Abstract
Lasp-1 (LIM and SH3 domain protein 1) is a multidomain actin-binding protein that is differentially expressed within epithelial tissues and brain. In the gastric mucosa, Lasp-1 is highly expressed in the HCl-secreting parietal cell, where it is prominently localized within the F-actin-rich subcellular regions. Histamine-induced elevation of parietal cell [cAMP]i increases Lasp-1 phosphorylation, which is correlated with activation of HCl secretion. To determine whether Lasp-1 is involved in the regulation of HCl secretion in vivo, we generated a murine model with a targeted disruption of the Lasp-1 gene. Lasp-1-null mice had slightly lower body weights but developed normally and had no overt phenotypic abnormalities. Basal HCl secretion was unaffected by loss of Lasp-1, but histamine stimulation induced a more robust acid secretory response in Lasp-1-null mice compared with wild-type littermates. A similar effect of histamine was observed in isolated gastric glands on the basis of measurements of accumulation of the weak base [14C]aminopyrine. In addition, inhibition of the acid secretory response to histamine by H2 receptor blockade with ranitidine proceeded more slowly in glands from Lasp-1-null mice. These findings support the conclusion that Lasp-1 is involved in the regulation of parietal HCl secretion. We speculate that cAMP-dependent phosphorylation of Lasp-1 alters interactions with F-actin and/or endocytic proteins that interact with Lasp-1, thereby regulating the trafficking/activation of the H+, K+-ATPase (proton pump).
Keywords: LIM and SH3 domain protein, transmission electron microscopy, [14C]aminopyrine, bone mineral density
lasp-1 [LIM and SH3 domain protein 1 (39)] is a highly conserved protein that is differentially expressed in certain F-actin-rich cells in peripheral tissues as well as in the brain (8, 9, 19, 35). The protein was first characterized as pp40, a 40-kDa phosphoprotein in the HCl-secreting gastric parietal cell, where elevation of intracellular cAMP led to increased phosphorylation (4). The gene encoding pp40 was later cloned and identified as Lasp-1 (9), an amplified gene product in some breast carcinomas (39, 40).
In addition to SH3 and LIM domains, Lasp-1 possesses two internal nebulin-like repeats (6, 34), signature motifs of the nebulin family of actin-binding proteins (6). Lasp-1 binds to F-actin in vitro in a phosphorylation-dependent manner (2, 6, 35) and is localized within dynamic F-actin-rich structures in epithelial cells and brain (9, 22, 28, 33). Lasp-1 is also present in focal adhesions, filopodia, and the leading edges of lamellipodia in fibroblasts and cell lines (6, 19, 35). Numerous associations between Lasp-1 and other proteins, mainly via the SH3 and actin-binding domains, have been reported including the endocytic proteins, dynamin 2 (31), Huntington interacting Protein 1 Related (Hip1r), and clathrin (5), and the focal adhesion proteins, zyxin (19, 21) and krp1 (36).
The physiological functions of Lasp-1 are not well characterized. There is evidence from cell lines linking the protein to cell migration, differentiation, and proliferation (12, 13, 15, 23, 36, 37). Lasp-1 may also play a regulatory role in cAMP-dependent membrane restructuring activities linked to ion transport (8). Within the gastric mucosa, histamine H2-receptor activation elevates cAMP and increases Lasp-1 phosphorylation with a time course that mirrors the initiation of parietal cell HCl secretion (4, 8, 9).
The parietal cell is the prototypical model of membrane recruitment and recycling (10). When the cell is not actively secreting HCl, the proton pump (H+, K+-ATPase) is sequestered within cytoplasmic tubulovesicles, which, upon stimulation, fuse with apically directed, intracellular canalicular membrane. This fusion event serves to translocate the proton pump to F-actin-rich microvilli, where it becomes active (10). Elevation of cAMP also induces the partial translocation of Lasp-1 to the canalicular region (8). The localization of dynamin, Hip1r, and clathrin within this region (5, 16, 29–31) and the potential association of these proteins with Lasp-1 further suggest that Lasp-1 serves as an adaptor protein to modulate interactions between the actin cytoskeleton and endocytic machinery. The goal of this study was to disrupt the Lasp-1 gene in vivo to determine 1) whether Lasp-1 is involved in the regulation of energy-dependent proton secretion and 2) whether loss of Lasp-1 affects growth or other developmentally related functions that rely on cell migration/differentiation/proliferation.
MATERIALS AND METHODS
Animals.
All animal experimental and breeding protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Medical College of Georgia. C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Antibodies and fluorescent probes.
Monoclonal anti-Lasp-1, clone 8C6, was produced and characterized as previously described for clone 3H8 (8, 16, 24). Rabbit polyclonal anti-Lasp-2 was a gift from Dr. Akira Terasaki, Chiba University, Chiba, Japan. Other antibodies and fluorescent probes were obtained commercially from the following sources: anti-Hip1r (clone #44), dynamin 1 (C-18, an antibody that recognizes all dynamin isoforms), and Clathrin heavy chain, from BD Transduction Labs; anti-zyxin, from ProteinTech Group, Chicago, IL; anti-H+, K+-ATPase, α-subunit (clone HK 12.18), from Calbiochem, La Jolla, CA; anti-β-actin, from Sigma-Aldrich, St. Louis, MO; cyanine (Cy)-5-tagged donkey anti-mouse IgG, from Jackson Immunoresearch Laboratories, Westgrove, PA; Alexa 568 phalloidin and Sytox Green, from Molecular Probes/Invitrogen, Eugene, OR.
Protein analyses.
Protein was quantified by using a fluorescence-based protein assay kit (Quant-iT, Molecular Probes/Invitrogen). Western blotting with enhanced chemiluminescence (ECL) detection and quantitation with a Syngene GeneGnome system was performed as previously described (6, 7).
Southern blots.
DNA was extracted from tail snips of 8–10-day-old mice with the use of standard molecular biological procedures (27). For Southern blotting, 10–15 μg of solubilized tail DNA was digested with 3–4 μl of 40 U/μl of EcoR I (Roche) in Buffer H (50 mM Tris·HCl, 100 mM NaCl, 10 mM MgCl2, and 1 mM dithiothreitol). Digestion products were resolved on 0.8% agarose gels and transferred to Hybond-N+ membranes (Amersham, Buckinghamshire, UK) with 1.5 M NaCl and 0.5 M NaOH. After neutralizing the membranes with 1.5 M NaCl and 0.5 M Tris, DNA was imaged under short wavelength UV light and then crosslinked (UV Stratalinker, 1,200 joules). For blotting, membranes were prehybridized by rinsing with 2× SSC buffer and incubation at 65°C (4 h) in prewarmed Church buffer (0.17% H3PO4, 0.25M Na2HPO4, 1% BSA, 1 mM EDTA, and 7% SDS) and were then incubated in Hybaid roller bottles with [α-32P] dCTP (6,000 Ci/nmol, prepared using the Amersham Ready-To-Go kit) in 25 ml of the same buffer (65°C, overnight). After sequential washes (30-min intervals, 65°C with 2X SSC, 1X SSC, 0.2× SSC containing 0.1% SDS throughout), blots were analyzed with a Phosphoimager and were then exposed to X-ray film.
