Abstract
Mice deficient in the organic solute transporter (Ost)-α subunit of the heteromeric organic solute and steroid transporter, Ostα-Ostβ, were generated and were found to be viable and fertile but exhibited small intestinal hypertrophy and growth retardation. Bile acid pool size and serum levels were decreased by more than 60% in Ostα−/− mice, whereas fecal bile acid excretion was unchanged, suggesting a defect in intestinal bile acid absorption. In support of this hypothesis, when [3H]taurocholic acid or [3H]estrone 3-sulfate were administered into the ileal lumen, absorption was lower in Ostα−/− mice. Interestingly, serum cholesterol and triglyceride levels were also ∼15% lower in Ostα−/− mice, an effect that may be related to the impaired intestinal bile acid absorption. After intraperitoneal administration of [3H]estrone 3-sulfate or [3H]dehydroepiandrosterone sulfate, Ostα−/− mice had higher levels of radioactivity in their liver and urinary bladder and less in the duodenum, indicating altered hepatic, renal, and intestinal disposition. Loss of Ostα was associated with compensatory changes in the expression of several genes involved in bile acid homeostasis, including an increase in the multidrug resistance-associated protein 3, (Mrp3)/Abcc3, an alternate basolateral bile acid export pump, and a decrease in cholesterol 7α-hydroxylase, Cyp7a1, the rate-limiting enzyme in bile acid synthesis. The latter finding may be explained by increased ileal expression of fibroblast growth factor 15 (Fgf15), a negative regulator of hepatic Cyp7a1 transcription. Overall, these findings provide direct support for the hypothesis that Ostα-Ostβ is a major basolateral transporter of bile acids and conjugated steroids in the intestine, kidney, and liver.
Keywords: Ostα−/− mouse, bile acid absorption, Fxr, Cyp7a1, cholesterol
bile acid and sterol reabsorption by ileal enterocytes, renal proximal tubular cells, and cholangiocytes is essential for cholesterol homeostasis, intestinal absorption of dietary fats and vitamins, and for proper regulation of bile flow and biliary lipid secretion (11, 20, 25). The initial step in bile acid reabsorption by these epithelial cells is mediated largely by the sodium-bile acid cotransporter (Asbt)/Slc10a2 and Na-taurocholate cotransporting polypeptide (Ntcp)/Slc10a1. In contrast to the uptake step, the mechanism for sterol export by these epithelial cells has not been established although recent studies provide strong evidence that export is mediated by the organic solute transporter (Ost)α-Ostβ heteromeric transporter (2). Ostα-Ostβ was initially identified by Wang and coworkers (26) from the liver of the little skate, Leucoraja erinacea, and the human and mouse orthologs were subsequently cloned and characterized by Seward and coworkers (23). This transporter is composed of a predicted seven-transmembrane (TM)-domain protein, Ostα, and a single TM-domain ancillary polypeptide, Ostβ. Ostα and Ostβ are encoded by genes on different chromosomes, they exhibit no sequence identity with each other, and appear to lack paralogs in mammalian genomes (2, 23, 26). Heterodimerization of Ostα and Ostβ is required for the delivery of the functional transport complex to the plasma membrane (17).
Several lines of evidence support the hypothesis that Ostα-Ostβ is the key basolateral exporter of bile acids and related molecules: 1) Ostα-Ostβ mediates the transport of bile acids and conjugated steroids, including taurocholate, estrone-3 sulfate, and dehydroepiandrosterone sulfate (DHEAS) (2, 3, 17, 23, 26); 2) transport occurs by a facilitated diffusion mechanism, and thus Ostα-Ostβ can mediate either efflux or uptake depending on the electrochemical gradient of a given substrate (3); 3) Ostα and Ostβ expression in various tissues and along the gastrointestinal tract parallels that of Asbt (3, 8, 23); 4) both Ostα and Ostβ proteins are localized to the basolateral plasma membrane of cells that are known to export bile acids, including enterocytes, cholangiocytes, and renal proximal tubular cells (3, 5, 8); and 5) expression of both Ost genes is positively regulated by bile acids through the bile acid-activated farnesoid X receptor (Fxr) consistent with a role of this transporter in bile acid export (5, 10, 15, 16, 27).
To more directly test the functional role of Ostα-Ostβ, the present study characterized an Ostα-deficient mouse model that was recently developed in our laboratory (17). As demonstrated by Li et al. (17), Ostα−/− mice lack both Ostα and Ostβ proteins because the individual subunits of this heteromeric transport complex are not stable in the absence of their obligate heterodimerization partner.
A recent study has also characterized the role of Ostα in intestinal bile acid absorption (21), and their findings in part confirm those presented in the present report. The study of Rao et al. (21) focused on the role of Ostα in intestinal bile acid transport, whereas the present study also indicates a role of Ostα in the kidney and liver, and it identifies a role in the transport of both bile acids and conjugated steroids (i.e., estrone 3-sulfate and DHEAS). In addition, the present study provides novel information on gene expression changes in kidney, liver, and small intestine, it provides direct in vivo evidence for impaired ileal bile acid and estrone 3-sulfate absorption, and documents a change in serum glucose levels in the Ostα−/− mice. Moreover, the gene-targeting vector used in the present study replaced exons 3-9 of the Ostα gene (or a replacement of about two-thirds of the encoded amino acids), whereas Rao et al. (21) replaced a portion of the proximal promoter and exons 1 and 2 (about one-third of the encoded amino acids). Thus the present results were generated with the use of a novel mouse model. The fact that a similar phenotype was generated with the use of these different mouse models provides confirmation of the important role of this transporter in bile acid and conjugated steroid homeostasis.
