LSM image software.
Western Blot Analysis
Protein from cells and tissue was extracted in 1 ml of homogenization buffer [1% Triton X-100 with (in mM) 50 Tris·HCl, 150 NaCl, 1 EDTA, and 1 EGTA]. Each sample was homogenized, and protein concentration was determined by Bradford assay. One hundred micrograms of protein was run per sample on a 4–20% Tris-glycine gel (Invitrogen). Gels were transferred to polyvinylidene difluoride plus membranes and were blocked with rabbit serum. Membranes were stained with a 1:1,000 diluted sheep anti-mouse α2 primary antibody (R and D Systems, Minneapolis, MN) and a 1:5,000 diluted peroxidase-conjugated rabbit anti-sheep IgG secondary antibody (Jackson ImmunoResearch, West Grove, PA).
Laser Capture Microdissection and Real-Time RT-PCR
Extrahepatic biliary sections were mounted on PEN membrane glass slides (Arcturus Bioscience, Mt. View, CA) and were placed on dry ice. Sections were thawed, stained with hematoxylin and eosin, and dried for laser capture microdissection (LCM). Using the Veritas Microdissection System LCC 1704 (Arcturus Bioscience), biliary epithelial cells were selectively captured. Total RNA was extracted by using the PicoPure RNA isolation kit (Arcturus Bioscience) according to the manufacturer's instructions. The quality and quantity of RNA was evaluated by using the Nanodrop ND-1000 UV-Vis spectrophotometer (Wilmington, DE). cDNA was generated by using Invitrogen reagents in a total reaction volume of 50 μl. cDNA pools were subjected to real-time kinetic PCR on a Mx-4000 Multiplex Quantitative PCR (Stratagene, La Jolla, Ca) using SYBR Green I as a double-stranded DNA-specific binding dye to quantify mRNA expression of α2-protein relative to GAPDH by using techniques previously described (31). Primers for α2 were as follows: forward, 5′-CGCTCCTTCTGTCATCAAGAGTGTC-3′ and reverse, 5′-GGAATGTGGATAGTCACCAATGCC-3′.
Statistical Analysis
Analysis of noncontinuous variables was done by using χ-square and Fisher Exact tests. Results of continuous variables were expressed as means ± SE and were analyzed by using ANOVA with post hoc testing where appropriate. A P value <0.05 was considered significant.
RESULTS
RRV Targets the Biliary Epithelial Cell
Consistent with previous reports in the literature, we have found that inoculation of newborn BALB/c mice with RRV results in extrahepatic biliary obstruction. To determine how infection resulted in biliary obstruction, we quantitated the amount of RRV in hepatobiliary tissue and found that the amount of RRV per milligram of tissue was 11 times greater in the extrahepatic biliary tree than in the liver [2.9 ± 1.4 × 105 vs. 2.7 ± 2.1 × 104 focus-forming units (FFU)·ml−1·mg−1; P < 0.05, respectively]. Recent studies using dual-staining immunohistochemistry of the liver and the extrahepatic biliary tract harvested from newborn mice infected with RRV revealed that the target of rotavirus infection was the biliary epithelial cell (1, 31). RRV was not found within parenchymal hepatocytes. To determine the pathogenic basis for infection, we established a novel in vitro system of rotavirus infection of cells of biliary epithelial or hepatocyte origin. The ability of RRV to infect in vitro the two dominant cell types within the liver (cholangiocytes and hepatocytes) are shown in Table 1. Cholangiocytes and H2.35 cells were inoculated with RRV at an MOI of 1, and both the CPE and the ability of RRV to replicate in the two cell lines were measured. RRV caused minimal CPE in mCl cells, but there was a 118-fold increase in virus, indicating that RRV was able to replicate within the cells. In H2.35 cells, there was little CPE with only threefold increase in virus, significantly less than in the cholangiocytes. When the two cell lines were infected with more viral particles (as indicated by increasing MOI), there was a greater viral yield in cholangiocytes than hepatocytes at all MOIs tested (Table 1).
Table 1.
Ability of RRV to replicate in cholangiocytes vs. hepatocytes: focus-forming units present 24 h after infection with RRV
| MOI | Cholangiocytes, FFU/ml | Hepatocytes, FFU/ml |
|---|---|---|
| 1 | 6.0±0.9 × 107* | 1.8±0.4 × 106 |
| 10 | 2.5±0.4 × 108* | 2.9±0.8 × 107 |
| 30 | 3.4±0.0 × 108* | 8.5±1.1 × 107 |
| 300 | 9.2±1.8 × 108* | 3.1±0.7 × 108 |
Confluent monolayers of cholangiocytes (mCl cells) and hepatocytes (H2.35 cells) grown in cell-culture tubes were infected with increasing multiplicities of infection (MOI) of Rhesus rotavirus (RRV), followed by a focus-forming assay run 24 h after infection. Values are expressed as focus-forming units (FFU)/ml ± SE.
P < 0.05.
