Abstract
Objectives
To determine the permeability of human metaphase II oocytes to ethylene glycol and water in the presence of ethylene glycol, and to use this information to develop a method to vitrify human oocytes.
Design
An incomplete randomized block design was used for this study.
Setting
A University-affiliated assisted reproductive center.
Patients
Women undergoing assisted reproduction in the Center for Reproductive Medicine at Shandong University.
Interventions
Oocytes were exposed to 1.0 molar ethylene glycol in a single step, and photographed during subsequent volume excursions.
Main outcome measures
A 2-parameter model was employed to estimate the permeability to water and EG.
Results
Water permeability ranged from 0.15 to 1.17 µm/(min·atm), and ethylene glycol permeability ranged from 1.5 to 30 µm/min between 7 °C at 36 °C. The activation energies for water and ethylene glycol permeability were 14.42 Kcal/mol and 21.20 Kcal/mol, respectively.
Conclusions
Despite the lower permeability of human MII oocytes to ethylene glycol compared to previously published values for propylene glycol and dimethylsulfoxide, methods to add and remove human oocytes with a vitrifiable concentration of ethylene glycol can be designed which prevent excessive osmotic stress and minimize exposure to high concentrations of this compound.
Keywords: Vitrification, ethylene glycol, human, oocytes, permeability, computer modeling
INTRODUCTION
Cryopreservation of gametes and gonadal tissue offers a means to preserve fertility among individuals undergoing cancer therapy (1, 2). Oocyte cryopreservation may also obviate cryopreservation of preimplantation embryos – a practice which is forbidden in some locales – when ovarian stimulation procedures are utilized and result in the generation of supernumerary oocytes (3–5). Having effective means to cryopreserve oocytes would also facilitate the development of oocyte banks, making therapy for patients more effective by eliminating the need for cycle synchrony between donor and patient; it would also allow more effective screening of donors for transmittable diseases, as is currently practiced for sperm donors (6, 7).
Progress in improving human oocyte cryopreservation has been made in recent years, evident by numerous reports describing pregnancies and live births after the application of various procedures (recently reviewed in (8–10)). Following successful reports in the mid-1990s (11–14), an equilibrium freezing method using propylene glycol (PG) as the penetrating cryoprotective agent (CPA) has become the most utilized method to freeze human oocytes (15). Fabbri and colleagues (16) demonstrated a significant improvement in survival when the concentration of sucrose in the freezing medium was increased to 0.3 mol/L; reports of live births using this method or slight variations followed (17–21). Despite these promising results, very recent reports, some using large cohorts of patients, suggest that the current methods are still sub-optimal (22–24). It continues to be argued that caution should be taken when applying oocyte cryopreservation in a therapeutic setting (1, 10, 25). In recent years, vitrification (cf. (26) for a definition of terms associated with vitrification procedures) has been applied to human oocyte cryopreservation as an alternative to equilibrium freezing (27–31); and some of these outcomes have been very promising. With further progress in applying vitrification to human oocytes, this approach may eventually surpass equilibrium freezing as the method of choice (32). Vitrification in the context of reproductive medicine, and the associated challenges, were recently reviewed (33).
Cryopreservation procedures constitute several steps, including CPA addition to and removal from cells, and cooling and warming. Vitrification methods are particularly challenging in regards to the application of CPA, as high concentrations of these compounds are needed to assure that a vitreous state is attained during cooling and maintained during warming (34). When developing a vitrification procedure, primary considerations include: (1) determining the appropriate solution to use; and (2) determining the manner in which it is to be used. Regarding the consideration of the appropriate solution, one method to reduce potential chemical toxicity is to replace permeating solutes with non-permeating solutes. However, the glass-forming, or vitrification properties of solutes are not identical, and the proportion of each type of solute in a solution affects the overall vitrification properties of a solution (35). Another consideration regarding the appropriate solution is the total concentration of solutes necessary to maintain a vitreous state during cooling and warming. When these considerations are taken together, designing a vitrification solution requires one to know the appropriate proportions of each solute in relation to the total concentration of all solutes necessary to prevent ice formation during cooling and warming. Regarding the second consideration mentioned above – the manner in which a solution is to be used – adding CPAs to cells and removing them from cells can be osmotically damaging. Fortunately, osmotic damage can be alleviated by using stepwise addition and removal procedures (36–41).
Ethylene glycol (EG) is frequently used as a permeating CPA for vitrification of mammalian embryos and oocytes (42), particularly due to its lower toxicity compared to other CPAs (43). It has been used in most studies with human oocytes, either alone (44, 45) or in conjunction with dimethylsulfoxide (DMSO) (27, 29, 30). Quantitative permeability values of metaphase II (MII) human oocytes to PG and DMSO have been determined (46, 47). While quantitative permeability values for EG have been reported for oocytes from some mammals (48–50), these values for human MII oocytes have only been investigated qualitatively (51). However, in order to design CPA addition and removal procedures in a rational manner, the quantitative membrane permeability values need to be known (cf. (52) for a discussion of these concepts).
The present study was designed to address some of these gaps in our present knowledge. First, we determined the quantitative permeability of mature human oocytes to EG and water in the presence of EG at different temperatures. Second, we assessed the relationship between the amount of EG and sucrose in saline necessary to maintain an ice-free state when cooling to and warming from cryogenic temperatures. Finally, we used computer modeling to investigate vitrification methods based upon the experimental results from the present study.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO USA) unless otherwise stated.
Experiment 1: Oocyte permeability to ethylene glycol
Oocyte collection
This study design was approved by the Shandong Provincial Hospital Institutional Review Board. Informed, written consent was obtained from all patients who donated oocytes to this study. The average age of the oocyte donors was 28 years (range: 22 – 33 years; n=19). Cumulus-oocyte complexes (COCs) derived from smaller follicles of the total follicle cohort were used in this study. Only oocytes having a normal appearance and a visible first polar body were used in this experiment. The ovarian stimulation protocol commenced with 150 IU human menopausal gonodotropin (HMG; lebaode, lizhu, China) by muscle injection from menstrual cycle day 5. Thirty-six hours after an injection of 10,000 IU human chorionic gonadotropin (hCG; lizhu, China), COCs were aspirated transvaginally. The COCs selected for the study were separated from the others and placed into 4-well dishes containing human tubal fluid medium (HTF; Irvine Scientific) + 10% serum substitute supplement (SSS; Irvine Scientific) in a culture incubator at 37 °C with a 5% CO2/air atmosphere. The cumulus cells were removed approximately 3–4 hours after collection using hyaluronidase (80IU/ml) and gentle pipetting. Oocytes were returned to HTF after cumulus stripping. The oocytes remained in culture for no more than 5–6 hours prior to use in the experiment.
Oocyte perfusion and image acquisition
Measurements of oocyte permeability were conducted at different temperatures on an inverted microscope (Leica DMIRB, China). Perfusion of the oocytes was performed as previously described and validated in our laboratory (53). Oocytes were placed in 20 µl drops of Hepes-HTF (Irvine Scientific) with 10% SSS in a petri dish covered with mineral oil (Vitrolife; Englewood, CO, USA) to prevent evaporation. An initial photograph was taken of the oocyte in order to calculate the initial volume. Then, approximately 3.5 ml of Hepes-HTF diluted with EG at a final concentration of 1.0 M was added to the dish. When the perfusate merged with the 20 µl drop of base medium, photographs of the oocytes were taken at regular intervals. The oocytes were held in place during the experiment using a glass holding pipette (Humagen Holding MPH-MED-30, I.D.−20µm, O.D.−95~120µm) and micromanipulators (#RI MODEL SAS11/2-E, Integra Research Instruments, UK). For temperatures lower than ambient, a 35mm Petri dish (Falcon 35–1008) was placed in an aluminum chamber that was cooled with circulating liquid via a cooling bath (Fisher model 9109) and pumped through the aluminum chamber using polymer tubing. The bottom of the dish was wrapped in aluminum foil and a square hole was cut in the foil to facilitate viewing the oocyte. This was performed so the dish would fit snugly in the chamber to facilitate heat transfer from the chamber to the dish. The temperature in the cooling bath was set to either −2 °C or +7 °C for the 2 cold temperatures. The actual temperature of the media in the dish was warmer than this due to heat exchange of the fluid as it circulated through the tubing and chamber. For the third treatment, oocyte permeability was measured at ambient temperature (~25 °C) where the perfusion took place in a 50mm Petri dish (Falcon 35–1006) placed on the microscope stage with no heating or cooling. Finally, the temperatures above ambient were attained in a similar fashion but the temperature of the media was raised by using a microscope stage warmer set to 39 °C. Variations in temperature of the media occurred due to day-to-day fluctuations in temperature in the laboratory. However, the actual temperature of the media for each replicate during the experiment was measured using a type-T (copper/constantan) thermocouple and an electronic thermometer (model 51 II, Fluke Corporation, Everett, WA USA). After the experimental run, the oocyte was released from the holding pipette on the micromanipulator and the temperature of the medium was measured such that the thermocouple was visible in the microscope field of view, ensuring that the temperature was recorded at the exact location of the oocyte. On several occasions the media was measured both during and after the experiment, and the initial and final temperatures did not vary by more than 0.5 °C. The media was either pre-warmed or pre-cooled prior to perfusion, depending upon the experimental conditions. For the experiments using the warm stage, photographs were taken every 3 seconds for the duration of the oocyte volume excursion. For the experiments at ambient temperature, photos were taken every 5 seconds. For the experiments when the cooling bath was set to 7 °C, photographs were taken every 5 seconds initially, and after the oocyte reached a nadir in volume and began to swell, the time duration was changed to 30 seconds. For the coldest temperature, photos were taken every 10 seconds initially, and every 60 seconds during swelling. The exact time of these transitions was recorded during each replication and was accounted for during the calculations to estimate the permeability parameters (please see below).
