Abstract
Stem cell factor (SCF) and its receptor c-kit are important in hematopoiesis and cellular proliferation. c-kit has also been identified as a cell surface marker for progenitor cells. We have previously shown that there is a large reservoir of hepatic SCF, and this molecule plays a significant role in liver regeneration after 70% hepatectomy. In the current study, we further examined the expression of SCF and c-kit in acetaminophen (APAP)-induced liver injury in C57BL/6J mice or SCF-deficient sl-sld mice and their appropriate wild-type controls. Following APAP-induced liver injury, c-kit mRNA expression increased, with peak levels detected 48 h postinjury. Hepatic SCF mRNA levels after APAP injury were also increased, with peak levels seen 16 h post-APAP. The mortality rate in SCF-deficient mice treated with APAP was significantly higher than that of wild-type mice; furthermore, administration of exogenous SCF significantly reduced the mortality of APAP-treated wild-type mice. Bromodeoxyuridine incorporation experiments showed that SCF significantly increased hepatocyte proliferation at 48 and 72 h in APAP-treated mice. SCF inhibited APAP-induced hepatocyte apoptosis and increased Bcl-2 and Bcl-xL expression, suggesting that this decrease in hepatocyte apoptosis is mediated through Bcl-2 and Bcl-xL. In summary, SCF and c-kit expression was increased after APAP-induced liver injury. Administration of exogenous SCF reduces mortality in APAP-treated mice, increases hepatocyte proliferation, and prevents hepatocyte apoptosis induced by APAP, suggesting that these molecules are important in the liver's recovery from these injuries.
Keywords: liver regeneration, apoptosis, hepatocyte proliferation, cytokines
acetaminophen, or Tylenol, is an over-the-counter analgesic with potentially hepatotoxic effects. A Tylenol overdose can cause acute liver failure in humans, as well as experimental animals, and acetaminophen poisoning has become the most common cause of acute liver failure in the United States (15, 24). Acetominophen-induced hepatic toxicity is a clinically important experimental model of drug-induced liver injury. The mechanism of acetominophen-induced liver injury has been attributed to the cytochrome P-450-generated metabolite, N-acetyl-p-benzoquinoneimine, which induces glutathione depletion, impairs mitochondrial respiration, and interferes with calcium homeostasis, although the actual cellular events that lead to cell death are still not well understood (4).
Stem cell factor (SCF) and its receptor, c-kit, play an important role in cell differentiation, proliferation, chemotaxis, cell adhesion, and apoptosis (16). c-kit is expressed on mast cells, some hematopoietic stem cells, germ cells, melanocytes, hepatic oval cells, and Cajal cells of the gastrointestinal tract (16). SCF exists in two forms, soluble and membrane bound. During the process of SCF activation and release, membrane-bound SCF is cleaved from the cell surface and becomes solubilized. It is then carried in serum to exert its biological effects at distant sites. Upon binding, c-kit dimerizes, activates its intrinsic tyrosine kinase, and autophosphorylates (38). This initiates additional specific signal transduction pathways, including the phosphatidylinositol 3-kinase (PI 3-kinase)/protein kinase B (Akt) pathway, Src family members, the JAK/STAT pathway, and the Ras-Raf-mitogen-activated protein kinase cascade (38). Previous studies in our laboratory have shown that there is a large reservoir of SCF in the liver and that hepatic SCF levels change dramatically following partial hepatectomy in mice; furthermore, administration of exogenous SCF enhanced liver regeneration in mice after 70% hepatectomy (37). Other investigators have also demonstrated proliferative or antiapoptotic effects for SCF in a variety of experimental models. For example, Dhandapani and colleagues (6) have shown that SCF attenuates camptothecin-induced apoptosis in rat cortical neurons. Similarly, Wang and others (41) illustrated that hydrogen peroxide-induced apoptosis in mouse vascular smooth muscle cells was decreased by SCF. Based on our prior studies in the 70% hepatectomy model, which demonstrated a pro-proliferative effect of SCF, we hypothesized that SCF and its receptor, c-kit, may also be involved in the liver's recovery from acetominophen-induced hepatotoxicity.
MATERIALS AND METHODS
Animal model.
SCF-deficient mice (sl/sl-d) were generated by crossing B6.D2-Kitlsl-d/J mice with WC/ReJKitlsl/J mice (Jackson Laboratory, Bar Harbor, ME) in the University of Michigan animal facility (44). These mice express low levels of SCF and are not completely SCF-deficient. Complete SCF knockout mice are very fragile animals and will not tolerate anesthesia or even low doses of acetaminophen. The genetically altered mice were used at 8–12 wk of age. For experiments not using genetically altered mice, 8- to 12-wk-old female C57BL/6J mice weighing ∼20 g (Jackson Laboratory) were used. All experiments in this study were approved by the University of Michigan's Committee on the Use and Care of Animals.
Acetaminophen was administered intraperitoneally in various doses; control animals received an equal volume of intraperitoneal PBS (21). Based on previous experiments in this strain of mouse, an acetominophen dose of 750 mg/kg is the approximate LD50 dose, and a dose of 375 mg/kg is the approximate LD25. The higher acetominophen dose was used in mortality experiments, and the lower dose was used for the remainder of the investigations. For experiments in which mice received exogenous SCF, Alzet pumps (Durect, Cupertino, CA) were implanted intraperitoneally. Implantation of the pumps occurred 2 h after administration of acetaminophen; a 2-h delay was used to assure that the intraperitoneal dose of acetaminophen was absorbed before opening the peritoneal cavity to surgically place the pumps. These pumps deliver 1 μl/h for up to 3 days. For mice receiving SCF, a solution of 160 ng/ml was placed within the pump reservoir; controls received an equivalent amount of PBS. The mount of SCF the mouse received through pump is 0.2 μg·kg−1·day−1. We chose the Alzet pump delivery system since we were interested in delivering a continuous level of SCF. Alternatively, SCF could have been administered one or two times a day by intraperitoneal injection; however, this method does not deliver a relatively constant level of SCF and results in peaks and troughs of this substance.