To determine why RRV was better able to replicate in the cholangiocyte, we used our in vitro model to dissect the mechanism by which a virus infects a cell. Viral infection of a host cell is dependent on viral attachment/binding to the cell surface followed by internalization, uncoating, replication, and viral release. To determine whether there was a difference in the ability of RRV to attach to cells of hepatobiliary origin, viral attachment assays were performed comparing mCl and H2.35 cells. The cell lines were exposed to RRV for 1 h at 4°C. By performing studies at 4°C, the subsequent steps of viral infection were blocked. Under these conditions, RRV attached to mCl cells fivefold greater than to H2.35 cells (12.5 ± 1.8% in mCl cells vs. 2.5 ± 1.3% in H2.35 cells; P < 0.05; n = 3–5 wells/assay; assay repeated in triplicate).
Cholangiocyte vs. Hepatocyte Cell-Surface Expression of Integrins
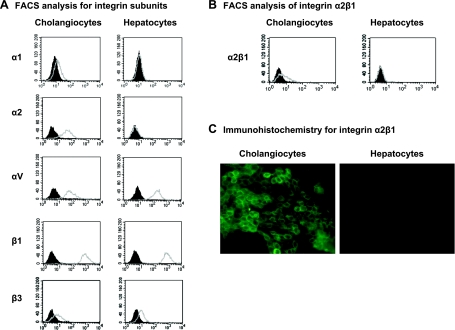
Cell-surface expression of the integrins α2β1, α4β1, αxβ2, and αvβ3 has been shown to play a role in the attachment and entry of rotaviruses into other cell lines (8, 13, 14, 16, 21). Flow cytometry was performed on the mCl and H2.35 cells to determine whether they express the integrin subunits α1, α2, α4, αv, αx, β1, β2, or β3. FACS analysis revealed that the mCl cells expressed α1, α2, αv, β1, and β3, whereas H2.35 cells expressed αv, β1, and β3 but not α2 (Fig. 1A). Neither mCl nor H2.35 cells expressed α4, αx, or β2 (see supplemental Fig. 1, available online at the American Journal of Physiology-Gastrointestinal and Liver Physiology website). The pattern of integrin subunit expression indicated that although both cell types expressed αvβ3, only mCl expressed the α2β1-integrin. To ensure that mCl express the heterodimer α2β1, FACS analysis using a primary antibody to the heterodimer α2β1 was performed and demonstrated presence of the integrin (Fig. 1B). Immunohistochemistry for the α2-integrin on the cell surface of the cholangiocytes confirmed the FACS results (Fig. 1C).
Fig. 1.
Presence of α2β1-integrin on the cholangiocyte cell surface. A: FACS analysis for α- and β-integrin subunits. Presence of the integrin subunits α1, α2, αv, β1, and β3 on cell surface of cholangiocytes and hepatocytes was determined by FACS analysis (x-axis, counts; y-axis, FITC or R-phycoerythrin intensity). Subunit antibody binding, open gray curves; isotype binding, filled black curves. Shift of gray from black indicated presence of subunit. B: FACS analysis for integrin heterodimer α2β1. Indirect FACS analysis was used to detect presence of integrin heterodimer α2β1 on cell surface of cholangiocytes or hepatocytes (x-axis, counts; y-axis, FITC intensity). Shift of gray target curve from black isotype curve demonstrated presence of integrin. C: presence of α2β1 subunit in cholangiocytes as demonstrated by immunohistochemistry. Cholangiocyte cell-surface staining for α2β1 using a FITC labeled antibody directed against α2-integrin (magnification ×40). No staining was noted in H2.35 cells.
The Role of α2β1 In Vitro
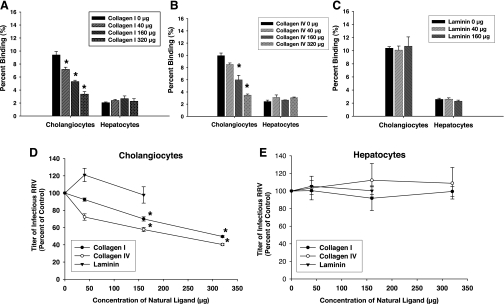
With the finding that cholangiocytes but not hepatocytes express the α2β1-integrin, we determined whether this integrin could be associated with the ability of RRV to attach to cholangiocytes. Cells were pretreated with increasing doses of type I human collagen, type IV mouse collagen, and laminin, natural ligands to α2β1, before exposure with RRV. Type I and IV collagen were both tested because they are natural ligands for α2β1 but are derived from different species. Type I collagen has a higher affinity for the α2β1-integrin (35). Pretreatment with both collagens resulted in a dose-dependent reduction in the ability of RRV to bind to mCl cells (Fig. 2, A and B). Neither affected RRV binding to the H2.35 cells. At the highest dose of collagen (320 μg/ml), RRV binding was reduced by an average of 71% (Fig. 2A). Higher concentrations could not be used as collagen precipitated out of solution. Laminin had no effect on RRV attachment to either cell line (Fig. 2C).
Fig. 2.