Image analysis, parameter estimation, and cell dynamic modeling
Only those oocytes remaining nearly spherical during the volume excursions were used to estimate the permeability parameters. Forty three of 72 oocytes fit this criterion (~60%). In several of the instances where the oocytes were rejected for analysis, the cells shrank close to spherical, but folds in the membranes prevented accurate determination of the cell volume. Image analysis was performed using image analysis software (Fovea Pro®, Reindeer Graphics, Asheville NC, USA, and Photoshop®, Adobe Systems, Inc. San Jose, CA, USA). To determine the cell volume in each image, the following process was performed (see Fig 1). Initially, the image was thresholded by grey scale to isolate the oolemma, as it is noticeably darker than the area in its immediate surroundings (Fig 1A and 1B). Imperfections in this step were manually corrected. The next step involved filling in areas completely surrounded by black pixels (Fig 1C). The next step involved isolating the oocyte from the remainder of the features in the image by deleting objects other than the oocyte (1D). Finally, the area of the oocyte was calculated by counting the number of black pixels, and the diameter of a circle with this equivalent area was calculated. All of these processes were performed by the software, and the software was calibrated with an image of a stage micrometer. Details of these processes can be found in (54). The volume of the oocyte was calculated from this diameter assuming spherical geometry.
Figure 1.
The steps in the procedure used for image analysis are shown. Panel A shows an original photograph. Notice that the oolemma is darker than its surroundings. A threshold process was applied to the original image, isolating pixels based upon their grayscale value. The result is shown in Panel B. The next process involved filling in areas that are completely surrounded by black pixels (Panel C). Finally, all objects smaller than a minimum size (chosen as 3000 pixels) and those touching an edge of the image were rejected, leaving only the oocyte (Panel D). The area of this object was determined by summing the total number of pixels and multiplying this value by the area per pixel. From this area, the total volume of the oocyte was determined assuming spherical geometry.
For this study, we used a 2-parameter model to describe the cell dynamics (55). We adopted the 2-parameter model (vs. the 3-parameter model) as it has been argued that this model is more parsimonious, and many previous investigations into membrane permeability of cells including oocytes have shown that the interaction factor (σ) is insignificant (46, 56). This model uses a pair of coupled, linear ordinary differential equations to describe the change in cell water volume and moles of intracellular permeating solute (e.g. EG). Equation 1 describes the change in cell water volume (Vw) over time (t) as a function of the hydraulic conductivity (Lp), surface area (A), gas constant (R: 0.082 L Atm mol−1 K−1), temperature (T, in K), intracellular permeating (me s) and non-permeating (me n) solute concentration in osmoles, and the extracellular permeating and non-permeating solute concentration (osmoles of solute (nis and nin respectively) / cell water volume (Vw)):
| (1) |
Equation 2 describes the change in intracellular moles of permeating solute (nis) over time as a function of the solute permeability (Ps):
| (2) |
All other terms are equivalent to those in equation 1.
A spreadsheet was created to estimate the permeability parameters using the Solver tool in Microsoft Excel® (Microsoft, Redmond WA, USA). Briefly, for each experimental run, the volume change data from the image analysis was imported into the spreadsheet and compared to the theoretical model of volume change data calculated from the above equations. The initial volume of cell water in the oocyte was determined by subtracting the osmotically inactive fraction of the cell volume (0.19x isotonic volume; from (57)) from the total cell volume estimated from the initial image of the oocyte. Similarly, the total cell volume from the model calculations was determined by summing the cell water volume calculated from equation 1, the volume of EG in the cell (calculated as the product of the partial molar volume of EG (0.056 L mol−1) and the intracellular moles of EG from equation 2) and the osmotically inactive cell volume as described above. The solver tool in Excel® was used to estimate the permeability parameter values by minimizing the sum of squared errors between the experimental cell volume and the volume calculated from equation 1 and equation 2 for each run (58). Once these parameters have been estimated, they can be applied in equation 1 and equation 2 to calculate the change in volume and intracellular CPA concentration an oocyte will undergo for any CPA addition and removal procedure (59).
The activation energy for Lp and Ps was determined assuming an Arrhenius relationship between the parameter and temperature (60). This relationship can be described as in Equation 3 for Lp:
| (3) |
where Lp0 and T0 are reference parameters (e.g. Lp at a specific temperature T0). When 1000/RT (on the abscissa) is plotted against ln Lp (on the ordinate), the slope of the linear regression through these data gives the value for −Ea. In this instance, the appropriate value for R is 1.987 cal mol−1 K−1. The equation describing Ea of Ps is similar, with Ps substituted for Lp in (3).
Experimental Design and Statistical Analysis
An incomplete randomized block design was used in this experiment (61), the blocking factor being a patient. Each oocyte was randomly assigned to one of the treatments. The order of the four temperatures on each day was randomized for each replicate. All randomization procedures were conducted using the random number generator in Excel®. Linear regression analysis was performed with the statistical analysis system (SAS ®, Cary NC, USA). A total of 43 oocytes were analyzed for this experiment. For the four experimental treatments (~ 33, 26, 14, and 9 °C), 13, 13, 9, and 8 oocytes were analyzed, respectively.
Experiment 2: Assessing the amount of EG necessary to achieve and maintain a vitreous state during cooling and warming for different concentrations of sucrose in saline
This experiment was designed to determine the amount of EG necessary to maintain an ice-free (i.e. vitreous) state during cooling to cryogenic temperatures (~ 160 °C; below the glass transition temperature of these solutions) and warming, when the sucrose concentration ranged from 0.1 to 1.1 molal (mol/kg; equivalent to 0.053 to 0.53 molar (mol/L)). In studies of the physical properties of solutions, components of the solutions are usually measured by weight, not volume. A primary reason for this is because weight (in comparison to volume) does not change with temperature. Furthermore, concentrations are usually reported in weight fractions (weight percent (w/w, or wt %)). We will use these conventions here and make references to the equivalent molar concentrations when comparing the results to previously published studies.
A Diamond Differential Scanning Calorimeter (DSC; Perkin-Elmer Waltham, MA, USA) outfitted with an autosampler and CryoFill liquid nitrogen cooling system was employed for the thermal analysis. The solutions were composed of water (5 g) and NaCl (0.0045 g) to which various amounts of sucrose and EG were added. For this experiment, spectrophotometric grade (>99%) EG was utilized. All components were weighed on an analytical balance. Errors between the target and actual weight percent were less than 0.1 % in all instances. To test the vitrification properties of the solutions, 5 µl (~ 0.0055 grams) were loaded into standard 10 µl aluminum DSC pans (Perkin-Elmer Part # BO14-3015), and the pans were hermetically sealed. Embryo-grade water and HPLC-grade (> 99.9% purity) cyclohexane were also run as calibration standards. Analyses were conducted at a cooling rate of 100 °C/min and a warming rate of 10 °C/min to be consistent with previous studies (62, 63). In instances when crystallization and melting peaks were not clearly evident on the curves, plots of the heat flow as a function of temperature were analyzed by fitting a polynomial curve to the data and determining if a crystallization or melting peak was evident above the random noise of the signal. Random noise was estimated by determining the standard deviation of the actual signal from the expected value from the polynomial fit. When an apparent crystallization peak followed by a melting peak in the expected temperature range was noted, and the maximum point on the melting peak was beyond 3 standard deviations from the expected value, it was classified as a thermal event (64). Only when three independent solutions confirmed the absence of crystallization and melting was the solution classified as having achieved and maintained a vitreous state throughout the cooling and warming procedure.
Experiment 3: Computer modeling toward an optimal vitrification method for human oocytes
Using the experimental data from the present study and osmotic tolerance data from our previous study (65), we have investigated methods to vitrify human oocytes using computer modeling. Our goal in the present work was to develop a method which should ensure ice-free cryopreservation (i.e. achieve vitrification and preclude devitrification during cooling and warming) and prevent osmotic damage to the cell during the CPA addition and removal processes using an optimal combination of sucrose and EG.
The first step was to determine the appropriate composition of a vitrification solution using EG and sucrose as the cryoprotectants. There were two criteria used to make this determination: (1) the total solute concentration should be high enough to preclude ice formation during cooling and warming at rates applicable to devices used for cryopreservation currently in practice; and (2) the sucrose concentration should be as high as possible (high enough just to reach the oocyte osmotic tolerance threshold, which will allow the lowest amount of EG to be used, reducing the chemically-toxic properties of the solution).
It is known that the concentration of solutes necessary to achieve vitrification and avoid devitrification decreases with increasing cooling and warming rates (66, 67). Therefore, it was necessary to account for this property when estimating the appropriate solution composition. Baudot and Odagescu (63) analyzed cooling and warming rates necessary for vitrification of solutions containing EG. Their analysis confirmed that warming rates are more critical than cooling rates when trying to maintain a vitreous state. Therefore, we focused on the warming rates necessary to avoid devitrification from this point forward (for a discussion on why this is the case, please see reviews on vitrification (33, 34)). In their analysis, Baudot and Odagescu (63) determined that a solution containing 50 wt % EG should maintain a vitreous state when the warming rate is on the order of 1×103 °C/min, and 48 wt % when the warming rate is on the order of 1×104 °C/min. It has previously been shown that 59 wt % EG is necessary to maintain a vitreous state during cooling and warming at the rates that we used in our DSC analysis (62). Cooling and warming rates using standard ¼ cc straws and the so-called ultra-rapid cooling devices (e.g. cryotops, open-pulled straws) can achieve cooling and warming rates on the order of 1×103 and 1×104 °C/min, respectively (28, 68). Because the difference in wt % necessary to avoid devitrification with a warming rate of ~ 1×104 °C/min compared to 1×103 °C/min is relatively minor (48 vs. 50 wt %), we focused on the lower warming rate, applicable to ¼ cc straws for the remainder of our analysis (please see below for a more complete discussion of this choice). This analysis suggests that the weight percent of solutes can be reduced by 15% (59 vs. 50 wt %) when the warming rates are increased from ~ 1×101 to ~ 1×103 °C/min. Therefore, for our investigation of an ideal vitrification solution, we focused on solutions across the range of sucrose molalities used in experiment 2, yet having a total solute composition reduced by 15 wt %.