Primary hepatocyte isolation and culture.
Primary hepatocyte isolation was performed by collagenase perfusion. Briefly, general anesthesia was induced with isoflurane inhalation, midline laparotomy performed, and the inferior vena cava was exposed and cannulated with a 26-gauge angiocatheter. The liver was perfused with warm liver perfusion buffer (GIBCO, Grand Island, NY) at a rate of 3 ml/min for 10 min to flush the liver of intravascular blood. Next, warm liver digest buffer (GIBCO) was infused at a rate of 3 ml/min for 10 min to digest the liver. The liver was removed to a petri dish, minced into 1-mm pieces, and gently agitated to disperse the cells in wash buffer (GIBCO). The cell suspension was then filtered and washed two times at 500 rpm at 4°C for 5 min. The hepatocytes were plated on collagen I-coated plates and incubated in DMEM-F-12 medium with 10% FBS, 50 μg/ml gentamycin, 2 mM glutamine, 10 mM HEPES, 132 ng/ml dexamethasone, and 1% insulin-transferrin-selenium-X at 37°C under 5% CO2.
Real-time quantitative RT-PCR.
mRNA was extracted from 30 mg liver tissue using the Micro-FastTrack 2.0 Kit (Invitrogen, Carlsbad, CA) as per the manufacturer's instructions. The extracted mRNA was DNase I (Amplification Grade; Invitrogen) treated and then reverse transcribed into cDNA with TaqMan Reverse Transcription Reagent (Applied Biosystems, Branchburg, NJ). Real-time PCR was performed to determine the expression of c-kit and SCF in a SYBR Green PCR Master mix (Applied Biosystems) using the Bio-Rad iCycler iQ Multicolor Real-Time PCR Detection System (version 3.0 software) using the following protocol: initial activation at 95°C for 15 min, 40 cycles at 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s. Specificity of the amplification was checked by melting curve analysis and agarose gel electrophoresis. Primers were purchased from Qiagen [glyceraldehyde-3-phosphate dehydrogenase (GAPDH): QT00309099, SCF: QT00124887, c-kit: QT00145215]. Relative levels of mRNA expression were calculated according to the comparative threshold cycle method. Gene-specific expression values were normalized to levels of GAPDH (endogenous control) within each sample and relative to control values.
Bromodeoxyuridine incorporation in vivo.
Hepatocyte proliferation in vivo was estimated by bromodeoxyuridine (BrdU) incorporation. Animals were injected intraperitoneally with 30 μg BrdU/g body wt 2 h before death. Animals were then killed, and liver specimens were obtained, fixed overnight in 4% paraformaldehyde, processed for histological analysis, and stained using the Amersham cell proliferation kit (Amersham Pharmacia Biotech, Hertfordshire, UK). Four to six mice were used per treatment group per time point. A minimum of 500 cells (BrdU positive cells and negative cells) were counted in at least five separate fields for each animal, and the overall percentage of BrdU positive cells was calculated.
Immunohistochemical staining for c-kit and SCF.
Paraffin-embedded liver sections were deparaffinized with xylene and rehydrated in a series of graded ethanol washes and deionized water. Antigen retrieval was performed in sodium citrate buffer. Endogenous peroxidase was inhibited in 1.6% hydrogen peroxide for 30 min; 10% goat serum and 1% BSA in TBS buffer was used to block nonspecific binding. The slides were incubated with anti-CD117 antibody (clone: ACK2; e-Bioscience, San Diego, CA) overnight at 4°C with gentle agitation. Biotinylated goat antirat IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used to detect the first antibody. Horseradish peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA) was added to bind biotin. Diaminobenzidine substrate (Vector Laboratories, Burlingame, CA) was applied to tissue for color development. Slides were then counterstained with hematoxylin, dehydrated, and mounted. Immunostaining for SCF was performed in an identical fashion except for the primary and secondary antibodies that were used. For SCF staining, slides were incubated with primary rabbit polyclonal SCF antibody (Santa Cruz Biotechnology) at a dilution of 1:20 overnight at 4°C. Slides were washed three times in PBS and then incubated with goat biotinylated antirabbit secondary antibody for 30 min at room temperature. The remainder of the procedure is otherwise identical to staining for c-kit.
Western blots.
Perfused liver homogenate (100 μg) was subjected to 15% SDS-PAGE under reducing conditions and transferred to polyvinylidene difluoride membrane. The blots were blocked in 5% milk solution and then exposed to anti-Bcl-2 or anti-Bcl-xL antibody (anti-Bcl-2; R&D Systems, Minneapolis, MN and Cell Signaling Technology, Danvers, MA), anti-Bcl-xL (R&D Systems) overnight at 4°C. Because there is some controversy as to whether the liver expresses Bcl-2 during liver regeneration (40), we used two different antibodies to Bcl-2 to further document that we were detecting Bcl-2 in our Western blots. The first antibody was detected with horseradish peroxidase-conjugated goat antirat IgG or goat antimouse IgG (Santa Cruz Biotechnology). All membranes were visualized using West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL).
MTT cell proliferation assay.
