Abstract
Several lines of evidence indicate that Group II metabotropic glutamate receptor (mGluR) activation can depress sensory transmission. We have reported the expression of Group II mGluRs on unmyelinated axons, many of which were presumed to be nociceptors, in the rat digital nerve (Carlton et al., 2001b). The goals of the present study are to further our understanding of Group II modulation of nociceptor processing in the periphery, documenting behavioral changes using inflammatory models and documenting, for the first time, cutaneous single fiber activity following exposure to a Group II agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC) and antagonist LY341495 (LY). The data indicate that peripheral Group II mGluR activation does not depress nociceptive behaviors or nociceptor fiber responses in the non-sensitized state (i.e. following brief nociceptive mechanical or thermal stimulation) but can depress these responses when nociceptors are sensitized by exposure to formalin or inflammatory soup. Group II mGluR agonist-induced inhibition can be blocked by a selective Group II antagonist. Peripheral Group II mGluR-induced inhibition evoked in these studies occurs through activation of local receptors and not through spinal or supraspinal mechanisms. The data indicate that administration of selective Group II agonists may be potent therapeutic agents for prevention of peripheral sensitization and for treatment of inflammatory pain.
Keywords: Group II mGluR, pain, APDC, LY341495, formalin, inflammation, inflammatory soup
Glutamate and glutamate receptors play an important role in peripheral pain mechanisms in animals (Carlton, 2001; Carlton et al., 2003) and humans (McNearney et al., 1999; McNearney et al., 2000; Cairns et al., 2001; Alfredson et al., 2001; Alfredson and Lorentzon, 2002; Svensson et al., 2003; Cairns et al., 2003). To date, ionotropic glutamate receptors (iGluRs) including N-methyl-D-aspartate (NMDA) and non-NMDA receptors are implicated in peripheral cutaneous pain mechanisms (Carlton et al., 2003; Bleakman et al., 2006).
More recent studies indicate that metabotropic glutamate receptors (mGluRs) can modulate peripheral nociceptor function. In contrast to iGluRs, which are ligand-gated ion channel receptors, mGluRs mediate their function via G-protein coupled receptors which trigger intracellular second-messenger systems (Pin and Duvoisin, 1995). According to sequence similarity, transduction mechanisms and pharmacology, mGluRs have been classified into 3 groups: Group I receptors (mGluRs 1 and 5) are coupled to Gq/11 proteins and activate phospholipase C, enhancing neuronal excitability; Group II (mGluRs 2 and 3) and III receptors (mGluRs 4, 6, 7 and 8) are coupled to Gi/o proteins, lead to inhibition of adenylate cyclase, inhibiting cyclic adenosine monophosphate (cAMP) formation and cAMP-dependent protein kinase A, enhancing neuronal inhibition (Schoepp and Conn, 1993; Pin and Duvoisin, 1995; Conn and Pin, 1997).
Our lab has been elucidating the role of Group II mGluRs expressed by dorsal root ganglion (DRG) cells. Approximately 40–50% of lumbar DRG cells express Group II mGluRs and these are mainly small to medium sized cells (mean diameter = 23μM) (Carlton et al., 2001b; Carlton and Hargett, 2007). The majority of DRG cells expressing Group II mGluRs (76%) stain positively for isolectin Griffonia Simplicifolia (I-B4) (Carlton et al., 2001b), suggesting that these cells either express low levels of peptides or they are non-peptidergic (Silverman and Kruger, 1990). Furthermore, 32% and 28% of unmyelinated and myelinated primary afferent axons, respectively, label for Group II mGluRs in the rat digital nerve (Carlton et al., 2001b), indicating that the receptors are transported out of the cell bodies to the peripheral terminals. Consistent with this anatomical localization, intraplantar injection of a Group II agonist blocks prostaglandin E2-induced thermal (Yang and Gereau, 2002) and mechanical (Yang and Gereau, 2003) sensitization in mice. The goals of the present study are to further our understanding of Group II modulation of nociceptor processing in the periphery, documenting behavioral changes and single fiber activity using 2 different inflammatory models and a Group II agonist and antagonist. Some of these data have been previously presented in abstract form (Zhou and Carlton, 2005; Du and Carlton, 2005).
EXPERIMENTAL PROCEDURES
All experiments were approved by the University Animal Care and Use Committee and followed the IASP guidelines for the ethical care and use of laboratory animals (Zimmermann, 1983). Steps were taken to minimize both the number of animals and their discomfort. All rats were male, Sprague Dawley (250–300g) obtained from Harlan, Indianapolis, IN.
Behavioral studies
Habituation
Rats were housed in groups of three in plastic cages with soft bedding under a reversed light/dark cycle of 12h/12h. Following arrival at the animal care facility, they were acclimated for at least 3 days before any behavioral testing was initiated. To determine whether the Group II agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC) altered mechanical sensitivity, naïve rats were habituated to testing with a modified Randall Selitto apparatus (Analgesy-Meter, Ugo Basile, Italy), by being placed in a plastic cone and held upright so that the hind quarters extended from the cone and were supported in the palm of the investigators’ hand. One hind paw was placed on a small plinth and a mechanical force on the dorsal surface of the paw was slowly increased until the rat withdrew its paw. This habituation was repeated 2 times on 2 consecutive days. To determine whether APDC altered formalin-induced behaviors or inflammatory soup (IS)-induced mechanical sensitivity (von Fry testing), rats were placed on a wire screen platform in Plexiglas cages (8 × 8 × 18 cm) for 1 hr. Each rat was habituated two times before being placed in an experimental group. To determine whether APDC altered thermal sensitivity in naïve or IS-injected rats, they were habituated by being placed in a Plexiglas cage on a 1/4″ thick glass plate maintained at 23°C. One hind paw of each rat was tested for heat sensitivity using a laser stimulus (see below) every 10 min for 40 min on each of 5 consecutive days.
Drug Injections
Intraplantar drug injections were performed using a 28-gauge needle attached to 50 μl Hamilton syringe with PE20 tubing. For formalin testing, awake rats received a needle puncture in the plantar skin proximal to the footpads and the needle was guided into the subcutaneous space to the center of the hind paw. For all other hind paw injections, rats were briefly anesthetized (2–3 min) with 4% halothane. In experiments testing mechanical sensitivity, the skin of the paw proximal to the footpads was penetrated and the needle tip advanced about 1 cm to the base of the third toe. In experiments testing thermal sensitivity, the needle punctured the plantar skin near the heel and was guided into the subcutaneous space to the center of the hind paw. To ensure that anesthetic effects had worn off, rats were not tested for mechanical or thermal sensitivity until at least 10 min following drug injection. For all experiments, each rat was used only once and the experimenter was unaware of which drug was being injected into each animal. All behavior data were assessed in real time and recorded by direct observation by an investigator.
The Group II agonist APDC (Tocris Cookson Ltd., Ellisville, MO) was dissolved in 1N NaOH (made in 100mM as a stock solution). The highly selective Group II antagonist, (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495, [LY], Tocris Cookson) (Thomas et al., 1996; Schoepp et al., 1999), was used to demonstrate receptor specificity of the APDC effect. LY was dissolved in 1.2N NaOH (made as a 100 mM stock solution). All stock solutions were diluted with PBS (GibcoTM) to different concentrations as needed in the studies and adjusted to pH 7.4. A 2% formalin solution (FM) was made by diluting the FM stock solution with PBS. An inflammatory soup (IS) cocktail was made in synthetic interstitial fluid (SIF) that contained 10 nM bradykinin, 1 μM 5HT, 1 μM histamine, 1 μM PGE2, and 7mM potassium chloride (Bolyard et al., 2000).
Group II mGluR modulation of acute mechanical and thermal sensitivity
To determine if activation of Group II mGluRs affected acute mechanical nociception, rats received 20 μl intraplantar injections of 0.1 or 100 μM APDC or vehicle and mechanical sensitivity was assessed using the modified Randall Selitto test. The paw withdrawal threshold (PWT) was determined before and then at 10 min intervals for 40 min following drug injection and is reported as percent change from baseline.
To determine if activation of Group II mGluRs affected acute thermal nociception, rats received 20 μl intraplantar injections of 0.1 or 100 μM APDC or vehicle and thermal sensitivity was assessed by measuring paw withdrawal latency (PWL) (Hargreaves et al., 1988). The laser system (assembled in-house) consisted of a microprocessor-controlled 980 nm (near infrared) continuous wave, solid-state laser (4 watt) with a spot diameter of 2 mm. Parameters were set to obtain ~8 sec PWLs (within 10 sec the laser reached a maximum of 51°C at a glass surface maintained at 23°C, with a cutoff at 15 sec). A red (670 nm) sighting beam was coupled to the invisible laser to allow positioning. The PWL was determined before and then at 10, 30 and 50 min following drug injection.