MATERIALS AND METHODS
Materials.
[3H(G)]Taurocholic acid (2 Ci/mmol), [1,2,6,7-3H(N)] DHEAS (74 Ci/mmol), and [6,7-3H(N)]estrone 3-sulfate (46 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). All other chemicals and reagents were purchased from Ambion (Austin, TX), Amersham Biosciences (Piscataway, NJ), Bio-Rad (Hercules, CA), Fermentas (Burlington, ON, Canada), Integrated DNA Technologies (Coralville, IA), Invitrogen (Carlsbad, CA), Jackson Immunology Research Laboratory (Bar Harbor, ME), Mallinckrodt Baker (Phillipsburg, NJ), New England Biolab (Beverly, MA), Perkin-Elmer (Foster City, CA), Qiagen (Valencia, CA), Roche (Mannheim, Germany), Sigma-Aldrich (St. Louis, MO), or Stratagene (La Jolla, CA).
Generation of Ostα−/− mice.
These mice were generated as previously described (17). Briefly, a targeting vector was designed to replace exons 3-9 of Ostα with a neomycin-containing cassette (Caliper Life Sciences/Xenogen, Cranbury, NJ). The final FtNwCD vector was linearized with NotI before electroporation into C57BL/6 embryonic stem (ES) cells (Caliper Life Sciences/Xenogen). G418-resistant ES clones were analyzed by Southern blot analysis, the selected clones injected into FVB blastocysts, and the blastocysts implanted into uteri of pseudo-pregnant females for generation of chimeras. Male chimeric mice were bred, and the resulting progeny were genotyped until heterozygous germline transmission was achieved. Heterozygous C57BL/6 animals (Ostα+/-) were bred to generate Ostα−/− and Ostα+/+ mice, and all animals were maintained on a standard laboratory diet (LabDiet; PMI Nutrition International, Saint Louis, MO) at the University of Rochester School of Medicine and Dentistry Vivarium. All experimental protocols were approved by the University of Rochester Animal Care and Use Committee, according to criteria outlined in the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences, as published by the National Institutes of Health (NIH publication 86-23, revised 1985). Genotyping was performed by PCR analysis of DNA isolated from tail biopsies. Genomic DNA was isolated from 0.5 cm of tail with DirectPCR-Tail lysis reagent (Viagen Biotech, Los Angeles, CA). A sample (1 μl) of crude lysate was used in a one-step genotyping PCR with primers for amplification of a ∼600-bp segment of the neomycin cassette (Fwd 5′-CTGTGCTCGACGTTGTCACTG.-3′ and Rev 5′-GATCCCCTCAGAAGAACTCGT.-3′), and an ∼800-bp region of Ostα genomic DNA containing exons 4 and 5 (Fwd 5′- ATTTGTGGTGTCAGTTCTCCTGTCT.-3′ and Rev 5′-TATTATTGGCTTTGCCCTACACAAG.-3′ designed with Primer Express 1.5).
Clinical chemistry analysis.
Female mice that had been fasted overnight were anesthetized with pentobarbital sodium (50 mg/kg ip), and blood was collected by cardiac puncture. Serum was separated by centrifugation for 15 min at 1,700 g at 4°C and was frozen at −20°C. Clinical chemistry analysis was performed by Charles River Laboratories (Wilmington, MA).
Bile acid analysis.
To assess total bile acid pool size, the liver, gallbladder, bile duct, and small intestine were removed from anesthetized Ostα+/+ and Ostα−/− female mice that had been fasted overnight. Feces were collected over a 24-h period from Ostα+/+ and Ostα−/− female mice that had unrestricted access to their normal diet. The tissues and feces were homogenized in ten volumes of 10 N NaOH using an Ultra-Turrax homogenizer (Janke & Kunkel IKA Labortechnik, Staufen im Breisgau, Germany). The insoluble material was removed through centrifugation at 1,700 g, and fecal lysates were also passed through a 0.45-μm nitrocellulose filter (Millipore, Billerica, MA). The samples were neutralized with HCl before analysis of total bile acids using an enzymatic assay (19).
Total mRNA isolation from mouse tissues.
Wild-type and Ostα−/− female mice that had been fed ad libitum were anesthetized with pentobarbital sodium (50 mg/kg ip). Liver, kidney, and small intestine were collected. The small intestine was divided into three equal sections along the cephalocaudal axis, and total mRNA was isolated using RNEasy Midi Kit (Qiagen).
Real-time quantitative reverse-transcriptase PCR analyses.
Gene-specific oligonucleotide primers (Table 1) were designed using Primer Express 1.5 (Applied Biosystems). Relative gene expression was determined on a Corbett Rotor-Gene 3000 real-time cycler (San Francisco, CA). All samples were analyzed in triplicate using Bio-Rad's iScript one-step reverse-transcriptase PCR kit with SYBR Green (catalog no. 170-8893). A sample (10 ng) of total RNA was analyzed per reaction with the expression of each gene being quantified via a standard curve containing 102–107 copies diluted in yeast total RNA. Expression levels were quantified as a ratio to mouse β-actin within each sample and reported as percent of control.
Table 1.
