Abstract
Hypertension induced by high-salt diet in Dahl salt-sensitive rats leads to compensatory cardiac hypertrophy by ∼11 wk, cardiac dysfunction at ∼17 wk, and death from cardiac dysfunction at ∼21 wk. It is unclear what molecular hallmarks distinguish the compensatory hypertrophy from the decompensated cardiac dysfunction phase. Here we compared the gene expression in rat cardiac tissue from the compensatory hypertrophic phase (11 wk, n = 6) with the cardiac dysfunction phase (17 wk, n = 6) and with age-matched normotensive controls. Messenger RNA levels of 93 genes, selected based on predicted association with cardiac dysfunction, were measured by quantitative real-time PCR. In the hypertrophic phase, the expression of three genes, atrial natriuretic peptide (ANP; P = 0.0089), brain natriuretic peptide (P = 0.0012), and endothelin-1 precursor (P = 0.028), significantly increased, whereas there was decreased expression of 24 other genes including SOD2 (P = 0.0148), sarco(endo)plasmic reticulum Ca2+-ATPase 2a (P = 0.0002), and ryanodine receptor 2 (P = 0.0319). In the subsequent heart cardiac dysfunction phase, the expression of an additional 20 genes including inducible nitric oxide synthase (NOS; P = 0.0135), angiotensin I-converting enzyme (P = 0.0082), and IL-1β (P < 0.0001) increased, whereas the expression of seven genes decreased compared with those of age-matched controls. Furthermore, the expression of 22 genes, including prepro-endothelin-1, ANP, angiotensin I-converting enzyme, β1-adrenergic receptor, SOD2, and endothelial NOS, significantly changed in the cardiac dysfunction phase compared with the compensatory hypertrophic phase. Finally, principal component analysis successfully segregated animals with decompensatory cardiac dysfunction from controls, as well as from animals at the compensated hypertrophy phase, suggesting that we have identified molecular markers for each stage of the disease.
Keywords: quantitative polymerase chain reaction, diagnosis, heart failure, left ventricular
currently, five million people suffer from heart failure in the US alone (26), and mortality associated with heart failure increased by 20% from 1993 to 2003, despite considerable advances in pharmacological therapy, device technology, and heart transplantation. Hypertension is one of the major risk factors for left ventricular (LV) dysfunction and heart failure (5, 22). Several studies correlate the physiology of cardiac dysfunction with mRNA expression profiling, using different models and examining different stages of the disease (1, 10, 14, 27). These studies employed microarrays, which provide information on the expression of a large number of genes although the information is not quantitative (29).
Here we used the quantitative real-time PCR method to analyze specific genes of interest and to identify markers of compensatory hypertrophy and markers of decompensated cardiac dysfunction using the salt-sensitive Dahl rat as a model. Our study of candidate genes represents a search for markers for the disease rather than identifying previously unknown genes that contribute to the molecular basis of the disease. The choice of genes with expressions that may be modulated by the disease state was based on a literature search; a list of publications that supported the inclusion of each gene in this study is provided in the supplementary methods (all supplemental material can be found with the online version of this article).
Dahl salt-sensitive rats provide a reliable animal model of hypertension-induced cardiac dysfunction. When the rats were placed on an 8% NaCl-containing diet from the age of 6 wk, the blood pressure of the rats increased from ∼150 to ∼240 mmHg (13). Compensatory LV hypertrophy develops by the age of 11 wk, followed by cardiac dysfunction at 17 wk and death from cardiac dysfunction by 21 wk (11–13). We used this relatively synchronized model of the disease to correlate the changes in the expression of select genes with the changes in cardiac size and function of each individual animal.
As mentioned above, the genes for the study were selected based on their implication in cardiac dysfunction (see supplementary methods). We also focused on the protein kinase C (PKC) signaling pathway, which plays a role in cardiac dysfunction as evidenced by studies using transgenic mice (3, 7–9, 18, 28) and animal models in which PKC was regulated by pharmacological agents (11), as well as a study on changes in PKC activity in humans (2, 23).
METHODS
Hypertension-induced cardiac dysfunction rat model.
Animal protocols were approved by the Stanford University Institutional Animal Care and Use Committee. Male Dahl salt-sensitive rats were placed on an 8% NaCl-containing diet (Dahl high salt) or a 0.3% NaCl low-salt diet (n = 6) from the age of 6 wk onward, as previously reported (11–13). Survival rate, fractional shortening (FS), and blood pressure were evaluated in these groups. Systolic blood pressure was measured by the tail-cuff method (BP-2000; Visitech Systems), and FS was measured by transthoracic echocardiography (Vivid 7; GE).
Real-time PCR.