For the next step, we calculated the degree to which solutions containing the various concentrations of EG and sucrose from the previous step will affect the equilibrium volume of a human oocyte. This was done by solving equation 1 and equation 2 using the appropriate values of the variables for each solution. For the modeling work reported here, we assumed that the oocyte would have properties of an average human oocyte, namely a cell radius of 63 µm (57), a permeability to water of 0.69 µm/min/atm, and a permeability to EG of 9.16 µm/min (average values at 25 °C from the present work).
From this analysis we could determine a solution that would have the optimal combination of EG and sucrose for vitrification, meaning that it would have the maximum amount of sucrose, and hence the minimum amount of EG, yet would not result in the oocyte exceeding the estimated osmotic tolerance. Osmotic tolerance data for human oocytes are relatively scarce compared to data for oocytes from other taxa (69, 70). To our knowledge, our previous report on the osmotic tolerance of human oocytes using MII spindle morphology as an endpoint (65) is the most comprehensive published data set. Therefore, we used this information as a guide for estimating the osmotic tolerance. In that study, we determined the relationship between osmolality and the morphology of the MII spindle as an experimental endpoint using a logistic regression analysis. For the present work, we used those data and calculated the 95 % confidence interval for the mean using the logistic regression model (71). We defined the osmotic tolerance as the range of osmolalities which 90% of oocytes should tolerate, and then estimated the corresponding cell volume utilizing the previously published Boyle van’t Hoff relationship for human oocytes (57). The results from this analysis suggest that 90% of human oocytes should tolerate changes in cell volume ranging from 57 % to 154 % of their isotonic volume. Thus, of the solutions analyzed, we chose the one which contained a combination of sucrose and EG such that the cell volume would be reduced to 57 % of the isotonic volume upon equilibration.
Finally, after determining the optimal solution using these criteria, we proceeded to model EG addition and removal procedures which will keep the oocytes within the osmotic tolerance limits defined above. Cell dynamic modeling was performed using Mathematica® (Wolfram Research, Champaign IL, USA) with a program written by one of the authors (SFM).
For the cell dynamic modeling carried out in the experiments described in this report, several simplifying assumptions were used as commonly employed in previous cryobiology studies (cf. (72, 73)). These include: 1) the extracellular space is assumed to be infinite and the intracellular space is the volume of a sphere (V = 4πr3/3); 2) the surface area of the cell is constant and determined by the initial cell radius (A = 4πr2); 3) the intracellular and extracellular solutions are assumed to be ideal and dilute; 4) the hydrostatic pressure across the cell membrane is assumed to be zero. We also assumed that the osmotic coefficients for the solutes were equal to 1, such that molalities and osmolalities are equivalent, and the additivity of solute osmolalities are linear.
RESULTS
Experiment 1: Oocyte permeability to ethylene glycol
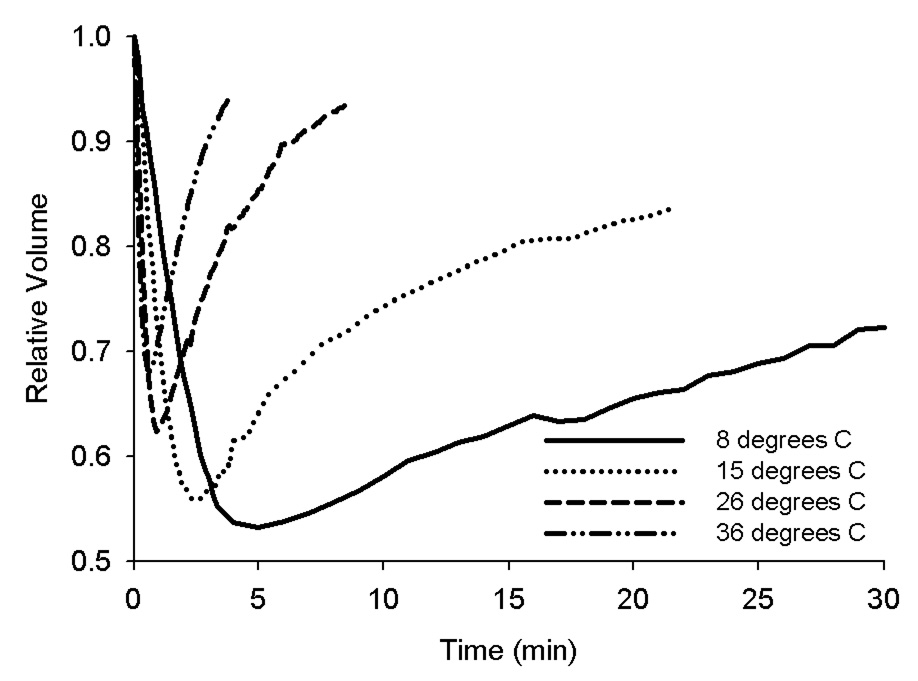
When the oocytes were abruptly exposed to a solution containing 1.0 mol/L EG, they responded osmotically, first by shrinking, then swelling, as expected. Furthermore, the degree of shrinkage and time for swelling was influenced by the temperature of the solution (Fig 2). This response is dictated by the permeability of the oolemma to both water and EG, as described by equation 1 and equation 2. The average radius of human oocytes in an isotonic solution was previously reported by Newton et al. to be 63 µm (57). The mean radius of human oocytes analyzed in this study was nearly identical (63.7 µm). The population was slightly skewed toward higher values, and this is reflected by a slight decrease in the median value (63.4 µm).
Figure 2.
Examples of the volume changes that representative oocytes experienced during equilibration with 1.0 mol/L EG at 4 different temperatures are shown. Notice that lower temperatures are associated with a greater total volume loss and a slower return to isotonic volume.
Figure 3 shows the relationship between temperature and the two permeability parameters for the oocytes in the present study. As expected, temperature and permeability were strongly correlated (r = −0.97 for both Lp and Ps). The values of Lp differed by an order of magnitude across the temperature range (from 0.15 µm/(min·atm) at 6.7 °C to 1.17 µm/(min·atm) at 35.7 °C). For the four experimental treatments (~ 33, 26, 14, and 9 °C), the coefficients of variation for Lp were 21%, 34%, 17%, and 15%, respectively. For Lp, the activation energy was 14.42 Kcal/mol (95% confidence interval: 13.19 to 15.65 Kcal/mol). The values of Ps also differed by an order of magnitude across the temperature range in the present study (from 1.5 µm/min at 6.7 °C to 30.0 µm/min at 35.7 °C). For the four experimental treatments (~ 33, 26, 14, and 9 °C), the coefficients of variation for Ps were 24%, 22%, 27%, and 19%, respectively. For Ps, the activation energy was 21.20 Kcal/mol (95% confidence interval: 19.49 to 22.91 Kcal/mol). Linear regression allows the calculation of the expected value of these parameters at any temperature T (in K). In this instance, Lp can be calculated by solving the following equation:
| (4) |
and Ps can be calculated by solving the following equation:
| (5) |
Figure 3.
Arrenhius plots of the relationship between temperature and Lp (left) and Ps (right) are shown along with linear regression lines.
Experiment 2: Assessing the amount of EG necessary to achieve and maintain a vitreous state during cooling and warming for different concentrations of sucrose
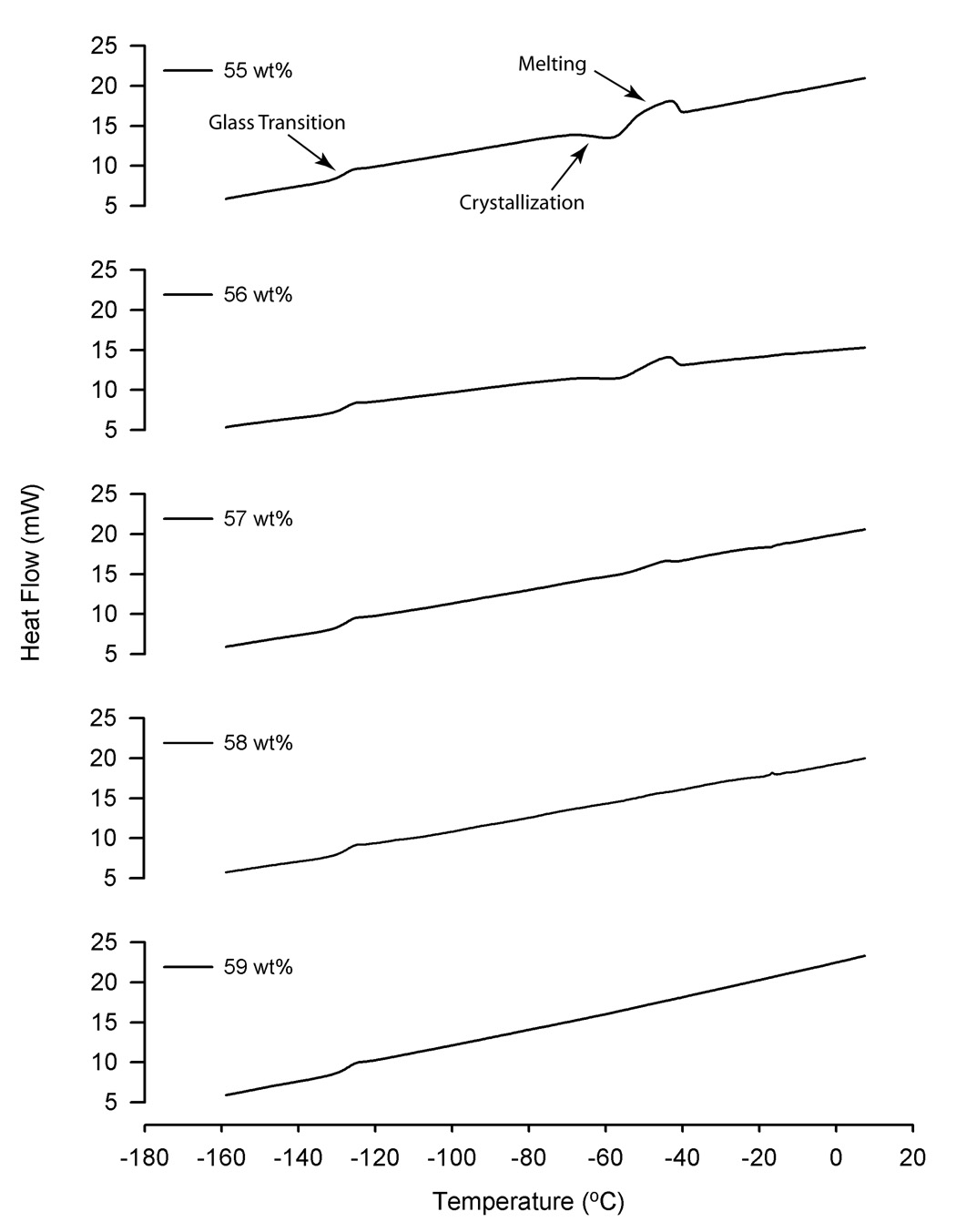
Figure 4 shows examples of DSC thermograms during warming for solutions containing 0.3 mol/kg sucrose with varying total solute concentrations. The thermal transition on the far left of the curve, near −130 °C, represents the glass transition. Two other transitions are evident as the solution warms and approaches −60 °C. The first change, when the heat flow decreases, represents the heat of fusion of water as ice crystallization occurs. Approximately 20 °C warmer, another transition occurs, as the ice crystals melt. It is evident that the magnitude of these later transitions becomes smaller as the total weight percent increases, until no evidence for crystallization or melting is apparent. In all of the solutions tested, there was no evidence for crystallization during cooling (data not shown). The total concentration of the solution necessary to prevent crystallization during warming increased as the sucrose concentration increased from 0.1 to 1.1 mol/kg; solutions containing 0.1, 0.3, 0.5, 0.7, 0.9, and 1.1 mol/kg sucrose required 59, 59, 59, 60, 61, and 61 wt %, respectively.