Group II mGluR modulation of FM-induced nociceptive behaviors
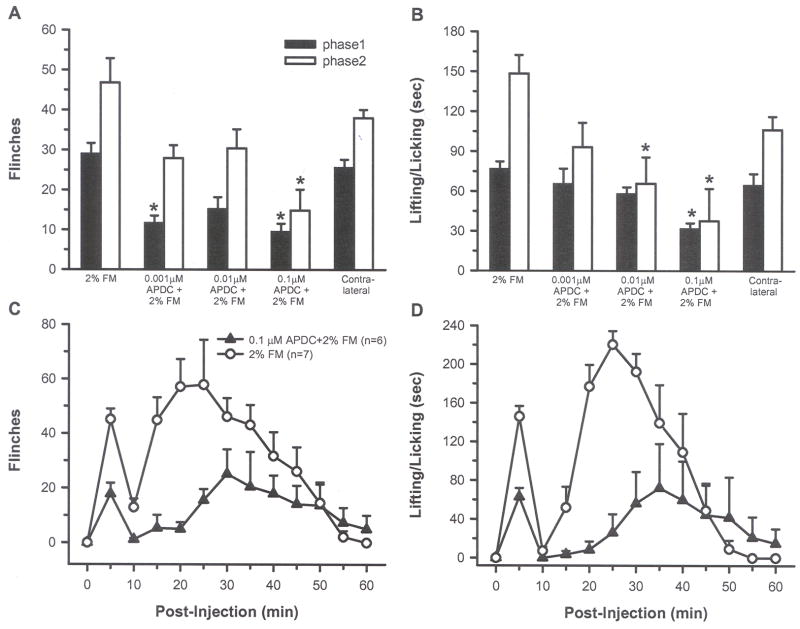
To demonstrate the effect of Group II mGluR activation on acutely sensitized nociceptors, and to establish a working dose of APDC, rats were injected with either 20 μl of 2% FM alone (n = 7), or a 20 μl cocktail containing 2% FM+APDC (0.001, 0.01, or 0.1 μM, n = 6/group). Possible systemic effects produced by intraplantar APDC were evaluated by injection of 20μl of the working dose of APDC (0.1 μM) in one paw, followed immediately by injection of 20 μl of 2% FM into contralateral paw (n = 6). To demonstrate that actions of APDC were receptor specific, a 20 μl cocktail of 100 μM LY (LY341494, a Group II antagonist) + 0.1 μM APDC + 2% FM was injected in the hind paw (n = 6/group). To determine if LY alone produced any effects on FM-induced nociception, a 20 μl cocktail containing 100 μM LY + 2% FM was injected in the hind paw (n = 6). PBS was injected in some groups to keep volumes equivalent. Formalin-induced nociception was assessed by quantitating two behaviors: counting the number of flinches and the number of sec an animal spent lifting and/or licking (L/L) the injected hind paw in 5-min intervals. Flinching was defined as a rapid jerk of the whole paw whether the paw was on the screen or held in the air. The data for the time course was recorded as the number of flinches and number of sec of L/L per 5 min interval over 60 min.
There are two phases in FM-induced pain. The first phase is from 0–10 min after FM injection, the second phase is from 10 to approximately 40 min. The data for each phase are reported as the average number of flinches or number of sec spent L/L per 5 min interval in each phase and the following formulas were used to calculate these averages: 1) first phase: [total number of flinches or L/L time (sec)] /2 (two 5 min intervals in 10 min); 2) second phase: [total number of flinches or L/L time (sec)] /6 (six 5 min intervals between 10–40 min).
Group II mGluR modulation of IS-induced behavioral sensitization
An intraplantar injection of IS was used to induce sensitization of peripheral terminals. To determine if activation of Group II mGluRs attenuated the thermal hyperalgesia, rats were briefly anesthetized and given a 20 μl injection of vehicle (n = 7), IS alone (n = 7), 0.1 μM APDC + IS (n = 7), or IS in one paw and 0.1 μM APDC in the contralateral paw (n = 6). All volumes and concentrations were kept constant by adding PBS. Thermal sensitivity was assessed by measuring paw withdrawal latency (PWL) at 10, 30 and 50 min using the solid state laser system described above. To determine if activation of Group II mGluRs attenuated the IS-induced mechanical allodynia, a cocktail of APDC + IS was given intraplantar but the APDC did not effectively block the IS-induced mechanical sensitivity. Using in a different approach where the drugs were injected separately, rats were briefly anesthetized and given 2 10μl injections, 10 min apart: PBS followed by PBS, PBS followed by IS, or 0.5 μM APDC followed by IS (n = 6 in each group). In one group (n = 6), IS was injected in one paw and 0.5 μM APDC in the contralateral paw. Mechanical sensitivity was assessed by measuring paw withdrawal threshold (PWT) using the Dixon up-down method (Chaplan et al., 1994) every 10 min for 60 min.
Electrophysiological studies
In vitro skin-nerve preparation
Rats were sacrificed with an overdose of CO2. The glabrous skin from the ankle to the tips of the toes was dissected from the hind paw. The medial and lateral plantar nerves were dissected free and kept intact with the glabrous skin. All muscle and tendon tissues were removed from the preparation. The preparation was placed corium side up in an organ bath and superfused (15 ml/min, 34°C) with an oxygen-saturated, modified synthetic interstitial fluid solution (SIF, in mM: NaCl, 123; KCl, 3.5; MgSO4, 0.7; CaCl2, 2.0; Na gluconate, 9.5; NaH2PO4, 1.7; Glucose, 5.5; Sucrose, 7.5; and HEPES, 10; pH 7.45 ± 0.05). The plantar nerves were moved into a separate chamber containing a superficial layer of mineral oil and a bottom layer of SIF. The nerves were desheathed and teased apart on a mirror stage. Small filaments from either the medial or the lateral plantar nerve were repeatedly split with sharpened forceps until single unit activity was obtained by recording with gold wire electrodes. This preparation has been used successfully to record from peripheral primary afferents in our laboratory (Carlton et al., 2001a; Du et al., 2003; Carlton et al., 2004; Du et al., 2006) and others (Lang et al., 1990; Koltzenburg et al., 1992; Rueff and Mendell, 1996).
Neurophysiological Recordings
Neural activity was recorded using a DAM80 Differential Amplifier (World Precision Instruments, New Haven, CT). Action potentials were acquired and later analyzed with the CED 1401 (Cambridge, UK) using Spike 2 (v5.08) software. The conduction velocity of each unit was determined by monopolar electrical stimulation (1 ms duration, 1Hz) at the most mechanosensitive site in the receptive field of each unit using a Teflon-coated steel electrode (5 MΩ impedance, 250 μm shaft diameter with an uninsulated tip) that was gently lowered into the receptive field. The conduction velocity of each unit was determined from the latency of the action potential and the distance from the stimulating electrode to the recording site. Based on measurements using our in vitro chamber with a 34°C bath temperature and isolated sciatic nerves (n=3), the average conduction velocity for C-mechanoheat (CMH) fibers was ≤ 1.2 ± 0.1 m/s. This agrees with previous in vitro nerve recordings in rat (Koltzenburg et al., 1992; Kress et al., 1992).
Thermal, mechanical and chemical testing procedures
Only units responding to mechanical probing of the glabrous skin with a blunt glass rod with a clearly defined receptive field were studied in detail. For thermal stimulation, radiant heat was applied to each receptive field by a feedback-controlled lamp placed beneath the organ bath. The beam was focused through the bottom of the bath onto the epidermal surface of the skin. A thermocouple was placed into the corium above the light beam to measure intracutaneous temperature. A standard heat ramp starting from an adapting temperature of 34°C and rising to 51°C in 10 sec was applied to each unit from the epidermal side (51°C on the epidermal side resulted in a thermal probe reading of 47°C on the corium side). The heating ramp was monitored by positioning a thermal probe in the corium and displaying the temperature readout on an oscilloscope (inset Fig. 1C).
Fig. 1.
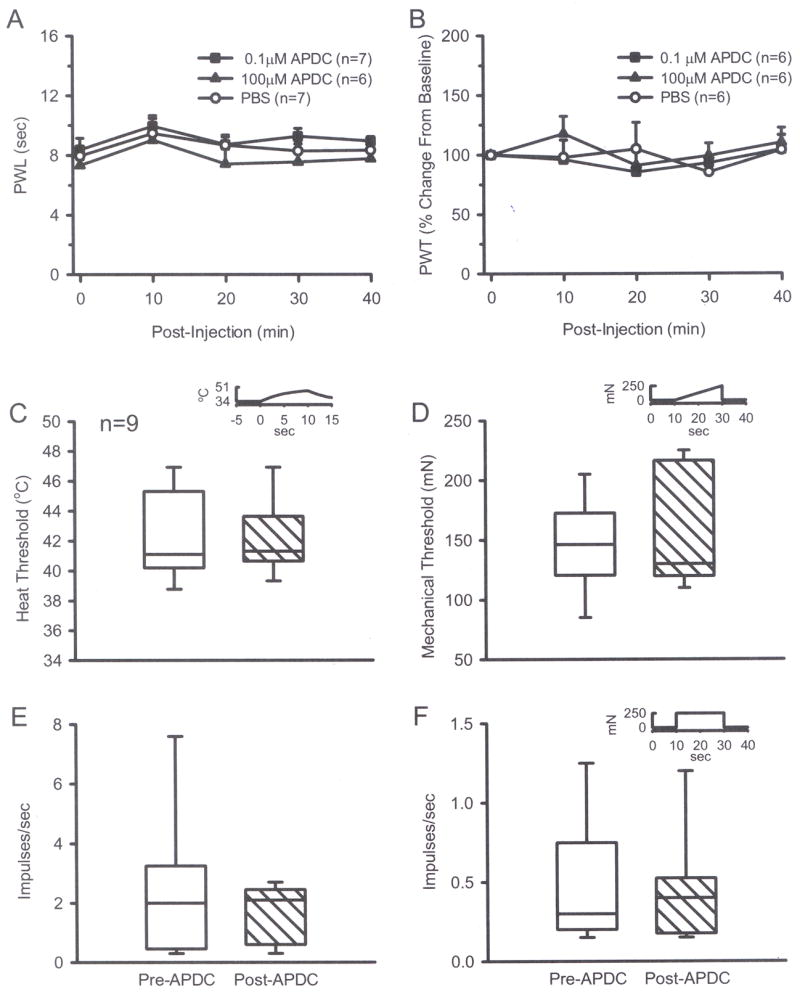
Intraplantar injection of 0.1 μM or 100 μM APDC alone did not change thermal (A) or mechanical (B) thresholds compared to PBS groups (one-way ANOVA). A 2 min exposure of CMH fibers in normal skin to 0.5 μM APDC had no effect on thermal threshold (C) or discharge rate (E). There was no effect on mechanical threshold (D) or on discharge rate (F) in response to a suprathreshold mechanical stimulus (Wilcoxon test). Insets in C, D, and F show stimulus parameters. Median evoked responses are plotted in C–F. Box plot values: the thick horizontal line within each box is the median value, the upper and lower edges of each box represent the 75th and 25th percentiles, respectively; the upper and lower whiskers represent the 90th and 10th percentiles, respectively.