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell proliferation assay kit (Molecular Probes, Eugene, OR) was used to determine hepatocyte proliferation in vitro as per the manufacturer's instructions. Cells were seeded at densities of 2,000 cells/well in 96-well plates. Absorbance was read at 570 nm.
Statistical analysis.
Statistical analysis was performed using an upaired Student's t-test. Differences were considered significant if P < 0.05. Survival rates are presented using Kaplan-Meier curves, and significance was calculated by the Log Rank test. All statistical analysis was performed using Prism 3.0 computer software.
RESULTS
SCF reduces acetaminophen-induced mortality.
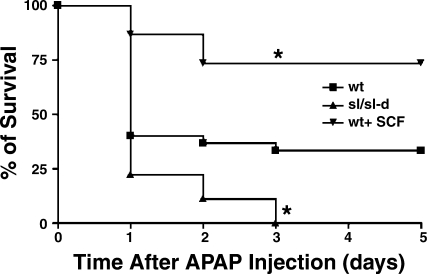
Ourinitial experiments investigated the effect of SCF on acetaminophen's hepatotoxicity by measuring mortality rates. For these experiments, all mice received 750 mg/kg acetaminophen, and mortality was recorded every 24 h out to 5 days. Three groups were studied: wild-type mice receiving acetaminophen alone (n = 30), sl/sl-d mice receiving acetaminophen alone (n = 9), and wild-type mice receiving acetaminophen and exogenous SCF (n = 15). The results are shown in Fig. 1. The mortality rate for sl/sl-d mice treated with acetominophen was significantly increased compared with wild-type mice receiving acetaminophen alone and wild-type mice receiving acetaminophen and exogenous SCF (P < 0.05 for both groups); furthermore, mortality was significantly decreased in wild-type mice receiving acetaminophen and SCF compared with wild-type mice receiving only acetaminophen (P < 0.05).
Fig. 1.
The effect of stem cell factor (SCF) on mortality in mice treated with toxic doses of acetaminophen. All mice received 750 mg/kg of acetaminophen, and mortality was observed. Three groups were studied: wild-type mice given acetaminophen alone (n = 30), sl/sl-d mice given acetaminophen alone (n = 9), and wild-type mice given acetaminophen and exogenous SCF (n = 15). The number of survivors was measured every 24 h, and survival curves were generated with Kaplan-Meier Analysis. Wild-type mice had a significantly increased survival compared with sl/sl-d mice (*P < 0.05); furthermore, wild-type mice treated with acetaminophen and SCF had a significantly increased survival compared with both sl/sl-d mice and wild-type mice treated with acetaminophen alone.
Exogenous SCF does not decrease liver injury after acetominophen as measured by serum aspartate aminotransferase and alanine aminotransferase activities.
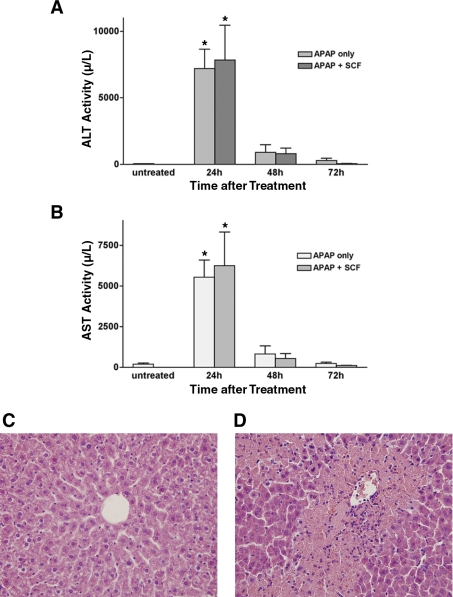
Mice receiving 375 mg/kg acetaminophen demonstrated hepatocyte necrosis on standard hematoxylin and eosin (H&E) staining of liver tissues within 24 h of acetominophen dosing, as well as significant increases in serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Serum AST and ALT activities were measured at 24, 48, and 72 h after administration of 375 mg/kg acetaminophen with and without SCF treatment. Serum AST and ALT activities were determined with the ADVIA 2400 Chemistry System (Bayer). Figure 2 demonstrates that both AST and ALT activities dramatically increased after acetaminophen administration, with peak levels occurring 24 h after acetaminophen dosing. More significantly, SCF treatment did not have a significant effect on serum AST and ALT activities; no differences were seen in serum AST or ALT activities between SCF-treated mice and SCF-untreated mice at any time point. This suggests that SCF does not directly affect the liver injury that follows acetaminophen hepatotoxicity and that the decrease in mortality that is seen in mice treated with acetaminophen and SCF is related to a mechanism that is unrelated to the initial hepatocyte toxicity.
Fig. 2.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels after acetaminophen (APAP) dosing with or without SCF administration. Mice were treated with 375 mg/kg of acetaminophen alone or 375 mg/kg acetaminophen and exogenous SCF. A and B: serum ALT and AST levels were determined at 24, 48, and 72 h after acetaminophen dosing, with peak levels occurring 24 h after acetaminophen administration. Treatment with SCF had no effect on AST and ALT levels. *P < 0.001 vs. control for both AST and ALT. C and D: standard hematoxylin and eosin (H&E) staining is shown for liver specimen obtained 24 h after acetominophen (D) or vehicle (C) dosing; there is obvious hepatic necrosis in the section from the acetominophen-treated animal. The animal treated with vehicle clearly has normal hepatic histology.
SCF does not prevent acetaminophen- or hydrogen peroxide-induced decreases in primary mouse hepatocyte proliferation in vitro.