Figure 4.
Examples of DSC thermograms during warming for solutions containing 0.3 mol/kg sucrose, EG and saline, with a total solute concentration ranging from 55 to 59 wt % (adjusted by increasing the amount of EG). The primary thermal transitions, including the glass transition, crystallization, and melting are noted on the top graph. Notice how the magnitude of the crystallization and melting transitions diminish as the concentration increases from 55 to 59 wt %.
Experiment 3: Computer modeling toward an optimal vitrification method
Having established the permeability of human oocytes to EG and water in the presence of EG in experiment 1 and the appropriate proportions of EG and sucrose to include in vitrification solutions in experiment 2, we calculated the effect of solutions with the same proportions, yet with a total concentration reduced by 15 %, on the equilibrium volume of human oocytes. As expected, the volumes that human oocytes attained upon equilibration were reduced in direct proportion to the sucrose concentration (Figure 6). From this analysis it was determined that a solution containing 0.75 mol/kg (0.40 mol/L) sucrose would dehydrate the cell just to the point of the osmotic tolerance threshold (57 % of the isotonic volume). According to the results from the second experiment, the concentration of EG necessary to maintain a vitreous state during cooling and warming in a solution containing 0.75 mol/kg sucrose is 12.49 mol/kg (6.72 mol/L). Thus, we chose this combination as the optimal solution for further analysis.
We proceeded to determine an appropriate stepwise method to expose human oocytes to EG with this optimal solution as the final target, in preparation for vitrification. Sucrose is only necessary in the final vitrification solution; in fact, having sucrose in the initial solutions would be less efficient because it would cause osmotic shrinkage of the cell and reduce the amount of EG in the initial solutions to which oocytes can safely be exposed (safely in this context refers to preventing osmotic damage). Using equation 1 and equation 2 described above, and the osmotic tolerance limits discussed, we determined an appropriate EG addition and removal procedure for human oocytes. The procedure requires a 4-step CPA addition (5 minutes for the first 3 steps) and a 2-step CPA removal. The details of this procedure can be found in Table 1.
Table 1.
| Table 1a. The solution parameters for the proposed 4-step addition of EG to MII human oocytes and intracellular EG concentration values are shown. Each of the first 3 steps should proceed for 5 minutes at 25 °C. | |||||
|---|---|---|---|---|---|
| EG addition step | Hepes-HTF mass (g) | Water supplement mass (g)a | EG mass (g) | Sucrose mass (g) | Final intracellular EG concentration (molarity/molality) |
| Solution 1 | 58.3 | 41.7 | 9.2562 | 0 | 0.96 / 1.34 |
| Solution 2 | 58.3 | 41.7 | 23.4490 | 0 | 2.23 / 3.54 |
| Solution 3 | 58.3 | 41.7 | 47.8236 | 0 | 3.90 / 7.36 |
| Solution 4 | 58.3 | 41.7 | 77.1349 | 25.5227 | 5.02 / 12.5 b |
| Table 1b. The solution parameters for the CPA removal steps are shown. Step 1 should proceed for 4 minutes 25 °C. | ||
|---|---|---|
| EG removal step | Hepes- HTF mass (g) | Sucrose mass (g) |
| Solution 1 | 100 | 50.8316 |
| Solution 2 | 100 | 0 |
A lower concentration of non-permeating components (i.e. salts) was utilized as a means to allow an increase in the total concentration of EG exposure during each step while keeping the cells within their osmotic tolerance limits. Such a reduced concentration of non-permeating solutes has been shown to be tolerated by human MII oocytes (65).
Values after exposure for 12 seconds, after which the straw could be plunged into liquid nitrogen.
DISCUSSION
Equilibrium freezing (i.e. slow-cooling (74)) is the predominant method in practice for human oocyte cryopreservation (15). Despite approximately twenty years of effort, the results from this approach remain highly variable (cf. (75)), and human oocyte cryopreservation is still considered an experimental procedure (1). The relatively poor performance of equilibrium freezing has been highlighted in recently published large trials (22–24). During the past few years, vitrification methods have been applied to cryopreserve human oocytes to determine if improvements can be made (27–31). Some of these investigations have demonstrated remarkable success (28), but, to date, too few reports on vitrification have been published to reach definitive conclusions.
Ethylene glycol permeability and its use for vitrification
Ethylene glycol is one of the primary permeating cryoprotectants used in vitrification methods, principally due to its relatively low toxicity compared to other compounds (76). Because vitrification procedures necessitate the use of high solute concentrations, making toxic and osmotic damage more likely, it is somewhat surprising that the quantitative permeability values for EG have yet to be determined for MII human oocytes. The results from the present study fill in this important gap in the human oocyte cryopreservation literature.
Concern about osmotic damage is particularly important when using EG, as mammalian oocytes generally have a lower permeability to EG relative to other permeating CPAs. For example, it has been shown that the oolemma in mouse oocytes has a lower permeability to EG relative to PG and DMSO (48, 77, 78). In the 1999 study by Paynter and colleagues, the average permeability to EG was 0.24 µm/s at 30 °C (48). This value is very similar to the estimated average permeability of human oocytes at this temperature from the present study (0.27 µm/s). In contrast, the permeability of Rhesus macaque oocytes to EG at this temperature was reported to be much lower (0.14 µm/s; (50)). Although quantitative permeability values were not determined in the study by Pedro et al., of the 5 cryoprotectants tested, the volumetric response of the oocytes suggests that only glycerol had a permeability value lower than EG.
The results from the present study also show that in vivo matured MII human oocytes have a lower permeability to EG in comparison to PG and DMSO. At 24 °C, the average permeability values for the three CPAs are 8.2, 15.0, and 16.8 µm/min, respectively (46, 47). The effect of this difference on the volume response of human oocytes to cryoprotectant solutions can be calculated using equation 1 and equation 2 from above. For example, a single-step exposure to 1.5 mol/L PG, DMSO, and EG at 24 °C will result in the reduction of cell volume to approximately 67 %, 61 %, and 53 % of the isotonic volume for the respective cryoprotectants. Although the difference in cell volume response suggests that EG may be an inferior permeating agent for cryopreservation of human oocytes, the volume changes associated with permeating cryoprotectants are only one of many important factors to consider when designing cryopreservation procedures.
In fact, because volume changes can be modulated by changing the method used to expose the cells to such compounds, the inherent toxicity of the permeating cryoprotectant may be a more important consideration. Very recently, a report on the permeability properties of in vitro matured human oocytes was published (79). In that study, the average permeability of oocytes to EG at 30, 22, and 8 °C was reported to be 28.5, 11.7, and 3.7 µm/min (these values were converted from the reported units of cm/min to be consistent with the present study). Overall, these permeability values are higher than the expected average permeability of in vivo matured human oocytes at the respective temperatures as determined from our study (17.1, 6.4, and 1.0 µm/min).
Osmotic stress, and the resulting cell volume excursions, is one of the primary theories of injury during cryopreservation (80, 81). Previous studies have shown that mammalian oocytes are sensitive to solutions with high osmolalities. Agca et al. (70) documented the effects on bovine oocytes of exposures to anisotonic solutions, and determined that exposure to concentrations of 1200 milliosmolal or greater (equivalent to a reduction in volume to ~ 40 % of the isotonic volume (determined from the analysis of (82))) reduced the potential to develop in vitro to blastocysts by ~50 % relative to untreated cells. Rhesus macaque oocytes suffered membrane damage after exposure to concentrations of EG at 3 mol/L or higher using a single-step addition and removal (50), although it was not determined if this damage occurred specifically as a result of the volume excursions, or from a chemical effect of the EG, or an interaction between these 2 factors. While it has been shown that human oocytes can tolerate exposure to fairly high concentrations of mono- and disaccharides (up to 1.5 mol/L; equivalent to a reduction in volume to ~35 % of isotonic (57)) as measured by immediate viability using membrane integrity and enzyme activity (83), a reduced tolerance was reported as measured by MII spindle morphology (65). To our knowledge, viability after osmotic stress, as measured by developmental potential, has not been reported for in vivo matured MII human oocytes.