A Dual Mode Lever System (Aurora Scientific Inc. Ontario, Canada) was used to determine mechanical threshold. The system has a motor-driven stylus that delivered a force in the form of a continuous ascending ramp (from 0–250 mN in 20 sec, inset Fig. 1D). The stylus (1.5 mm tip diameter) was placed on the corium side in the most sensitive region of the receptive field of the unit. This same instrument was used to determine magnitude of response of units to a suprathreshold mechanical stimulus. The stylus was placed on the corium side in the most sensitive region of the receptive field and a square wave pulse was used to deliver a 250 mN constant stimulus for 20 sec (inset Fig. 1F).
To document unit responses to drugs, a small cylinder (5 mm diameter) was placed over the receptive field of each unit. The SIF in the cylinder was replaced with the drug(s) made in SIF and buffered to pH 7.40 ± 0.05.
Determination of threshold and discharge rate in response to a stimulus
As previously described (Reeh, 1986), the majority of units from normal skin had little or no spontaneous activity. However, units exposed to IS had considerable activity (> 0.1 imp/s), that was driven by inflammatory mediators. We established criteria in order to determine heat and mechanical thresholds. The mean background instantaneous frequency + 2SDs for units from naïve rats was calculated over a 10 s period. Following initiation of a heat or mechanical stimulus, when a unit exceeded this criteria (mean background instantaneous frequency + 2SDs), this was considered threshold for that unit and this latency was converted into the corresponding temperature or mN (threshold to activation). To analyze discharge rate in response to heat or a mechanical square wave stimulus, the mean background discharge rate was determined over a period equivalent to the stimulation period and this was subtracted from the discharge rate obtained during the stimulus period.
Group II mGluR modulation of thermal and mechanical thresholds of CMH fibers
To document at the single fiber level whether Group II activation affected background nociceptor activity or affected nociceptor responses to acute thermal or mechanical stimulation, the receptive fields of CMH fibers were exposed to 0.5 μM APDC by replacing SIF in the cylinder with drug and background discharge rates of each fiber recorded for 2 min and then responses to a thermal stimulus (10 sec heat ramp) or a mechanical stimulus (in the form of a ramping force or a suprathreshold square wave pulse) were recorded before (designated H1 or M1) and after (designated H2 or M2) the 2 min drug exposure.
Group II mGluR modulation of IS-induced excitation and sensitization
To determine whether activation of Group II mGluRs attenuated sensitization of nociceptors, IS was used to induce an acute heat sensitization of CMH fibers (Kessler et al., 1992; Bennett et al., 1998). The CMH units were exposed to IS alone, IS + 0.5 μM APDC or IS + 0.5 μM APDC + 100 μM LY (a Group II antagonist) for 2 min and unit activity recorded as well as responses to thermal or mechanical stimulation before (H1, M1, respectively) and after (H2, M2, respectively) exposure to these agents. Parallel in vitro studies with formalin could not be done because formalin is a strong fixative that cross-links membrane proteins, preventing normal axonal responses.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) or as medians and SigmaStat v3.1 software was used for statistical analysis. The PWLs and PWTs were analyzed with a one-way ANOVA. In the formalin test, the average number of flinches or L/L behavior in each phase for each group of rats was plotted as bar graphs versus treatment. Differences in behavior between groups or phases (formalin) were evaluated using a one-way ANOVA followed by a student Newman-Keuls test. For the electrophysiological studies, differences between two groups were determined using the Wilcoxon Rank test, and differences between multiple groups were determined using a Kruskal-Wallis test followed by a Dunn’s post hoc analysis; p < 0.05 was considered significant.
RESULTS
Behavioral studies
Group II mGluR modulation of mechanical and thermal sensitivity
Intraplantar injection of 0.1 μM or 100 μM APDC in normal rats did not affect baseline thermal sensitivity, inducing no change in PWL compared to vehicle over the subsequent 40 min testing period (Fig. 1A). Similarly, intraplantar injection of either 0.1 μM or 100 μM APDC did not affect mechanical sensitivity, inducing no change in PWT compared to vehicle (Fig. 1B). The results suggest that APDC in this range does not modulate behavioral responses to acute thermal or mechanical stimulation in normal rats.
Group II mGluR modulation of FM-induced nociceptive behaviors
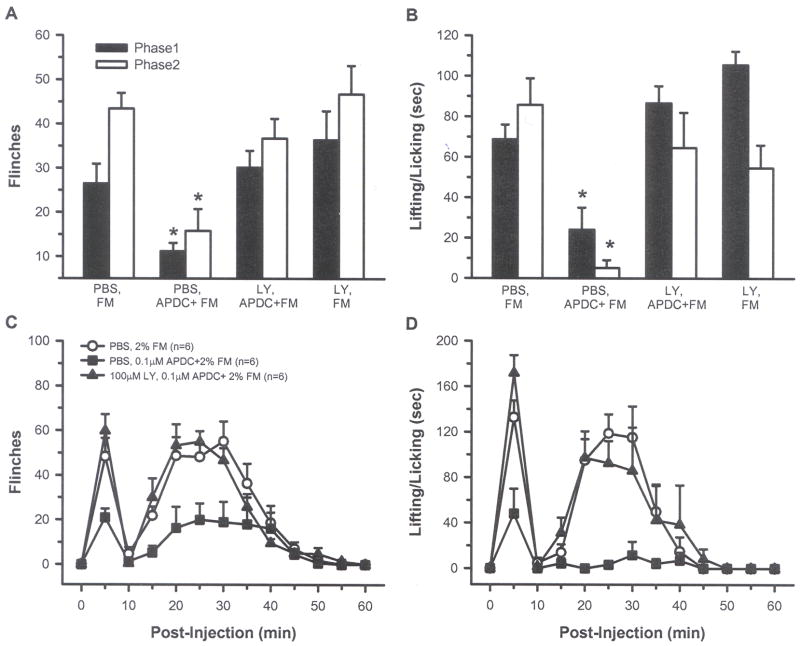
Intraplantar injection of 2% FM resulted in biphasic nocifensive behaviors, with the rats showing flinching and L/L behaviors in both phase 1 and 2. Compared with 2% FM alone, injection of each of three APDC concentrations (0.001, 0.01 and 0.1 μM) attenuated phase 1 and 2 behaviors. Inhibition of L/L was dose dependent and only the highest dose (0.1 μM) significantly inhibited both phase 1 and 2 flinching and L/L behaviors (Fig. 2A and B, p < 0.05, one-way ANOVA). This concentration produced a 67% and 68% reduction in flinching and a 59% and 75% reduction in L/L in phases 1 and 2, respectively. When plotted over time, flinching and L/L behaviors were reduced at virtually all time points following injection of 0.1 μM APDC + FM (Fig. 2C and D). Intraplantar injection of 2% FM in one paw and 0.1 μM APDC in the contralateral hind paw resulted in behaviors similar to those seen in rats injected with 2% FM alone, indicating that local peripheral injection of APDC was not exerting its effects through a systemic action (Fig. 2A and B). Co-injection of the Group II antagonist LY (100 μM), blocked the APDC-induced inhibition of FM pain behaviors, confirming that the APDC-induced inhibition was most likely mediated by activation of Group II mGluRs (Fig. 3, p< 0.05, one-way ANOVA). Animals injected with LY + FM showed behaviors similar to those produced by FM alone.
Fig. 2.
A and B) Intraplantar injection of 0.001, 0.01 or 0.1 μM APDC + 2% FM reduced flinching and L/L compared to FM alone, but only 0.1 μM APDC significantly inhibited both behaviors in both phases (*indicates significant difference comparing phase 1 from each group to phase 1 FM alone or comparing phase 2 from each group to phase 2 FM alone, p < 0.05, one-way ANOVA followed by a student-Newman-Keuls test). Injection of 2% FM in one paw and 0.1 μM APDC in the contralateral paw did not reduce FM-induced behaviors (contralateral, 2A and B). The inhibition of FM-induced flinching (C) and L/L (D) produced by 0.1 μM APDC is shown over a 60 min period.
Fig. 3.
Intraplantar injection of LY blocked the APDC-induced inhibition so that rats in this group had flinching (A) and L/L (B) behaviors that were no different from those receiving PBS, FM. The LY effects on flinching (C) and L/L (D) are shown over a 60 min period. (*indicates significant difference comparing phase 1 from each group to phase 1 PBS, FM group or comparing phase 2 from each group to phase 2 PBS, FM group, p < 0.05, one-way ANOVA followed by a student-Newman-Keuls test ).
Group II mGluR modulation of IS-induced behavioral sensitization
Intraplantar injection of 20 μl IS did not result in nocifensive behaviors (flinching or L/L) but did result in enhanced thermal sensitivity. The IS-injected rats had a decreased PWL to heat compared vehicle-injected animals, but activation of Group II mGluRs with APDC blocked this thermal hyperalgesia (Fig. 4A and B, p < 0.05, one-way ANOVA). The injection of 0.1 μM APDC had a local and not a systemic effect since injection of IS in the ipsilateral hind paw followed by injection of APDC in the contralateral hind paw resulted in a decrease in PWL that was similar to animals receiving IS alone. IS-injected rats also showed changes in mechanical sensitivity with decreased PWTs to von Frey stimulation compared to vehicle-injected rats (Fig. 5A, p < 0.05, one-way ANOVA). The time course study demonstrated that activation of Group II mGluRs with 0.5 μM APDC blocked this mechanical sensitivity for at least 60 min (Fig. 5B). Injection of APDC in the paw contralateral to the IS had no effect on IS behaviors. These results indicate that IS-induced thermal hyperalgesia and mechanical allodynia can be blocked by local activation of Group II mGluRs.
Fig. 4.