Blocking assays using natural ligands to α2β1-integrin. A: type I collagen. Cholangiocytes and hepatocytes were pretreated with increasing amounts of type I collagen followed by attachment assays with rhesus rotavirus (RRV; *P < 0.05 vs. serum-free media). B: type IV collagen. Cholangiocytes and hepatocytes were pretreated with increasing amounts of type IV collagen followed by attachment assays with RRV (*P < 0.05 vs. serum-free media). C: mouse laminin. Cholangiocytes and hepatocytes were pretreated with increasing amounts of laminin followed by attachment assays that revealed no effect on ability of RRV to bind to either cell type. D: viral yield in cholangiocytes following blocking with natural ligands. Cholangiocytes were pretreated with type I collagen, type IV collagen, and laminin followed by RRV inoculation performed at 4°C. Cells were washed and then allowed to warm to 37°C for one replication cycle (18 h). Yield of virus was measured by FFA and represented as %control, which showed that cholangiocytes pretreated with type I and IV collagen produced less virus compared with nonblocked RRV-infected cells (*P < 0.05). Pretreatment with laminin produced no reduction in viral yield in cholangiocytes. E: viral yield in hepatocytes following blocking with natural ligands. Hepatocytes, following similar treatment to cholangiocytes, expressed no reduction in viral yield after pretreatment with type I collagen, type IV collagen, and laminin.
The yield of replication-competent virus following blockade was reduced in a dose-dependent fashion following pretreatment with type I and IV collagen. A 50% decrease in viral yield was observed at the highest concentration of collagen (Fig. 2D). Laminin had no effect on viral replication in mCl or H2.35 cells (Fig. 2, D and E).
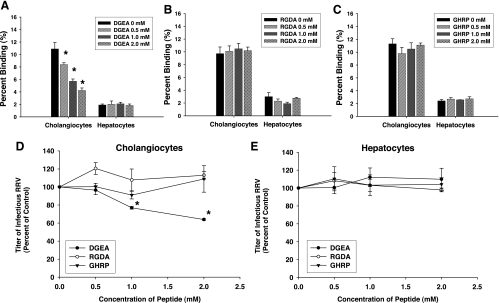
Previous studies have shown that the sequence of peptides found within rotavirus VP4 protein that binds to the α2β1-integrin consists of the amino acids DGE. This sequence is the collagen-binding domain for the α2β1 integrin (33). A synthetic peptide, DGEA, was generated. Pretreatment of cholangiocytes with increasing concentrations of DGEA followed by RRV attachment was tested. Blocking with this peptide resulted in a significant reduction of RRV binding to mCl cells (Fig. 3A). DGEA had no effect on H2.35 cells. At the highest doses of DGEA (2 mM), RRV attachment was decreased by 32% in mCl cells. To ensure the specificity of these results, we tested the effect of two other short peptide sequences, RGDA and GHRP. RGD is the laminin binding domain to the α2β1-integrin (28), whereas GHRP served as a negative peptide control. When these synthetic peptides RGDA and GHRP were used as blocking agents, they had no effect on RRV binding to cholangiocytes or hepatocytes (Fig. 3 B and C).
Fig. 3.
Blocking assays using synthetic peptides. A: aspartic acid-glycine-glutamic acid-alanine (DGEA). Cholangiocytes and hepatocytes were pretreated with increasing amounts of DGEA followed by attachment assays with RRV (*P < 0.05 vs. serum-free media). B: arginine-glycine-aspartic acid-alanine (RGDA). Cholangiocytes and hepatocytes were pretreated with increasing amounts of RGDA followed by attachment assays with RRV. C: glycine-histidine-arginine-proline (GHRP). Pretreatment of cholangiocytes and hepatocytes with increasing amounts of GHRP followed by attachment assays revealed no effect on ability of RRV to bind to either cell type. D: viral yield in cholangiocytes following blocking by peptide sequences. Cholangiocytes were pretreated with peptide sequences DGEA, RGDA, and GHRP followed by RRV inoculation performed at 4°C. Cells were washed and then allowed to warm to 37°C for 1 replication cycle (18 h). Yield of virus was measured by FFA and was represented as %control, which showed that cholangiocytes pretreated with peptide sequence DGEA produced less virus compared with nonblocked RRV-infected cells (*P < 0.05). Pretreatment with sequences RGDA and GHRP both produced no reduction in viral yield in cholangiocytes. E: viral yield in hepatocytes following blocking with peptide sequences. Hepatocytes, following similar treatment to cholangiocytes, expressed no reduction in viral yield after pretreatment with peptide sequences DGEA, RGDA, or GHRP.
The cholangiocytes blocked with 2 mM DGEA produced 52% less virus than control (Fig. 3D). The peptides RGDA and GHRP had no effect on replication yield in either mCl or H2.35 cells (Fig. 3, D and E).
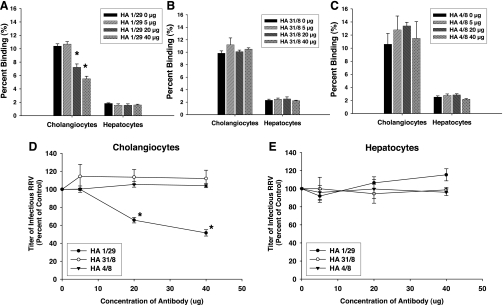
Blocking assays using a monoclonal antibody directed at the α2-subunit confirmed the effect of the natural ligands. An anti-α2 monoclonal antibody, Ha1/29, reduced the ability of RRV to attach to mCl cells by 47% (Fig. 4A). In contrast, an anti-α1-subunit monoclonal antibody Ha31/8 and an isotype control, Ha4/8, had no effect on viral attachment (Fig. 4, B and C). Viral yield after anti-α2 pretreatment of mCl cells also decreased significantly after one replication cycle (Fig. 4D), whereas Ha31/8 and Ha4/8 had no effect.