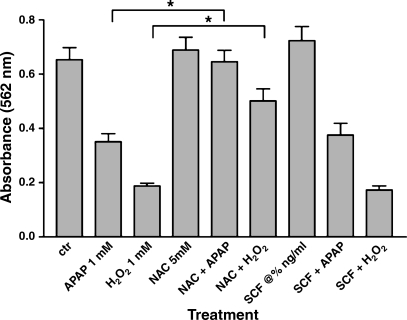
To further investigate whether SCF can prevent acetaminophen-induced hepatotoxicity, we measured primary mouse hepatocyte proliferation in vitro using an MTT assay after treatment with acetaminophen. Rates of hepatocyte proliferation were used as an estimate of cellular health. Because a significant component of acetaminophen's toxicity has been attributed to free radical-mediated damage, hepatocytes were also treated with hydrogen peroxide to produce a direct free radical-mediated injury. N-actylcysteine (NAC) is a well-known antioxidant that is used to treat acetaminophen overdose and toxicity. Hepatocytes were also treated with NAC in conjunction with hydrogen peroxide and acetaminophen; because NAC has known protective effects against both free radical-mediated injury and acetaminophen-mediated injury, it was used as a comparative agent to help judge the effects of SCF. Primary mouse hepatocytes were isolated from C57BL/6 mice and were treated with 1 mM acetaminophen, 1 mM hydrogen peroxide, 5 mM NAC, 25 ng/ml SCF, or various combinations of these agents, and proliferation was measured at 24 h using an MTT assay. These experiments were performed in triplicate and repeated three times.
As shown in Fig. 3, treatment with either acetaminophen or hydrogen peroxide results in a significant decrease in cellular proliferation. Treatment with NAC provides significant protection against both a hydrogen peroxide- and acetaminophen-induced insult, as illustrated by increases in cellular proliferation in those cells treated with either hydrogen peroxide and NAC or acetaminophen and NAC. In contrast, cells treated with hydrogen peroxide and SCF or acetaminophen and SCF illustrated no change in proliferation compared with cells treated with hydrogen peroxide or acetaminophen alone.
Fig. 3.
Primary hepatocyte proliferation after treatment with acetaminophen or hydrogen peroxide. Primary mouse hepatocytes were isolated from C57BL/6 mice and were treated with 1 mM acetaminophen, 1 mM hydrogen peroxide, 5 mM N-actylcysteine (NAC), 25 ng/ml SCF, or various combinations of these agents, and proliferation was measured at 24 h using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. These experiments were performed in triplicate and repeated three times. Hepatocytes that were treated with hydrogen peroxide and NAC or acetaminophen and NAC had a significantly increased rate of proliferation compared with cells treated with hydrogen peroxide alone or acetaminophen alone (*P < 0.01); treatment with SCF did not result in a similar effect.
Acetaminophen administration increases hepatic SCF and c-kit expression.
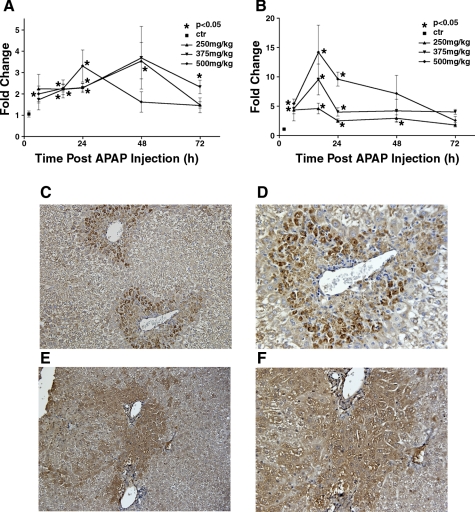
Because SCF-deficient mice have an increased mortality rate after an acetaminophen overdose compared with wild-type mice and wild-type mice receiving exogenous SCF had a decreased mortality rate after acetaminophen overdose compared with wild-type mice treated with acetaminophen and vehicle, we postulated that SCF is important in the liver's response to this injury. In light of this, our next experiments measured the expression of SCF and c-kit in the liver after acetaminophen-induced liver injury. Mice were treated with 250, 375, or 500 mg/kg acetaminophen and were killed at 6, 16, 24, 48, and 72 h after acetaminophen administration; 5–10 mice were used per group per time point. Hepatic SCF and c-kit mRNA expression was quantified by real-time PCR. As shown in Fig. 4A, following an acetaminophen overdose, hepatic c-kit mRNA expression begins to increase 6 h following the acetominophen dose and reaches peak levels 48 h postadministration, at which point the c-kit mRNA levels were more than threefold higher than untreated control mice. Similarly, as shown in Fig. 4B, following acetaminophen overdose, hepatic SCF mRNA levels also begin to increase 6 h after initial acetaminophen administration; peak levels occur 16 h after acetaminophen dosing, with SCF mRNA levels being more than 10-fold higher than untreated control mice.
Fig. 4.
Hepatic SCF and c-kit mRNA expression after acetaminophen treatment. C57BL/6 mice were treated with increasing doses of acetaminophen (250, 375, or 500 mg/kg) and were killed at 6, 16, 24, 48, and 72 h after acetaminophen dosing. Liver samples were obtained, mRNA was extracted, and real-time PCR was performed to determine c-kit (A) and SCF (B) expression. SCF and c-kit mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels for the same sample. C (magnification ×20) and D (magnification ×40) show immunohistochemical staining for hepatic c-kit expression at 48 h after a 375 mg/kg dose of acetaminophen. E (magnification ×20) and F (magnification ×40) show immunohistochemical staining for SCF expression at 12 h after a 375 mg/kg dose of acetaminophen.
c-kit expression on the cell surface was also qualitatively measured by immunohistochemical staining. These experiments demonstrated an increase in c-kit receptor expression beginning 24 h after acetaminophen administration and peaking at 48 h after acetaminophen dosing; c-kit expression was mainly noted around the portal triads (Fig. 4C). These data are consisted with the mRNA data.