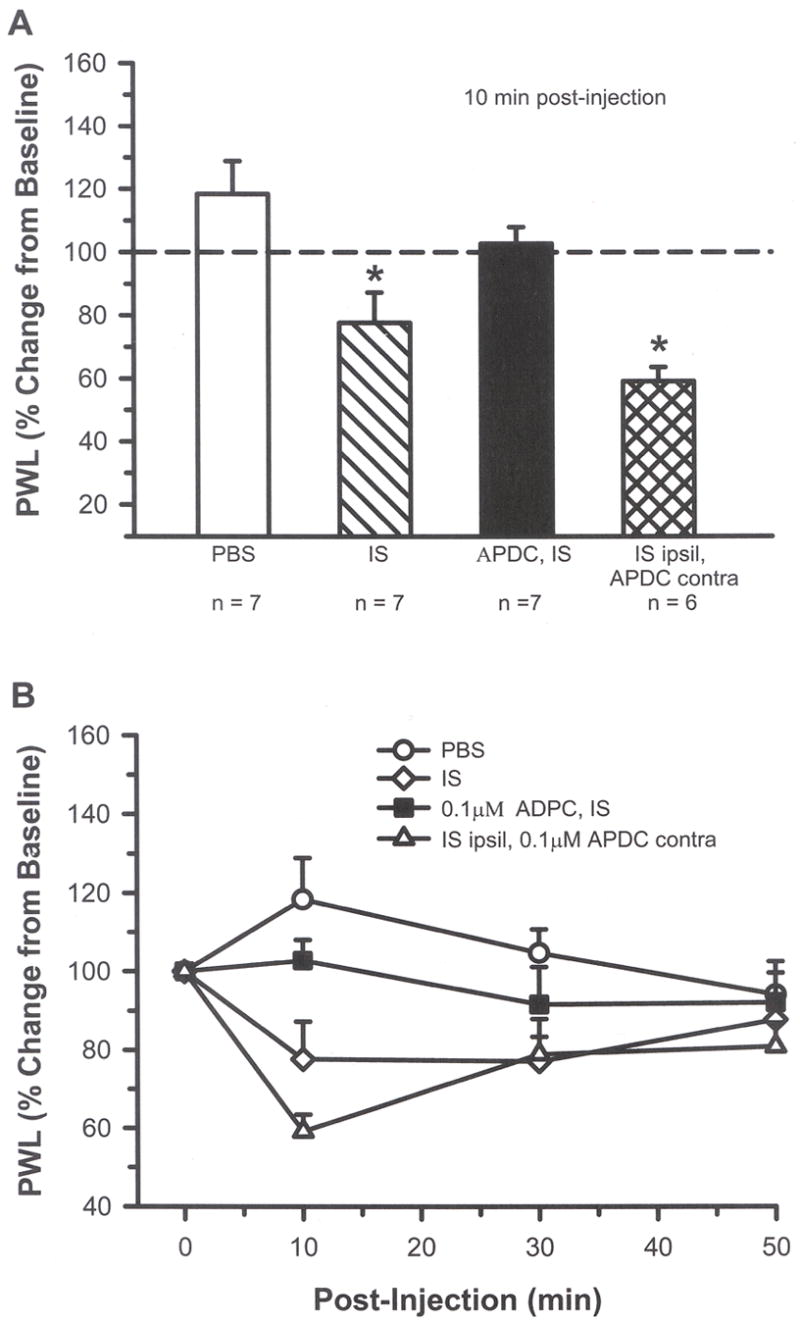
A) At 10 min post-injection, intraplantar IS (20 μl) resulted in a significant change from baseline with shorter paw withdrawal latency (PWL) compared to vehicle (PBS), indicating the presence of thermal hyperalgesia. Addition of 0.1 μM APDC blocked the IS-induced thermal hyperalgesia. Behavior resulting from injection of IS in the ipsilateral (ipsil) paw and APDC in the contralateral (contra) paw confirmed that APDC was not having a systemic effect (* indicates significant difference compared to PBS and APDC/IS, p < 0.05, one-way ANOVA followed by a student-Newman-Keuls test). B) The time course for these drug effects is shown for a 50 min period.
Fig. 5.
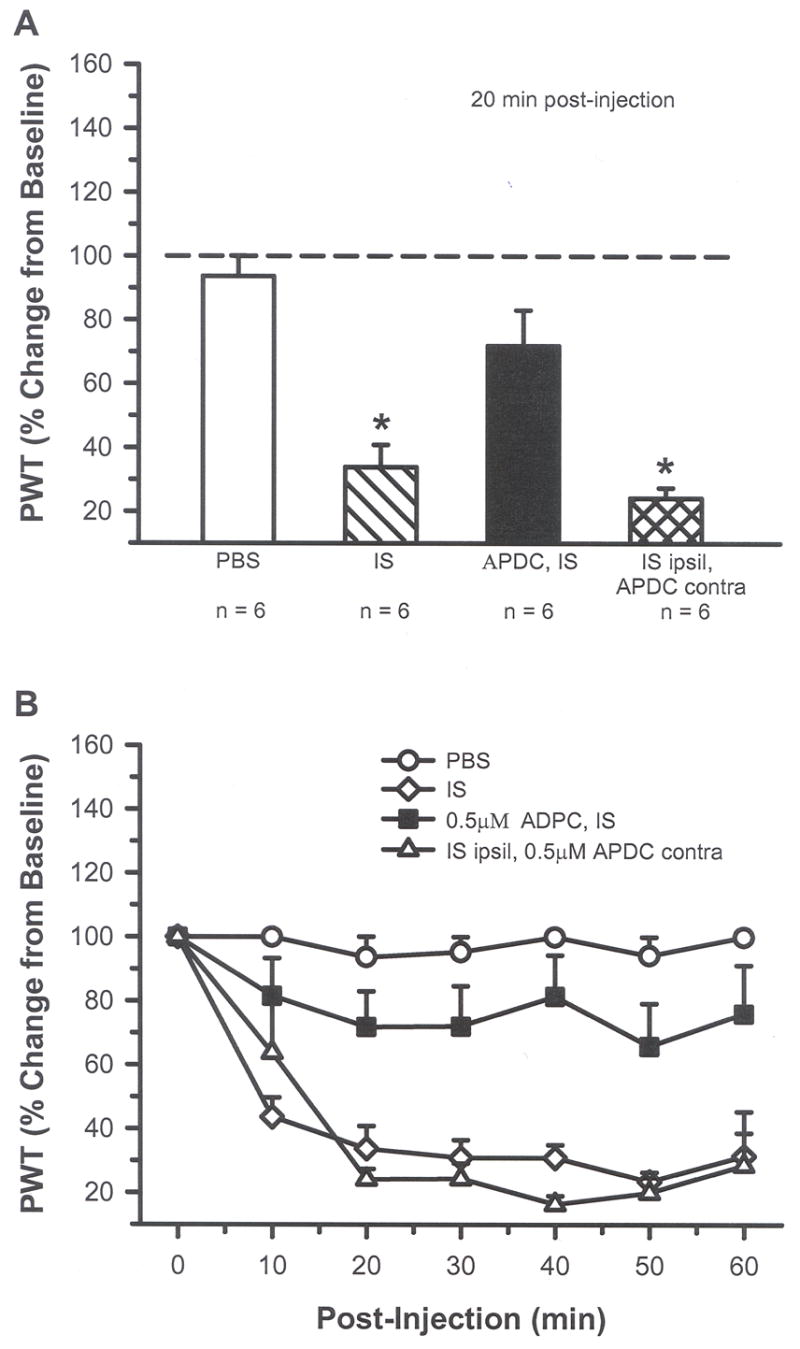
A) At 20 min post-injection, intraplantar IS (20 μl) resulted in a significant change from baseline with a decrease in threshold compared to vehicle (PBS), indicating the presence of mechanical allodynia. Addition of 0.5 μM APDC blocked the IS-induced mechanical sensitivity. IS in one paw (ipsil) and APDC in the contralateral (contra) paw resulted in behavior no different from IS alone (* indicates significant difference compared to PBS and APDC/IS group; p < 0.05, one-way ANOVA followed by a student-Newman-Keuls test). B) The APDC-induced inhibition of mechanical sensitivity lasted for at least 60 min post-injection.
Electrophysiological studies
In the in vitro single fiber recordings (from 44 rats), units that had mechanical thresholds ≥ 25 mN, thermal thresholds ≥ 42 °C and had conduction velocities ≤ 1.2m/s were classified as C-mechanoheat (CMH) fibers. During 2 min background recordings in skin, the majority of units were quiet in the absence of stimulation, with only an occasional action potential (mean background activity was 0.05 ± 0.01 imp/s in 110 CMH units). Application of 0.5 μM APDC (n = 7) or 100 μM LY (n = 10) to the receptive field of CMH units for 2 min had no effect on mean background activity (0.04 ± 0.01 pre-drug compared to 0.04 ± 0.02 imp/s post-drug for APDC group, and 0.04 ± 0.01 pre-drug compared to 0.04 ± 0.01 imp/s post-drug for LY group).
Group II mGluR modulation of thermal and mechanical sensitivity of CMH fibers
Activation of Group II mGluRs had no effect on baseline nociceptive responses. Neither the thermal or mechanical thresholds nor discharge rates changed significantly following exposure of CMH receptive fields to 0.5 μM APDC for 2 min. The median value for the thermal threshold was 41.1 °C pre-APDC compared to 41.3 °C post-APDC (Fig. 1C); the median value for discharge rate was 2.0 imp/s pre-APDC compared to 2.1 imp/s post-APDC (Fig. 1E). The median value for mechanical threshold was 146 mN pre-APDC and 130 mN post-APDC (Fig. 1D); the median value for discharge rate in response to a suprathreshold mechanical stimulus (250 mN, 20 sec) was 0.3 imp/s pre-APDC compared to 0.4 imp/s post-APDC (Fig. 1F). The data demonstrate that activation of Group II mGluRs did not modulate background activity or peripheral responses of nociceptors to acute thermal or mechanical stimulation in naïve rats.