Fig. 4.
Blocking assays using monoclonal antibodies. A: Ha1/29. Cholangiocytes and hepatocytes were pretreated with increasing amounts of Ha1/29 followed by attachment assays with RRV (*P < 0.05 vs. serum-free media). B: Ha31/8. Cholangiocytes and hepatocytes were pretreated with increasing amounts of Ha31/8 followed by attachment assays with RRV. C: Ha4/8. Pretreatment of cholangiocytes and hepatocytes with increasing amounts of Ha4/8 followed by attachment assays revealed no effect on ability of RRV to bind to either cell type. D: viral yield in cholangiocytes following blocking by monoclonal antibodies. Cholangiocytes were pretreated with monoclonal antibodies Ha1/29, Ha31/8, and Ha4/8 followed by RRV inoculation performed at 4°C. Cells were washed and then allowed to warm to 37°C for 1 replication cycle (18 h). Yield of virus was measured by focus-forming unit (FFU) assay and is represented as %control, which showed that cholangiocytes pretreated with antibody Ha1/29 produced less virus compared with nonblocked RRV-infected cells (*P < 0.05). Pretreatment with antibodies Ha31/8 and Ha4/8 both produced no reduction in viral yield in cholangiocytes. E: viral yield in hepatocytes following blocking with monoclonal antibodies. Hepatocytes, following similar treatment to cholangiocytes, expressed no reduction in viral yield after pretreatment with the antibodies Ha1/29, Ha31/8, and Ha4/8.
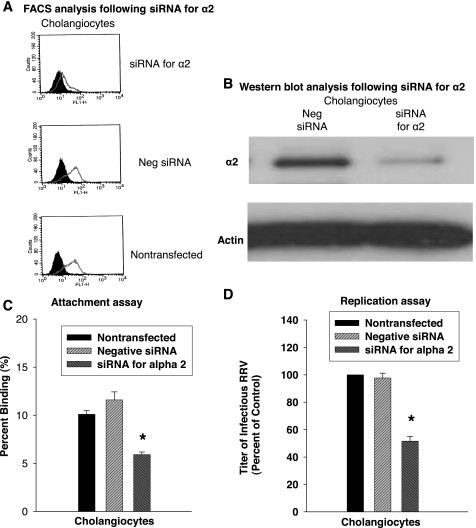
Although blocking assays with natural ligands reduced the ability of RRV to bind to the cholangiocyte, assays using ligands are not always specific. To precisely evaluate the role of α2β1, we suppressed the expression of the α2-subunit by using siRNA. mCl cells were transfected with siRNA against α2 or nontargeting siRNA (negative siRNA) control. Suppression of α2 expression was confirmed by flow cytometry (Fig. 5A) and Western blotting (Fig. 5B), demonstrating a nearly 70% downregulation of α2 expression. This suppression resulted in a 49% decrease in viral binding compared with cells treated with the nontargeting siRNA or to nontransfected cells during attachment assays (Fig. 5C). Infectivity assays demonstrated a 47% reduction in viral yield in α2-silenced cells when compared with mCl cells treated with nontargeted siRNA (Fig. 5D).
Fig. 5.
α2 RNA interference reduces rotavirus infection of cholangiocytes. A: FACS analysis for α2-integrin after RNA interference. A significant decrease in α2-protein expression was demonstrated by direct FACS analysis compared with nontargeted (negative) small interfering RNA (siRNA) and nontransfected controls. B: Downregulation of α2-protein in cholangiocytes after RNA interference. Western blotting for α2-protein demonstrated a significant reduction in protein amount in cells treated with siRNA directed against α2. C: binding to cholangiocytes after RNA interference against α2. Attachment assays performed on cholangiocytes transfected with siRNA against mouse α2 revealed a reduction in amount of RRV bound to the cells compared with both nontransfected cells and cells transfected with a nontargeted (negative) siRNA (*P < 0.05). D: viral yield after RNA interference directed at α2-subunit. A decrease in viral yield was seen (*P < 0.05).
Because mCl and H2.35 cells expressed the αvβ3-integrin, its role was tested in both cell lines by using a short peptide directed against the binding domain of the αvβ3 and siRNA directed against the αv-subunit. Pretreatment with the short peptide had no effect on rotaviral binding or replication in either cell line (see supplemental Fig. 2, A and B, available online at the American Journal of Physiology-Gastrointestinal and Liver Physiology website). Interestingly, siRNA against αv had no effect on viral binding to cholangiocytes but reduced by 25% replication yield (see supplemental Fig. 2, C and D, available online at the American Journal of Physiology-Gastrointestinal and Liver Physiology website).