Library screening for Lasp-1 genomic DNA.
A mouse genomic λ-Dash II phage library (a gift from Dr. Brian Condie, University of GA) was screened with a 500-bp Lasp-1 cDNA probe (from ATG start site). Three clones that contained DNA encompassing the complete open reading frame for Lasp-1 were identified; however, each had a gap in the coding sequence, an arrangement reminiscent of inactive, processed pseudogenes. This sequence was entered into the GenBank database (accession number, AY302250). A pBeloBAC11 129/SvJ embryonic stem (ES) cell library (Incyte Genomics, Wilmington, DE) was used to isolate genomic DNA encoding for exons 1 and 2 plus noncoding DNA upstream of these exons. A positive clone was identified and partially mapped by restriction endonuclease digestion to verify correct intron/exon structure corresponding to the sequence of GenBank accession number AL596446 (mouse chromosome 11).
Design and production of the targeting vector.
To disrupt exon 1, which encodes the first half of the LIM domain, a PCR-based approach using the bacterial artificial chromosome clone as a template was employed (Supplemental Fig. 1, A–D). (Supplemental material for this article is available online on the American Journal of Physiology Gastrointestinal Physiology website.) First, a 2,812-bp fragment upstream of exon 1, with flanking Not I and Spe I sites corresponding to positions 75,378 to 78,188 in the Lasp-1 mouse genomic sequence (AL596446.11), was amplified by PCR. Next, a 3,222-bp fragment downstream of exon 1 containing flanking Xho I and Sal I sites corresponding to positions 78,426 to 81,624 was generated. 5′ and 3′ probes (229 and 277 bp, respectively) corresponding to positions 74,349–74,577 and 81,674–81,950 were amplified for ES cell screening. Finally, a 1,738-bp insert adjacent to the downstream Lasp-1 homology domain, flanked by Sal I sites and encoding for the negative selection marker, diphtheria toxin-A (DT-A), was PCR amplified from DT-A-pUC18.
PCR products were cut with the appropriate enzymes and progressively ligated along with the pACN cloning vector (Bgl II cut sites; AF169416, a gift from Dr. Kirk R. Thomas, University of Utah) into Bluescript KS−, with transformation into JM109 bacteria as outlined in Supplemental Fig. 1. The pACN vector contains a self-excision cassette with a testes-specific promoter from the angiotensin-converting enzyme gene that drives expression of the Cre-recombinase gene, t-ACE-Cre, which is linked to the selectable marker gene, Neor. LoxP sites flank the two genes. This design allows for deletion of the DT-A marker in ES cells and the deletion of the Neor gene (floxed neo) in the mouse F1 generation (Supplemental Fig. 1D).
ES cell transfection, screening, and founder generation.
The targeting construct was linearized with Not I and electroporated into a 129SvJ ES cell line by the Transgenic Core Facility at Medical College of Georgia (MCG). One hundred and thirty-eight ES cell clones were isolated and screened by Southern blot hybridization with 3′ and 5′ probes.
PCR analyses and DNA quantitation.
Routine genotyping was performed with 50–100 ng mouse-tail DNA using an Invitrogen Thermal Ace DNA Polymerase kit (standard conditions, 30 cycles). Initially, DNA was extracted as described above. Subsequently, extraction was performed using DirectPCR (tail) lysis reagent for crude lysates (Viagen Biotech, Los Angeles, CA) per manufacturer's instructions. Sense and anti-sense primers for PCR reactions were as follows (see diagram in Fig. 1) : 5′ CAC ACT CGC GTC TGT TTC TCC AGC 3′; (corresponding to nucleotides 77,991-77,934 in the mouse genomic sequence); 5′ GCA CCT CTA ACC TCC TGC ACA CAC C 3′ (nucleotides 78,656-78,680). Quant-IT fluorescence-based assay kits (Molecular Probes) were used for DNA quantitation.
Fig. 1.
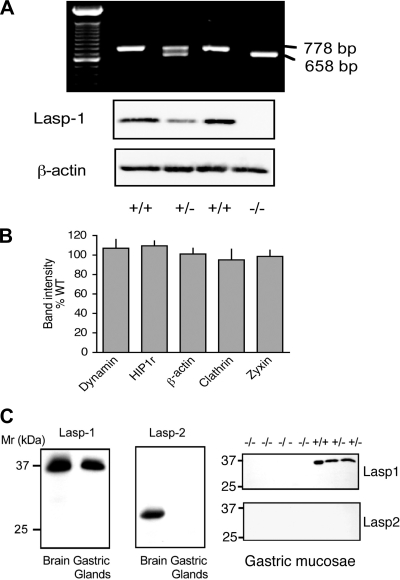
PCR and Western blot analyses of F6 generation mice. A: Top: PCR analysis of tail DNA from the same litter identifying two wild-type (WT) (+/+), one heterozygous (+/−), and one Lasp-1 knockout (KO) (−/−) mouse based on criteria described in materials and methods. 100-bp DNA ladder (Invitrogen) is included in the first lane. Bottom: Western blots of gastric mucosal extracts from the same mice showing differences in the Lasp-1 signal (8C6 antibody; 1:2,000 dilution) compared with the β-actin signal (1:5,000 dilution). B: quantitation of Western blot signals from gastric mucosal extracts from F6-F10 generation littermates obtained with antibodies directed against β-actin and several endocytosis-associated proteins. Antibody dilutions were as follows: dynamin, 1:1,000; Huntington Interacting Protein 1 Related (Hip1r), 1:1,000; β-actin, 1:5,000; clathrin heavy chain, 1:1,000; zyxin, 1:500. Values from Lasp-1-null mice expressed as a percentage of band densities from WT littermates. N = 5–9, P > 0.1. C: Western blot analyses of Lasp-1 and Lasp-2 expression in gastric mucosa and brain. Left: Lasp-1, which migrates on SDS PAGE gels with an apparent molecular ratio (Mr) of ∼37 kDa, was detected in both tissues. Lasp-2 (LIM-nebulette), which migrated with an apparent Mr of ∼30 kDa, was detected only in brain. Right: in littermate comparisons, no Lasp-1 expression was detected in gastric mucosal extracts from Lasp-1−/− mice. In other experiments, signals were quantified and found to be ∼50% lower in Lasp-1+/− compared with Lasp-1+/+ mice (not shown). Lasp-2 was not detected in any of these extracts.