Primer sequences used for real-time RT-PCR analysis
| Primer Pair | Accesion Number | Temperature,°C | Forward Sequence (5′–3′) | Reverse Sequenece (5′–3′) | Amplicon Size, bp |
|---|---|---|---|---|---|
| Ntcp | NM_011387 | 62 | GCCACACTATGTACCCTACGTC | TTTAGTCGGAAGAGAGCAGAGA | 201 |
| Asbt | NM_011388 | 62 | GCACAAGCAGTGATGGATAAC | CCTAGGAACTTGTGGACTTCC | 191 |
| Shp1 | NM_011850 | 62 | AAGATACTAACCATGAGCTCCG | GTCTTGGCTAGGACATCCAAG | 218 |
| Fgf15 | NM_008003 | 60 | CAGTCTGTGTCAGATGAAGATCC | GATGGCAATCGTCTTCAGAG | 201 |
| Fxr | NM_009108 | 55 | TCCGGACATTCAACCATCAC | TCACTGCACATCCCAGATCTC | 101 |
| Cyp7a1 | NM_007824 | 62 | GACATGGAGAAGGCTAAGACG | CCAAGTAAATGGCATTCCCT | 193 |
| Bsep | NM_021022 | 62 | TGGTAGAGAAGAGGCGACAAT | TGAGGTAGCCATGTCCAGAA | 208 |
| Mrp2 | NM_013806 | 62 | TATAAATCTCAGTGGCGGTCAG | GAAGTGAATGCCATGTGTAACC | 199 |
| Mrp3 | NM_029600 | 62 | TGAAGACTGCACCGTACTGAC | AGAAACCCTTGGAATGCATC | 191 |
| Ostβ | NM_178933 | 60 | AGATGCGGCTCCTTGGAATTA | TGGCAGAAAGACAAGTGATG | 277 |
| β-actin | NM_007393 | 59 | ACCCTGTGCTGCTCACCGA | CTGGATGGCTACGTACATGGCT | 110 |
Ntcp, sodium-taurocholate cotransporting polypeptide; Asbt, apical sodium-bile acid transporter; Shp1, small heterodimer partner 1; Fgf15, fibroblast growth factor 15; Fxr, faresoid X receptor; Cyp7a1, cholesterol 7α-hydroxylase; Bsep, bile salt export pump; Mrp, multidrug resistance-associated protein; Ost, organic solute transporter.
Intraileal and intraperitoneal administration of [3H]estrone 3-sulfate, [3H]DHEAS, or [3H]taurocholic acid to anesthetized mice.
Mice that had been fed ad libitum were anesthetized by intraperitoneal administration of pentobarbital sodium (50 mg/kg), and additional anesthetic was administered as required for the duration of the experiment. For the intraileal injection, a median laparotomy was performed and 100 μl of 50 μM [3H]estrone 3-sulfate (1 μCi/100 μl) or 100 μM [3H]taurocholic acid (1 μCi/100 μl) was injected with a 27-gauge needle directly into the ileum (∼3 cm above the junction between small intestine and cecum). The experiment was terminated 15 min after [3H]taurocholic acid administration and 30 min after [3H]estrone 3-sulfate administration. [3H]estrone 3-sulfate [200 μl of 500 μM (1 μCi/200 μl), or 400 μl of 1 mM (1 μCi/400 μl)] and [3H]DHEAS (400 μl of 1 mM; 1 μCi/400 μl) were also administered intraperitoneally, and 2 h later tissues were collected. At the end of the experiments, 150–300 μl of blood were collected from the abdominal aorta in a tared 1-ml syringe containing 50 μl of heparin (1,000 U/ml). The small intestine was removed and segmented into three parts, where the first third after the stomach comprised the duodenum, the middle third the jejunum, and the section before the ileo-ceco-colic junction comprised the ileum. Colon, liver, gallbladder, kidney, and the urinary bladder were also removed and weighed. The contents of the small intestine, colon, gallbladder, and urinary bladder were included in the analysis. For the liver, ∼0.2–0.4 g of tissue was taken for analysis of radioisotope content. Tissue and blood samples were placed in tared 20-ml glass vials, and Solvable (Perkin-Elmer, Life and Analytical Sciences) was added to each vial (1.0 ml/0.1 g tissue or 2.0 ml/1.0 ml blood), which was then heated to 60°C for 2 to 3 h. The vials were allowed to cool to room temperature, and 0.1 ml of 0.1 M EDTA/1.0 ml of Solvable was added to each vial of blood. H2O2 (30%) was then added in 0.1-ml aliquots (0.3 ml/1.0 ml Solvable for blood or 0.1 ml/1.0 ml Solvable for tissues). After standing at room temperature for 15 to 30 min, the vials were capped tightly and heated to 60°C for 1 h and allowed to cool back to room temperature. After the addition of scintillation fluid (4 ml of Opti-Fluor to 200 μl of each sample) (Perkin-Elmer, Life and Analytical Sciences), the samples were allowed to stand for at least 2 h at room temperature before counting. Samples were counted in a Beckman LS 6500 scintillation counter (Beckman Coulter, Fullerton, CA). Total blood volume was estimated as 6% of body weight.
Statistical analyses.
Data are given as means ± SE. Mean values were considered to be significantly different, when P < 0.05 by use of a one-way ANOVA followed by Bonferroni's multiple comparison test or a Student's t-test where applicable.
RESULTS
Ostα-deficient mice are viable and fertile but exhibit growth retardation and small intestinal hypertrophy.
As described in a recent report (17), a conventional gene-targeting approach was used to replace exons 3-9 of Ostα with a neomycin-containing cassette in the C57BL/6 background to generate Ostα−/− mice. Western blotting confirmed that these mice lack Ostα protein, but, interestingly, these animals also lack the obligate heterodimerization partner of the transporter, namely Ostβ protein, indicating that Ostβ is not stable in the absence of Ostα (17).