The Role of α2β1 In Vivo
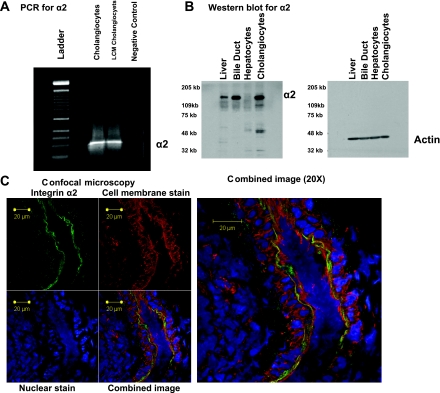
The in vitro results indicated that the α2β1-integrin played a role in cholangiocyte susceptibility to RRV infection. To determine whether it played a similar role in vivo, we surveyed the extrahepatic biliary tissue for mRNA for the α2-subunit. RT-PCR performed on extracts obtained from liver and extrahepatic biliary tissue revealed the presence of mRNA for α2 (data not shown). Because extrahepatic biliary tissue contained a variety of cells, LCM was used to isolate the biliary epithelium and RT-PCR was performed on extracted mRNA. Within these samples, mRNA for the α2-subunit was detected (Fig. 6A). Western blot analysis was performed on liver and extrahepatic biliary tissue homogenates for the presence of α2-protein (Fig. 6B). The α2 protein was detected in the extrahepatic biliary tree, with less expression found in liver samples. Confocal immunohistochemical analysis demonstrated the presence of the α2β1-integrin on the apical surface of the biliary epithelium (Fig. 6C, 1 and 2).
Fig. 6.
Localization in vivo in murine liver of α2β1-integrin. A: laser capture microdissection (LCM) for α2-subunit. LCM was used to isolate cholangiocytes, and RT-PCR for α2 was performed on cDNA generated from these cells. Agarose gel (2%) containing reaction products revealed a single band at expected length. B: Western blot analysis for α2-subunit. Extracts from liver, extrahepatic bile duct, H2.35 cells (negative control), and mCl cells in vitro (positive control) were probed for α2-protein. α2-Subunit was present in extrahepatic biliary and mCl samples, minimally expressed in liver samples, and absent in H2.35 cells. Actin was used as a loading control. C: immunohistochemistry for α2-subunit. Immunostaining of common bile duct from a 7-day-old pup visualized with a Zeiss LSM-510 confocal microscope with a ×40 water objective. Top left: FITC anti-mouse α2-antibody amplified by Cy2 anti-FITC demonstrated staining of biliary epithelium along apical side of lumen, shown here in green. Top middle: rhodamine-conjugated wheat germ agglutinin stained cell membranes, shown here in red. Bottom left: cell nuclei in blue were stained with TO-PRO-3. Bottom middle: overlay of all 3 images. Right: image at bottom middle magnified ×20 where α2β1-integrin is localized to apical surface of cholangiocyte.
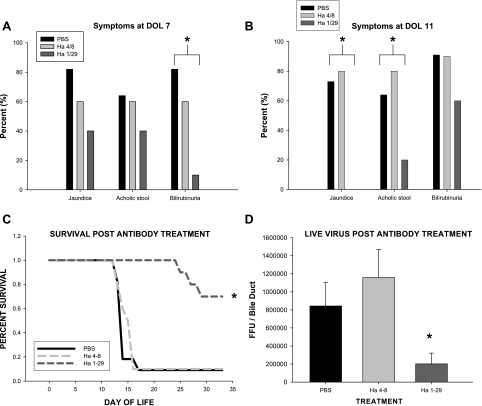
To determine whether α2β1 played a role in the murine model of biliary atresia, newborn BALB/c mice were pretreated with monoclonal antibody against α2 followed by infection with RRV. The clinical manifestations of bile duct obstruction in mice pretreated with anti-α2 antibody was markedly reduced at postinfection days 7 and 11, the typical time at which infected mice exhibit the maximal amount of symptomatology (Fig. 7, A and B). Antibody at a dose of 100 μg/injection decreased mouse mortality by >60% following RRV infection (Fig. 7C). The amount of live virus found within the bile duct after pretreatment with α2-antibody was significantly reduced when compared with isotype and saline controls (Fig. 7D).
Fig. 7.
Effect on murine model of biliary atresia of monoclonal antibody inhibition of α2β1. A: symptoms of pups at day of life (DOL) 7 pretreated with Ha1/29, Ha4/8, or saline followed by inoculation with RRV. Mice pretreated with Ha1/29 had lower incidence of jaundice, acholic stool, and bilirubinuria than mice pretreated with Ha4/8 or saline at DOL 7 (n = 10–11 pups/group; *P < 0.05 by χ-square analysis among groups). B: symptoms of pups at DOL 11 pretreated with Ha1/29, Ha4/8, or saline followed by inoculation with RRV. Mice pretreated with Ha1/29 continued to have lower incidence of jaundice, acholic stool, and bilirubinuria than mice pretreated with Ha4/8 or saline at a DOL 11 (*P < 0.05 by χ-square analysis). C: survival curve of newborn pups pretreated with Ha1/29, Ha4/8, or saline followed by inoculation with RRV. Survival after RRV infection was significantly higher in mice treated with monoclonal antibody directed against α2 (Ha1/29) compared with isotype control (Ha4/8) or negative control (n = 10–13 pups/group, *P < 0.05 by χ-square analysis). D: live virus found in extrahepatic biliary tract harvested from mice followed by inoculation with RRV. A significant reduction in live RRV was found in extrahepatic biliary samples harvested 7 days after viral infection in mice treated with monoclonal antibody directed against α2 (Ha1/29) compared with isotype control (Ha4/8) or negative control (PBS) (n = 5–8 bile ducts/group; *P < 0.05).