Gastric acid secretion.
Mice (3–6 mo of age) were fasted overnight in metabolic cages with free access to water, anesthetized with Nembutal (50 mg/kg ip), and injected with a histamine H1-receptor antagonist (chlorpheniramine, 0.3 mg/kg sc) followed by histamine (10 mg/kg). Forty-five minutes after histamine injection, stomachs were ligated, excised, rinsed with 10 ml 0.9% NaCl, and titrated to pH 6.5 with 0.1–0.01 N NaOH. Gastric acid production was expressed as μeq HCl. In some experiments, gastric secretions were collected from anesthetized mice following esophageal ligation by gravity feed using a catheter (polyethylene tubing, 2 mm ID) inserted immediately below the pylorus. Before collection, stomachs were rinsed repeatedly with normal saline (20 × 500 μl) via the cannula. For acid secretory measurements, 300-μl aliquots of 0.9% NaCl were injected through the cannula and then withdrawn at 15-min intervals. Acid secretion was measured as described above and expressed as μeq HCl/15 min.
Isolation of gastric glands and measurement of acid secretory-related responses.
Gastric glands were isolated from 3-mo-old mice using a technique similar to that previously described for rabbit (3). In brief, stomachs were perfused in situ after snipping the liver by injecting 5–10 ml warm, oxygenated saline into the left ventricle. Stomachs were opened by an incision along the lesser curvature. After being rinsed in PBS, the mucosa was scraped away from the underlying muscularis and placed in a 2 1/2-in. polystyrene weigh dish (Fisher Scientfic, Pittsburg, PA) containing oxygenated PBS (room temperature). This step was repeated until mucosal scrapings from 8–10 mice were collected. Pooled mucosal scrapings were finely minced, placed in a medium composed of bicarbonate-free (BF)-DMEM/F12 (Sigma-Aldrich) plus 2% BSA and digested for 5 min with pronase (clostridiopeptidase A, Calbiochem, 0.25 mg/ml). Partially digested mucosal scrapings were collected by brief centrifugation and then digested with collagenase [Type II, Sigma-Aldrich (C-6885), 0.5 mg/ml] in BF-DMEM plus 2% BSA for 30 min. All digestion steps were performed in a 37°C water bath using a 200-ml round-bottomed flask (25–50-ml volumes). Flask contents were continuously stirred using a smooth, egg-shaped magnetic stir bar while gassing with 100% O2 with a Pasteur pipette suspended above the medium. Digestions were terminated by the addition of two volumes of BF-DMEM, 2% BSA containing 1 mM dithiothreitol. Gastric glands were collected in this medium by gravity settling in 15-ml conical tubes (2 × 10 min, room temperature) followed by a brief centrifugation (1×) before dilution at a ratio of ∼0.1 ml packed glands/20 ml medium. Acid secretion was assessed indirectly using the [14C]aminopyrine (AP) accumulation technique (3) in which 1.5–3 ml of diluted glands were incubated in capped 25-ml Erlenmeyer flasks (37°C, shaking water bath), and 0.25-ml aliquots were withdrawn for each sample.
Histology and immunofluorescent localization.
Paraffin-embedded sections were prepared after retrograde cardiac perfusion of Nembutal-anesthetized mice with PBS followed by fresh 10% formalin (Fisher Scientific). Stomachs were excised, opened along the greater curvature, rinsed, secured on dental wax, and stored flat in 10% formalin. Fixed tissues were processed and embedded in paraffin, sectioned, and stained with hematoxylin and eosin or toluidine blue by the Histology Core at MCG using standard histological procedures.
For transmission electron microscopy (TEM), stomachs were excised, placed in Tissue Tek compound, and then immediately frozen in isopentane cooled in dry ice/ethanol. Frozen fundic sections were processed in the Electron Microscopy Core at MCG. In brief, tissues were fixed with 2% glutaraldehyde, 2% paraformaldehyde in 0.1 M cacodylate buffer, pH 7.2, rinsed in 7% sucrose in 0.1 M cacodylate buffer, pH 7.2, postfixed in 4% osmium tetroxide, dehydrated in a graded acetone series, embedded in Epon/Araladite, and stained with 2% uranyl acetate and lead citrate. Sections (80–100 nm) were examined and photographed on a Jeol 1010 TEM.
Analyses with fluorescently tagged antibodies and probes were performed as previously described (7, 8). In brief, cells were fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and stained with various antibodies plus fluorescently tagged secondary antibodies (1:100 dilution), the nuclear stain, Sytox green (0.5 μM), and/or fluorescently tagged phalloidin (1:40 dilution) to label F-actin. Stained cells were mounted on slides with Prolong Gold anti-fade reagent (Invitrogen/Molecular Probes), and images were acquired with a Zeiss LSM 510 confocal microscope system using Meta 3.2 software with controls for fluorescent emission crossover and nonspecific binding (6, 7).
Osteoclast and bone analyses.
Bone marrow-derived osteoclasts were produced by in vitro differentiation of bone marrow macrophages from 6–8-wk-old C57BL/6J mice using macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANK-L) as previously described (43). Differentiation into osteoclasts was confirmed by tartrate-resistant acid phosphatase (TRAP) staining using a leukocyte acid phosphatase kit (Sigma-Aldrich).
Bone mineral densities (BMD) and mineral content (BMC) of femurs from 3-mo-old Lasp-1−/− and Lasp-1+/+ littermates were measured by dual-energy X-ray absorptiometry (PIXImus system; GE LUNAR, Madison, WI) as previously described (14). Dynamic changes in bone histomorphometry were analyzed by injecting mice with tetracycline hydrochloride (Sigma, 20 mg/kg ip). Nine days later mice were injected with demeclocycline (Sigma, 20 mg/kg ip) and euthanized 24 h later. Tibiae were fixed in 70% ethyl alcohol, dehydrated, embedded in methyl methacrylate, sectioned at 30 μm across the proximal third of the shaft (20), and then analyzed with a Zeiss Axioplan2 fluorescent microscope equipped with a SPOT camera. Total endosteal perimeter and labeled endosteal perimeter were measured, and forming endosteal perimeter was calculated as a percentage of noneroded, single-labeled endosteal perimeter/total endosteal perimeter × 100. The mineral apposition rate (μm/day) was determined by measuring the linear distance between the two labels and then dividing the mean width of the double labels by the interlabel time (10 days) (32).