SCF increases hepatocyte proliferation in vivo after acetaminophen overdose.
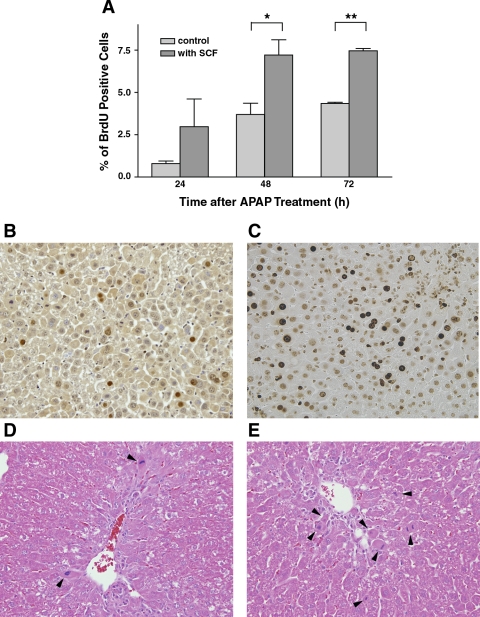
We have previously shown that SCF increases hepatocyte proliferation after 70% hepatectomy (37). We hypothesize that SCF may also increase hepatocyte proliferation after acetaminophen overdose, particularly in light of the fact that SCF does not appear to offer any protective effects from the initial hepatic injury, based on the observation that SCF does not protect against the increases in serum AST and ALT that occur after acetaminophen overdose. To test this hypothesis, mice were treated with 375 mg/kg acetaminophen and exogenous SCF or PBS (vehicle control). Mice were killed at 24, 48, or 72 h after acetaminophen administration, and livers were harvested for determination of hepatocyte BrdU incorporation. As shown in Fig. 5A, SCF significantly enhanced hepatocyte proliferation at 48 and 72 h; representative histological sections stained with BrdU are also shown in Fig. 5, B and C. Beginning at 48 h, despite the presence of significant residual areas of injury, well-defined areas of restoration could be identified, and cell mitoses could also be seen on standard H&E staining (Fig. 5, D and E). The number of cell mitoses in mice receiving exogenous SCF was significantly higher than that seen in mice treated with PBS.
Fig. 5.
Effect of SCF on hepatocyte proliferation in vivo after acetaminophen treatment. C57BL/6 mice were treated with 375 mg/kg acetaminophen and SCF or PBS (vehicle control). Animals were killed at 24, 48, and 72 h postacetaminophen administration, and in vivo hepatocyte proliferation was estimated using a bromodeoxyuridine (BrdU) incorporation assay. A: animals treated with acetaminophen and SCF have a significantly increased rate of hepatocyte proliferation, compared with animals treated with acetaminophen and PBS, at 48 and 72 h (*P < 0.05 and **P < 0.01). B and C: representative histological sections after BrdU staining; B represents the liver from a mouse treated with 375 mg/kg acetaminophen with vehicle, and C represents liver from a mouse treated with 375 mg/kg of acetaminophen and SCF (48-h time point). Similarly, D and E are representative H&E staining of liver sections from mice treated with acetaminophen and PBS (D) or acetaminophen and SCF (E; both at 48-h time point). Arrowheads indicate the cells that are in mitosis; there are significantly more mitotic cells in the animal treated with SCF compared with the animal treated with PBS.
SCF inhibits hepatocyte apoptosis after acetaminophen administration.
Acetaminophen toxicity is often associated with hepatic centrilobular necrosis. In the current study, apoptotic hepatocytes were also seen at the tissue interface between necrotic and healthy hepatic tissue 24 h after acetaminophen treatment (Fig. 6A). Because other investigators have shown that SCF prevents Fas-induced apoptosis in human erythroid precursor cells in vitro, we hypothesized that SCF may also have antiapoptotic effects in acetaminophen-treated mouse livers (30).
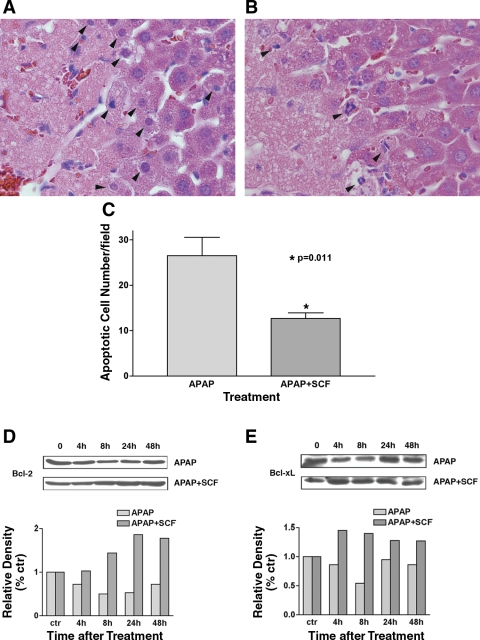
Fig. 6.