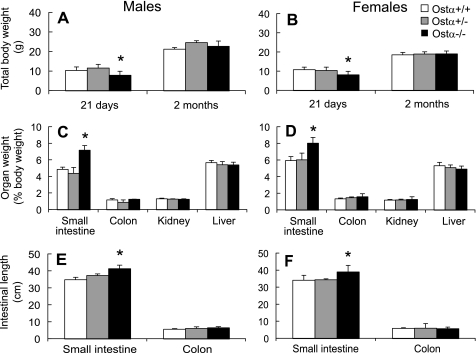
The Ostα−/− mice were found to be viable and fertile, and matings between heterozygous animals produced the predicted Mendelian distribution of genotypes. Ostα−/− mice exhibited no gross abnormalities up to 1.8 years of age and were able to thrive on normal rodent laboratory chow. However, preweanling Ostα−/− mice of both sexes were about 25% smaller than wild-type or heterozygous (Ostα+/−) littermates, although this difference in weight disappeared shortly after weaning (Fig. 1, A and B). Analysis of organ size revealed that the small intestine was heavier and longer in the Ostα−/− mice, suggesting intestinal hypertrophy, whereas the other organs analyzed were similar in weight (Fig. 1). No gross pathology was noted in the small intestine of Ostα−/− mice (data not shown).
Fig. 1.
Preweanling organic solute transporter (Ost)α−/− mice are smaller than Ostα+/+ mice, and Ostα−/− mice have heavier and longer small intestines. A and B: weights of male and female Ostα+/+, Ostα+/-, and Ostα−/− mice at 21 days of age and as adults (2 mo of age). C and D: organ weights expressed as a percentage of body weight. E and F: length of the intestines. Values are means ± SE, n > 3. *Statistically significant from Ostα+/+ animals, P < 0.05.
Bile acid pool size and serum levels are markedly lower in Ostα-/- mice, but fecal bile acid excretion is unchanged.
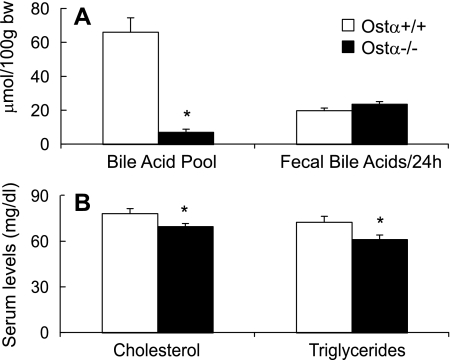
If Ostα-Ostβ is the critical intestinal basolateral bile acid transporter, one would predict that Ostα−/− mice should have higher fecal bile acid excretion rates and lower tissue bile acid levels. Because the vast majority of the bile acids are present in the liver, gallbladder, bile duct, and small intestine, these tissues were combined to assess the total bile acid pool size. The results reveal that the bile acid pool size in Ostα−/− mice was ∼90% smaller (Fig. 2A) and that serum levels were about 60% lower compared with wild-type mice (Table 2). However, fecal bile acid excretion rates were similar (Fig. 2A), suggesting a defect in intestinal bile acid absorption.
Fig. 2.
The bile acid pool size, serum cholesterol, and triglycerides are decreased in Ostα−/− mice. A: bile acid pool size was assessed by measuring total bile acids in the liver, gallbladder, bile duct, and small intestine of female mice that were fasted overnight. Fecal bile acids were measured in female mice that had been fed ad libitum and are expressed as μmol of bile acids excreted per 24 h, per 100 g body wt. B: cholesterol and triglyceride levels in serum of overnight fasted female mice. Values are means ± SE, n > 4. *Statistically significant from Ostα+/+ animals, P < 0.05.
Table 2.
Serum clinical chemistry profile of Ostα+/+ and Ostα−/− mice
| Ostα+/+ | Ostα−/− | |
|---|---|---|
| Bile acids, μmol/l | 7.2±1.0 | 2.8±0.3* |
| Total bilirubin, mg/dl | 0.4±0.1 | 0.3±0.1 |
| Glucose, mg/dl | 205±15 | 248±13* |
| Phosphorus, mg/dl | 8.7±0.6 | 8.7±0.5 |
| Total protein, g/dl | 5.0±0.2 | 4.9±0.2 |
| Calcium, mg/dl | 8.8±0.2 | 9.0±0.1 |
| Blood urea nitrogen, mg/dl | 31±2 | 27±1 |
| Creatinine, mg/dl | 0.19±0.03 | 0.20±0.01 |
| Albumin, g/dl | 2.8±0.1 | 2.8±0.1 |
| Alanine aminotransferase, U/l | 85±26 | 90±23 |
| Aspartate aminotransferase, U/l | 462±107 | 590±128 |
| Alkaline phosphatase, U/l | 158+17 | 149+10 |
Values are means ± SE, n = 8 female mice for each genotype. The animals were starved overnight.
Statistically significant from Ostα+/+ mice, P < 0.05.
Decreased ileal absorption of [3H]taurocholate and [3H]estrone 3-sulfate in Ostα−/− mice.