To measure total section area, marrow area, cortical area, cortical thickness, trabecular number, trabecular thickness, and trabecular bone volume, tibiae were cut across the proximal third of the shaft and decalcified, along with L4 vertebrae, in 4% EDTA and were then washed, dehydrated, and embedded in paraffin. Cross sections (5 μm) were cut transversely with a rotary microtome through proximal tibiae and horizontally across lumbar vertebrae then stained with hematoxylin and eosin to yield crosssections that were used for measurements. Sections were also stained for TRAP (Sigma leukocyte acid phosphatase kit) to identify active osteoclasts. Osteoclasts were counted on the trabecular bone surface within a 0.50-mm2 area of interest and expressed as number of osteoclasts per bone surface. Section images were captured using a Leica compound DMLS microscope equipped with a digital camera and computer interface.
Statistical analyses and curve fitting.
All analyses were performed using Graph Pad Prism, version 4. Chi-squared analyses were used to analyze Mendelian frequencies. One- and two-way ANOVA were used to analyze multiple data sets in conjunction with Bonferroni's multiple comparison test. Curve fitting was performed using one-phase exponential decay or Boltzmann Sigmoidal equations as appropriate.
RESULTS
Generation of Lasp-1-null mice.
A targeting vector designed to disrupt exon 1 was produced as outlined in Supplemental Fig. 1. Screening of ES cell clones by Southern blotting identified three positive clones, one of which was used for blastocyst injection (Supplemental Fig. 2A). A single founder was obtained and bred to C57BL/6J females. Heterozygotes from these crosses were interbred to produce homozygous offspring. Subsequent generations were produced by alternate male/female outcrosses between Lasp-1-null mice and the C57BL/6J strain.
Southern blot and PCR analyses for a representative litter sired by the founder are shown in Supplemental Fig. 2B. Homozygote production and genotyping were confirmed by PCR and Western blotting (See Fig. 1A for a representative example). To determine whether loss of Lasp-1 led to the upregulation of endocytosis-associated proteins, dynamin, Hip1r and clathrin, and/or the focal adhesion protein zyxin, Western blots of gastric mucosal extracts from F6 generation littermates were analyzed. As shown in Fig. 1B, there was no significant difference in the level of expression of these proteins or β-actin. Because Lasp-1 and Lasp-2 (LIM-nebulette) are the only known nebulin family members that are expressed in nonmuscle tissues, we also probed Western blots of gastric mucosal extracts for Lasp-2 to determine whether this protein might be upregulated to compensate for the loss of Lasp-1. This did not appear to be the case because Lasp-2 was readily detected in brain extracts but not in gastric mucosal extracts from either wild-type or Lasp-1−/− mice (Fig. 1C).
General phenotypic analyses of Lasp-1−/− mice.
There was no overt mutant phenotype in the F2 or in subsequent generations (through F10) that were outcrossed to the C57BL/6J strain. Lasp-1-null mice were fertile, developed normally, and displayed normal Mendelian frequencies (F6 generation mice; 54.5% male, 45.2% female; N = 279; NS vs. expected 50/50 ratio; P > 0.1) with the expected wild-type:heterozygous:homozygous phenotypes (males, 36:85:32; females, 38:57:31; NS vs. expected 1:2:1 ratio). Although not extensively analyzed, no loss of coordination or aberrant behavior was detected on the basis of visual observations of littermates.
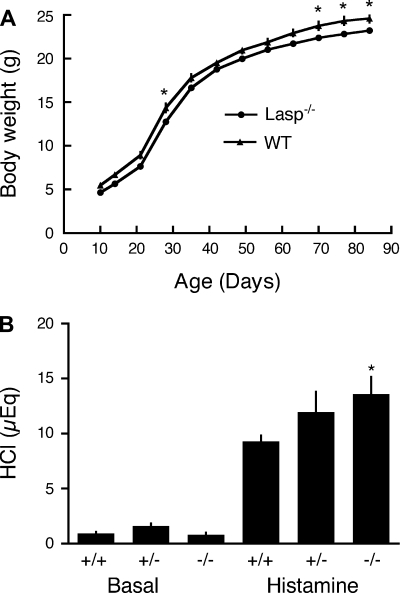
Comparisons of organ weights from matched male littermates revealed enlarged testes, slightly enlarged hearts, and smaller seminal vesicles in the F2 generation, but these differences were not evident in F6 generation mice (Table 1). Growth curve analyses in F6 generation mice revealed similar patterns of weight gain with slightly lower body weights in male Lasp-1-null mice (0.8–1.6 g) compared with wild-type littermates (Fig. 2A). Growth patterns were similar in matched female littermates with Lasp-1-null mice weighing 0.5–1.5 g less than their wild-type littermates but were less pronounced (0.3–0.5 g) at 10–12 wk of age. The small differences in body weights persisted in both sexes for at least 6 mo [males: wild-type, 24.1 ± 0.86; −/−, 22.8 ± 0.67 (N = 6–9). Females: wild-type 23.8 ± 2.32; Lasp-1−/− 21.4 ± 1.14 (N = 4–6)].
Table 1.
Comparison of weights (g) of representative organs from 3-mo-old F2 and F6 generation littermates
| Generation | Testes | Seminal Vesicles | Kidneys | Spleen | Stomach | Heart |
|---|---|---|---|---|---|---|
| F2 | 149±16.9* | 62.0±3.5† | 95.4±8.6 | 114.0±11.6 | 110.9±14.5 | 115.6±3.2* |
| F6 | 110.4±5.1 | 101.5±4.1 | 98.8±5.5 | ND | 97.6±7.7 | ND |
Values are means ± SE expressed as % of wild-type littermates. ND, not determined; F2, N = 5; F6, N = 5–8.
P < 0.05,
P < 0.001.
Fig. 2.