SCF inhibits acetaminophen-induced hepatocyte apoptosis. A: mice received 375 mg/kg acetaminophen and PBS (vehicle control), and liver tissue was obtained at 24 h. H&E staining demonstrates apoptotic hepatocytes between normal and necrotic hepatocytes (arrowheads indicate apoptotic cells). B: H&E staining of liver tissue obtained at 24 h from mice treated with 375 mg/kg acetaminophen and SCF; a decreased number of apoptotic hepatocytes are noted (arrowheads indicate apoptotic cells). C: apoptotic cells/high-power field in liver tissue 24 h after treatment with 375 mg/kg acetaminophen with (n = 6) and without (n = 6) SCF treatment. Ten fields were counted in each sample. Treatment with SCF results in a significant decrease in apoptotic cells. D and E: expression of Bcl-2 (D) and Bcl-xL (E) was examined with Western blotting. The gels represent 1 of 3 repeated experiments (all with similar results). The relative densities of Bcl-2 and Bcl-xL bands were normalized to those of GAPDH in the same samples.
As described above, mice were treated concurrently with acetaminophen and SCF or acetaminophen and PBS as a control. Hepatocyte apoptosis was reduced in animals treated with acetaminophen and SCF compared with animals treated with acetaminophen and PBS (Fig. 6B). This was further quantitated by counting apoptotic cells in liver tissues 24 h after treatment with 375 mg/kg acetaminophen with (n = 6) and without (n = 6) SCF treatment. This demonstrated a significant decrease (P = 0.011) in the number of apoptotic cells in animals treated with acetaminophen and SCF compared with animals treated with acetaminophen alone (Fig. 6C). To understand how SCF may inhibit apoptosis, we also examined Bcl-2 and Bcl-xL expression after administration of acetaminophen with or without SCF treatment. Because there is some controversy as to whether Bcl-2 is expressed in the liver during regeneration, we used two different antibodies in our Western blot analysis for Bcl-2. Both antibodies resulted in essentially identical Western blots, further suggesting that we were detecting changes in Bcl-2 in these experiments. These studies demonstrated that Bcl-2 expression decreased after acetaminophen administration alone, with decreases beginning 4 h after acetaminophen dosing and nadiring 8 h after acetaminophen dosing (Fig. 6C). In contrast, in mice receiving acetaminophen and SCF, Bcl-2 expression did not decrease at 4 h and actually increased 8 h after drug administration (Fig. 6C). Bcl-xL expression had a similar pattern of expression to Bcl-2 in mice treated with acetaminophen alone (Fig. 6D). In addition, for mice treated with acetaminophen and SCF, Bcl-xL expression also increased at 4 h, and these increases were maintained for 48 h (Fig. 6D).
DISCUSSION
The present study investigates the hypothesis that the SCF/c-kit ligand/receptor system plays a role in attenuating liver damage and/or liver regeneration in a murine model of acetaminophen-induced hepatic injury. Our experiments have shown that hepatic SCF and c-kit mRNA are elevated in acetaminophen-injured livers. In addition, SCF-deficient mice have an increased mortality rate compared with wild-type mice after acetaminophen poisoning, and, furthermore, administration of exogenous SCF to normal mice also decreases mortality associated with acetaminophen intoxication. SCF's protective effects in this model appear to be associated with enhanced hepatocyte proliferation and suppressed hepatocyte apoptosis. These observations suggest that SCF and c-kit are important for the antiapoptotic and proliferative hepatic responses to liver damage.
SCF and its type III receptor tyrosine kinase, c-kit, have been shown to promote survival (7), proliferation (5, 9, 25), mobilization (10), and adhesion (26) in a variety of hematopoietic progenitor cells. Our results illustrate that c-kit mRNA transcription and c-kit protein expression in the liver were substantially increased in response to acetaminophen administration. Although c-kit is known to be expressed on oval cells in the liver, it has also been suggested that hepatocytes may temporarily express c-kit in response to hepatic injury (11). Investigators have reported upregulation of c-kit mRNA in human fulminant hepatic failure patients, acetaminophen-treated rat liver, and in bile duct-ligated mice (1, 12, 32). In our model, the upregulation of SCF and its receptor, c-kit, suggests that this receptor/ligand system is involved in hepatic repair mechanisms. Okumoto and colleagues (31) have shown that low serum levels of SCF were linked to a poor prognosis in patients with severe liver injury. This supports our findings that administration of exogenous SCF in the context of acetaminophen poisoning decreased mouse mortality, inhibited hepatocyte apoptosis, and enhanced hepatocyte proliferation. This suggests that SCF may be a possible treatment for acute liver failure induced by acetaminophen or other factors.
The role of apoptosis in acetaminophen-induced hepatotoxicity is controversial. It is generally accepted that the ultimate form of hepatic damage caused by acetaminophen is necrosis (13, 33). Acetaminophen can induce apoptosis both in vivo and in vitro by triggering the release of mitochondrial cytochrome c and activating the apoptotic pathway (2, 8, 35). Our studies found apoptotic cells in the areas between normal and necrotic hepatic tissues (Fig. 6). Several investigators have suggested that cell death induced by acetaminophen, either apoptotic or necrotic, may be related to overall acetaminophen tissue concentrations. Kon and colleagues (22) found that intracellular concentrations of ATP correlated with necrosis vs. apoptosis in the setting of acetaminophen-induced hepatotoxicity. When ATP levels were low, oncotic necrosis occurred. In contrast, when ATP levels were sustained at normal levels, necrosis was prevented, and caspase-dependent apoptosis occurred. Similarly, ATP depletion in human T cells converts the type of cell death caused by two classic apoptotic triggers, staurosporine and CD95 stimulation, from apoptosis to necrosis (28, 29). The acetaminophen concentration around the central vein is likely to be higher, resulting in ATP depletion and subsequent hepatocyte necrosis. In contrast, acetaminophen tissue concentrations likely decrease as the portal triad is approached, therefore resulting in more normal ATP levels and the development of apoptosis, which is seen in the interface between necrotic and normal cells.