Group II mGluR modulation of IS-induced excitation and sensitization
To study the effect of Group II activation on sensitized nociceptors, inflammatory soup (IS) was applied to the receptive field of CMH units. In this population (n = 9), IS alone caused a significant change in the median discharge rate from 0.08 to 0.32 imp/s (Fig. 6D, 7A, p < 0.05, Wilcoxon test). In separate populations of CMH fibers, co-application of IS + APDC significantly attenuated this increased activity (median value was 0.08 imp/s, Figs. 6E, 7B, p < 0.05, Kruskal-Wallis test), and addition of the Group II antagonist LY (100 μM) with IS + APDC blocked the APDC-induced inhibition such that these responses were not different from IS alone (median value was 0.77 imp/s, Figs. 6F, 7B, p < 0.05, Kruskal-Wallis test). Using the paradigm shown in Fig. 8A, IS alone enhanced thermal responses when comparing the pre-drug heat response (H1) to the post-drug heat response (H2) (median value for H1 = 0.7, H2 = 2.4 imp/s, Figs. 8B inset, p < 0.05, Wilcoxon test). Thermal threshold was also significantly decreased following IS alone (median value for H1 = 43.0, H2 = 39.8 °C, Fig. 8C inset, p < 0.05, Wilcoxon test). In a separate population of CMH fibers, co-application of IS + APDC blocked the IS-induced increases in discharge rate (median value for H2 = 1.5 imp/s) and the decreases in threshold (median value for H2 = 41.9 °C, Figs. 6, 8B and C, 9, p < 0.05, Kruskal-Wallis test). In a third set of CMH fibers, co-application of IS + APDC with LY resulted in thermal discharge rates similar to that obtained following IS alone (median value for H2 = 2.4 imp/s) and thermal thresholds were again decreased (median value for H2 = 40.4 °C) such that post-drug responses were similar to those following IS alone (Figs. 6, 8B and C, 9, p < 0.05, Kruskal-Wallis test). The data demonstrate that activation of Group II mGluRs on peripheral nociceptors prevents thermal sensitization following acute inflammation.
Fig. 6.
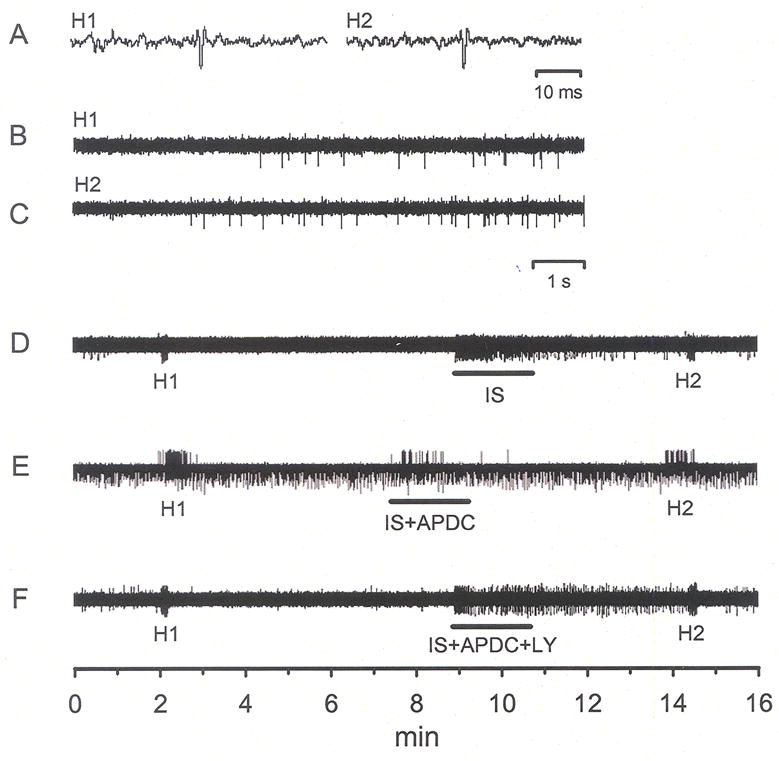
Traces of raw, unfiltered data demonstrating responses of CMH units following activation by heat and inflammatory soup (IS). Traces in panels A, B and C were expanded from panel D and are all from the same unit. Panels D, E and F are recordings from 3 different units. H1 = pre-drug heat response, H2 = post-drug heat response. A) The waveforms of the unit under study in D. B) Response of unit to H1. C) Response of unit to H2; note enhanced activity to heat following a 2 min exposure to IS. D) Application of IS to the receptive field of a CMH fiber induced a robust excitation and heat sensitization (compare H1 to H2 in B and C). Exposure to 0.5 μM APDC (IS+APDC) prevented IS-induced excitation and blocked heat sensitization (E), 100 μM LY blocked the expected APDC-induced inhibition (F).
Fig. 7.
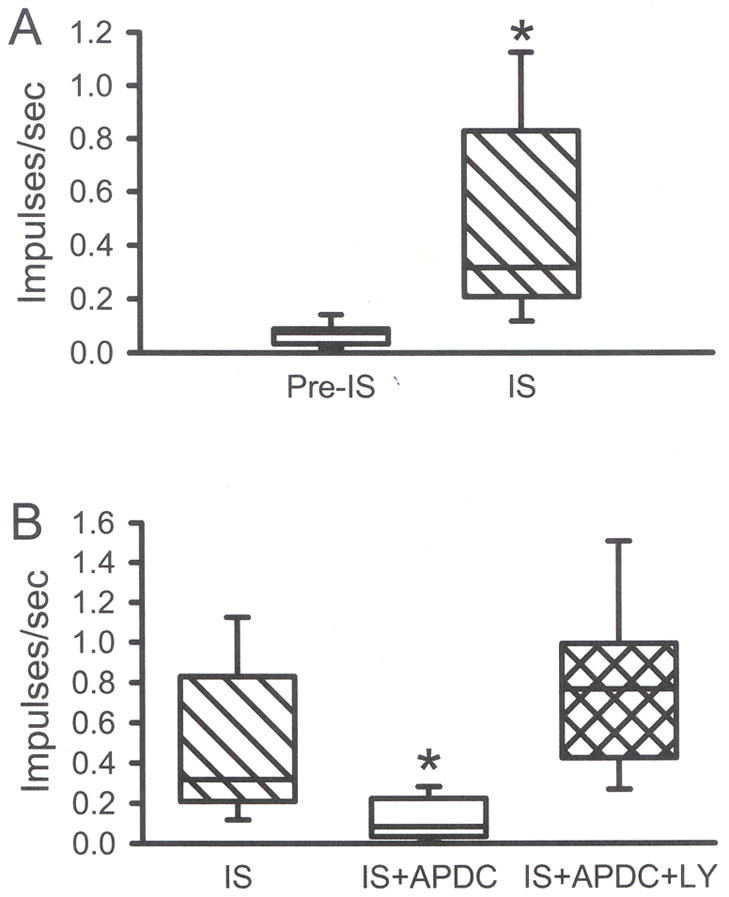
A) Compared to pre-IS baseline, IS induced a robust increase in the discharge rate of CMH fibers (*p<0.05, Wilcoxon test,). B) Co-application of 0.5 μM APDC + IS blocked this increase and 100 μM LY (IS+APDC+LY) blocked the APDC-induced inhibition (B, p<0.05, *significantly different from all other groups, Kruskal-Wallis test). Median evoked responses are plotted. Box plot values same as in Fig. 1.
Fig. 8.
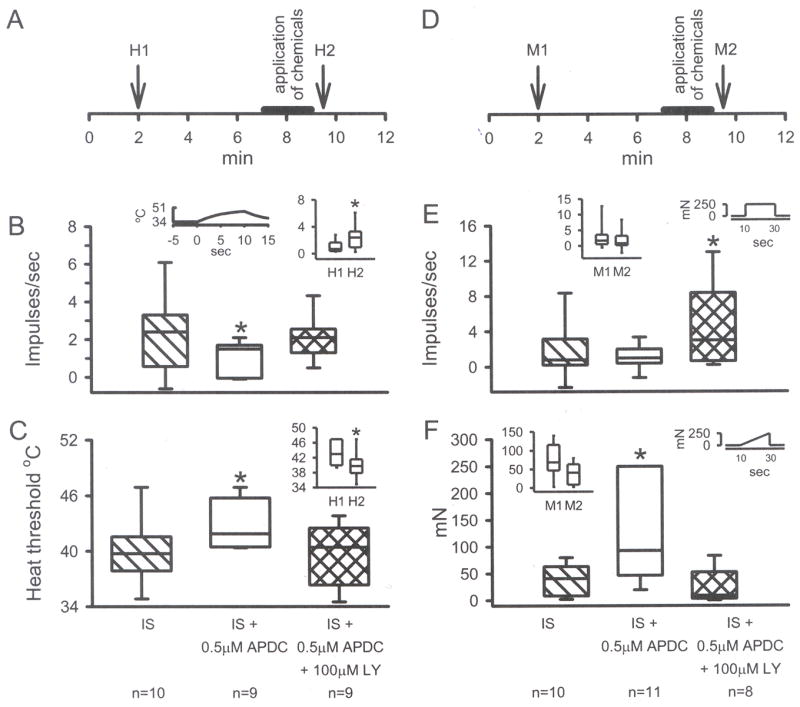
A) Standard in vitro paradigm with unit responses to heat recorded before (H1) and after (H2) drugs are applied for 2 min. IS increased the discharge rate of H2 compared to H1 (B inset) and decreased heat threshold to firing for H2 compared to H1 (C inset, *p<0.05, Wilcoxon test). Addition of 0.5 μM APDC blocked the IS-induced changes and 100 μM LY prevented the APDC-induced inhibition (B and C, p<0.05, *significantly different from all other groups, Kruskal-Wallis test). D) Standard paradigm with unit mechanical responses recorded before (M1) and after (M2) drugs are applied for 2 min. IS did not change the discharge rate (E, inset) or the threshold (F, inset) to mechanical stimulation. However, addition of APDC in the presence of IS increased the threshold (F, p<0.05, *different from all other groups, Kruskal-Wallis test) while addition of LY increased the discharge rate (E, p<0.05, * different from IS + APDC group only, Kruskal-Wallis test). Box plot values same as in Fig. 1.