Growth curves and HCl secretion in F6 generation Lasp-1-null mice. A: matched male littermates, F6 generation. Body weights of Lasp-1-null mice were slightly lower (0.8–1.6 g) than their WT littermates. Values are means ± SE. N = 14 animals/group. Two-way ANOVA, significantly different by time, P < 0.001 and by genotype, P < 0.01; Bonferroni posttest, *P < 0.05 significantly different from WT. B: basal and histamine-stimulated gastric acid secretion in male littermates. Mice (3 mo of age) were fasted overnight then injected with the H1-receptor antagonist, chlorimpiramine (0.3 mg/kg), followed by saline (basal) or histamine (45 min, 10 mg/kg). Gastric contents were titrated to pH 6.5 with NaOH (B). +/+, WT; +/− heterozygotes; −/− Lasp-1 KO mice. *P < 0.05, N = 8–10.
In vivo gastric acid secretion and gastric mucosal histology.
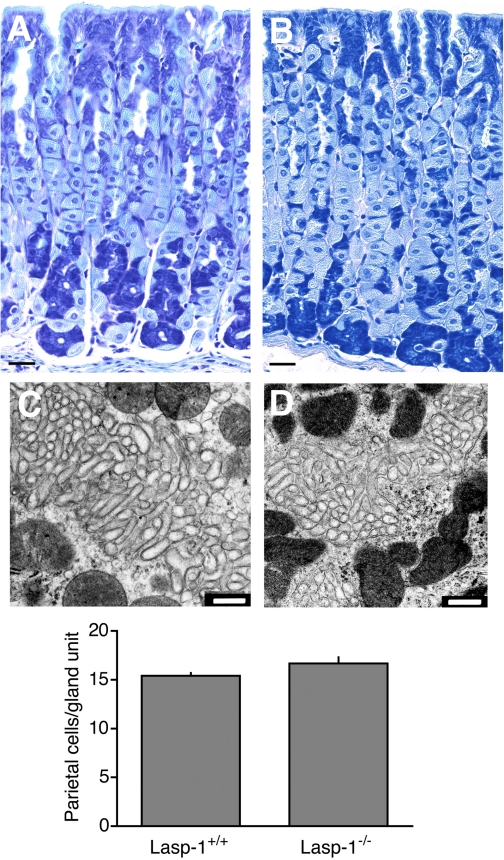
In preliminary experiments with F2 generation mice, the acid secretory response to histamine was more robust in Lasp-1 mice compared with wild-type controls and peaked more rapidly, but basal secretion was similar in the two groups (data not shown). Similar data were obtained with F6 generation mice in which HCl secretion was measured in unstimulated animals (basal secretion) and 45 min after histamine stimulation (Fig. 2B). Histamine-stimulated HCl secretion in heterozygotes was also elevated compared with wild-type littermates, but this upward trend did not attain statistical significance (Fig. 2B). The more robust acid secretory response in Lasp-1-null mice did not appear to be attributable to changes in the numbers of HCl-secreting parietal cells because the average number of parietal cells in glandular units were similar and there was no significant difference in the expression of H+, K+−ATPase on the basis of Western blot analyses of gastric mucosal extracts using anti-H+, K+−ATPase-α antibody at a 1:10,000 dilution (Lasp-1−/−, 96.8 ± 11.9% of wild-type littermates, N = 5). Histological analyses also revealed no overt differences in gastric mucosal morphologies in mice ranging from 1 to 12 mo of age (Fig. 3, A and B). On the basis of TEM analyses of samples for several different mice of both sexes, no differences in parietal cell morphologies were detected in Lasp-1-null mice compared with wild-type (Lasp-1+/+) littermates (See Fig. 3, C and D for representative images).
Fig. 3.
Histology, transmission electron microscopy (TEM) analyses, and parietal cell counts from gastric mucosal sections of 3 to 12-mo-old Lasp-1−/− and Lasp-1+/+ mice (A and B). Representative images of toluidine blue-stained gastric mucosal sections from Lasp-1−/− (A) and Lasp-1+/+ (B) male littermates (12 mo of age). Bars = 50 μm. C and D: representative TEM images showing intracellular canaliculi of parietal cells in gastric mucosae of histamine-stimulated mice. Three-mo-old Lasp-1+/+ (C) and Lasp-1−/− (D) littermates were injected as described in Fig. 2. Stomachs were processed 45 min later. Only the canalicular region, which is known to undergo changes in morphology in which F-actin-rich microvilli become elongated and cytoplasmic tubulovesicular numbers decrease, is shown. No differences in morphology were identified in other regions of the cells or in parietal cells from unstimulated controls. Bars = 500 nm. Graph: parietal cell numbers in gland units from Lasp-1+/+ and Lasp-1−/− male and female mice. Counts were performed on sections from 3-mo-old littermates: 1–3 sections/mouse, 5–25 glandular units counted/animal. No significant difference in parietal cell numbers was detected. N = 7 animals/group, P > 0.07.
In vitro analyses of parietal cell acid secretory responses.
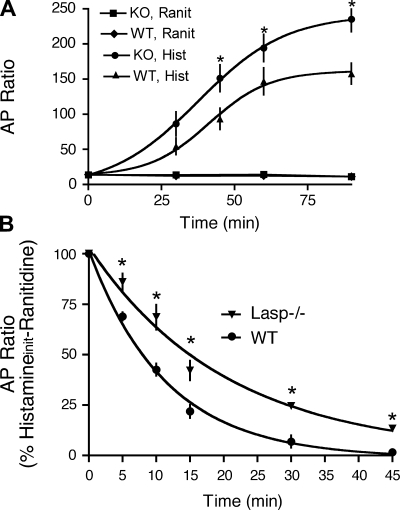
Isolated gastric glands were used to analyze acid secretory responses in Lasp-1-null mice (F6–F10 generations) in more detail. As shown in Fig. 4A, histamine-stimulated AP accumulation in glands from Lasp-1-null mice was significantly greater than in wild-type mice. In contrast, when glands were preincubated with the histamine H2-receptor antagonist ranitidine, basal AP accumulation was similar in the two groups. Thus these indirect in vitro measurements appeared to accurately reflect in vivo measurements of HCl secretion.
Fig. 4.
Aminopyrine (AP) accumulation in gastric glands isolated from WT and Lasp-1−/− mice. A: time courses of basal (ranitidine-inhibited) and histamine-stimulated AP accumulation. Ranitidine (Ranit, 50 μM) was added immediately before incubation. Histamine (Hist, 10 μM) was added to glands after a 10-min temperature equilibration at 37°C. Values shown are means ± SE, N = 5 independent experiments, 8–10 mice/experiment, *P < 0.05. B: time course of decline in histamine-stimulated AP accumulation upon addition of the H2-receptor antagonist, ranitidine. Drug concentrations were the same as in A. Values are means ± SE, N = 4–6 independent experiments, 8–10 mice/experiment, *P < 0.05. Average T1/2 values for ranitidine inhibition in Lasp-1−/− and Lasp-1+/+ glands were 14.1 and 7.9 min, respectively (P < 0.05).