Two micrograms of total RNA extracted from the LV tissues of each 17-wk-old rat (n = 6 for each group) were converted to first-strand cDNA by reverse transcription using the high-capacity cDNA Archive kit (Applied Biosystems). The primers and probes for PCR were obtained from Applied Biosystems, which has a wide collection of TaqMan gene expression assays to address the rat genome. Sixty-four of the primer-probe sets were predesigned by the Applied Biosystems bioinformatic pipeline, which uses standard optimized thermal cycling condition. Twenty-nine primer-probe sets were custom designed because predesigned assays were not available for these genes at the time. To obtain optimal assay performance, the Applied Biosystems design pipeline used a set of algorithms to generate assays meeting specific criteria including melting temperature (Tm), amplicon size, and minimal primer-dimer formation. An assay was then selected for each gene based on the in silico quality score determined from transcript blast and genome blast results. Real-time PCR was performed with TaqMan assays and TaqMan Universal Master Mix. Each PCR was set up in quadruplicate. After 2 min at 50°C and 10 min at 95°C, 40 cycles of amplification were performed, each at 95°C for 15 s and 60°C for 1 min, using the 7900HT Sequence Detection System (Applied Biosystems). Data were collected using SDS 2.1 software (Applied Biosystems). The fluorescence signals were normalized to the ubiquitously expressed housekeeping gene peptidylprolyl isomerase A (PPIA; cyclophilin A). Fold changes of mRNA levels relative to the corresponding controls were determined using cycle threshold values (6, 16).
Mathematical analyses of the acquired data, which included principal component analysis (PCA), were performed using Genesis software (25). Instead of genes and animals being clustered by heat map or tree view, the similarity between the gene expression profiles of each animal is presented as three-dimensional PCA in this study. PCA reduces the variables of the data set while retaining those characteristics of the data set that contribute most to its variance. The dimensions on the figures do not represent any biological feature of the animals but instead indicate through the tight clustering the similarity of gene expression profiles between the animals in each treatment group. Three-dimensional PCA presents the similarity of gene expression profiles with three mathematically calculated components, which is an appropriate way to compare several animals at a time.
Statistics.
Data are expressed as means ± SD (Table 1) or means ± SE (for the rest of the data). Statistical analysis was assessed by one-way factorial ANOVA with Fishers test. A value of P < 0.05 was considered significant.
Table 1.
Variability in expression of housekeeping genes
| Average Cycle Threshold Value | SD | |
|---|---|---|
| β-Glucuronidase | 24.87 | 0.600 |
| β2-Microglobulin | 16.31 | 0.402 |
| β-Actin | 18.23 | 0.625 |
| Peptidylprolyl isomerase A | 19.18 | 0.368 |
Values are means with SD. Variability in expression of 4 housekeeping genes was evaluated by SD in samples starting with the same RNA amounts. Peptidylprolyl isomerase A (cyclophilin A), which presented lowest deviation, was used for the gene expression analysis.
RESULTS
Selection of genes to be analyzed.
We selected genes that were previously implicated in cardiac dysfunction as well as in the PKC-related cellular signaling pathway, a pathway that our laboratory has been studying for many years (see supplementary methods for references that support the inclusion of these genes in the study). Genes encoding 17 neurohormones, 6 cytokines, 5 growth factors, 31 cell signaling proteins, 5 apoptosis-related proteins, 4 sarcoplasmic reticulum-related proteins, 3 contractile proteins, 9 oxidative stress-related proteins, 3 nitric oxide (NO)-related proteins, 5 extracellular matrix-related enzymes, and 5 cytoskeletal proteins were selected. Most of them were chosen as well-known genes from textbooks or recent studies. Others were selected as gene families of well-known genes. They may participate in the same biological process with similar or opposing roles.
Selection of housekeeping genes to be used as gene expression internal controls.
Four housekeeping genes, β-glucuronidase (NM_017015), β2-microglobulin (NM_012512), β-actin (NM_031144), and PPIA (cyclophilin A, NM_017101), were evaluated to identify a control gene with the least variation in gene expression. The expression of PPIA was the least variable in the 54 animals that was tested in the exploratory phase of our study and thus was selected as the internal control for this study (Table 1).
Gene expression changes correlate with compensated and decompensated hypertrophy.