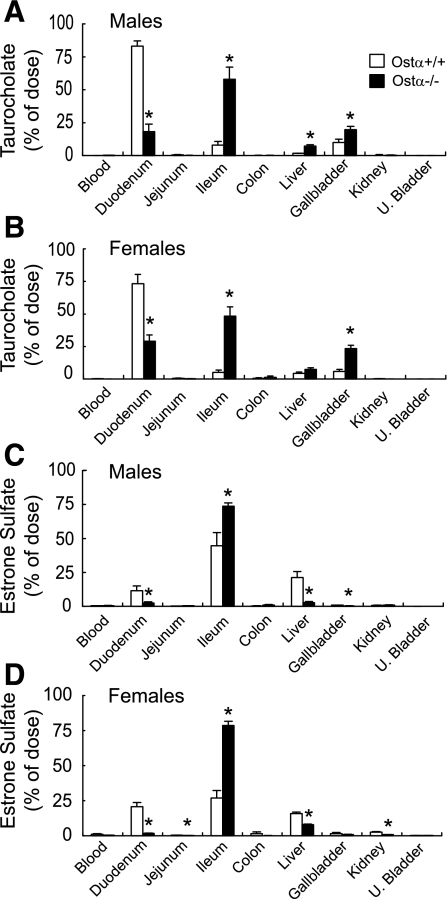
To examine the role of Ostα-Ostβ in the ileal absorption of a bile acid and a steroid conjugate, [3H]taurocholic acid and [3H]estrone 3-sulfate were injected directly into the ileal lumen of anesthetized male and female Ostα+/+ and Ostα−/− mice, and tissue distribution of radioactivity was measured at short time points thereafter (15 min for taurocholate and 30 min for estrone 3-sulfate). Ileal absorption of both compounds was lower in Ostα−/− mice (Fig. 3), indicating that Ostα-Ostβ is important for this process. In particular, the amount of taurocholate absorbed from the ileum of Ostα−/− mice was 60–80% lower than in the wild-type animals (Fig. 3, A and B). Of the taurocholate that was absorbed by the Ostα−/− mice, a significant fraction was found in the liver and gallbladder at the 15-min time point (Fig. 3, A and B), whereas, in the wild-type animals, most of the taurocholate had already reached the duodenum by this time point. Estrone sulfate was absorbed more slowly than taurocholate by both wild-type and Ostα−/− mice: only about 60–70% of the estrone sulfate was removed from the ileum of wild-type animals in 30 min vs. >90% absorption for taurocholate in only 15 min (Fig. 3). Estrone sulfate absorption was markedly lower in the Ostα−/− mice compared with the wild-type mice (Fig. 3, C and D). Given that only a small fraction of the estrone sulfate was absorbed from the ileum of Ostα−/− mice (i.e., 20–30%), relatively little radioactivity was recovered in the tissues from these animals (Fig. 3, C and D).
Fig. 3.
Decreased ileal absorption of taurocholate and estrone 3-sulfate in Ostα-deficient mice. [3H]taurocholate (0.01 μmol/100 μl) was injected directly into the ileal lumen of Ostα+/+ and Ostα−/− male (A) and female (B) mice that had been fed ad libitum. Tissue distribution of [3H]taurocholate was determined after 15 min. [3H]estrone 3-sulfate (0.005 μmol/100 μl) was injected directly into the ileal lumen of Ostα+/+ and Ostα−/− male (C) and female (D) mice. Tissues were collected for analysis of radioactivity after 30 min. Values are means ± SE, n > 3; *statistically significant from Ostα+/+ animals, P < 0.05.
Note, however, that these data also demonstrate that alternate or compensatory mechanisms are present in the Ostα−/− mice that allowed taurocholate and estrone sulfate to still be absorbed, albeit less efficiently than in wild-type animals.
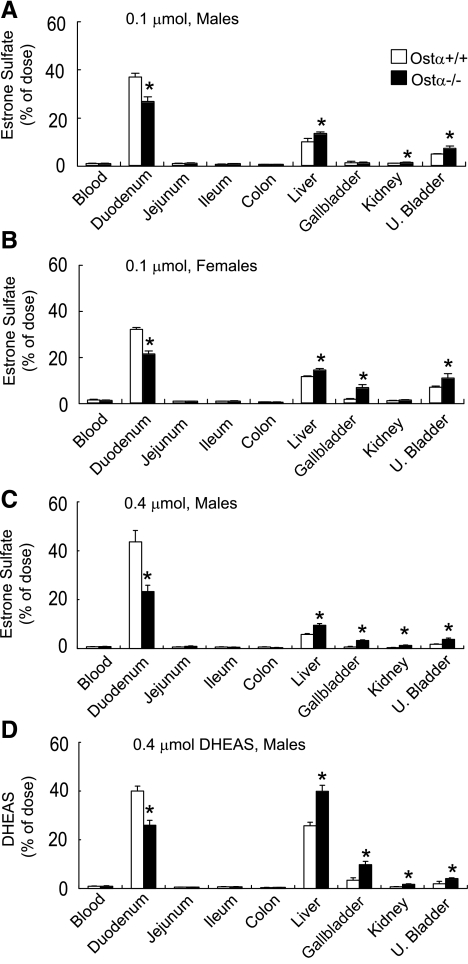
Altered tissue distribution of [3H]estrone 3-sulfate and [3H]DHEAS in Ostα−/− mice.
To characterize the role of Ostα-Ostβ in the in vivo disposition of known substrates for this transporter, the tissue distribution of [3H]estrone 3-sulfate and [3H]DHEAS was measured at 2 h after their intraperitoneal administration (Fig. 4). The Ostα−/− mice had less radioactivity in their small intestine and more in liver, kidney, and urinary bladder (Fig. 4), indicating altered disposition of these steroids and/or their metabolites. These observations support the hypothesis that Ostα-Ostβ is a major sterol transporter in the intestine, kidney, and biliary epithelia (3). In the kidney, the absence of Ostα-Ostβ is expected to diminish sterol reabsorption and to thus increase urinary excretion of these compounds, and this hypothesis is supported by the data illustrated in Fig. 4. Although expression of Ostα-Ostβ is low in the whole mouse liver, these proteins are present in mouse cholangiocytes (3), and thus this transporter may contribute to hepatobiliary disposition of bile acids and related molecules in the mouse (Fig. 4).
