Abstract
Pulmonary hypertension, characterized by elevated pulmonary arterial pressure (PAP) and right ventricular hypertrophy, is caused by decreased atmospheric oxygen at high altitude. We hypothesized that maternal undernutrition programs right ventricle gene expression and sensitivity to increasing PAP at high altitude (2,183 m). On day 30 of gestation, forty Angus × Gelbvieh cows received diets to induce either gain (Control) or loss of body weight (Restricted) until day 125 of gestation. On day 126 of gestation, Restricted cows were realimented to achieve the same body weight as Controls by day 250. Parturition occurred naturally. PAP, which ranged from 40 to 114 mmHg, was determined in 15-mo-old steers from Control or Restricted cows before necropsy. At necropsy, hearts were collected from steers, separated into right and left ventricles, atria, and septa and weighed. Ventricular thickness was recorded. Eight Affymetrix bovine microarrays were screened [four high PAP (two Control and two Restricted) and four low PAP (two Control and two Restricted)] with right ventricle mRNA. This analysis revealed that pentraxin-related protein, interferon-related developmental regulator, and peroxisome proliferator-activated receptor-γ coactivator-1α were differentially expressed (P < 0.05) in steer right ventricle from high-PAP cows compared with low-PAP cows. Also, activation peptide and pancreas cationic trypsinogen, α-actin, similar to ubiquitin carboxylesterase, were differently expressed (P < 0.05) in steers from Restricted cows compared with those from Control cows. Upregulated genes in high-PAP right ventricle have been associated with pathological cardiac hypertrophy. It is concluded that right ventricle gene expression may be differentially programmed by maternal undernutrition in the fetus during early gestation and may be detrimental to health and longevity of offspring, particularly at high altitude.
Keywords: right ventricle, high altitude
hypoxia at high elevation is known to cause pulmonary hypertension in cattle. Limitation of oxygen at high elevations induces increased resistance to blood flow in small arteries in the lungs. The right ventricle (RV) of the heart compensates for higher blood pressure through hypertrophic response. Bovine and porcine species are known to be more susceptible to pulmonary hypertension at elevated altitude than other mammalian species (34), but the reason is unclear. However, the amount of smooth muscle present in the media of small pulmonary arteries is positively correlated with pulmonary hypertension and RV hypertrophy in species highly susceptible to pulmonary hypertension (40). For example, onset of RV hypertrophy due to the pulmonary arterial hypertension without pulmonary disease is common in steers.
Adequate nutrient supply during early gestation is critical for fetal organogenesis. Epidemiological studies suggest that maternal undernutrition induces growth retardation and raises blood pressure in offspring (20, 33, 44). A global 50% nutrient restriction during the first half of gestation in ewes causes fetal growth restriction and an increase in left ventricle (LV) and RV weight per unit fetal weight by day 78 of gestation (17). Also, mean arterial pressure was greater in 9-mo-old male offspring from these ewes (15).
Undernutrition is known to impact cardiac function and systemic blood pressure. However, the impact of undernutrition on pulmonary hypertension and cardiac function has not been described. Because some breeds of cattle are more susceptible to high-altitude stress than others and exhibit RV hypertrophy more readily in response to elevated pulmonary arterial pressure (PAP), they serve as a good model for studying the effect of maternal undernutrition and programmed cardiac gene response to this stress. The altitude at Laramie, Wyoming is 2,183 m, and the atmospheric pressure at elevation 2,183 m decreases to 74% of sea level, which is ∼583 mmHg. Atmospheric pressure at sea level is 759 mmHg. We hypothesized that maternal undernutrition impacts RV gene expression and sensitivity to increasing PAP at high altitude (2,183 m). To examine this hypothesis, cows with low and high arterial pressure were subjected to nutrient stress during pregnancy. Offspring (finished steers) were examined for RV gene expression using microarray and RT-PCR approaches.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee before the initiation of this study. Forty multiparous Angus × Gelbvieh cows (initial body weight = 576.8 ± 7.7 kg) from the University of Wyoming herd were artificially inseminated to a single South Devon sire. These cows had produced at least two previous calves at the University of Wyoming without difficulty and had never exhibited signs of pulmonary hypertension. From day 30 to day 125 of gestation, cows were distributed by body weight and assigned to one of two dietary treatments. Cows within a dietary treatment were housed in groups of five, with feed bunks and heated waterers assigned to each group. Twenty cows (4 pens) were allotted to a control diet (Control) consisting of native grass hay (12.1% crude protein, 70.7% in vitro dry matter digestibility on a dry matter basis) fortified with vitamins and minerals and fed at National Research Council (29) recommendations for a nonlactating, mature cow to gain 0.72 kg/day during the first 125 days of gestation. The other 20 cows (4 pens) were allotted to a nutrient-restricted (Restricted) diet, which consisted of feeding one-half of the control diet's vitamins and minerals and millet straw (9.9% crude protein, 54.5% in vitro dry matter digestibility on a dry matter basis) to provide 68.1% net energy for maintenance and 86.7% of metabolizable protein requirements during the first 125 days of gestation. The diet formulation resulted in a global nutrient restriction for Restricted cows, including deficiency in protein, energy, minerals, and vitamins. All cows were weighed every 14 days to adjust rations for changes in body weight throughout the experiment. On approximately day 80 of gestation, all cows were confirmed pregnant with bull calves. Control cows were fed the control diet to maintain a body condition score of 5.75 from day 125 to day 250 of gestation. The Restricted cows were fed the hay and the control diet minerals and vitamins plus a corn-based supplement to achieve a body condition score equal to their Control contemporaries by day 250 of gestation. After day 250 of gestation, all cows were managed to ensure that the cows were in average body condition score (5–6) at calving. After calving, calves were vaccinated and branded, and bull calves were castrated at ∼50 days of age. Once weaned, the male progeny from Control and Restricted cows were placed in the feed lot at the University of Wyoming Livestock Center. Steer calves were divided into eight pens by treatment and blocked by body weight. Steers were fed a three-step background ration for 60 days to allow for adjustment to a high-energy diet. The background ration began with 47% roughage and decreased stepwise to the final diet containing 13% roughage. Dietary net energy for growth increased from 1.06 to 1.37 Mcal/kg in the final diet. Steers remained on the final diet until slaughter.