Identifying optimal combinations of EG and sucrose for vitrification
When designing a vitrification procedure, one consideration is whether the cryoprotectant solution can form and maintain a stable glass during cooling and warming. Additionally, the lowest concentration of solutes necessary to meet this condition should be used to minimize the potential toxicity of the solution. However, a review of the literature suggests that these criteria are rarely used in an explicit manner when different vitrification methods have been tested. Because solutes have different physical properties, the relative amount of each solute in a solution will affect the ability of a solution to vitrify (35). Ethylene glycol and sucrose are commonly used as permeating and non-permeating solutes for vitrification of mammalian oocytes, yet few investigations have been undertaken to determine the glass-forming properties of aqueous solutions containing these solutes. Kuleshova and colleagues conducted a similar analysis to the one undertaken in this study, but with fewer concentrations of sucrose (0, 0.1, 0.5, and 1.0 mol/kg) (62). The results from the study by Kuleshova et al. and the present study are similar. Kuleshova et al. determined that the solutions required 59, 60, 61, and 65 wt % at the respective sucrose concentrations to maintain a vitreous state during cooling and warming. In both studies, the total weight percent necessary to avoid crystallization increased as EG was replaced with sucrose. This is expected, as aqueous solutions containing only sucrose as the solute require higher concentrations (> 70 wt %) (84) to maintain a vitreous state during slow warming compared to solutions containing EG as the only solute (~ 59 wt %) (62).
Investigating methods for vitrification using computer modeling based upon fundamental principles
Cellular damage can occur during any one of the steps in a cryopreservation procedure. Optimizing such a multi-step procedure through purely empirical means would require a very large experiment – the number of treatment combinations could easily be in the thousands. By using fundamental principles and computer modeling, estimates of optimal methods can be arrived at through rational analysis, with the potential to save a significant amount of resources by narrowing the choices to test via empirical methods. Such an approach is particularly appealing for human oocyte cryopreservation, where experimental material is scarce, and experimental design is significantly influenced by ethical considerations. In the present study we have used such principles to make an initial prediction of an optimal vitrification method for human oocytes using EG and sucrose as cryoprotectants. We based these predictions on several criteria, including the necessity of maintaining a stable vitreous state during cooling and warming, using the minimum amount of permeating cryoprotectant, and designing the method to reduce the potential for osmotic damage to the cell.
The reasons for moving away from equilibrium freezing methods and toward the use of vitrification include reducing cell damage associated with chilling injury and ice formation. If the later is a true goal, then one should use a solution that maintains a stable vitreous state during cooling and warming. However, using solutions with higher concentrations of solutes than is necessary to maintain a vitreous state exposes cells to unnecessary risks of chemical and osmotic damage. In our modeling, we used the measured vitrification properties of solutions from the second experiment as a guide for determining the appropriate concentration of the various solutes. If solutions are used that are not true vitrification solutions, the degree to which ice forms and the size of the resulting crystals are difficult to control. Having little control over an important variable such as ice formation is likely to add to the variability of a method (85).
Vitrification methods for mammalian oocytes have evolved toward the use of devices to achieve so-called “ultra-rapid cooling” following their application with bovine oocytes (86, 87). Because of the results from these and similar studies, an implicit assumption seems to have developed in the field of human oocyte cryopreservation that successful vitrification requires cooling rates which can only be attained with such devices. In fact, most of the reports on human oocyte vitrification have used such devices, including open-pulled straws (88), electron microscope grids (31), cryoloops (89) and cryotops (27–30). However, this assumption may not be valid. Firstly, the use of different devices in an experimental comparison introduces potentially confounding variables other than cooling and warming rates (e.g. the time for which the cells are exposed to the vitrification solution prior to plunging into liquid nitrogen). Secondly, in particular reference to human oocytes, very high cooling rates may not be necessary because previous studies have suggested that human oocytes are more tolerant to chilling than bovine oocytes (90). One possible reason for this is the relatively low level of intracellular lipids in human oocytes compared to oocytes from other domestic animals (e.g. cattle and swine), a property which makes these oocytes chilling sensitive (91).
Current vitrification methods have also evolved to rely upon a very brief exposure to the final vitrification solution, and it is often assumed that the damage from longer exposures is due to chemical toxicity of the solutes. However, such data are equivocal, as the chemical effect is completely confounded by the osmotic effect. Several reports with mammalian oocytes have shown that cryopreservation outcomes can be improved if the osmotic effect of exposure to the solutions is modulated by prolonged CPA addition and/or removal (92–97) suggesting that consideration of the osmotic effects are at least as important as the chemical effects.
We focused our modeling on the use of a solution that could maintain a stable vitreous state during cooling and warming when using a standard ¼ cc freezing straw for several reasons. First, there are years of experience in the clinical IVF community with the use of these devices for cryopreservation of other samples. The use of some of the ultra-rapid cooling devices has been reported to be cumbersome, potentially increasing the likelihood of damage to the oocytes during their use, losing oocytes during handling, and requiring extensive technical training periods (27). In addition, unlike many of the other devices used, standard freezing straws can be safely sealed, thus avoiding the potential for contamination through direct contact with liquid nitrogen (98, 99). We acknowledge that, more recently, some of the ultra-rapid cooling devices have been modified to be fully sealed systems and should alleviate this problem; however, to our knowledge no reports of the use of these devices for human oocytes have been published. Furthermore, the design of some of these sealed systems makes it likely that the cooling and warming rates will not be as fast as the open systems. Although seemingly paradoxical, the larger thermal mass of a ¼ cc straw may have benefits. It is well known that devitrification and recrystallization are serious risks when vitrification solutions are used that are near the threshold of thermodynamic stability (33). For a device with a low thermal mass, accidental warming, and the concomitant risk of devitrification, is higher than a device with a higher thermal mass. Finally, it is often argued (frequently without supporting evidence) that the higher cooling rates attainable with ultra-rapid cooling devices allow a significant reduction in the concentration of cryoprotectant necessary to maintain stable vitrification (up to 30 % according to one recent report (100)). However, the analysis by Baudot and Odagescu (2004), based upon measured physical properties of solutions, suggests that the actual reduction is quite small (~ 2 %), at least for EG, given the difference in cooling rates between standard freezing straws and the other devices.
Since the work by Martino et al. in 1996 (86), few investigations of bovine oocyte vitrification using standard freezing straws have been published, presumably because of the assumption stated above. However, even for bovine oocytes the necessity of ultra-rapid cooling has been successfully challenged. Otoi and colleagues attempted to refine methods for bovine oocyte vitrification using ¼ cc straws to determine if success rates comparable to those achieved by ultra-rapid cooling methods could be attained (97). The best results they achieved rivaled those originally achieved with EM grids (86) and open-pulled straws (87). The method by which Otoi and colleagues were able to achieve their success is strikingly similar to what we have proposed to be an optimal method based upon computer modeling. Otoi et al. achieved the best results using a solution they called ES40. This solution contains EG at a concentration of 7.15 mol/L whereas our proposed optimal solution contains EG at 6.72 mol/L. The sucrose concentration in ES40 is 0.35 mol/L whereas in our solution the sucrose concentration is 0.4 mol/L. Otoi et al. were also able to achieve a significant improvement in survival by changing the method for CPA addition to include a prolonged addition procedure (3 steps total vs. 1 or 2 steps). This is very similar to the conclusions from our analysis. Overall, the results by Otoi et al.. support our argument that osmotic stress is a significant concern when vitrifying mammalian oocytes, yet can be overcome by changing the CPA addition and removal procedures.
To our knowledge, the report by Kuwayama and colleagues is the only publication where an experiment was conducted to test different vitrification methods directly on human oocytes (28). These investigators tested 2 solutions with the cryotop method, one contained 6.8 mol/L EG and the other contained 5.0 mol/L EG. Each solution also contained sucrose at 1.0 mol/L. The proportion of oocytes that showed normal morphology and blastocyst formation was higher in the solution containing the lower concentration of EG. Our proposed optimal solution contains EG at 6.72 mol/L, which at first suggests that it will perform poorly compared to a solution with a lower concentration. However, our solution also contains considerably less sucrose (0.4 mol/L), and this, coupled with our proposed method for CPA addition and removal, should reduce the damaging osmotic effects associated with its use compared to the method used in the study by Kuwayama et al. (28).
There are several aspects of our modeling that may influence the predictive accuracy. We have focused much of the model restrictions on the estimates of osmotic tolerance based upon MII spindle morphology. We recognize that the developmental potential of human oocytes may be a more sensitive indicator of osmotic tolerance than spindle morphology, as has been shown with bovine oocytes (cf. (70, 101)). However, to date, for human oocytes this is the best estimate of osmotic tolerance available. In addition, our previous experiment on the effect of osmotic stress on human oocytes was conducted in the absence of intracellular cryoprotectants which may have an effect on the spindle microtubules, and hence the estimates of osmotic tolerance (102). One of the assumptions in our model is that achieving and maintaining a true vitreous state is a prerequisite for a robust procedure. It is known that cells can survive freezing in the presence of ice (both extracellular and intracellular). Therefore, solutions with lower solute concentrations than the one we propose may allow successful cryopreservation, despite not being able to vitrify. However, the tolerance of cells to ice formation (particularly intracellular ice formation) is not well understood. Furthermore, controlling ice formation and growth is difficult. Therefore, we believe that, if true vitrification can be achieved and the potential damage from the solution used to achieve vitrification can be managed, preventing ice formation during cooling and warming is preferable.