Fig. 4.
Altered distribution of estrone 3-sulfate and dehydroepiandrosterone sulfate (DHEAS) in Ostα-deficient mice. Tissue distribution of [3H]estrone 3-sulfate in male (A) and female (B) mice that were injected intraperitoneally with 0.1 μmol/200 μl of this compound. Tissues were collected for analysis of radioactivity after 2 h. [3H]Estrone 3-sulfate (0.4 μmol/400 μl) was injected intraperitoneally into male mice (C), and tissues were collected for analysis of radioactivity after 2 h. [3H]DHEAS (0.4 μmol/400 μl) was injected intraperitoneally into male mice (D), and tissues were collected for analysis of radioactivity after 2 h. All of the animals for these studies were fed ad libitum until the time of experimentation. Values are means ± SE, n > 3; *statistically significant from Ostα+/+ animals, P < 0.05.
Serum cholesterol and triglyceride levels are also lower in Ostα−/− mice.
Because bile acids are required for intestinal lipid absorption, the diminished ability to absorb bile acids in Ostα−/− mice may lead to a decrease in lipid absorption and possibly to decreased serum lipid levels. Indeed, as illustrated in Fig. 2B, serum cholesterol and triglyceride levels were lower in the Ostα−/− mice (∼15% lower), whereas other serum parameters were generally similar (Table 2).
Gene expression profiling in Ostα−/− intestine, liver, and kidney.
To further examine the functional significance of Ostα, and to assess whether expression of other putative bile acid and organic anion transporters is altered to compensate for the loss of Ostα-Ostβ in Ostα−/− mice, real time RT-PCR was used to analyze transcript abundance of some of the key genes involved in bile acid homeostasis in the small intestine, kidney, and liver (Fig. 5). Because of the marked functional differences along the length of the small intestine, this tissue was divided into three equal sections, with the distal section representing mainly the ileum, and transcript abundance was measured in each section (Fig. 6).
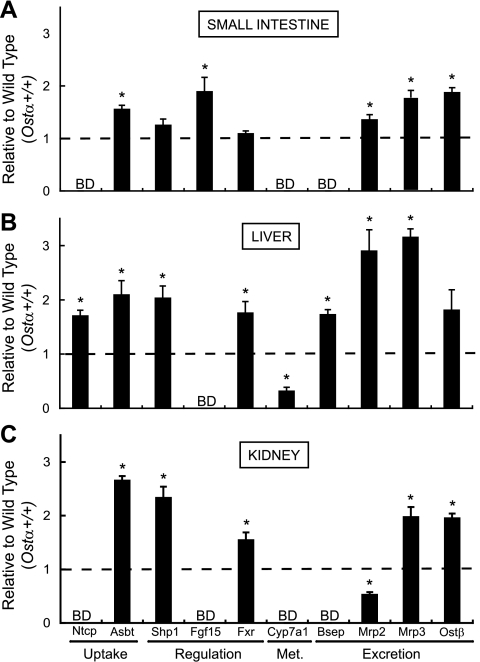
Fig. 5.
Gene expression analysis of small intestine, liver, and kidney in Ostα−/− animals. Total RNA of these tissues was isolated from Ostα+/+ and Ostα−/− female mice that had been fed ad libitum, and mRNA levels were analyzed via real time RT-PCR. mRNA expression levels in the small intestine (A), liver (B), and kidney (C) are functionally categorized into uptake, regulation, metabolism (Met), and excretion groupings and reported relative to the expression in Ostα+/+ animals. All samples were analyzed in triplicate, and values are means ± SE, n = 4 experiments. *Statistically significant from Ostα+/+ animals, P < 0.05; BD, below detection limit; Ntcp, sodium-taurocholate cotransporting polypeptide; Asbt, apical sodium-bile acid transporter; Shp1, small heterodimer partner 1; Fgf15, fibroblast growth factor 15; Fxr, farnesoid X receptor; Cyp7a1, cholesterol 7α-hydroxylase; Bsep, bile salt export pump; Mrp, multidrug resistance-associated protein.
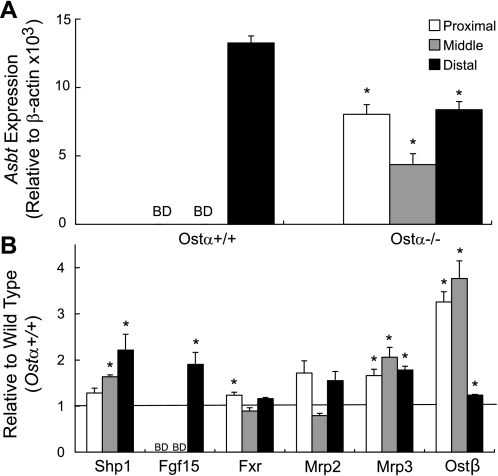
Fig. 6.
Alterations of intestinal gene expression patterns in Ostα−/− animals. The small intestine of female Ostα+/+ and Ostα−/− mice was divided into 3 sections of equal length. Total RNA was isolated, and expression levels of the indicated genes were subsequently determined by real-time RT-PCR. A: Asbt mRNA expression levels in the proximal, middle, and distal segments of the small intestine. Changes in Asbt expression in the Ostα+/+ and Ostα−/− mice are reported relative to β-actin. B: individual gene expression levels along the intestinal tract are reported relative to wild-type animals. Samples were analyzed in triplicate, and values are means ± SE, n = 4. *Statistically significant from Ostα+/+ animals, P < 0.05.