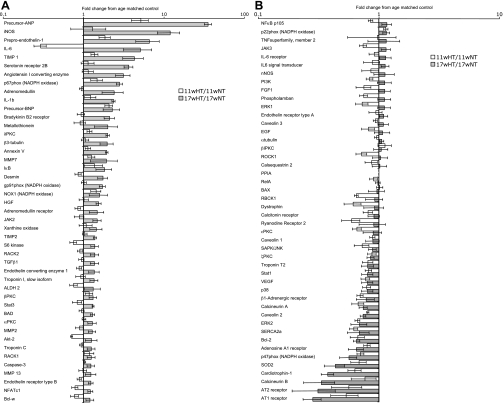
When placed on an 8% salt diet from the age of 6 wk, Dahl rats develop hypertension-induced compensatory hypertrophy by week 11 and subsequently present cardiac dysfunction around week 17 (11–13). When compared with that of the age-matched normotensive controls, the expression analysis of the 11-wk-old hypertensive animals showed an increased expression of the genes for precursor atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and prepro-endothelin-1 and a decreased expression of 24 genes, including SOD2, sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a), and ryanodine receptor 2 (Table 2 and Fig. 1). The analysis of 17-wk-old hypertensive rats with age-matched controls presented 23 upregulated genes, including inducible NO synthase (iNOS), tissue inhibitor of metalloprotease (TIMP)-1 and -2, and nuclear factor of activated T-cells (NFAT)c1 and 7 downregulated genes, including Bcl-2 and adenosine A1 receptor (Table 3 and Fig. 1). To focus on the transition from the compensatory hypertrophic phase to the decompensated phase, the gene expression in 17-wk-old and 11-wk-old hypertensive rats was compared using ANOVA analysis. There was a statistically significant increase in the expression of 17 genes and a decrease in the expression of 5 genes when comparing the compensatory hypertrophy phase with the cardiac dysfunction phase. Additional increases in the gene expression of endothelin-1 and precursor ANP and an additional decrease in SOD2 expression were noted through the progression of hypertrophy to decompensated cardiac dysfunction (Table 4).
Table 2.
Differentially expressed genes in hypertrophy
| Gene Name |
Gene Expression |
Correlation With LVW
|
||||||
|---|---|---|---|---|---|---|---|---|
| Fold Change | P Value | RC | P Value | |||||
| Increased expression with hypertrophy | ||||||||
| Precursor ANP | 5.18 | 0.0089 | 329.0 | 0.0003 | ||||
| Precursor BNP | 2.08 | 0.0012 | 312.0 | 0.0023 | ||||
| Prepro-endothelin-1 | 1.86 | 0.0228 | 168.7 | 0.0018 | ||||
| Decreased expression with hypertrophy | ||||||||
| NF-κB p105* | 0.85 | 0.0297 | NS | |||||
| STAT 1* | 0.82 | 0.0264 | NS | |||||
| Caveolin 2 | 0.81 | 0.0038 | −248.7 | 0.0069 | ||||
| p38* | 0.79 | 0.0130 | NS | |||||
| NFATc1* | 0.78 | 0.0324 | NS | |||||
| VEGF* | 0.78 | 0.0249 | NS | |||||
| S6 kinase† | 0.75 | 0.0219 | −200.4 | 0.0303 | ||||
| Phospholamban* | 0.75 | 0.0076 | −213.3 | 0.0233 | ||||
| JAK3* | 0.74 | 0.0050 | −262.9 | 0.0123 | ||||
| SAPK/JNK* | 0.73 | 0.0256 | NS | |||||
| ROCK1* | 0.72 | 0.0216 | NS | |||||
| Akt-2* | 0.71 | 0.0010 | −229.0 | 0.0153 | ||||
| p47phox | 0.71 | 0.0011 | −390.8 | 0.0171 | ||||
| β1-Adrenergic receptor* | 0.70 | 0.0009 | −508.0 | 0.0196 | ||||
| SOD2 | 0.69 | 0.0148 | NS | |||||
| Calcineurin A | 0.67 | 0.0035 | NS | |||||
| Bcl-2 | 0.67 | 0.0075 | NS | |||||
| ERK2 | 0.67 | 0.0017 | NS | |||||
| ɛPKC* | 0.66 | 0.0045 | −416.2 | 0.0056 | ||||
| RBCK1* | 0.65 | 0.0046 | −418.6 | 0.0265 | ||||
| Cardiotrophin-1 | 0.63 | 0.0027 | NS | |||||
| SERCA2a* | 0.62 | 0.0002 | −506.2 | 0.0059 | ||||
| Dystrophin* | 0.61 | 0.0062 | −438.3 | 0.0384 | ||||
| Ryanodine receptor 2* | 0.59 | 0.0319 | NS | |||||
Values are indicated with means with P values. List of genes with expression in the myocardium that changed in 11-wk-old hypertensive (HT) rats relative to age-matched normotensive rats is shown at left. Individual regression analysis between change in gene expression and the ratio in left ventricular weight (LVW)/body weight (BW) was carried out. Genes that significantly correlated (P < 0.05) with change in LVW/BW are listed at right. RC, regression coefficient; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; NS, not significant; NFAT, nuclear factor of activated T-cells; ROCK, Rho-associated kinase-β; RBCK, RBCC protein interacting with PKC; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase 2a.