Fig. 9.
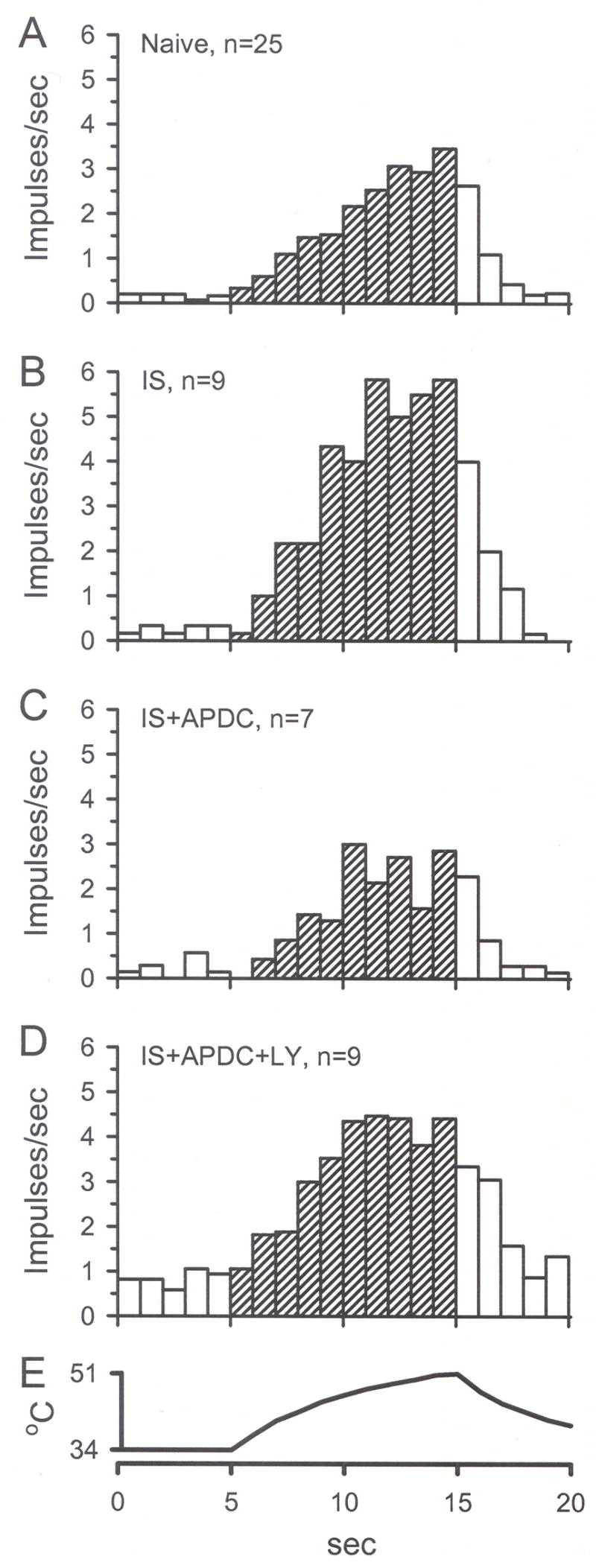
Unit responses to thermal stimulation (H2) are represented by mean instantaneous frequency in 1 sec bins. A) H2 response of units in naïve skin. B) A 2 min application of IS enhanced (sensitized) H2 compared to naïve H2. C) Addition of 0.5 μM APDC blocked the IS-induced enhancement of H2. D) Addition of 100 μM LY blocked APDC-induced inhibition of H2. E) The standard heat stimulus that ramps from 34 to 51°C in 10 sec.
Using the paradigm shown in Fig. 8D to test changes in mechanical sensitivity, IS alone did not enhance discharge rates when comparing the pre-drug mechanical response (M1) to the post-drug mechanical response (M2) (median value for M1 = 1.7, M2 = 0.8 imp/s, Figs. 8E inset). Although there was a trend for the mechanical threshold to be lowered by IS, this did not reach significance (median value for M1 = 68.9, M2 = 40.8 mN, Fig. 8F inset). In a separate population of CMH fibers, co-application of IS + APDC did not significantly change the M2 discharge rate (median value for M2 = 1.0 imp/s) compared to the IS group but it significantly increased the M2 threshold (median value = 141.0 mN) compared to IS threshold (Figs. 8F, 10, 11, p < 0.05, Kruskal-Wallis test). In a third set of CMH fibers, co-application of IS + APDC with LY resulted in M2 discharge rates that were similar to IS alone, but were significantly higher compared to the IS + APDC group discharge rates (median value for M2 = 3.0 imp/s, Figs. 8E, 10 and 11, p<0.05, Kruskal-Wallis test). The M2 mechanical threshold for this group was decreased (median value = 11 mN), such that it was not different from IS alone (Figs. 8F, 10 and 11, p < 0.05, Kruskal-Wallis test). The data demonstrate that activation of Group II mGluRs on peripheral nociceptors can increase mechanical threshold in the absence of mechanical sensitization and this action is blocked by a Group II antagonist.
Fig. 10.
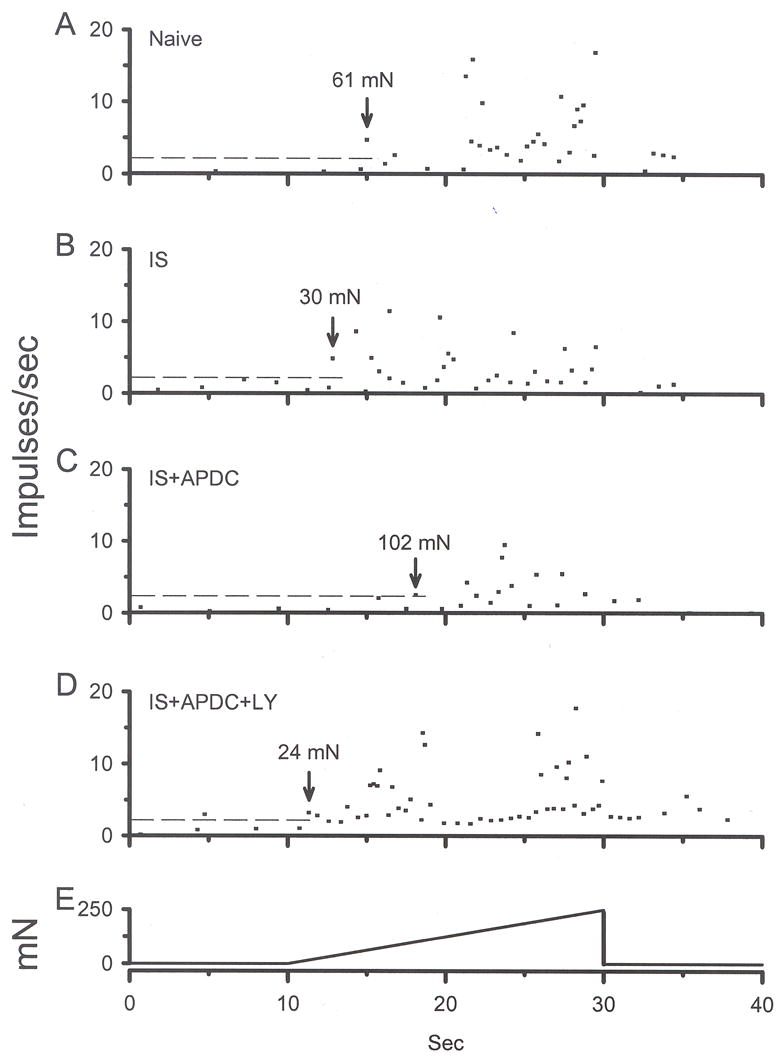
Instantaneous frequency was used to determine threshold to activation to a mechanical ramp stimulus. Each panel represents a different CMH unit: A) naïve, B) exposed to IS for 2 min, C) exposed to IS + APDC for 2 min, D) exposed to IS + APDC + LY for 2 min, E) standard mechanical ramp stimulus. Dashed lines in each panel represent mean baseline instantaneous frequency + 2SD (calculated from naïve rats in panel A). Following activation of the ramp, when the instantaneous frequency of a unit exceeded the mean + 2SD, this was considered threshold for that unit (marked by arrow in each panel).
Fig. 11.