The present modeling has focused on a vitrification method using a single permeating cryoprotectant (EG). As was stated above, EG is generally less toxic than other compounds. Bautista and colleagues showed that mouse oocyte developmental potential was reduced by only 30 % after exposure to 7 mol/L EG in a 2-step manner (95). Hotamisligil and colleagues showed that mouse oocytes could tolerate 8 mol/L EG fairly well if the exposure time was less than 1 minute (mean blastocyst development rate of ~ 50 % vs. ~ 62 % for controls) , and exposure to 6 mol/L EG for 5 minutes had no effect on development compared to untreated oocytes (103). Martino et al. showed a significant reduction in bovine oocyte developmental potential when the cells were exposed to 4 or 5.5 mol/L EG in a single step (86). However, because the exposure was conducted in a one-step manner, osmotic effects could account for much of the damage. Our procedure would expose human oocytes to less than 4 mol/L EG throughout the EG loading steps up until transfer to the vitrification solution. The oocytes should reach near equilibrium with the EG after exposure to the final solution within 12 seconds. Thus, the cells needn’t be exposed to the final solution for long before transfer to liquid nitrogen.
In summary, the present research was conducted to examine fundamental cryobiological properties of human oocytes and vitrification solutions in a first step to optimize vitrification methods. The results from our analysis suggest that successful vitrification in standard freezing straws can be achieved, as the resulting theoretically-optimized method is similar to one shown to improve vitrification with bovine oocytes. Future work should be directed at testing this method with other methods currently in practice and further research into the fundamental cryobiological properties of human oocytes is warranted.
Figure 5.
This plot demonstrates changes in the relative cell volume of MII human oocytes resulting from equilibration in vitrification solutions containing varying compositions of EG and sucrose in saline (black circles) having a total solute concentration necessary to maintain a vitreous state as described in the results from experiment 3. The equilibrium volume was calculated by solving equation 1 and equation 2 using the concentrations of permeable (EG) and non-permeable (sodium chloride and sucrose) solutes in the respective solutions (please see text for further details). As the sucrose concentration is increased, the amount of EG in the solution necessary to achieve and maintain a vitreous state during cooling and warming decreases. However, as the sucrose concentration increases, a greater effect on the cell volume will occur. For our choice of an optimal solution, we wanted a combination of EG and sucrose such that the solution would have the maximum amount of sucrose (reducing the EG concentration) yet would not result in excessive cell shrinkage. The lower osmotic tolerance chosen for this analysis would result in the cell shrinking to 57 % of its isotonic volume (horizontal line). Therefore, the optimal solution will occur where the curve intersects the horizontal line. This occurs when the sucrose concentration is 0.75 mol/kg (0.40 mol/L). The corresponding EG concentration is 12.5 mol/kg (6.72 mol/L).
Acknowledgements
The authors would like to thank the following individuals for their assistance with the experiments: Jing-Mei Hu (B.S.), Shui-Ying Ma (B.S.), Shan Liu (B.S.), Na Zhang (B.S.), Hui-Jun Yang (M.S.), and Wan-Xia Zhong (B.S.). Special thanks go to Jun-Hao Yan (MD, Ph.D.). Locksley McGann (Ph.D., Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, Canada) provided us with an example of the spreadsheet used to determine the permeability parameters in this study, and his assistance in this matter is kindly appreciated. We thank Igor Katkov (Ph.D. Project Leader, Level V, Stem Cell Center, UCSD and Burhnam Institute, La Jolla, California, USA.) for helpful discussions of the thermodynamics of cell permeability.
This work was supported by the National High Technology Research and Development Program (“863 Program) of China (2006AA02Z4A4) and the Cryobiology Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Some of the results from this study were presented at the 62nd annual meeting of the American Society for Reproductive Medicine, 21–25 October 2006, in New Orleans, LA USA.
Conflict of Interest: None.
Capsule
Human metaphase II oocyte permeability to ethylene glycol was determined. A method for vitrification, based upon fundamental principles, was developed from these data.
References
- 1.ASRM Practice Committee. Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2004;82:993–998. doi: 10.1016/j.fertnstert.2004.07.925. [DOI] [PubMed] [Google Scholar]
- 2.Sonmezer M, Oktay K. Fertility preservation in female cancer patients: a comprehensive approach. In: Tulandi T, Gosden RG, editors. Preservation of Fertility. London: Taylor and Francis; 2004. pp. 177–190. [Google Scholar]
- 3.Fugger EF. Clinical status of human embryo cryopreservation in the United States of America. Fertil Steril. 1989;52:986–990. doi: 10.1016/s0015-0282(16)53163-x. [DOI] [PubMed] [Google Scholar]
- 4.Benagiano G, Gianaroli L. The new Italian IVF legislation. Reprod Biomed Online. 2004;9:117–125. doi: 10.1016/s1472-6483(10)62118-9. [DOI] [PubMed] [Google Scholar]
- 5.Bankowski BJ, Lyerly AD, Faden RR, Wallach EE. The social implications of embryo cryopreservation. Fertil Steril. 2005;84:823–832. doi: 10.1016/j.fertnstert.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 6.Karow AM. Implications of Tissue Banking for Human Reproductive Medicine. In: Karow AM, Critser JK, editors. Reproductive Tissue Banking, Scientific Principles. San Diego: Academic Press; 1997. pp. 441–464. [Google Scholar]
- 7.Hornicek FJ, Woll JE, Kasprisin D, editors. Standards for Tissue Banking. McLean, VA: American Association of Tissue Banks; 2002. [Google Scholar]
- 8.Leibo SP. Cryopreservation of mammalian oocytes. In: Tulandi T, Gosden RG, editors. Preservation of Fertility. London: Taylor and Francis; 2004. pp. 141–155. [Google Scholar]
- 9.Smith GD, Silva ESCA. Developmental consequences of cryopreservation of mammalian oocytes and embryos. Reprod Biomed Online. 2004;9:171–178. doi: 10.1016/s1472-6483(10)62126-8. [DOI] [PubMed] [Google Scholar]
- 10.Stachecki JJ, Cohen J. An overview of oocyte cryopreservation. Reprod Biomed Online. 2004;9:152–163. doi: 10.1016/s1472-6483(10)62124-4. [DOI] [PubMed] [Google Scholar]
- 11.Gook DA, Osborn SM, Bourne H, Johnston WI. Fertilization of human oocytes following cryopreservation; normal karyotypes and absence of stray chromosomes. Hum Reprod. 1994;9:684–691. doi: 10.1093/oxfordjournals.humrep.a138572. [DOI] [PubMed] [Google Scholar]
- 12.Gook DA, Osborn SM, Johnston WI. Cryopreservation of mouse and human oocytes using 1,2-propanediol and the configuration of the meiotic spindle. Hum Reprod. 1993;8:1101–1109. doi: 10.1093/oxfordjournals.humrep.a138201. [DOI] [PubMed] [Google Scholar]
- 13.Gook DA, Schiewe MC, Osborn SM, Asch RH, Jansen RP, Johnston WI. Intracytoplasmic sperm injection and embryo development of human oocytes cryopreserved using 1,2-propanediol. Hum Reprod. 1995;10:2637–2641. doi: 10.1093/oxfordjournals.humrep.a135759. [DOI] [PubMed] [Google Scholar]
- 14.Kazem R, Thompson LA, Srikantharajah A, Laing MA, Hamilton MP, Templeton A. Cryopreservation of human oocytes and fertilization by two techniques: in-vitro fertilization and intracytoplasmic sperm injection. Hum Reprod. 1995;10:2650–2654. doi: 10.1093/oxfordjournals.humrep.a135761. [DOI] [PubMed] [Google Scholar]
- 15.Koutlaki N, Schoepper B, Maroulis G, Diedrich K, Al-Hasani S. Human oocyte cryopreservation: past, present and future. Reprod Biomed Online. 2006;13:427–436. doi: 10.1016/s1472-6483(10)61449-6. [DOI] [PubMed] [Google Scholar]
- 16.Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum Reprod. 2001;16:411–416. doi: 10.1093/humrep/16.3.411. [DOI] [PubMed] [Google Scholar]
- 17.Quintans CJ, Donaldson MJ, Bertolino MV, Pasqualini RS. Birth of two babies using oocytes that were cryopreserved in a choline-based freezing medium. Hum Reprod. 2002;17:3149–3152. doi: 10.1093/humrep/17.12.3149. [DOI] [PubMed] [Google Scholar]
- 18.Boldt J, Cline D, McLaughlin D. Human oocyte cryopreservation as an adjunct to IVF-embryo transfer cycles. Hum Reprod. 2003;18:1250–1255. doi: 10.1093/humrep/deg242. [DOI] [PubMed] [Google Scholar]
- 19.Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82:601–605. doi: 10.1016/j.fertnstert.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Chen SU, Lien YR, Chen HF, Chang LJ, Tsai YY, Yang YS. Observational clinical follow-up of oocyte cryopreservation using a slow-freezing method with 1,2-propanediol plus sucrose followed by ICSI. Hum Reprod. 2005;20:1975–1980. doi: 10.1093/humrep/deh884. [DOI] [PubMed] [Google Scholar]