If the proposed function of Ostα-Ostβ is correct, loss of this transporter should lead to the accumulation of bile acids in ileocytes, the major site of bile acid absorption, and thus to altered expression of bile acid-regulated genes. As expected, Asbt expression was decreased in the terminal portion of the small intestine (composed primarily of ileum) but was increased in proximal segments of the small intestine (Fig. 6A), as well as in liver and kidney (Figs. 5, B and C, respectively). Expression of multidrug resistance-associated protein 3 (Mrp3), a known alternative bile acid export pump, was upregulated in all three tissues analyzed (Fig. 5). In addition, Ntcp, short heterodimer partner (Shp)1, Fxr, bile salt export pump (Bsep), and Mrp2 were increased in liver (Fig. 5B), and Shp1, Fxr, and Ostβ were increased in kidney (Fig. 5C), indicating altered bile acid homeostasis. Note that Mrp2 mRNA was higher in liver but was lower in kidney (Fig. 5), indicating differential tissue regulation of this organic anion export pump.
Of significance, these data also demonstrate that intestinal expression of fibroblast growth factor 15 (Fgf15), a key negative transcriptional regulator of the rate-limiting enzyme in bile acid synthesis (i.e., cholesterol 7α-hydroxylase, Cyp7a1), was increased (Fig. 5), an effect that was seen mainly in the distal portion of the small intestine (Fig. 6B).
Because Fgf15 is a negative regulator of Cyp7a1 expression (12–14), one would predict that the increased levels of this growth factor may then decrease hepatic Cyp7a1, despite the smaller bile acid pool size in Ostα−/− mice. Indeed, as illustrated in Fig. 5B, hepatic Cyp7a1 transcript abundance was markedly lower in Ostα−/− mice. Overall, these changes in gene expression support the functional data described above and provide insight into possible compensatory mechanisms.
DISCUSSION
The present study provides direct support for the hypothesis that Ostα-Ostβ is a major basolateral transporter of bile acids and structurally related molecules and demonstrates that impairment of this transport step leads to a marked decrease in the bile acid pool size and serum bile acid levels and is associated with lower serum cholesterol and triglyceride levels. These observations provide key insights into mechanisms of bile acid and sterol disposition and have significant implications for human conditions related to imbalances in bile acid or lipid homeostasis.
Given the importance of bile acids to overall lipid balance, much effort has been devoted to the identification of mechanisms of bile acid transport and to the development of strategies for altering bile acid disposition. The present observations in Ostα−/− mice demonstrate that Ostα-Ostβ plays a key role in bile acid homeostasis but also indicate that other transport mechanisms are able to compensate for the loss of this function.
The present findings also identify Ostα-Ostβ as a potential therapeutic target for interrupting the enterohepatic circulation of bile acids. In this regard, the one approach that has been used successfully in humans since the 1960s is the oral administration of nonabsorbable bile acid-binding resins or polymers, which sequester the bile acids in the intestinal lumen and thus prevent their absorption (4). Other agents have also been evaluated as either inhibitors of intestinal bile acid absorption, including direct Asbt inhibitors, or as agonists/antagonists of the transcription factors that regulate bile acid levels, but none are presently available for human use (4). When intestinal bile acid uptake is interrupted with either bile acid sequestrants or with Asbt inhibitors, this leads to an increase in hepatic bile acid synthesis (7, 22), whereas the converse is seen in the Ostα−/− mice, namely hepatic Cyp7a1 expression is decreased (Fig. 5B). As described in more detail below, these contrasting effects are most likely explained by the enhanced intestinal expression of Fgf15, an important negative regulator of hepatic bile acid synthesis (12), when Ostα-Ostβ function is abrogated.
Bile acid concentrations are normally controlled by a feedback regulatory mechanism, whereby bile acid activation of Fxr represses hepatic transcription of Cyp7a1 levels and thus leads to a decrease in bile acid synthesis. Bile acid activation of Fxr also leads to decreased expression of the bile acid uptake transporters Asbt and Ntcp, and to increased expression of the bile acid exporters Bsep and Ostα-Ostβ. Collectively, these transport proteins, along with the enzyme Cyp7a1, mediate a decrease in intracellular bile acid concentrations back to basal levels. Ileocytes, however, also express Fgf15, another key regulator of bile acid synthesis (12). When intracellular bile acid levels in ileocytes are elevated (as they are expected to be in the Ostα−/− mice), this leads to the Fxr-mediated induction of Fgf15 (12). Fgf15 is then delivered to the liver, where it represses Cyp7a1 expression through a mechanism that involves Fgf receptor 4 (Fgfr4) and the orphan nuclear receptor Shp (12). As illustrated in Fig. 5, intestinal expression of Fgf15 was higher and hepatic expression of Cyp7a1 was lower in Ostα−/− mice, as predicted by this model.
These observations in Ostα−/− mice contrast with those seen in Asbt−/− mice (7). The absence of Asbt leads to a diminished ability of the enterocytes to take up bile acids across their apical membranes and thus to relatively low intracellular bile acid levels. These low bile acid levels downregulate Fgf15 expression and thus relieve Fgf15-mediated repression of Cyp7a1 transcription. In addition, the low hepatocellular bile acid levels in Asbt−/− mice also relieve the Fxr- and Shp-mediated repression of hepatic Cyp7a1 activity, with the net result being a fivefold increase in Cyp7a1 expression and in bile acid synthesis rate in Asbt−/− mice (7).