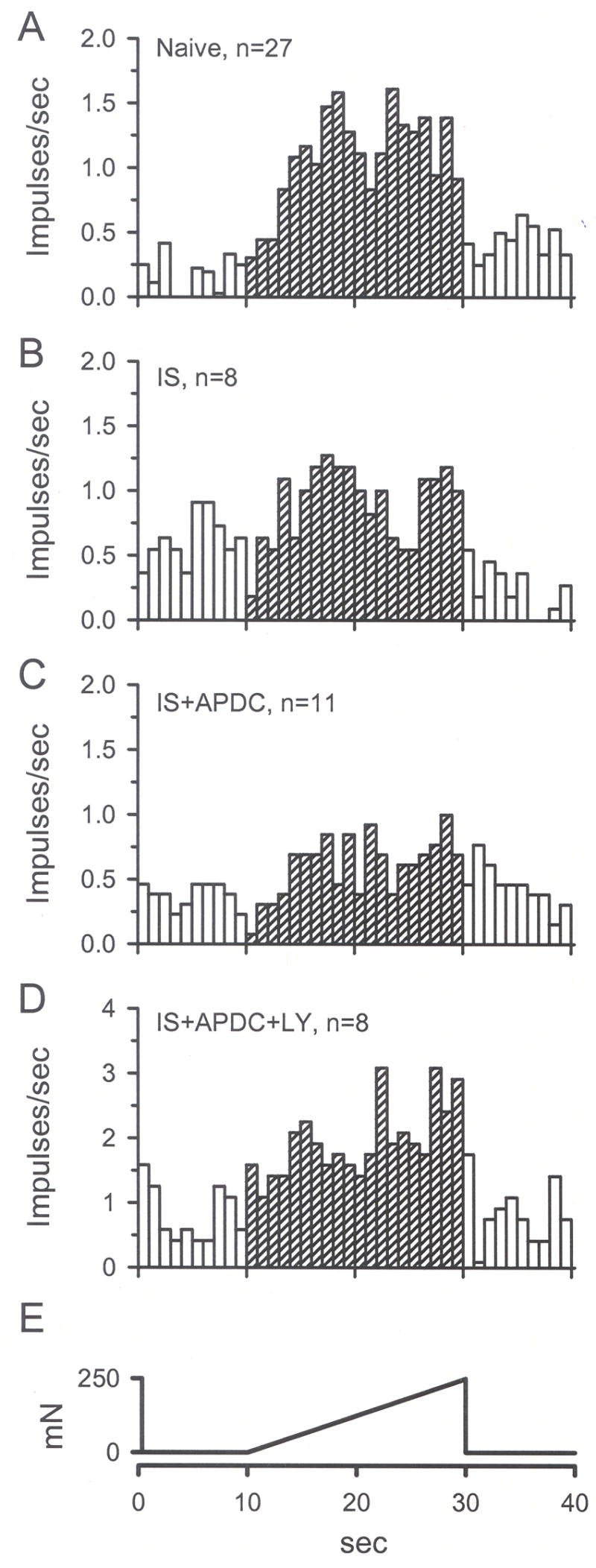
Unit responses to mechanical stimulation (M2) are represented by mean instantaneous frequency in 1 sec bins. A) M2 response of units in naïve skin. B) A 2 min application of IS did not change M2 threshold compared to naïve M2. C) Addition of 0.5 μM APDC reduced the M2 threshold compared to naïve and IS M2 thresholds. D) Addition of 100 μM LY blocked APDC-induced inhibition of M2. E) The standard mechanical stimulus that ramps from 0 to 250 mN in 20 sec.
DISCUSSION
These studies demonstrate several key aspects of peripheral Group II mGluR function. Activation of peripheral Group II mGluRs 1) does not depress nociceptive behavioral responses or nociceptor activity in the non-sensitized state (i.e. following brief nociceptive mechanical or thermal stimulation), 2) can depress these responses when the nociceptors are sensitized by inflammation, 3) can produce an inhibitory effect through local receptor activation that can be blocked by a local Group II antagonist.
Differences in mechanisms underlying thermal vs mechanical sensitization
The data indicated that acute inflammation with IS resulted in a decrease in the PWL and PWT in the behavioral studies and this is consistent with previous studies using acute inflammatory mediators (Taiwo et al., 1987; Taiwo et al., 1989; Simone et al., 1989b; Gilchrist et al., 1996). Sensitization to heat was confirmed at the single fiber level, with CMH units showing a decrease in threshold and an increase in discharge rate in response to IS. However, IS did not result in a decrease in mechanical threshold or an increase in discharge rate in the CMH fibers. Thus, mechanical sensitization was not confirmed at the single fiber level. There are at least 2 explanations for this discrepancy: 1) the changes in mechanical sensitivity were due to central and not peripheral sensitization and/or 2) a population other than CMH fibers wase sensitized by IS to mechanical stimulation. In relation to the first possibility, central sensitization of dorsal horn neurons to mechanical input has been reported and can occur very quickly after application to the hind paw of chemicals such as capsaicin (Simone et al., 1989a; Dougherty and Willis, 1992) or mustard oil (Woolf and King, 1990). Thus, it is probable that central changes contributed to the increased behavioral responses following mechanical stimulation. In relation to the second possibility, Lang et al (1990) used a similar in vitro preparation and reported that bradykinin did not change mechanical thresholds of CMH or Aδ fibers. Thus, the possibility that Aδ but not CMH fibers were responsible for the mechanical sensitization in this study seems remote. We analyzed mechanical thresholds as well as responses to suprathreshold mechanical stimuli because it has been shown that mechanical thresholds are inadequate predictors of sensitization (Cooper et al., 1991; Andrew and Greenspan, 1999). In neither paradigm did CMH fibers show a change in mechanical sensitivity. We (Du et al., 2006) and others (Andrew and Greenspan, 1999) reported mechanical sensitization of cutaneous CMH fibers 16–48 hrs following intraplantar CFA. It is highly likely that a 2 min exposure to IS (current protocol) did not initiate the long term processes that led to the peripheral afferent sensitization reported in these longer term CFA studies. Thus, we conclude that central sensitization was the underlying mechanism for the acute mechanical sensitization observed in the behavioral studies.
Action of Group II mGluRs in the sensitized versus non-sensitized state
Previous behavioral studies demonstrate that intraplantar (Yang and Gereau, 2002; Yang and Gereau, 2003) or systemic injection (Sharpe et al., 2002) of Group II agonists does not alter mechanical or thermal thresholds, suggesting that peripheral Group II mGluRs do not modulate basal mechanical or thermal sensation. However, peripheral injection of Group II agonists can prevent or delay carrageenan- (Sharpe et al., 2002; Yang and Gereau, 2003) and prostaglandin E2-induced (Yang and Gereau, 2002) decreases in mechanical and thermal behavioral thresholds. We confirm these findings using the FM and IS models. Importantly, we extend these findings demonstrating at the single fiber level that activation of Group II mGluRs has no effect on background activity or mechanical and thermal thresholds of nociceptors, but can reduce IS-induced excitation and as well as thermal sensitization, demonstrating an anti-hyperalgesic effect on nociceptor function that can be blocked by a selective Group II antagonist. Furthermore, the concentrations of APDC (0.1 μM and 0.5 μM) needed to achieve these effects are very close to the ED50 (0.4 μM, based on an assay analyzing inhibition of forskolin-stimulated cAMP in non-neural cells, [Schoepp et al., 1999]) and these concentrations are at least 100-fold less than that used in previous studies (Yang and Gereau, 2002; Yang and Gereau, 2003). Although Group I and III mGluRs are also expressed by primary afferents (Ohishi et al., 1995; Zhou et al., 2001; Azkue et al., 2001; Carlton and Hargett, 2007), it is unlikely that they contribute to these actions since APDC, used at these low concentrations, does not bind to Group I or Group III mGluRs (Schoepp et al., 1996, 1999). The Group II antagonist LY does have some affinity for Group I and III mGluRs (Schoepp et al., 1999); however, 100 μM LY has no significant inhibitory effect on forskolin-activated cAMP in mGluR8-expressing cells and LY was shown to enhance forskolin-activated cAMP in mGluR7- and mGluR4-expressing cells (Kingston et al., 1998). When used at 100 μM, LY can inhibit basal phosphoinositide hydrolysis in mGluR1- and mGluR5-expressing cells (Kingston et al., 1998). Thus, if Group I mGluRs were activated in our paradigms, we could not rule out an action of LY on these two mGluR subtypes.
Peripheral site of action for the Group II agonist
Sharpe et al., 2002 previously reported in rats that pre-treatment with a Group II agonist delayed carrageenan-induced thermal hyperalgesia. The agonist was injected systemically and can cross the blood-brain barrier (Bond et al., 2000), so the site of action was unknown. The site of action may have been spinal since application of Group II agonists to dorsal horn neurons inhibits their responses to peripheral input (Stanfa and Dickenson, 1998; Neugebauer et al., 2000). The site of action in the present study was most certainly in the periphery since intraplantar APDC injection in the contralateral paw had no effect on ipsilateral pain behaviors. Thus, the data indicate that peripheral activation of Group II mGluRs can have an anti-hyperalgesic effect. If given systemically, the agonists could have a dual site of action (CNS and PNS), increasing the therapeutic value of these drugs.
Mechanisms by which Group II mGluRs reduce sensitization
There are at least 3 mechanisms by which Group II mGluRs may reduce inflammation-induced hypersensitivity. They may reduce nociceptor activation through membrane stabilization. It is clear from our peripheral nerve recordings that APDC can significantly reduce IS-induced excitation. This may occur through Group II agonist-induced inhibition or activation of various ion channels in the nociceptor axonal membrane such as voltage-sensitive Ca2+ and voltage-dependent K+ channels (see Pin and Duvoisin, 1995 for review). If working by this mechanism, Group II activation would be beneficial in controlling nociceptor activity in a variety of other painful conditions including, but not limited to, neuropathic, migraine and burn pain. Group II mGluRs may reduce inflammatory mediator-induced sensitization. We have shown using peripheral nerve recordings that IS-induced heat sensitization of nociceptors is blocked by APDC and this is reflected by reduced nociceptive behaviors (Yang and Gereau, 2002; Sharpe et al., 2002; Yang and Gereau, 2003). This may occur through Group II mGluR interaction with bradykinin, prostaglandin, serotonin and/or histamine receptors directly but most likely occurs through indirect mechanisms, i.e. modulation of second messenger pathways. In this regard, Group II mGluRs inhibit a forskolin-stimulated cAMP pathway (Tanabe et al, 1992), a pathway activated by many inflammatory mediators leading to hyperalgesia (Taiwo et al. 1989, 1991; Yang and Gereau, 2002). Group II mGluRs may reduce peripheral glutamate release. It has been demonstrated that Group II activation can inhibit glutamate release in several supraspinal sites (Anwyl, 1999; Cartmell and Schoepp, 2000) and may do so in the periphery as well. When nociceptors are activated, peripheral glutamate is released (Omote et al., 1998; deGroot et al., 2000), thus, intraplantar APDC may attenuate this release, curtailing the subsequent autocrine and/or paracrine activation of excitatory GluRs on nociceptors. This would dampen nociceptor activity and peripheral sensitization (Carlton, 2001).
As discussed above, IS resulted in thermal but not mechanical sensitization of CMH fibers. In this sensitized state, activation of Group II mGluRs resulted in significant elevation of both thermal and mechanical thresholds. We and others have presented evidence that Group II mGluRs are only effective in the sensitized state, that their action is anti-hyperalgesic. How then could APDC produce a significant increase in mechanical threshold even though this modality is not sensitized? As discussed above, a change in the over-all excitability level of the terminal membrane may result from Group II activation, reducing nociceptor activity in response to mechanical as well as thermal stimulation. Furthermore, if Group II mGluR activation reduces glutamate release, this would reduce the probability of activation of nearby mechanoreceptive terminals, raising mechanical threshold.