Because there is evidence that Lasp-1 interacts with F-actin as well as with several endocytosis-associated proteins in vitro, including dynamin 2 and Hip1r, which are highly expressed in parietal cells and, like Lasp-1, are partially localized within the intracellular canalicular region (5, 6, 16, 31), we examined the rate of decline in histamine-stimulated AP accumulation after ranitidine addition. If Lasp-1 does play a role in modulating the function(s) of one or more of these proteins, we reasoned that this interaction might be involved in regulating the rate of proton pump retrieval from the canalicular membrane. Loss of this interaction might, therefore, affect this process. To assess this possibility, glands from several different groups of Lasp-1−/− and Lasp-1+/+ mice were incubated with histamine (10 μM) for 60 min followed by an excess of ranitidine (50 μM). AP accumulation was measured immediately before and at 5 to 15-min intervals after ranitidine addition. As shown in Fig. 4B, the average half-time for ranitidine inhibition was increased by 78% in the Lasp-1-null mice (14.1 min) compared with that for wild-type mice (7.9 min, P < 0.05 significantly different from Lasp-1-null mice). In addition, AP accumulation in glands from Lasp-1-null mice was not suppressed to the same level as that in wild-type mice within the 45-min sampling time (Fig. 4B). The combined data from all of our the experiments with isolated gastric glands, therefore, support a role for Lasp-1 in modulating the kinetics as well as the magnitude of the acid secretory response to histamine.
Lasp-1 expression in osteoclasts and bone remodeling analyses.
A recent mouse genome-wide microarray study detected high levels of Lasp-1 gene expression in bone (44). The main cell types within bone are bone-forming osteoblasts and mineralized bone-degrading osteoclasts. In many respects osteoclasts exhibit properties similar to parietal cells of the stomach. Both cell types secrete large quantities of acid from a specialized region of the plasma membrane (the intracellular canalicular membrane in parietal cells and the ruffled membrane/sealing zone in osteoclasts), and acid secretion is similarly regulated by dramatic membrane rearrangements (trafficking) that lead to the insertion of proton pumps into the appropriate subcellular region (1, 41).
To determine whether Lasp-1 is expressed in osteoclasts, we probed Western blots of bone marrow macrophages extracted after overnight culture with M-CSF, harvesting nonadherent cells and enrichment on a Ficoll-Hypaque gradient and osteoclast extracts from the same isolates after RANK-L induction. A single band of ∼37 kDa was detected in both extracts with an ∼1.7-fold increase in the Lasp-1 signal in the osteoclast enriched fraction (not shown). To define the subcellular localization of Lasp-1, osteoclasts were probed with the 8C6 Lasp-1 antibody plus Cy-5-tagged secondary antibody along with fluorescently tagged phalloidin to localize F-actin. Sytox Green, a nuclear probe, was used to confirm that the cells were multinucleate, a signature characteristic of osteoclasts. As shown in Fig. 5, Lasp-1 was immunolocalized to punctate podosomal and F-actin-rich internal vesicular structures (Fig. 5, A–D) as well as to F-actin rings (Fig. 5, E–G) in large, multinucleate osteoclasts. The localization to podosomes and F-actin rings was of particular interest because these structures are thought to arise from podosomes (17). In some respects, podosomes are similar to focal adhesions and have been implicated not only in bone resorption but also in osteoclast migration and adhesion (18).
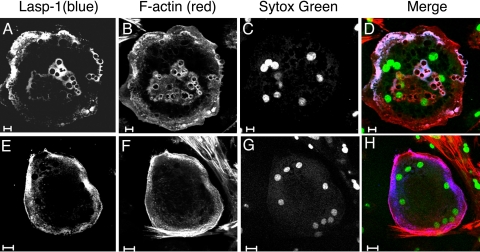
Fig. 5.
Immunolocalization of Lasp-1 in cultured mouse osteoclasts. Bone marrow cells were placed in culture and induced to differentiate into osteoclasts with macrophage colony-stimulating factor/receptor activator of NF-κB ligand. Cells were stained with Lasp-1 8C6 antibody (1:200) and cyanine (Cy)-5 donkey anti-mouse secondary antibody (1:100) and counterstained with Alexa 568 phalloidin (1:40) and Sytox Green (0.5 μM) to localize F-actin and nuclei, respectively. The presence of multiple nuclei and large size are hallmark signatures of differentiated osteoclast morphology. A–D: bars = 10 μM. E–H: bars = 20 μM.
Because basal gastric HCl secretion in Lasp-1-null mice was not elevated and these mice did not develop gastric or duodenal ulcers (data not shown), it seemed unlikely that either osteoclast activity or bone formation would be significantly affected in unchallenged Lasp-1-null mice. To determine whether this was indeed the case, bone formation in Lasp-1-null mice and wild-type littermates was analyzed with several different approaches. As shown in Table 2, there were no significant differences in BMD or content in 3-mo-old F6 generation wild-type and Lasp-1-null male littermates (Table 2). There were also no significant differences in osteoclast numbers in spines or tibiae, nor were bone sizes or bone-forming surfaces affected (N = 6 mice/group, P > 0.1).
Table 2.
BMD and BMC in 3-mo-old male littermates, F6 generation
| Genotype | BMD, g/cm2 | BMC, g | Area, mm2 | N |
|---|---|---|---|---|
| WT | 0.04752±0.00160 | 0.01640±0.0006 | 0.35±0.04 | 10 |
| −/− | 0.04864±0.00146 | 0.01721±0.0007 | 0.36±0.04 | 14 |
| +/− | 0.04773±0.00157 | 0.01736±0.0011 | 0.36±0.05 | 14 |
Values are means ± SE. BMD, bone mineral densities; BMC, bone mineral content; WT, wild-type. N denotes the number of animals in each group.