The Bcl-2 family proteins are antiapoptotic; Bcl-2 and Bcl-xL play important roles in inhibiting mitochondrial-dependent extrinsic and intrinsic cell death by inhibiting the translocation and subsequent oligomerization of Bax/Bak, which leads to the release of mitochondrial cytochrome c (18, 19, 42). After acetaminophen administration in murine tubular epithelial cells and mouse hepatocytes, Bcl-xL protein levels decreased without a significant change in Bcl-xL mRNA levels (27, 34). The changes in Bcl-xL protein levels in these model systems were related to Bcl-xL phosphorylation, which inactivates Bcl-xL (34, 36). Similarly, other investigators have found that Bcl-2 is phosphorylated in lymphoid cells exposed to the chemotherapeutic drug taxol, with subsequent inhibition of its ability to interfere with apoptosis (14). In rat cortical neurons, rat oocytes, and mouse vascular smooth muscle cells, SCF suppresses apoptosis through upregulation of Bcl-2 and Bcl-xL via the PI 3-kinase-Akt pathway (6, 17, 41). In the current study, we found that both Bcl-2 and Bcl-xL proteins declined after acetaminophen administration, and this decrease was prevented by SCF administration. Although Bcl-xL has clearly been shown to function as a delayed early response gene in liver regeneration, the role of Bcl-2 is more controversial (40). Other investigators have shown a potential role for Bcl-2 in the liver's recovery from injury. More specifically, studies in wild-type and interleukin (IL)-6 knockout mice subjected to a carbon tetrachloride-induced injury demonstrated that IL-6 knockout mice had preexisting reduced levels of Bcl-2, with subsequent defective liver regeneration and increased hepatocyte apoptosis (23). Similarly, other studies have shown a reduced susceptibility to hepatocarcinoma by sensitizing hepatocytes to increased levels of apoptosis via downregulation of Bcl-2 (3, 43). More specifically, our findings suggest that SCF may exert its antiapoptotic effects through activation of c-kit tyrosine kinase and Akt, which then results in an increase in intracellular Bcl-2 protein, which then prevents mitochondrial cytochrome-c release, attenuates caspase-3 activation, and ultimately inhibits hepatocyte apoptosis.
Recently Simpson and others (39) reported that SCF attenuated acetaminophen-induced liver damage by reducing the level of mRNA for cytochrome P-450 Cyp2E1, one of the primary hepatic enzymes that is responsible of protection against acetaminophen-induced hepatotoxicity. Although this finding may be one of the mechanisms associated with the SCF-related decreased acetaminophen-induced mortality, our data do not support this finding. If SCF can prevent acetaminophen-induced hepatotoxicity, serum AST and ALT levels should be decreased in SCF-treated mice. However, our data demonstrated there were no difference between SCF-treated and untreated mice exposed to toxic doses of acetaminophen. In addition, in vitro SCF did not reverse acetaminophen-induced inhibition of proliferation within the first 24 h of cell culture in vitro.
In summary, our data suggest that SCF and c-kit are important in the liver's recovery from acetaminophen-induced hepatic damage. Our data further suggest that SCF's function in this system by increasing hepatocyte proliferation and decreasing hepatocyte apoptosis following this injury, thus enhancing recovery, rather than protecting directly from the injury.
GRANTS
This work was support by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK-53224, “CXC Chemokines in Liver Regeneration.”
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bauman U, Crosby HA, Ramani P, Kelly DA, Strain AJ. Expression of the stem cell factor receptor c-kit in normal and diseased pediatric liver: identification of a human hepatic progenitor cell? Hepatology 30: 112–117, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Boulares AH, Zoltoski AJ, Stoica BA, Cuvillier O, Smulson ME. Acetaminopeh induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol 90: 38–50, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Christensen JG, Romach EH, Healy LN, Gonzales AJ, Anderson SP, Malarkey DE, Corton JC, Fox TR, Cattley RC, Coldsworthy TL. Altered bcl-2 family expression during non-genotoxic carcinogenesis in mice. Carcinogenesis 20: 1583–1590. [DOI] [PubMed]
- 4.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA 81: 1327–1331, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deCastro CM, Denning SM, Langdon S, Vandenbark GR, Kurtzber J, Scearce R, Haynes BF, Kaufman RE. The c-kit proto-oncogene receptor is expressed on a subset of human CD3-CD4-CD8- (triple-negative) thymocytes. Exp Hematol 22: 1025–1033, 1994. [PubMed] [Google Scholar]
- 6.Dhandapani KM, Wade FM, Wakade C, Mahesh VB, Brann DW. Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappa B. J Neurochem 95: 9–19, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med 192: 1707–1718, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PD, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: role of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol 191: 118–129, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Escribano L, Ocqueteau M, Almeida J, Orfao A, San Miguel JF. Expression of the c-kit (CD117) molecule in normal and malignant hematopoiesis. Leuk Lymphoma 30: 459–466, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Weissman IL. Steel factor influences the distribution and activity of murine hematopoietic stem cells in vivo. Proc Natl Acad Sci USA 90: 3760–3764, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujio K, Evarts RP, Hu Z, Marsden ER, Thorgeirsson SS. Expression of stem cell factor and its receptor, c-kit, during liver regeneration from putative stem cells in adult rat. Lab Invest 70: 511–516, 1994. [PubMed] [Google Scholar]