- 21.Fosas N, Marina F, Torres PJ, Jove I, Martin P, Perez N, et al. The births of five Spanish babies from cryopreserved donated oocytes. Hum Reprod. 2003;18:1417–1421. doi: 10.1093/humrep/deg297. [DOI] [PubMed] [Google Scholar]
- 22.Borini A, Sciajno R, Bianchi V, Sereni E, Flamigni C, Coticchio G. Clinical outcome of oocyte cryopreservation after slow cooling with a protocol utilizing a high sucrose concentration. Hum Reprod. 2006;21:512–517. doi: 10.1093/humrep/dei346. [DOI] [PubMed] [Google Scholar]
- 23.Levi Setti PE, Albani E, Novara PV, Cesana A, Morreale G. Cryopreservation of supernumerary oocytes in IVF/ICSI cycles. Hum Reprod. 2006;21:370–375. doi: 10.1093/humrep/dei347. [DOI] [PubMed] [Google Scholar]
- 24.La Sala GB, Nicoli A, Villani MT, Pescarini M, Gallinelli A, Blickstein I. Outcome of 518 salvage oocyte-cryopreservation cycles performed as a routine procedure in an in vitro fertilization program. Fertil Steril. 2006;86:1423–1427. doi: 10.1016/j.fertnstert.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Gosden RG. Prospects for oocyte banking and in vitro maturation. J Natl Cancer Inst Monogr. 2005:60–63. doi: 10.1093/jncimonographs/lgi007. [DOI] [PubMed] [Google Scholar]
- 26.Shaw JM, Jones GM. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 2003;9:583–605. doi: 10.1093/humupd/dmg041. [DOI] [PubMed] [Google Scholar]
- 27.Antinori M, Licata E, Dani G, Cerusico F, Versaci C, Antinori S. Cryotop vitrification of human oocytes results in high survival rate and healthy deliveries. Reprod Biomed Online. 2007;14:72–79. doi: 10.1016/s1472-6483(10)60766-3. [DOI] [PubMed] [Google Scholar]
- 28.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–308. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 29.Kyono K, Fuchinoue K, Yagi A, Nakajo Y, Yamashita A, Kumagai S. Successful pregnancy and delivery after transfer of a single blastocyst derived from a vitrified mature human oocyte. Fertil Steril. 2005;84:1017. doi: 10.1016/j.fertnstert.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 30.Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril. 2006;85:108–111. doi: 10.1016/j.fertnstert.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2003;79:1323–1326. doi: 10.1016/s0015-0282(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 32.Kuleshova LL, Lopata A. Vitrification can be more favorable than slow cooling. Fertil Steril. 2002;78:449–454. doi: 10.1016/s0015-0282(02)03305-8. [DOI] [PubMed] [Google Scholar]
- 33.Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil (Camb) 2005;8:231–239. doi: 10.1080/14647270500054803. [DOI] [PubMed] [Google Scholar]
- 34.Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- 35.Fahy GM, Levy DI, Ali SE. Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology. 1987;24:196–213. doi: 10.1016/0011-2240(87)90023-x. [DOI] [PubMed] [Google Scholar]
- 36.Fuku EJ, Liu J, Downey BR. In vitro viability and ultrastructural changes in bovine oocytes treated with a vitrification solution. Mol Reprod Dev. 1995;40:177–185. doi: 10.1002/mrd.1080400206. [DOI] [PubMed] [Google Scholar]
- 37.Gao DY, Liu J, Liu C, McGann LE, Watson PF, Kleinhans FW, et al. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod. 1995;10:1109–1122. doi: 10.1093/oxfordjournals.humrep.a136103. [DOI] [PubMed] [Google Scholar]
- 38.Papis K, Shimizu M, Izaike Y. Factors affecting the survivability of bovine oocytes vitrified in droplets. Theriogenology. 2000;54:651–658. doi: 10.1016/S0093-691X(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 39.Rojas C, Palomo MJ, Albarracin JL, Mogas T. Vitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilaments. Cryobiology. 2004;49:211–220. doi: 10.1016/j.cryobiol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Shaw PW, Fuller BJ, Bernard A, Shaw RW. Vitrification of mouse oocytes: improved rates of survival, fertilization, and development to blastocysts. Mol Reprod Dev. 1991;29:373–378. doi: 10.1002/mrd.1080290409. [DOI] [PubMed] [Google Scholar]
- 41.Songsasen N, Yu I, Murton S, Paccamonti DL, Eilts BE, Godke RA, et al. Osmotic sensitivity of canine spermatozoa. Cryobiology. 2002;44:79–90. doi: 10.1016/S0011-2240(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 42.Bautista JA, Kanagawa H. Current status of vitrification of embryos and oocytes in domestic animals: ethylene glycol as an emerging cryoprotectant of choice. Jpn J Vet Res. 1998;45:183–191. [PubMed] [Google Scholar]
- 43.Fahy GM, Wowk B, Wu J, Paynter S. Improved vitrification solutions based on the predictability of vitrification solution toxicity. Cryobiology. 2004;48:22–35. doi: 10.1016/j.cryobiol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Yoon TK, Chung HM, Lim JM, Han SY, Ko JJ, Cha KY. Pregnancy and delivery of healthy infants developed from vitrified oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2000;74:180–181. doi: 10.1016/s0015-0282(00)00572-0. [DOI] [PubMed] [Google Scholar]
- 46.Paynter SJ, Cooper A, Gregory L, Fuller BJ, Shaw RW. Permeability characteristics of human oocytes in the presence of the cryoprotectant dimethylsulphoxide. Hum Reprod. 1999;14:2338–2342. doi: 10.1093/humrep/14.9.2338. [DOI] [PubMed] [Google Scholar]
- 47.Paynter SJ, O'Neil L, Fuller BJ, Shaw RW. Membrane permeability of human oocytes in the presence of the cryoprotectant propane-1,2-diol. Fertil Steril. 2001;75:532–538. doi: 10.1016/s0015-0282(00)01757-x. [DOI] [PubMed] [Google Scholar]
- 48.Paynter SJ, Fuller BJ, Shaw RW. Temperature dependence of Kedem-Katchalsky membrane transport coefficients for mature mouse oocytes in the presence of ethylene glycol. Cryobiology. 1999;39:169–176. doi: 10.1006/cryo.1999.2199. [DOI] [PubMed] [Google Scholar]
- 49.Agca Y, Liu J, Peter AT, Critser ES, Critser JK. Effect of developmental stage on bovine oocyte plasma membrane water and cryoprotectant permeability characteristics. Mol Reprod Dev. 1998;49:408–415. doi: 10.1002/(SICI)1098-2795(199804)49:4<408::AID-MRD8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 50.Songsasen N, Ratterree MS, VandeVoort CA, Pegg DE, Leibo SP. Permeability characteristics and osmotic sensitivity of rhesus monkey (Macaca mulatta) oocytes. Hum Reprod. 2002;17:1875–1884. doi: 10.1093/humrep/17.7.1875. [DOI] [PubMed] [Google Scholar]
- 51.Coticchio G, Bianchi V, De Santis L, Flamigni C, Borini A, Paynter S. Volume changes of human oocytes on exposure to cryoprotectants. Human Reproduction. 1994;19:i78. doi: 10.1093/humrep/deh742. (Abstract) [DOI] [PubMed] [Google Scholar]
- 52.Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online. 2004;9:680–691. doi: 10.1016/s1472-6483(10)61780-4. [DOI] [PubMed] [Google Scholar]
- 53.Gao DY, McGrath JJ, Tao J, Benson CT, Critser ES, Critser JK. Membrane transport properties of mammalian oocytes: a micropipette perfusion technique. J Reprod Fertil. 1994;102:385–392. doi: 10.1530/jrf.0.1020385. [DOI] [PubMed] [Google Scholar]
- 54.Russ J. The image processing and analysis cookbook. 2002. Available from Reindeer Graphics ( http://reindeergraphics.com) [Google Scholar]
- 55.Kleinhans FW. Membrane permeability modeling: Kedem-Katchalsky vs. a two-parameter formalism. Cryobiology. 1998;37:271–289. doi: 10.1006/cryo.1998.2135. [DOI] [PubMed] [Google Scholar]
- 56.Agca Y, Liu J, Critser ES, McGrath JJ, Critser JK. Temperature-dependent osmotic behavior of germinal vesicle and metaphase II stage bovine oocytes in the presence of Me2SO in relationship to cryobiology. Mol Reprod Dev. 1999;53:59–67. doi: 10.1002/(SICI)1098-2795(199905)53:1<59::AID-MRD7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 57.Newton H, Pegg DE, Barrass R, Gosden RG. Osmotically inactive volume, hydraulic conductivity, and permeability to dimethyl sulphoxide of human mature oocytes. J Reprod Fertil. 1999;117:27–33. doi: 10.1530/jrf.0.1170027. [DOI] [PubMed] [Google Scholar]
- 58.Billo EJ. Excel for Chemists: a comprehensive guide. Second ed. New York: John Wiley and Sons; 2001. [Google Scholar]
- 59.Mazur P. Principles of Cryobiology. In: Fuller BJ, Lane N, Benson EE, editors. Life in the Frozen State. Boca Raton: CRC Press; 2004. pp. 3–65. [Google Scholar]
- 60.Mazur P, Rall WF, Leibo SP. Kinetics of water loss and the likelihood of intracellular freezing in mouse ova. Influence of the method of calculating the temperature dependence of water permeability. Cell Biophys. 1984;6:197–213. doi: 10.1007/BF02788619. [DOI] [PubMed] [Google Scholar]
- 61.Zolman JF. Biostatistics: Experimental Design and Statistical Inference. New York: Oxford University Press; 1993. [Google Scholar]
- 62.Kuleshova LL, MacFarlane DR, Trounson AO, Shaw JM. Sugars exert a major influence on the vitrification properties of ethylene glycol-based solutions and have low toxicity to embryos and oocytes. Cryobiology. 1999;38:119–130. doi: 10.1006/cryo.1999.2153. [DOI] [PubMed] [Google Scholar]