Genes with expression that went back to normal in 17-wk-old HT rats;
genes with expression upregulated over control in 17-wk-old HT rats.
Fig. 1.
A and B: relative change in gene expression compared with that of age-matched controls. Two micrograms of total RNA extracted from the left ventricular (LV) tissues of each 17-wk (w)-old rat (n = 6 for each group) were converted to cDNA. The primers for PCR were used in real-time PCR using TaqMan assays, each in quadruplicate. The fluorescence signals were normalized to the ubiquitously expressed housekeeping gene peptidylprolyl isomerase A (PPIA; cyclophilin A). Fold changes in gene expression (6 animals/group) using real-time PCR are presented using log scale (data are means ± SE). Numerical data are provided in supplementary Table 1. HT, hypertensive; NT, normotensive; ANP, atrial natriuretic peptide; iNOS, inducible nitric oxide synthase; TIMP, tissue inhibitor of metalloprotease; BNP, brain natriuretic peptide; NOX, NADPH oxidase; HGF, hepatocyte growth factor; RACK, receptor for activated C kinase; TGF, transforming growth factor; ALDH, aldehyde dehydrogenase; BAD, bcl-xl/bcl-2-associated death promoter; NFAT, nuclear factor of activated T-cells; TNF, tumor necrosis factor; nNOS, neuronal NOS; PI3K, phosphatidylinositol 3-kinase; ROCK, Rho-associated kinase-β; BAX, bcl-2-associated X protein; RBCK, RBCC protein interacting with PKC; SERCA2a, sarco(endo)plasmic reticulum Ca2+-ATPase; AT1, ANG II type 1.
Table 3.
Differentially expressed genes in heart failure
| Gene Name |
Gene Expression |
Correlation With FS
|
||||||
|---|---|---|---|---|---|---|---|---|
| Fold Change | P Value | RC | P Value | |||||
| Increased expression with failing heart | ||||||||
| Precursor ANP* | 35.54 | <0.0001 | −0.028 | 0.0013 | ||||
| iNOS* | 12.02 | 0.0135 | −0.027 | 0.0088 | ||||
| Prepro-endothelin-1* | 6.62 | 0.0257 | −0.016 | 0.0073 | ||||
| TIMP 1* | 4.32 | 0.0234 | −0.015 | 0.0004 | ||||
| Serotonin receptor 2B* | 3.66 | 0.0003 | −0.018 | 0.0039 | ||||
| ACE* | 3.15 | 0.0082 | −0.012 | 0.0270 | ||||
| p67phox* | 2.82 | <0.0001 | −0.014 | 0.0051 | ||||
| IL-1β* | 2.37 | <0.0001 | −0.016 | 0.0011 | ||||
| Precursor BNP | 2.28 | 0.0416 | NS | |||||
| Bradykinin B2 receptor | 2.12 | 0.0061 | NS | |||||
| δPKC* | 2.01 | <0.0001 | −0.014 | 0.0031 | ||||
| β3-tubulin | 1.98 | 0.0259 | NS | |||||
| Annexin V* | 1.95 | <0.0001 | −0.014 | 0.0007 | ||||
| Desmin | 1.75 | 0.0143 | NS | |||||
| gp91phox* | 1.72 | 0.0011 | −0.009 | 0.0486 | ||||
| HGF* | 1.55 | 0.0011 | −0.008 | 0.0016 | ||||
| JAK2 | 1.48 | 0.0204 | NS | |||||
| TIMP 2* | 1.39 | 0.0005 | −0.006 | 0.0151 | ||||
| S6 kinase | 1.39 | 0.0320 | NS | |||||
| Stat 3* | 1.32 | 0.0028 | −0.007 | 0.0136 | ||||
| BAD | 1.30 | 0.0081 | NS | |||||
| Endothelin receptor type B | 1.24 | 0.0293 | NS | |||||
| NFATc1 | 1.22 | 0.0412 | NS | |||||
| Decreased expression with failing heart | ||||||||
| Calcineurin A* | 0.74 | 0.0055 | 0.010 | 0.0189 | ||||
| Caveolin 2* | 0.74 | 0.0008 | 0.010 | 0.0060 | ||||
| Bcl-2* | 0.66 | 0.0004 | 0.014 | 0.0028 | ||||
| Adenosine A1 receptor | 0.65 | 0.0268 | NS | |||||
| p47phox* | 0.57 | 0.0008 | 0.020 | 0.0056 | ||||
| SOD2* | 0.44 | 0.0027 | 0.037 | 0.0190 | ||||
| Cardiotrophin-1* | 0.38 | <0.0001 | 0.043 | 0.0021 | ||||
Values are indicated with means with P values. List of genes with expression in the myocardium that changed in 17-wk-old HT rats relative to age-matched normotensive rats is shown at left. Individual regression analysis between changes in gene expression and fractional shortening (FS) was carried out. Genes that significantly correlated (P < 0.05) with change in FS are listed at right. iNOS, inducible nitric oxide synthase; TIMP, tissue inhibitor of metalloprotease; ACE, angiotensin I-converting enzyme; HGF, hepatocyte growth factor; BAD, bcl-xl/bcl-2-associated death promoter.