DISCUSSION
In this study, we report the production of a new mutant mouse line carrying a targeted disruption of the Lasp-1 gene. Lasp-1-null mice are viable and fertile and display no overt phenotypic or developmental abnormalities. Because changes in Lasp-1 phosphorylation have been linked to the activation of gastric HCl secretion, and because Lasp-1 is highly expressed within parietal cells in the gastric mucosa, we examined the stomachs of Lasp-1 mutants in depth. We found that chronic absence of Lasp-1 led to a modest elevation in histamine-stimulated gastric acid secretion in vivo but had no effect on normal gastric mucosal growth or development. In vitro experiments with isolated gastric glands supported the in vivo findings and further suggested that the return to the basal, or resting secretory state, is delayed in the absence of Lasp-1. Because reversal of the activation process is linked to the endocytic retrieval of the proton pump from intracellular canalicular microvilli to the cytosolic tubulovesicular compartment (10, 30, 31), our observations lend credence to the hypothesis that Lasp-1 modulates proton pump trafficking through actions on endocytic and/or F-actin-dependent processes. The finding that basal HCl secretion is unchanged by the chronic loss of Lasp-1 supports a regulatory, rather than an essential, role for Lasp-1 in modulating the activation of parietal cell HCl secretion. This regulatory role is similarly reflected in osteoclasts, which appear to function normally in unchallenged Lasp-1-null mice, based on our BMC and BMD measurements. Despite these negative in vivo findings, the localization of Lasp-1 to F-actin-rich subcellular compartments that have been linked to acid secretion in osteoclasts is intriguing and warrants further investigation at the cellular level where subtle changes in osteoclast functions can be more readily identified and characterized. It will also be important to analyze bone structure in aged mice in which developmental alterations may become apparent and to determine whether environmental and/or hormonal alterations affect bone formation in Lasp-1-null mice.
Functional redundancies in gene families and/or the upregulation of proteins with similar or parallel functions may obscure overt phenotypic changes in gene knockout studies. Lasp-1 is a member of the nebulin protein family, all members of which possess varying numbers of 35-residue nebulin “repeats.” In addition to Lasp-1, this family is known to include nebulin (42), nebulette (26), nebulin-related anchoring protein (N-RAP) (25), and LIM-nebulette (Lasp-2) (21, 38). Nebulin, nebulette, and N-RAP are muscle-specific and are thus unlikely candidates. Lasp-2 (LIM-nebulette) is a potential candidate because both Lasp-1 and Lasp-2 appear to be expressed in similar cellular compartments in the brain (9, 38), and LIM-nebulette has a similar subcellular distribution in fibroblasts (21); however, we were unable to detect Lasp-2 expression in the gastric mucosa of either wild-type or Lasp-1-null mice. These findings have led us to tentatively conclude that Lasp-2 does not to perform this function. In similar analyses, we found no changes in the level of expression of several candidate Lasp-1 interacting proteins including β-actin, dynamin, clathrin, Hip1r, and zyxin in gastric mucosal extracts. Thus it appears that compensatory changes in the expression of these proteins are also unlikely to offset the loss of Lasp-1. Further work at the cellular level will be required to determine whether other as yet unidentified proteins play a role in the process.
Because Lasp-1 is overexpressed in some cancers (11, 40) and is upregulated by activation of Sonic Hedgehog signaling (15), which plays an important role not only in gastric mucosal development but also in oncogenesis, there is considerable interest in defining the mechanisms linking Lasp-1 to cancer progression. Lasp-1 overexpression has recently been associated with increased invasiveness in some cancer cell lines (11); however, findings from overexpression and siRNA-based gene silencing studies in cell lines are complex. In HEK293 and COS-7 cells, as well as in the breast cancer cell line, MCF-7, both Lasp-1 overexpression and Lasp-1 gene silencing suppress cell migration (23). In contrast, in the nontransformed PTK-2 cell line, Lasp-1 overexpression has been linked to increased cell migration (12). It will be important to test the Lasp-1-null mouse model to determine whether loss of Lasp-1 alters cancer progression and/or metastasis. It will also be important to analyze further the effects of in vivo Lasp-1 gene disruption on normal cell motility and other cellular functions in vitro.
GRANTS
This work was supported by National Institutes of Health grant DK31900.
Acknowledgments
We thank Dr. Brian Condie for assistance in designing the gene knockout strategy and for providing the mouse λ-Dash II phage library used in the initial phase of this study, Dr. Kirk Thomas for providing the pACN vector, Dr. Wen-Cheng Xiong for providing mouse osteoclasts, Dr. Asako Terasaki for providing the Lasp-2 antibody, and Dr. Mark Hamrick and Ms. Cathy Pennington for performing bone histomorphometry analyses. We also thank Dr. John Parente for invaluable input during the initiation this project and Ms. Hai-Yan Qin and Ms. Kristina Andrews for expert technical assistance. We greatly appreciate the expert guidance of Dr. Katz Miyake and Mr. Darren Baker in the MCG Imaging Core with imaging applications and Ms. Penny Roon in the EM Core for TEM analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245: 855–857, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Butt E, Gambaryan S, Gottfert N, Galler A, Marcus K, Meyer HE. Actin binding of human LIM and SH3 protein is regulated by cGMP- and cAMP-dependent protein kinase phosphorylation on serine 146. J Biol Chem 278: 15601–15607, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Chew CS cAMP technologies, functional correlates in gastric parietal cells. Methods Enzymol 191: 640–661, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Chew CS, Brown MR. Histamine increases phosphorylation of 27- and 40-kDa parietal cell proteins. Am J Physiol Gastrointest Liver Physiol 253: G823–G829, 1987. [DOI] [PubMed] [Google Scholar]
- 5.Chew CS, Okamoto C, Chen X, Yang A, Qin H. Lasp-1 associates with endocytosis-related proteins in the gastric parietal cell (Abstract). FASEB J 18: A1026, 2004. [Google Scholar]