Localization of Group II mGluRs on peripheral primary afferents
Having shown that a Group II agonist, given systemically, could reduce thermal hyperalgesia, a peripheral site of action was not seriously considered by Sharpe et al., (2000), who concluded that Group II mGluRs played a role in the central sensitization process associated with persistent pain. Group II mGluRs have been localized in dorsal root ganglion cells (Petralia et al., 1996) and on central processes of presumed primary afferent fibers (Jia et al., 1999). Our lab has localized Group II receptors on peripheral cutaneous processes and lumbar DRG cell bodies (Carlton et al., 2001b; Carlton and Hargett, 2007). Thus, it is highly likely that Group II mGluRs can modulate primary afferent mechanisms that lead to peripheral sensitization, as well as spinal mechanisms that lead to central sensitization. Approximately 32% of unmyelinated and 28% of myelinated axons in rat digital nerves are positively labeled with antibodies directed against mGluR2/3. Since mRNA for mGluR3 has not been observed in DRG (Ohishi et al., 1993), it is highly likely that the effects reported here represent activation of mGluR2 exclusively.
It is most likely that the inhibitory effect of APDC was due to activation of Group II mGluRs constitutively expressed on nociceptors. In the in vitro studies, the mGluR-induced inhibition was evoked quickly (within seconds), allowing too little time for transport and insertion of new receptors into peripheral terminal endings. In the behavioral studies, immune cells expressing Group II mGluRs could invade the inflamed hindpaw territory and contribute to the anti-hyperalgesia via an indirect pathway; however, this could not be the case in the in vitro paradigm, where there is no blood flow and few blood products.
CONCLUSIONS
The results presented here demonstrate that Group II activation has no effect on normal (non-sensitized) nociceptor function. In contrast, when conditions arise where CMH nociceptors are sensitized by inflammatory mediators, activation of peripheral Group II mGluRs can dramatically reduce evoked nociceptive behaviors, and importantly, can effectively inhibit inflammatory mediator-induced excitation and sensitization, indicating that Group II mGluR activation is anti-hyperalgesic. Group II mGluRs can also significantly elevate mechanical thresholds of CMH sensitized units, even though the units are not sensitized to mechanical input.
Based on the current, as well as previous data (Sharpe et al., 2002), it is likely that activation of peripheral Group II receptors plays a role in modulating the development of inflammatory hyperalgesia by reducing excitatory transmission in nociceptors. Activation of Group II receptors reduces both peripheral (current study) and central (Neugebauer et al., 2000) neuronal sensitization; thus, systemic administration of selective Group II agonists may be potent therapeutic agents for treatment of inflammatory pain and possibly other types of pain resulting from sensitized states.
Acknowledgments
The authors would like to thank Ms. Lyn Schilling for her assistance in preparing this manuscript. This work was supported by NIH grants NS27910, NS40700 and NS54765 to SMC.
List of Abbreviations
- APDC
(2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate, Group II agonist
- CMH
C-mechanoheat
- DRG
dorsal root ganglia
- FM
formalin H1, pre-drug heat response
- H2
post-drug heat response
- iGluR
ionotropic glutamate receptor
- IS
inflammatory soup
- L/L
lifting and licking
- LY
LY341495, (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid, a Group II antagonist
- M1
pre-drug mechanical response
- M2
post-drug mechanical response
- mGluR
metabotropic glutamate receptor
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- PBS
phosphate buffered saline
- SIF
synthetic interstitial fluid
Footnotes
Section Editor: Dr. Linda S. Sorkin, Ph.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfredson H, Forsgren S, Thorsen K, Lorenz B. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orth Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Lorentzon R. Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Current Drug Targets. 2002;3:43–54. doi: 10.2174/1389450023348028. [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peirpheral inflammation in the rat. J Neurophys. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci. 1998;10:1282–1291. doi: 10.1046/j.1460-9568.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- Bolyard LA, Van Looy JW, Vasko MR. Sensitization of rat sensory neurons by chronic exposure to forskolin or “inflammatory soup” does not downregulate and requires continuous exposure. Pain. 2000;88:277–285. doi: 10.1016/S0304-3959(00)00341-9. [DOI] [PubMed] [Google Scholar]
- Bond A, Jones NM, Hicks CA, Whiffin GM, Ward MA, O’Neill NF, Kingston AE, Monn JA, Ornstein PL, Schoepp DD, Lodge D, O’Neill MJ. Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: Investigations into possible mechanisms of action in vivo. J Pharm Exp Ther. 2000;294:800–809. [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophys. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003;90:2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Carlton SM. Peripheral excitatory amino acids. Current Opinions in Pharm. 2001;1:52–56. doi: 10.1016/s1471-4892(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S, Coggeshall RE. Tonic control of peripheral cutaneous nociceptors by somatostatin receptors. J Neurosci. 2001a;21:4042–4049. doi: 10.1523/JNEUROSCI.21-11-04042.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Co-localization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neurosci. 2001b;105:957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Carlton SM, McNearney T, Cairns BE. Peripheral glutamate receptors: novel targets for analgesics?. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain; Seattle. 2003. pp. 125–139. [Google Scholar]
- Carlton SM, Zhou S, Du J, Hargett GL, Ji G, Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain. 2004;110:616–627. doi: 10.1016/j.pain.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Ann Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cooper B, Ahlquist M, Friedman RM, Labanc J. Properties of high-threshold mechanoreceptors in the goat oral mucosa. II Dynamic and static reactivity in carrageenan-inflamed mucosa. J Neurophysiol. 1991;66:1280–1290. doi: 10.1152/jn.1991.66.4.1280. [DOI] [PubMed] [Google Scholar]
- deGroot JF, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. NeuroReport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capasicin-induced sensitization in the monkey. J Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Carlton SM. Program No. 511. 5. Abstract Viewer/Itinerary Planner. Washington, D. C: Society for Neuroscience; 2005. Group II mGluRs modulate peripheral nociception: electrophysiological studies. Online. [Google Scholar]
- Du J, Zhou S, Carlton SM. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neurosci. 2006;137:999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neurosci. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 1999;410:627–642. [PubMed] [Google Scholar]
- Kessler W, Kirchhoff C, Reeh PW, Handwerker HO. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp Brain Res. 1992;91:467–476. doi: 10.1007/BF00227842. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Kress M, Reeh PW. The nociceptor sensitization by bradykinin does not depend on sympathetic neurons. Neurosci. 1992;46:465–473. doi: 10.1016/0306-4522(92)90066-b. [DOI] [PubMed] [Google Scholar]
- Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- Lang E, Novak A, Reeh PW, Handwerker HO. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990;63:887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- McNearney T, Speegle D, Lawand NB, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;27:739–745. [PMC free article] [PubMed] [Google Scholar]
- McNearney TA, Goel N, Lisse J, Speegle D, Baethge B, Westlund K. Temporal fluctuations in excitatory and inhibitory amino acid profiles of synovial fluids derived from patients with arthropathies. J Invest Med. 1999;47:109A. [Google Scholar]
- Neugebauer V, Chen P-S, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J Neurophys. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Nomura S, Ding YQ, Shigemoto R, Wada E, Kinoshita A, Li J-L, Neki A, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neurosci Lett. 1995;202:85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787:161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang Y-X, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neurosci. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pin J-P, Duvoisin R. Review: Neurotransmitter receptors I. The Metabotropic Glutamate Receptors: Structure and Functions. Neuropharm. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Rueff A, Mendell LM. Nerve growth factor and NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophys. 1996;76:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. TIPS. 1993;14:13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharm. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Sharpe EF, Kingston AE, Lodge D, Monn JA, Headley PM. Systemic pre-treatment with a group II mGlu agonist, LY379268, reduces hyperalgesia in vivo. Br J Pharmacol. 2002;135:1255–1262. doi: 10.1038/sj.bjp.0704583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytology. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, Collins JG, LaMotte RH. Sensitization of cat dorsal horn neurons to innocuous mechanical stimulation after intradermal injection of capsaicin. Brain Res. 1989a;486:185–189. doi: 10.1016/0006-8993(89)91293-6. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989b;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Dickenson AH. Inflammation alters the effects of mGlu receptor agonists on spinal nociceptive neurones. Eur J Pharm. 1998;347:165–172. doi: 10.1016/s0014-2999(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Taiwo Y, Goetzl EJ, Levine JD. Hyperalgesia onset latency suggests a hierarchy of action hyperalgesia nociception bradykinin leukotriene B4 norepinephrine prostaglandin D2. Brain Res. 1987;423:333–337. doi: 10.1016/0006-8993(87)90858-4. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neurosci. 1989;32:577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- Thomas NK, Jane DE, Tse H-W, Watkins JC. αmethyl derivatives of serine-O-phosphate as novel, selective competitive metabotropic glutamate receptor antagonists. Neuropharm. 1996;35:637–642. doi: 10.1016/0028-3908(96)84635-1. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci. 1990;10:2717–2726. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors mediate endogenous anti-allodynia in inflammation. Pain. 2003;106:411–417. doi: 10.1016/j.pain.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Yang D, Gereau RW. Peripheral group II metabotropic glutamate receptors (mGluR2/3) regulate prostaglandin E2-mediated sensitization of capasicin responses and thermal nociception. J Neurosci. 2002;22:6388–6393. doi: 10.1523/JNEUROSCI.22-15-06388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Carlton SM. Program No. 511. 4. Abstract Viewer/Itinerary Planner. Washington, D. C: Society for Neuroscience; 2005. Group II mGluRs modulate peripheral nociception. Online. [Google Scholar]
- Zhou S, Komak S, Du J, Carlton SM. Metabotropic glutamate 1α receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 2001;913:18–26. doi: 10.1016/s0006-8993(01)02747-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]