DISCUSSION
Perinatal infection with a virus has been proposed as a mechanism contributing to the pathogenesis of biliary atresia. Recent studies have shown that in the murine model of biliary atresia, RRV targets the biliary epithelium for infection, resulting in extrahepatic biliary obstruction (1, 31). The goal of the current study was to begin to define the mechanism by which RRV has tropism for the biliary epithelium. Using a novel in vitro model of RRV-cholangiocyte infection and a murine model of biliary atresia, we found that cell surface expression of the integrin α2β1 was an important determinant governing cholangiocyte vulnerability to RRV.
The establishment of an in vitro model using immortalized cholangiocytes and hepatocytes allowed us to dissect the basis for RRV tropism for cells of hepatobiliary origin. Both cell lines were derived from a BALB/c background, which was important because previous studies have shown that the murine model of biliary atresia is strain dependent, with BALB/c mice the most susceptible to RRV-induced biliary obstruction (30). When these two cell lines were exposed to RRV, it was found that yield of virus after a replication cycle was 33-fold greater in mCl than H2.35 cells. While the finding that cholangiocytes were susceptible to rotavirus infection was novel, the finding that hepatocytes were resistant was consistent with a previous study by Ciarlet et al. (6) who demonstrated similar results by using the hepatocyte cell line Hep G2. The differential susceptibility to infection between cholangiocytes and hepatocytes recapitulated the findings in the murine model of biliary atresia where RRV targets the biliary epithelial cell for infection.
The ability of a virus to infect a cell (viral tropism) is dependent on attachment to the cell surface, internalization, and replication using host intracellular machinery. Because previous studies have shown that viral tropism is governed in part by attachment (6, 22, 34), the finding that RRV attached to cholangiocytes sixfold better than hepatocytes was important. Recently, progress has been made in understanding how rotavirus binds to a cell. Coulson et al. (8) found that the rotavirus capsid protein VP4 contained the collagen peptide-binding sequence DGE, which binds to the α2β1-integrin in MA104 and Caco-2 cells. Expression of the α2β1-integrin conferred vulnerability to rotavirus infection, suggesting that this integrin served as a rotavirus receptor. Subsequent studies identified the integrins αxβ2, αvβ3, and α4β1 as other surface proteins that play a role in rotavirus attachment (13, 14, 16, 21). A survey of the cholangiocyte and hepatocyte cell surface using FACS analysis revealed that cholangiocytes uniquely expressed α2β1, providing a potential mechanistic basis for RRV tropism to cholangiocytes.
In vitro blocking assays supported a role for α2β1 as a determinant governing cholangiocyte vulnerability to RRV infection. Collagen and DGEA reduced the ability of RRV to attach to the cholangiocytes. Because H2.35 cells did not express this integrin, pretreatment with these proteins had no effect. Laminin, another natural ligand of α2β1, had no effect on RRV binding to cholangiocytes or hepatocytes. The binding site of collagen to α2β1 uses the peptide sequence DGE, whereas the binding site of laminin to α2β1 uses the sequence RGD (28). The DGE sequence is present within the peptide sequence of the RRV VP4 protein found on the outer layer of the rotaviral capsid (8). In contrast, the RGD sequence is absent, thus providing a basis for the differential effects of the natural ligands. Consistent with this, blocking assays with the peptide sequence RGDA had no effect of RRV attachment to the cholangiocyte.
The blocking assays using ligands provided important information. However, to precisely determine the role of α2β1, blocking studies using a monoclonal antibody directed against the α2-subunit and RNA suppression studies inhibiting the production of the α2-subunit were performed. Integrins are heterodimeric transmembrane proteins consisting of α- and β-subunits. The α2-subunit is only able to dimerize with the β1-subunit (19). Therefore, inhibiting α2 effectively reduces the expression of the α2β1-integrin. Both the monoclonal antibody-blocking assays and the RNA suppression studies confirmed the findings using natural ligands. Overall, the results from the attachment assays, FACS analysis, blocking assays, and RNA-suppression studies indicated that the expression of α2β1 was an important determinant governing cholangiocyte susceptibility to RRV.
The role of the αvβ3-integrin was tested in vitro by using a short peptide that blocks its binding site and siRNA against the αv-subunit. We found that suppression of αv had no effect on viral attachment, but it caused a small reduction in viral replication yield. This finding is consistent with recent studies (14, 22), where it was shown that the αvβ3-integrin served as an important determinant in viral entry but not initial attachment to the cell surface. Attachment and entry are thought to be two separate but complimentary steps in the viral infectious cycle.