Genes with expressions that correlated with FS.
Table 4.
List of genes with expression in the myocardium that changed in 17-wk-old HT rats relative to 11-wk-old HT rats
| Gene Name | Fold Change | P Value |
|---|---|---|
| Prepro-endothelin-1* | 3.81 | 0.0476 |
| Serotonin receptor 2B* | 3.20 | 0.0006 |
| Precursor ANP* | 2.83 | 0.0007 |
| ACE* | 2.78 | 0.0133 |
| Adrenomedullin receptor | 1.97 | 0.0394 |
| Bradykinin B2 receptor | 1.96 | 0.0108 |
| Annexin V | 1.80 | <0.0001 |
| FGF1 | 1.79 | 0.0092 |
| Desmin | 1.66 | 0.0251 |
| HGF | 1.65 | 0.0044 |
| Stat 3 | 1.54 | 0.0030 |
| S6 kinase | 1.53 | 0.0201 |
| gp91phox | 1.43 | 0.0135 |
| Endothelin receptor type B | 1.40 | 0.0050 |
| NFATc1 | 1.33 | 0.0278 |
| δPKC | 1.25 | 0.0249 |
| RelA | 1.21 | 0.0479 |
| Caveolin 2 | 0.84 | 0.0099 |
| α-tubulin | 0.79 | 0.0026 |
| β1-Adrenergic receptor | 0.67 | 0.0278 |
| Calcitonin receptor* | 0.55 | 0.0019 |
| SOD2* | 0.54 | 0.0276 |
Values are indicated with means with P values.
Genes with expression that changed twofold or greater.
Regression analysis correlating gene expression with indicators of cardiac dysfunction.
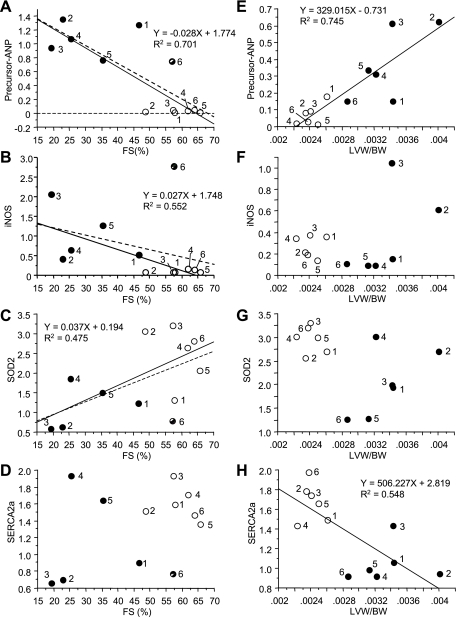
To further evaluate the potential role of individual genes in the progression of the disease, we carried out a simple regression analysis correlating gene expression with LV weight per body weight (LVW/BW) or FS as indicators of cardiac hypertrophy or cardiac dysfunction (Fig. 2 and Tables 2 and 3). Since the relative expression of many genes from animal No. 6 among 17-wk-old hypertensive rats differed significantly from the rest of the same group (Fig. 2, B and C, •), the regression analysis was performed without animal No. 6. Thirty differentially expressed genes correlated to LVW/BW, FS, or both. Four differentially expressed genes including precursor ANP were correlated with FS and LVW/BW (Tables 2 and 3 and Fig. 2, A and E). Sixteen differentially expressed genes including iNOS and SOD2 were correlated with FS only (Tables 2 and 3 and Fig. 2, B, C, F, and G). Ten differentially expressed genes including SERCA2a were correlated with LVW/BW only (Tables 2 and 3 and Fig. 2, D and H). Precursor ANP, which correlated to both FS and LVW/BW and showed a very low P value in both cases, indicated that it is a good marker for both hypertrophy and cardiac dysfunction.