- 6.Chew CS, Chen X, Parente JA Jr, Tarrer S, Okamoto C, Qin HY. Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci 115: 4787–4799, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Chew CS, Okamoto CT, Chen X, Thomas R. Drebrin E2 is differentially expressed and phosphorylated in parietal cells in the gastric mucosa. Am J Physiol Gastrointest Liver Physiol 289: G320–G331, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chew CS, Parente JA Jr, Chen X, Chaponnier C, Cameron RS. The LIM and SH3 domain-containing protein, lasp-1, may link the cAMP signaling pathway with dynamic membrane restructuring activities in ion transporting epithelia. J Cell Sci 113: 2035–2045, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Chew CS, Parente JA Jr, Zhou C, Baranco E, Chen X. Lasp-1 is a regulated phosphoprotein within the cAMP signaling pathway in the gastric parietal cell. Am J Physiol Cell Physiol 275: C56–C67, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Forte JG, Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol 6: 45–48, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Grunewald TG, Kammerer U, Kapp M, Eck M, Dietl J, Butt E, Honig A. Nuclear localization and cytosolic overexpression of LASP-1 correlates with tumor size and nodal-positivity of human breast carcinoma. BMC Cancer 7: 198, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunewald TG, Kammerer U, Schulze E, Schindler D, Honig A, Zimmer M, Butt E. Silencing of LASP-1 influences zyxin localization, inhibits proliferation and reduces migration in breast cancer cells. Exp Cell Res 312: 974–982, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Grunewald TG, Kammerer U, Winkler C, Schindler D, Sickmann A, Honig A, Butt E. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer 96: 296–305, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34: 376–383, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene 21: 8196–8205, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jain RN, Al-Menhali AA, Keely TM, El-Zaatari M, Chen X, Merchant JM, Ross TS, Chew CS, Samuelson LC. Loss of parietal cell tubulovesicles and gastrin-dependent hypertrophy in the Hip1r deficient mouse stomach. J Clin Invest. In press, 2008. [DOI] [PMC free article] [PubMed]
- 17.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol 85: 195–202, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kanehisa J, Yamanaka T, Doi S, Turksen K, Heersche JNM, Aubin JE, Takeuchi H. A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone 11: 287–293, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Keicher C, Gambaryan S, Schulze E, Marcus K, Meyer HE, Butt E. Phosphorylation of mouse LASP-1 on threonine 156 by cAMP- and cGMP-dependent protein kinase. Biochem Biophys Res Commun 324: 308–316, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif Tissue Int 66: 298–306, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Zhuang L, Trueb B. Zyxin Interacts with the SH3 Domains of the Cytoskeletal Proteins LIM-nebulette and Lasp-1. J Biol Chem 279: 20401–20410, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Hornshaw MP, van Minnen J, Smalla KH, Gundelfinger ED, Smit AB. Organelle proteomics of rat synaptic proteins: correlation-profiling by isotope-coded affinity tagging in conjunction with liquid chromatography-tandem mass spectrometry to reveal post-synaptic density specific proteins. J Proteome Res 4: 725–733, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Lin YH, Park ZY, Lin D, Brahmbhatt AA, Rio MC, Yates JR, III, Klemke RL. Regulation of cell migration and survival by focal adhesion targeting of Lasp-1. J Cell Biol 165: 421–432, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol 290: G970–G979, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Luo G, Zhang JQ, Nguyen TP, Herrera AH, Paterson B, Horowits R. Complete cDNA sequence and tissue localization of N-RAP, a novel nebulin-related protein of striated muscle. Cell Motil Cytoskeleton 38: 75–90, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Moncman CL, Wang K. Nebulette: a 107 kD nebulin-like protein in cardiac muscle. Cell Motil Cytoskeleton 32: 205–225, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd edition. New York: Cold Spring Harbor Laboratory, 2003.
- 28.Nakagawa H, Terasaki AG, Suzuki H, Ohashi K, Miyamoto S. Short-term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett 580: 3223–3228, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto CT, Duman JG, Tyagarajan K, McDonald KL, Jeng YY, McKinney J, Forte TM, Forte JG. Clathrin in gastric acid secretory (parietal) cells: biochemical characterization and subcellular localization. Am J Physiol Cell Physiol 279: C833–C851, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto CT, Forte JG. Vesicular trafficking machinery, the actin cytoskeleton, and H+−K+− ATPase recycling in the gastric parietal cell. J Physiol 532: 287–296, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto CT, Li R, Zhang Z, Jeng YY, Chew CS. Regulation of protein and vesicle trafficking at the apical membrane of epithelial cells. J Control Release 78: 35–41, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2: 595–610, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Phillips GR, Anderson TR, Florens L, Gudas C, Magda G, Yates JR 3rd, Colman DR. Actin-binding proteins in a postsynaptic preparation: Lasp-1 is a component of central nervous system synapses and dendritic spines. J Neurosci Res 78: 38–48, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber V, Masson R, Linares JL, Mattei MG, Tomasetto C, Rio MC. Chromosomal assignment and expression pattern of the murine Lasp-1 gene. Gene 207: 171–175, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber V, Moog-Lutz C, Regnier CH, Chenard MP, Boeuf H, Vonesch JL, Tomasetto C, Rio MC. Lasp-1, a novel type of actin-binding protein accumulating in cell membrane extensions. Mol Med 4: 675–687, 1998. [PMC free article] [PubMed] [Google Scholar]
- 36.Spence HJ, McGarry L, Chew CS, Carragher NO, Scott-Carragher LA, Yuan Z, Croft DR, Olson MF, Frame M, Ozanne BW. AP-1 differentially expressed proteins Krp1 and fibronectin cooperatively enhance Rho-ROCK-independent mesenchymal invasion by altering the function, localization, and activity of nondifferentially expressed proteins. Mol Cell Biol 26: 1480–1495, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun HJ, Bahk YY, Choi YR, Shim JH, Han SH, Lee JW. A proteomic analysis during serial subculture and osteogenic differentiation of human mesenchymal stem cell. J Orthop Res 24: 2059–2071, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Terasaki AG, Suzuki H, Nishioka T, Matsuzawa E, Katsuki M, Nakagawa H, Miyamoto S, Ohashi K. A novel LIM and SH3 protein (lasp-2) highly expressing in chicken brain. Biochem Biophys Res Commun 313: 48–54, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tomasetto C, Moog-Lutz C, Regnier CH, Schreiber V, Basset P, Rio MC. Lasp-1 (MLN 50) defines a new LIM protein subfamily characterized by the association of LIM and SH3 domains. FEBS Lett 373: 245–249, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Tomasetto C, Regnier C, Moog-Lutz C, Mattei MG, Chenard MP, Lidereau R, Basset P, Rio MC. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics 28: 367–376, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Vaananen HK, Karhukorpi EK, Sundquist K, Wallmark B, Roininen I, Hentunen T, Tuukkanen J, Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol 111: 1305–1311, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang K, Williamson CL. Identification of an N2 line protein of striated muscle. Proc Natl Acad Sci USA 77: 3254–3258, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Xie Y, Du QS, Wu XJ, Feng X, Mei L, McDonald JM, Xiong WC. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J Cell Biol 160: 565–575, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Morris QD, Chang R, Shai O, Bakowski MA, Mitsakakis N, Mohammad N, Robinson MD, Zirngibl R, Somogyi E, Laurin N, Eftekharpour E, Sat E, Grigull J, Pan Q, Peng WT, Krogan N, Greenblatt J, Fehlings M, van der Kooy D, Aubin J, Bruneau BG, Rossant J, Blencowe BJ, Frey BJ, Hughes TR. The functional landscape of mouse gene expression. J Biol 3: 21, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]