- 63.Baudot A, Odagescu V. Thermal properties of ethylene glycol aqueous solutions. Cryobiology. 2004;48:283–294. doi: 10.1016/j.cryobiol.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Draper NR, Smith H. Applied Regression Analysis. New York: John Wiley and Sons; 1966. [Google Scholar]
- 65.Mullen SF, Agca Y, Broermann DC, Jenkins CL, Johnson CA, Critser JK. The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod. 2004;19:1148–1154. doi: 10.1093/humrep/deh201. [DOI] [PubMed] [Google Scholar]
- 66.MacFarlane DR. Physical aspects of vitrification in aqueous solutions. Cryobiology. 1987;24:181–195. [Google Scholar]
- 67.MacFarlane DR. Devitrification in glass-forming aqueous solutions. Cryobiology. 1986;23:230–244. [Google Scholar]
- 68.Rall WF, Wood MJ, Kirby C, Whittingham DG. Development of mouse embryos cryopreserved by vitrification. J Reprod Fertil. 1987;80:499–504. doi: 10.1530/jrf.0.0800499. [DOI] [PubMed] [Google Scholar]
- 69.Adams SL, Kleinhans FW, Mladenov PV, Hessian PA. Membrane permeability characteristics and osmotic tolerance limits of sea urchin (Evechinus chloroticus) eggs. Cryobiology. 2003;47:1–13. doi: 10.1016/s0011-2240(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 70.Agca Y, Liu J, Rutledge JJ, Critser ES, Critser JK. Effect of osmotic stress on the developmental competence of germinal vesicle and metaphase II stage bovine cumulus oocyte complexes and its relevance to cryopreservation. Mol Reprod Dev. 2000;55:212–219. doi: 10.1002/(SICI)1098-2795(200002)55:2<212::AID-MRD11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 71.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 2000. [Google Scholar]
- 72.Devireddy RV, Raha D, Bischof JC. Measurement of water transport during freezing in cell suspensions using a differential scanning calorimeter. Cryobiology. 1998;36:124–155. doi: 10.1006/cryo.1997.2071. [DOI] [PubMed] [Google Scholar]
- 73.McGrath JJ. Preservation of biological material by freezing and thawing. In: Shitzer A, Eberhart RC, editors. Heat transfer in medicine and biology. Vol. 2. New York: Plenum Press; 1985. pp. 185–238. [Google Scholar]
- 74.Mazur P. Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys. 1990;17:53–92. doi: 10.1007/BF02989804. [DOI] [PubMed] [Google Scholar]
- 75.Paynter S. A rational approach to oocyte cryopreservation. Reprod Biomed Online. 2005;10:578–586. doi: 10.1016/s1472-6483(10)61664-1. [DOI] [PubMed] [Google Scholar]
- 76.Ali J, Shelton JN. Design of vitrification solutions for the cryopreservation of embryos. J Reprod Fertil. 1993;99:471–477. doi: 10.1530/jrf.0.0990471. [DOI] [PubMed] [Google Scholar]
- 77.Paynter SJ, Fuller BJ, Shaw RW. Temperature dependence of mature mouse oocyte membrane permeabilities in the presence of cryoprotectant. Cryobiology. 1997;34:122–130. doi: 10.1006/cryo.1996.1990. [DOI] [PubMed] [Google Scholar]
- 78.Pedro PB, Yokoyama E, Zhu SE, Yoshida N, Valdez DM, Jr., Tanaka M, et al. Permeability of mouse oocytes and embryos at various developmental stages to five cryoprotectants. J Reprod Dev. 2005;51:235–246. doi: 10.1262/jrd.16079. [DOI] [PubMed] [Google Scholar]
- 79.Van den Abbeel E, Schneider U, Liu J, Agca Y, Critser JK, Van Steirteghem A. Osmotic responses and tolerance limits to changes in external osmolalities, and oolemma permeability characteristics, of human in vitro matured MII oocytes. Hum Reprod. 2007 doi: 10.1093/humrep/dem083. In Press. [DOI] [PubMed] [Google Scholar]
- 80.Meryman HT. The exceeding of a minimum tolerable cell volume in hypertonic suspensions as a cause of freezing injury. In: Wolstenholme GEW, O'Connor M, editors. The Frozen Cell. London: J and A Churchill; 1970. pp. 51–64. [Google Scholar]
- 81.Meryman HT. Osmotic stress as a mechanism of freezing injury. Cryobiology. 1971;8:489–500. doi: 10.1016/0011-2240(71)90040-x. [DOI] [PubMed] [Google Scholar]
- 82.Ruffing NA, Steponkus PL, Pitt RE, Parks JE. Osmometric behavior, hydraulic conductivity, and incidence of intracellular ice formation in bovine oocytes at different developmental stages. Cryobiology. 1993;30:562–580. doi: 10.1006/cryo.1993.1059. [DOI] [PubMed] [Google Scholar]
- 83.McWilliams RB, Gibbons WE, Leibo SP. Osmotic and physiological responses of mouse zygotes and human oocytes to mono- and disaccharides. Hum Reprod. 1995;10:1163–1171. doi: 10.1093/oxfordjournals.humrep.a136112. [DOI] [PubMed] [Google Scholar]
- 84.Ablett S, Izzard MJ, Liliford PJ. Differential scanning calorimetric study of frozen sucrose and glycerol solutions. Journal of the chemical society faraday transactions. 1992;88:789–794. [Google Scholar]
- 85.Shaw PW, Bernard AG, Fuller BJ, Hunter JH, Shaw RW. Vitrification of mouse oocytes using short cryoprotectant exposure: effects of varying exposure times on survival. Mol Reprod Dev. 1992;33:210–214. doi: 10.1002/mrd.1080330214. [DOI] [PubMed] [Google Scholar]
- 86.Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod. 1996;54:1059–1069. doi: 10.1095/biolreprod54.5.1059. [DOI] [PubMed] [Google Scholar]
- 87.Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, et al. Open Pulled Straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev. 1998;51:53–58. doi: 10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 88.Kuleshova L, Gianaroli L, Magli C, Ferraretti A, Trounson A. Birth following vitrification of a small number of human oocytes: case report. Hum Reprod. 1999;14:3077–3079. doi: 10.1093/humrep/14.12.3077. [DOI] [PubMed] [Google Scholar]
- 89.Liebermann J, Tucker MJ. Effect of carrier system on the yield of human oocytes and embryos as assessed by survival and developmental potential after vitrification. Reproduction. 2002;124:483–489. doi: 10.1530/rep.0.1240483. [DOI] [PubMed] [Google Scholar]
- 90.Bernard A, Hunter JE, Fuller BJ, Imoedemhe D, Curtis P, Jackson A. Fertilization and embryonic development of human oocytes after cooling. Hum Reprod. 1992;7:1447–1450. doi: 10.1093/oxfordjournals.humrep.a137592. [DOI] [PubMed] [Google Scholar]
- 91.Nagashima H, Kashiwazaki N, Ashman RJ, Grupen CG, Seamark RF, Nottle MB. Removal of cytoplasmic lipid enhances the tolerance of porcine embryos to chilling. Biol Reprod. 1994;51:618–622. doi: 10.1095/biolreprod51.4.618. [DOI] [PubMed] [Google Scholar]
- 92.Wood MJ, Barros C, Candy CJ, Carroll J, Melendez J, Whittingham DG. High rates of survival and fertilization of mouse and hamster oocytes after vitrification in dimethylsulphoxide. Biol Reprod. 1993;49:489–495. doi: 10.1095/biolreprod49.3.489. [DOI] [PubMed] [Google Scholar]
- 93.Rayos AA, Takahashi Y, Hishinuma M, Kanagawa H. Quick freezing of unfertilized mouse oocytes using ethylene glycol with sucrose or trehalose. J Reprod Fertil. 1994;100:123–129. doi: 10.1530/jrf.0.1000123. [DOI] [PubMed] [Google Scholar]
- 94.Nowshari MA, Nayudu PL, Hodges JK. Effect of cryoprotectant concentration, equilibration time and thawing procedure on survival and development of rapid frozen-thawed mature mouse oocytes. Theriogenology. 1994;42:1193–1204. doi: 10.1016/0093-691x(94)90868-0. [DOI] [PubMed] [Google Scholar]
- 95.Bautista JA, Dela Pena EC, Katagiri S, Takahashi Y, Kanagawa H. In vitro viability of mouse oocytes vitrified in an ethylene glycol-based solution. Jpn J Vet Res. 1998;46:13–18. [PubMed] [Google Scholar]
- 96.Hamano S, Koikeda A, Kuwayama M, Nagai T. Full-term development of in vitro-matured, vitrified and fertilized bovine oocytes. Theriogenology. 1992;38:1085–1090. doi: 10.1016/0093-691x(92)90122-8. [DOI] [PubMed] [Google Scholar]
- 97.Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Cryopreservation of mature bovine oocytes by vitrification in straws. Cryobiology. 1998;37:77–85. doi: 10.1006/cryo.1998.2103. [DOI] [PubMed] [Google Scholar]
- 98.Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;46:146–152. doi: 10.1016/s0011-2240(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 99.Rall WF. Avoidance of microbial cross-contamination of cryopreserved gametes, embryos, cells, and tissues during storage in liquid nitrogen. The Embryologists' Newsletter. 2003;6:1–15. [Google Scholar]
- 100.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12:779–796. doi: 10.1016/s1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 101.Mullen SF, Agca Y, Critser JK. Modeling the probability of MII spindle disruption in bovine oocytes as a function of total osmolality using logistic regression, and its application toward improved CPA addition and removal procedures. Cell Preserv Technol. 2004;2:145–155. [Google Scholar]
- 102.Vincent C, Johnson MH. Cooling, cryoprotectants, and the cytoskeleton of the mammalian oocyte. Oxf Rev Reprod Biol. 1992;14:73–100. [PubMed] [Google Scholar]
- 103.Hotamisligil S, Toner M, Powers RD. Changes in membrane integrity, cytoskeletal structure, and developmental potential of murine oocytes after vitrification in ethylene glycol. Biol Reprod. 1996;55:161–168. doi: 10.1095/biolreprod55.1.161. [DOI] [PubMed] [Google Scholar]