Fig. 2.
Representative plots of individual regression analysis, correlating gene expression with cardiac size or function. Simple regression analysis correlating change in gene expression (y-axis) with fractional shortening (FS; x-axis) or with hypertrophy [LV weight/body weight (LVW/BW)] was carried out with data from hypertensive (•) and age-matched (○) controls (individual animals in each group are marked by numbers 1 through 6). Hearts from Dahl rats were analyzed at the cardiac dysfunction phase (at 17 wk) and correlated with FS (A–D) or LVW/BW (E–H). Linear fitting curves, equations, and R square values are indicated when gene expression significantly correlated with FS or LVW/BW values. The dashed line signifies a regression analysis that includes animal No. 6 of HT group, the FS of which did not decrease and in which the gene expression profile was substantially different from the rest of the group. The expression of these 4 genes shows correlation with both FS and LVW/BW (ANP), FS only (iNOS and SOD2), or LVW/BW only (SERCA2a) when analyzing individual animals.
PCA distinguishes between healthy rats and rats with lower cardiac functions and between rats with compensatory compared with decompensated hypertrophy.
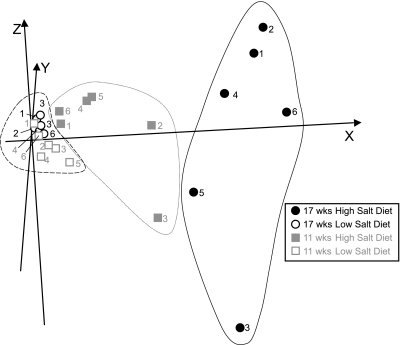
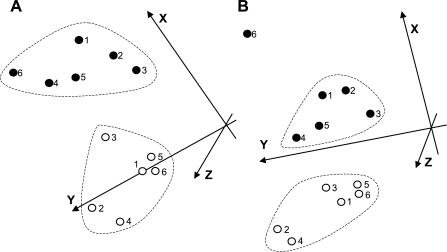
With the use of Genesis software (24, 25), the three-dimensional PCA of 20 selected genes, the expression of which was correlated with the level of cardiac dysfunction (Table 3, asterisk), successfully segregated 17-wk-old hypertensive rats from 11- and 17-wk-old normotensive rats (Fig. 3). Importantly, both control groups on the low-salt diet (11- and 17-wk-old rats; Fig. 3, ○ and □) segregated into one relatively tight cluster. Furthermore, such analysis using the 22 genes listed in Table 4 segregated between rats in the compensated and decompensated stage of the disease (Fig. 4A). A similar segregation between the two groups was achieved when using only six genes, the expression of which changed about twofold or more (indicated with asterisks in Table 4; Fig. 4B). The exception was one outlier (hypertensive animal No. 6), which had relatively normal FS and a gene expression profile that did not correlate well with the other animals in Fig. 2, A–D.
Fig. 3.
Segregation of animals with cardiac dysfunction from controls using 30 selected genes. The 20 out of 30 genes in Table 3 with expression were correlated with FS by regression analysis and were used for principal component analysis (PCA). PCA is used to present animals with similar gene expression profiles close in three-dimensional space. Because PCA is performed based on anonymously transformed gene expression data from each animal, each axis has only mathematical relevance for each spot (animal). Seventeen-week-old HT (area in far right black line) and NT (area in far left dotted line) animals were successfully segregated, and the 11-wk-old HT animals (area in middle gray line) were segregated to a third group between these 2 above groups. Individual animals are identified by numbers, as in Fig. 1.
Fig. 4.
Segregation of animals with cardiac dysfunction from animals with compensatory hypertrophy using selected genes. A: the 22 genes from Table 4 were used for PCA to provide more visual analysis of clustering of individual animals with similar changes (see methods for more details). Further analysis using genes with expression that changed twofold or greater (Table 4, asterisk) is provided in B. Individual animals are identified by numbers, as in Fig. 1.
DISCUSSION
In this study, we measured changes in the expression of select genes in heart tissue that were induced by severe hypertension. Changes in the levels of mRNA were determined during the compensated hypertrophy (at the age of 11 wk) and compared with the changes observed during the decompensated cardiac dysfunction stage (at the age of 17 wk). ANOVA analysis and simple regression analysis correlating these changes with heart weight or cardiac function enabled us to identify several marker genes that distinguish between compensatory hypertrophy and decompensated hypertrophy.