To examine whether the α2β1-integrin played a role regulating RRV tropism in vivo, we localized the integrin to cholangiocytes by using RT-PCR on LCM-captured cells, Western blot analysis, and immunohistochemistry. Pretreatment of newborn mice with monoclonal antibody directed against α2 confirmed that the integrin played a role in RRV-induced murine biliary atresia. The clinical manifestations of biliary obstruction were significantly diminished, survival was improved, and RRV titers in the extrahepatic bile duct were reduced in mice treated with the α2-antibody. These novel results indicate that inhibition of rotavirus attachment may be used to prevent clinical manifestations of disease.
The apical location of the α2β1-integrin as indicated by confocal microscopy raises interesting questions as to how the virus targets the biliary epithelial cell for infection. Its apical location suggests that for infection of the cholangiocyte to occur, RRV must gain access to the luminal portion of the extrahepatic biliary tract. In previous work from our laboratory, we showed that following RRV intraperitoneal inoculation, a systemic viremia takes place with rotavirus protein found not only in the liver but also in the intestine, brain, spleen, and kidney (1). The systemic viremia is consistent with the recent human-based findings, where it has been shown that during rotavirus infection virus can be detected in the blood (4). How rotavirus reaches the biliary lumen remains to be determined.
Although there was a correlation between the results obtained in vitro and in vivo, the precise mechanism by which the monoclonal antibody reduced the manifestations in vivo may differ from that found in vitro. We showed that the α2β1-integrin was present on the cholangiocyte cell surface, but it can also be found on other cells. Systemic inhibition of α2β1 cannot be excluded. To directly test the role of the α2β1-integrin at the biliary epithelial level, studies would need to be performed in a conditional α2β1 knockout mouse. An α2β1-integrin knockout has been generated but in a strain of mouse not susceptible to RRV-induced biliary atresia (5). Transfer of the knockout to the BALB/c background can be accomplished, but selective inhibition at the biliary epithelial level would still be needed.
The results in vitro and in vivo implicate the expression of α2β1 on the cell surface as a determinant governing susceptibility to viral infection; however, in both models blockade of the integrin did not completely abolish susceptibility to RRV infection. Rotavirus entry into a host cell is a complex process using interactions with surface molecules as the initial basis for viral attachment (2, 22). Sialic acid residues on cell-surface glycoproteins and other proteins may contribute to the mechanistic basis for how RRV infects a cholangiocyte (7, 20). In addition, integrins are transmembrane heterodimers that activate intracellular signaling pathways (11, 19). The role of the α2β1-integrin in cholangiocyte susceptibility to RRV infection may extend beyond simple cell-surface attachment.
Although the results of the current study were performed in a murine model, it remains to be determined whether these results contribute to the understanding of the disease process that occurs in infants. A viral basis of biliary atresia in infants remains the most plausible and well-supported etiology, and the model described most closely replicates both the morphological findings and the systemic inflammatory response seen in infants. Interestingly, α2β1 has been found on biliary epithelial cells in liver samples obtained from patients (36, 37). Because infection with rotavirus in children can result in systemic viremia (4), it is possible that a perinatal rotavirus infection could result in biliary epithelial injury in an infant, providing the basis for why rotavirus has been detected within the liver of children affected with biliary atresia (29). By establishing a mechanistic basis by which a virus contributes to the pathogenesis of biliary atresia, we hope to identify therapeutic alternatives that could ultimately change the treatment strategies used for this devastating childhood disorder.
DISCLOSURES
P. Rennert and P. Weinreb are employed by Biogen Idec, which manufactures the monoclonal antibodies Ha1/29, Ha4/8, and Ha31/8.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant K08-DK-728858-01 to G. Tiao.
Acknowledgments
Work from this manuscript was presented, in part, at the American Association for the Study of Liver Diseases Liver Meeting 2006 and received the American Liver Foundation Pediatric Research Abstract Award.