Another significant difference between Ostα−/− and Asbt−/− mice relates to contrasting effects on serum cholesterol levels: serum total cholesterol is decreased in Ostα−/− mice (Fig. 2) but increased in Asbt−/− mice (7). The reason for this difference remains to be elucidated but may also be related to altered bile acid and cholesterol homeostasis. In Asbt−/− mice, the increased Cyp7a1-mediated cholesterol utilization leads to a decrease in hepatic cholesteryl ester levels (7), which may stimulate either cholesterol synthesis or absorption, whereas the opposite is likely occurring in Ostα−/− mice. Additional studies are needed to test this hypothesis.
The present findings also indicate that serum glucose levels are elevated in Ostα−/− mice (Table 2). Although the mechanism for this increase is not clear, it may be related to the link between bile acid levels and glucose metabolism (6, 9). In particular, bile acids inhibit transcription of the gene encoding phosphoenolpyruvate carboxykinase (6, 9), the rate-limiting enzyme in gluconeogenesis, and thus the low bile acid levels in Ostα−/− mice may relieve this inhibition and result in higher glucose levels.
Although the present data indicate that Ostα-Ostβ is essential for sterol disposition, they also indicate that alternate absorptive mechanisms are able to partially compensate for the loss of this transporter, at least in adult mice. These compensatory mechanisms include intestinal hypertrophy (Fig. 1) and increased expression of alternate transport proteins (Figs. 5 and 6). The increased intestinal length, and presumably increased surface area, would provide a longer transit time in the intestine and thus would facilitate absorption by other mechanisms. The increased expression of Mrp3/ATP-binding cassette C (Abcc)3, an alternate basolateral bile acid export pump, and perhaps of other auxiliary transporters may subsume some of the sterol absorptive functions in the absence of Ostα-Ostβ.
In contrast to the adult mice, these compensatory gene expression changes may be inefficient in preweanling mice and may thus explain the growth delay of the Ostα−/− mice. Indeed, expression of Mrp3 and of some other Mrps is known to be very low in neonatal mice and reaches adult levels only after weaning (18, 24). Likewise, expression of the nuclear receptors that are involved in bile acid and sterol homeostasis, including Fxr, are expressed at low levels in neonatal animals (1). The low expression of these transcription factors and alternate sterol transporters in neonatal mice may account for the inability of Ostα-deficient mice to compensate for the lack of bile acid absorption; however, additional studies are needed to test these possibilities.
Taken together, the present findings indicate that Ostα-Ostβ is a major basolateral transporter of bile acids and conjugated steroids in the intestine, kidney, and liver and suggest that the resulting changes in bile acid levels have an effect on serum cholesterol, triglyceride, and glucose levels. In addition, these results indicate that targeted inhibition of Ostα-Ostβ may have advantages over other maneuvers that have been used to interrupt the enterohepatic circulation of bile acids.
GRANTS
This work was supported in part by National Institute of Health Grants DK067214 and DK48823, and National Institute of Environmental Health Sciences Training Grant ES07026 and Center Grants ES03828 and ES01247.
Acknowledgments
We thank Michael Madejczyk, Jin Young Lee, and Sylvia Notenboom for assistance with some of the analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Balasubramaniyan N, Shahid M, Suchy FJ, Ananthanarayanan M. Multiple mechanisms of ontogenic regulation of nuclear receptors during rat liver development. Am J Physiol Gastrointest Liver Physiol 288: G251–G260, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Ballatori N Biology of a novel organic solute and steroid transporter, OSTα-OSTβ. Exp Biol Med (Maywood) 230: 689–698, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTα-OSTβ, a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42: 1270–1279, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bays H, Stein EA. Pharmacotherapy for dyslipidaemia—current therapies and future agents. Expert Opin Pharmacother 4: 1901–1938, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JL, Trauner M, Mennone A, Soroka CJ, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTα-OSTβ in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol 290: G1124–G1130, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Claudel T, Staels B, Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol 25: 2020–2030, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278: 33920–33927, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ostα-Ostβ, is an ileal basolateral bile acid transporter. J Biol Chem 280: 6960–6968, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem 278: 39124–39132, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter alpha-beta, Ostα-Ostβ, by bile acids. Am J Physiol Gastrointest Liver Physiol 290: G912–G922, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Geyer J, Wilke T, Petzinger E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmiedebergs Arch Pharmacol 372: 413–431, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ, Moschetta A. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res 48: 2693–2700, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48: 2664–2672, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am J Physiol Gastrointest Liver Physiol 290: G476–G485, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res 47: 201–214, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking, and membrane topology of the two proteins, Ostα and Ostβ, that constitute the organic solute and steroid transporter. Biochem J 407: 363–372, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos 33: 947–955, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Mashige F, Tanaka N, Maki A, Kamei S, Yamanaka M. Direct spectrophotometry of total bile acids in serum. Clin Chem 27: 1352–1356, 1981. [PubMed] [Google Scholar]
- 20.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol 64: 635–661, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter α-β, Ostα-Ostβ, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci USA 105: 3891–3896, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell DW The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTα-OSTβ. J Biol Chem 278: 27473–27482, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Tomer G, Ananthanarayanan M, Weymann A, Balasubramanian N, Suchy FJ. Differential developmental regulation of rat liver canalicular membrane transporters Bsep and Mrp2. Pediatr Res 53: 288–294, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83: 633–671, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Seward DJ, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci USA 98: 9431–9436, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-α/β in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290: G923–G932, 2006. [DOI] [PubMed] [Google Scholar]