The expression of the precursor ANP and endothelin-1 genes, which increased in the compensatory phase, further increased in the decompensated phase (Tables 3 and 4). The expression of genes involved in calcium handling, including phospholamban, SERCA2a, and the ryanodine receptor 2, as well as gene products with antioxidative stress or apoptosis-related roles, including SOD2, Akt-2, and Bcl-2 decreased, suggesting a detrimental role of these changes in gene expression during pathological hypertrophy. We also found a decrease in the expression of several genes in the JAK/STAT pathway (including STAT1, JAK3, and cardiotrophin-1) and in the NFAT pathway (including NFATc1 and calcineurin A), as well as in the β1-adrenergic receptor [role in hypertrophy has already been documented (4); Table 2]. Additionally, the expression of other major cell signaling kinases, including p38, JNK, and ERK2, was downregulated. The decrease in the expression of these genes may reflect compensatory effects resulting in maintained normal contractile function. This is based on our observations that the expression of many of these genes returned back to normal levels (as seen in normotensive age-matched animals) during the decompensated phase (Table 3).
The number of genes with an expression that increased during the decompensated phase was higher compared with that of the compensated phase (Table 4). In addition to the marker genes for cardiac dysfunction (precursor ANP and BNP) and vasoconstriction-related genes [endothelin-1 and ACE(17)], there was an increase in the expression of NFATc1 and genes involved in remodeling (TIMP-1 and -2) and the JAK/STAT pathway (JAK2 and STAT3; Tables 3 and 4). We also found that the expression of 17 genes that decreased during the compensatory phase (Table 2, asterisk) increased back to normal or was further upregulated at the decompensatory phase (e.g., S6 kinase), demonstrating the importance of comparing several disease states in the same animal model (Table 4).
Two studies reported gene expression profiling of salt-induced cardiac dysfunction using the microarray method. Ueno et al. (27) determined that seven genes were induced during salt-induced hypertrophy in Dahl rats including ANP, β-actin, myosin heavy chain polypeptide 7, and aldolase A. Kong et al. (14) identified 369 genes including GATA4, RAB7, NRAS, GNA12, STAT3, STAT5B, FYN, CRKO, MYCN, PTEN, AKT1, and IL6ST/gp130, the expression of which was changed both in hypertrophy and heart failure (14). Those studies report changes in genes, such as ANP and STAT3, that are consistent with the data in our study. Because those two studies aimed at gene ontology analysis to measure the phenomenon of hypertension-induced cardiac dysfunction, the number of animals for each condition was one or three. Such small numbers are insufficient to establish statistical significance in the regulation of single genes. Another gene-profiling study for heart failure by Hwang et al. (10) focused on human dilated cardiomyopathy (DCM) or hypertrophic cardiomyopathy (HCM) using the microarray method (n = 3 or 2, respectively). Hwang et al. (10) identified several commonly induced genes in heart failure, such as ANP, and highlighted the methodological advantages of microarray in understanding heart failure. In our study, we focused on cell signaling pathways and those related genes in cardiac function and used a quantitative PCR method.
One of the significant changes in this study is the induction of the serotonin receptor 2B in 17-wk-old hypertensive rats. A role for serotonin receptor 2B levels in the regulation of development and function of the heart has already been suggested (19, 21), but there is limited data on its function in hypertrophy and cardiac dysfunction. Recent studies involving the genetic manipulation of the serotonin receptor 2B gene (20) or pharmacological inhibition of serotonin receptor 2B (15) have suggested its function in cardiac hypertrophy. Our study supports the finding that the serotonin receptor 2B has a detrimental role in the heart. Based on the finding from the genetic manipulation study by Nebigil et al. (19), an overexpression of this gene causes functional defects in mitochondria, which eventually induces cardiac hypertrophy. In our study, the induction of serotonin receptor 2B is observed during the later stage in decompensatory cardiac dysfunction. With the consideration that a mitochondrial defect could be caused by the overexpression of serotonin receptor 2B (20), this gene product could facilitate hypertrophy and/or cardiac dysfunction.
We showed here that the comparison of gene expression between compensatory hypertrophy and decompensated hypertrophy identified prepro-endothelin-1, serotonin receptor 2B, precursor ANP, angiotensin I-converting enzyme, calcitonin receptor, and SOD2 (Table 4) as potential hallmarks of cardiac dysfunction. Furthermore, PCAs of the quantitative comparison of gene expression using real-time PCR demonstrated that such analyses of multiple animals in each group provide a sensitive means to identify hallmark genes with an expression that correlates with the progression of the disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-76670.