We thank Dr. Jorge Bezerra for critical review of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen SR, Jafri M, Donnelly B, McNeal M, Witte D, Bezerra J, Ward R, Tiao GM. Effect of rotavirus strain on the murine model of biliary atresia. J Virol 81: 1671–1679, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias CF, Isa P, Guerrero CA, Mendez E, Zarate S, Lopez T, Espinosa R, Romero P, Lopez S. Molecular biology of rotavirus cell entry. Arch Med Res 33: 356–361, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology 23: 1682–1692, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Blutt SE, Kirkwood CD, Parreno V, Warfield KL, Ciarlet M, Estes MK, Bok K, Bishop RF, Conner ME. Rotavirus antigenaemia and viraemia: a common event? Lancet 362: 1445–1449, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161: 337–344, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciarlet M, Crawford SE, Cheng E, Blutt SE, Rice DA, Bergelson JM, Estes MK. VLA-2 (alpha2beta1) integrin promotes rotavirus entry into cells but is not necessary for rotavirus attachment. J Virol 76: 1109–1123, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciarlet M, Crawford SE, Estes MK. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J Virol 75: 11834–11850, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulson BS, Londrigan SL, Lee DJ. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc Natl Acad Sci USA 94: 5389–5394, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czech-Schmidt G, Verhagen W, Szavay P, Leonhardt J, Petersen C. Immunological gap in the infectious animal model for biliary atresia. J Surg Res 101: 62–67, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Domiati-Saad R, Dawson DB, Margraf LR, Finegold MJ, Weinberg AG, Rogers BB. Cytomegalovirus and human herpesvirus 6, but not human papillomavirus, are present in neonatal giant cell hepatitis and extrahepatic biliary atresia. Pediatr Dev Pathol 3: 367–373, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Farndale RW, Siljander PR, Onley DJ, Sundaresan P, Knight CG, Barnes MJ. Collagen-platelet interactions: recognition and signalling. Biochem Soc Symp: 81–94, 2003. [DOI] [PubMed]
- 12.Glaser JH, Balistreri WF, Morecki R. Role of reovirus type 3 in persistent infantile cholestasis. J Pediatr 105: 912–915, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Graham KL, Halasz P, Tan Y, Hewish MJ, Takada Y, Mackow ER, Robinson MK, Coulson BS. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. J Virol 77: 9969–9978, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrero CA, Mendez E, Zarate S, Isa P, Lopez S, Arias CF. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc Natl Acad Sci USA 97: 14644–14649, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemler ME, Huang C, Takada Y, Schwarz L, Strominger JL, Clabby ML. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem 262: 11478–11485, 1987. [PubMed] [Google Scholar]
- 16.Hewish MJ, Takada Y, Coulson BS. Integrins alpha2beta1 and alpha4beta1 can mediate SA11 rotavirus attachment and entry into cells. J Virol 74: 228–236, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jafri M, Donnelly B, McNeal M, Ward R, Tiao G. MAPK signaling contributes to rotavirual induced cholangiocyte injury and viral replication. Surgery 142: 192–201, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Jevon GP, Dimmick JE. Biliary atresia and cytomegalovirus infection: a DNA study. Pediatr Dev Pathol 2: 11–14, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Jung SM, Moroi M. Activation of the platelet collagen receptor integrin alpha(2)beta(1): its mechanism and participation in the physiological functions of platelets. Trends Cardiovasc Med 10: 285–292, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Kuhlenschmidt TB, Hanafin WP, Gelberg HB, Kuhlenschmidt MS. Sialic acid dependence and independence of group A rotaviruses. Adv Exp Med Biol 473: 309–317, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Londrigan SL, Graham KL, Takada Y, Halasz P, Coulson BS. Monkey rotavirus binding to alpha2beta1 integrin requires the alpha2 I domain and is facilitated by the homologous beta1 subunit. J Virol 77: 9486–9501, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez S, Arias CF. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol 12: 271–278, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Mack CL The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Semin Liver Dis 27: 233–242, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDiarmid S Liver transplantation. The pediatric challenge. Clin Liver Dis 4: 879–925, 2000. [DOI] [PubMed] [Google Scholar]
- 25.McNeal MM, Broome RL, Ward RL. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology 204: 642–650, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Morecki R, Glaser JH, Cho S, Balistreri WF, Horwitz MS. Biliary atresia and reovirus type 3 infection. N Engl J Med 307: 481–484, 1982. [DOI] [PubMed] [Google Scholar]
- 27.Petersen C, Biermanns D, Kuske M, Meyer-Junghanel L, Mildenberger H. New aspects in a murine model for extrahepatic biliary atresia. J Pediatr Surg 32: 1190–1195, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Pfaff M, Gohring W, Brown JC, Timpl R. Binding of purified collagen receptors (alpha 1 beta 1, alpha 2 beta 1) and RGD-dependent integrins to laminins and laminin fragments. Eur J Biochem 225: 975–984, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Riepenhoff-Talty M, Gouvea V, Evans MJ, Svensson L, Hoffenberg E, Sokol RJ, Uhnoo I, Greenberg SJ, Schakel K, Zhaori G, Fitzgerald J, Chong S, el-Yousef M, Nemeth A, Brown M, Piccoli D, Hyams J, Ruffin D, Rossi T. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis 174: 8–15, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, Ogra PL. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res 33: 394–399, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Shivakumar P, Campbell KM, Sabla GE, Miethke A, Tiao G, McNeal MM, Ward RL, Bezerra JA. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest 114: 322–329, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol RJ, Mack C. Etiopathogenesis of biliary atresia. Semin Liver Dis 21: 517–524, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem 266: 7363–7367, 1991. [PubMed] [Google Scholar]
- 34.Triantafilou K, Takada Y, Triantafilou M. Mechanisms of integrin-mediated virus attachment and internalization process. Crit Rev Immunol 21: 311–322, 2001. [PubMed] [Google Scholar]
- 35.Tulla M, Pentikainen OT, Viitasalo T, Kapyla J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J. Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J Biol Chem 276: 48206–48212, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Volpes R, van den Oord JJ, Desmet VJ. Distribution of the VLA family of integrins in normal and pathological human liver tissue. Gastroenterology 101: 200–206, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Volpes R, van den Oord JJ, Desmet VJ. Integrins as differential cell lineage markers of primary liver tumors. Am J Pathol 142: 1483–1492, 1993. [PMC free article] [PubMed] [Google Scholar]