- 12.Fujio K, Hu Z, Evarts RP, Marsden ER, Niu CH, Thorgeirsson SS. Coexpression of stem cell factor and c-kit in embryonic and adult liver. Exp Cell Res 224: 243–250, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67: 322–328, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc Natl Acad Sci USA 92: 4507–4511, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hart SG, Cartun RW, Wyand DS, Khairallah EA, Cohen SD. Immunohistochemical localization of acetaminophen in target tissues of the CD-1 mouse: correspondence of covalent binding with toxicity. Fundam Appl Toxicol 24: 260–274, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Heinemann A, Sturm GJ, Ofner M, Sturm EM, Weller C, Peskar BA, Hartnell A. Stem cell factor stimulates the chemotaxis, integrin upregulation, and survival of human basophils. J Allergy Clin Immunol 116: 820–826, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Jin X, Han CS, Yu FQ, Wei P, Hu ZY, Liu YX. Anti-apoptotic action of stem cell factor on oocytes in primordial follicles and its signal transduction. Mol Reprod Dev 70: 82–90, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BW, Cepero E, Boise LH. Bcl-Xl inhibits cytochrome c release but not mitochondrial depolarization during the activation of multiple death pathways by tumor necrosis factor-alpha. J Biol Chem 275: 31546–31553, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage. Oncol Rep 14: 595–599, 2005. [PubMed] [Google Scholar]
- 20.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Kofman Morgan G Kirschenbaum A Osbeck J Hussain M Swenson S Theise ND AV. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology 41: 1252–1261, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40: 1170–1179, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects again fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem 276: 26605–26613, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM. Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42: 1364–1372, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Leary AG, Zeng HQ, Clark SC, Ogawa M. Growth factor requirements for survival in G0 and entry into the cell cycle of primitive human hemopoietic progenitors. Proc Natl Acad Sci USA 89: 4013–4017, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levesque JP, Leavesley DI, Nuitta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med 181: 1805–1815, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorz C, Justo P, Sanz AB, Egido J, Ortiz A. Role of Bcl-xL in paracetamol-induced tubular epithelial cell death. Kidney Int 67: 595–601, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Nicotera P, Leist M, Ferrando-May E. Apoptosis and necrosis: different execution of the same death. Biochem Soc Symp 66: 69–73, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Nicotera P, Melino G. Regulation of the apoptosis-necrosis switch. Oncogene 23: 2757–2765, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Nishio M, Oda A, Koizumi K, Satoh I, Sato Y, Endoh T, Tsutsumi A, Fujihara M, Ikebuchi K, Ikeda H, Koike T, Sawada KI. Stem cell factor prevents Fas-mediated apoptosis of human erythoid precursor cells with Src-family kinase dependency. Exp Hematol 29: 19–29, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Okumoto K, Saito T, Onodera M, Sakamoto A, Tanaka M, Hattori E, Haga H, Ito JO, Sugahara K, Saito K, Togashi H, Kawata S. Serum levels of stem cell factor and thrombopoietin are markedly decreased in fulminant hepatic failure patients with a poor prognosis. J Gastroenterol Hepatol 22: 1265–1270, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Omori M, Omori N, Evarts RP, Teramoto T, Thorgeirsson SS. Coexpression of flt-3 ligand/flt and SCF/c-kit signal transduction system in bile duct-ligated Sl and W mice. Am J Pathol 150: 1179–1187, 1997. [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce RH, Franklin CC, Campbell JS, Tonge RP, Chen W, Fausto N, Nelson SD, Bruschi SA. Cell culture model for acetaminophen-induced hepatocyte death in vivo. Biochem Pharmacol 64: 413–424, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Ray SD, Jena N. A hepatotoxic dose of acetaminophen modulates expression of BCL-2, BCL-X(L), and BCL-X(S) during apoptotic and necrotic death of mouse liver cells in vivo. Arch Toxicol 73: 594–606, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ray SD, Mumaw VR, Raje RR, Fariss MW. Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate pretreatment. J Pharmacol Exp Ther 279: 1470–1483, 1996. [PubMed] [Google Scholar]
- 36.Ray SD, Parikh H, Hickey E, Bagchi M, Bagchi D. Differential effects of IH636 grape seed proanthocyanidin extract and a DNA repair modulator 4-aminobenzamide on liver microsomal cytochrome 4502E1-dependent aniline hydroxylation. Mol Cell Biochem 218: 27–33, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Ren X, Hogaboam C, Carpenter A, Colletti L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J Clin Invest 112: 1407–1418, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roskoski R Structure and regulation of Kit protein-tyrosine kinase. The stem cell receptor. Biochem Biophys Res Commun 338: 1307–1315, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Simpson K, Hogaboam CH, Kunkel SL, Harrison DJ, Bone-Larson C, Lukacs NW. Stem cell factor attenuates liver damage in a murine model of acetaminophen-induced hepatic injury. Lab Invest 83: 199–206, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Tzung SP, Fausto N, Hockenbery DM. Expression of Bcl-2 family during lier regeneration and identification of Bcl-x as a delayed early response gene. Am J Pathol 150: 1985–1995, 1997. [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CH, Verma S, Hsieh IC, Hung A, Cheng TT, Wang Sy Liu YC, Stanford WL, Weisel RD, Li RK, Cherng WJ. Stem cell factor attenuates vascular smooth muscle apoptosis and increases intimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol 27: 540–547, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng Ti Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondrial blocked. Science 275: 1129–1132, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Zhang GM, Feigenbaum L, Zhang YE. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell 9: 445–457, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeuner A, Pedini F, Signore M, Testa U, Pelosi E, Peschle C, De Maria R. Stem cell factor protects erythroid precursor cells from chemotherapeutic agents via upregulation of BCL-2 family proteins. Blood 102: 87–93, 2003. [DOI] [PubMed] [Google Scholar]