DISCLOSURES
D. Mochly-Rosen is the founder of KAI Pharmaceuticals, a company that plans to bring PKC regulators to the clinic. However, none of the work described in this study is based on or supported by the company.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ashrafian H, Watkins H. Reviews of translational medicine and genomics in cardiovascular disease: new disease taxonomy and therapeutic implications. Cardiomyopathies: therapeutics based on molecular phenotype. J Am Coll Cardiol 49: 1251–1264, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation 99: 384–391, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bowman JC, Steinberg SF, Jiang T, Geenen DL, Fishman GI, Buttrick PM. Expression of protein kinase C beta in the heart causes hypertrophy in adult mice and sudden death in neonates. J Clin Invest 100: 2189–2195, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bristow MR Beta-adrenergic receptor blockade in chronic heart failure. Circulation 101: 558–569, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Drazner MH The transition from hypertrophy to failure: how certain are we? Circulation 112: 936–938, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Furtado MR, Petrauskene OV, Livak KJ. Application of real-time quantitative PCR in the analysis of gene expression. In: DNA Amplification: Current Technologies and Applications, edited by Demidov VV and Broude NE. United Kingdom: Horizon Bioscience, 2004.
- 7.Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL, Solaro RJ, Buttrick PM. Protein kinase Cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res 95: 424–432, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, Kimball TF, Hewett TE, Dorn GW 2nd, Koch WJ, Molkentin JD. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation 114: 574–582, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambleton M, York A, Sargent MA, Kaiser RA, Lorenz JN, Robbins J, Molkentin JD. Inducible and myocyte-specific inhibition of PKCα enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol 293: H3768–H3771, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang JJ, Allen PD, Tseng GC, Lam CW, Fananapazir L, Dzau VJ, Liew CC. Microarray gene expression profiles in dilated and hypertrophic cardiomyopathic end-stage heart failure. Physiol Genomics 10: 31–44, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, Kihara Y. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol 34: 1377–1385, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol 39: 1384–1391, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Iwanaga Y, Kihara Y, Hasegawa K, Inagaki K, Yoneda T, Kaburagi S, Araki M, Sasayama S. Cardiac endothelin-1 plays a critical role in the functional deterioration of left ventricles during the transition from compensatory hypertrophy to congestive heart failure in salt-sensitive hypertensive rats. Circulation 98: 2065–2073, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Kong SW, Bodyak N, Yue P, Liu Z, Brown J, Izumo S, Kang PM. Genetic expression profiles during physiological and pathological cardiac hypertrophy and heart failure in rats. Physiol Genomics 21: 34–42, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Liang YJ, Lai LP, Wang BW, Juang SJ, Chang CM, Leu JG, Shyu KG. Mechanical stress enhances serotonin 2B receptor modulating brain natriuretic peptide through nuclear factor-kappaB in cardiomyocytes. Cardiovasc Res 72: 303–312, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Mann DL Pathophysiology of Heart Failure. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine (8th ed.), edited by Libby P, Bonow RO, Mann DL, and Zipes DP. Saunders Elsevier, 2007.
- 18.Montgomery DE, Rundell VL, Goldspink PH, Urboniene D, Geenen DL, de Tombe PP, Buttrick PM. Protein kinase Cɛ induces systolic cardiac failure marked by exhausted inotropic reserve and intact Frank-Starling mechanism. Am J Physiol Heart Circ Physiol 289: H1881–H1888, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Nebigil CG, Choi DS, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay JM, Maroteaux L. Serotonin 2B receptor is required for heart development. Proc Natl Acad Sci USA 97: 9508–9513, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebigil CG, Jaffre F, Messaddeq N, Hickel P, Monassier L, Launay JM, Maroteaux L. Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and cardiac hypertrophy. Circulation 107: 3223–3229, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Nebigil CG, Maroteaux L. Functional consequence of serotonin/5-HT2B receptor signaling in heart. Role of mitochondria in transition between hypertrophy and heart failure? Circulation 108: 902–908, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet 367: 356–367, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem 305: 103–111, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Sturn A, Mlecnik B, Pieler R, Rainer J, Truskaller T, Trajanoski Z. Client-server environment for high-performance gene expression data analysis. Bioinformatics 19: 772–773, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics 18: 207–208, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O′Donnell C, Kittner S, Lloyd-Jones D, Goff DC Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113: e85–e151, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Ueno S, Ohki R, Hashimoto T, Takizawa T, Takeuchi K, Yamashita Y, Ota J, Choi YL, Wada T, Koinuma K, Yamamoto K, Ikeda U, Shimada K, Mano H. DNA microarray analysis of in vivo progression mechanism of heart failure. Biochem Biophys Res Commun 307: 771–777, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci USA 94: 9320–9325, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuen T, Wurmbach E, Pfeffer RL, Ebersole BJ, Sealfon SC. Accuracy and calibration of commercial oligonucleotide and custom cDNA microarrays. Nucleic Acids Res 30: e48, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.