Tissue Collection
At 15 mo of age, PAP of steers from Control and Restricted cows was determined by catheterization of the heart in a standing and immobilized animal before tissue collection. A fine plastic catheter is first passed through a needle into the jugular vein. The catheter is then threaded down the jugular vein and superior vena cava into the right atrium. It is then passed though the tricuspid valve into the RV and finally through the pulmonary valve into the main pulmonary trunk. When the catheter has reached its final destination, characteristic pressure waves are observed on a heart monitor, giving an accurate measurement of the average PAP.
Steers from Control and Restricted cows were harvested at the University of Wyoming Meat Laboratory by accepted agricultural harvesting methods. Steers were stunned with a captive bolt followed by exsanguination, and live weights and hot carcass weights were collected from each steer. At slaughter, the heart was removed and weighed, and the RV and LV were dissected free of the septum, and each part weighed. RV and LV wall thickness was measured at three different locations, and these thicknesses were averaged. The middle of the RV section was cut, and a subsample was collected for isolation of mRNA and snap frozen in liquid nitrogen.
Total RNA extraction and Affymetrix bovine gene chip screening.
Total RNA from the RV was extracted using TRI reagent. Total RNA was further purified using an RNeasy kit treated with DNase I. Eight Affymetrix bovine gene chips were screened [four high PAP (two Control, two Restricted; average PAP 113 mmHg) and four low PAP (two Control and two Restricted; average PAP 42 mmHg)] with RV mRNA. The data were processed using GeneChip Operating Software. Low-PAP data files were imported into GeneSpring for data analysis. The data were analyzed to extract the flags (present, marginal, and absent calls). The data were normalized by robust multichip analysis and then filtered for a baseline level of signal (gene expression) in at least one replicate and filter of flags.
Semiquantitative RT-PCR.
To confirm the differential expression of mRNA from the gene chip screen, single-stranded cDNA was synthesized from RV total RNA using iScript reverse transcriptase (BioRad, Hercules, CA) at 25°C/5 min, 42°C/30 min, and then 85°C/5 min. The product was diluted 5× with DNase/RNase-free water. Diluted cDNA (10 μl) was used as a template for semiquantitative RT-PCR amplification using SYBRgreen (BioRad). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference for normalization of target gene mRNA expression. Genes of interest and GAPDH primers were designed to generate an amplicon size of ∼100 bp. RT-PCR for genes of interest and GAPDH cDNA amplification were performed at 95°C/30 s, 62°C/30 s, and 72°C/15 s for 40 cycles. Following RT-PCR amplification, the PCR products were melted (melting curve) to ensure the quality of amplification. For melting curve analysis, RT-PCR products were incubated for 10 s at each step, with an increase in temperature by 0.5°C from 55 to 95°C in each cycle.
Data Analysis
Correlation analysis was conducted between RV weight or thickness and PAP score by using PROC CORR procedure of SAS. After filtration, the bovine Affymetrix chip data were analyzed by two-way ANOVA to determine the effects of diet and PAP on differential gene expression. With ANOVA, a Welch t-test and Benjamini and Hochberg multiple-test correction were used. Two-way factorial comparisons were analyzed using ANOVA, and means were separated using a protected t-test at a significant level of P < 0.05.
Messenger RNA expression levels of genes of interest were calculated by relative gene expression within each group. Gene expression levels were log transformed. Gene expression level across the PAP classification was compared by Proc MIXED model of SAS with diet and PAP as a main factor. When no interaction was observed by treatment, the main effect was compared, and means were separated using a protected t-test at a significant level of P < 0.05. The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the paper as written.
RESULTS
PAP and RV Weights
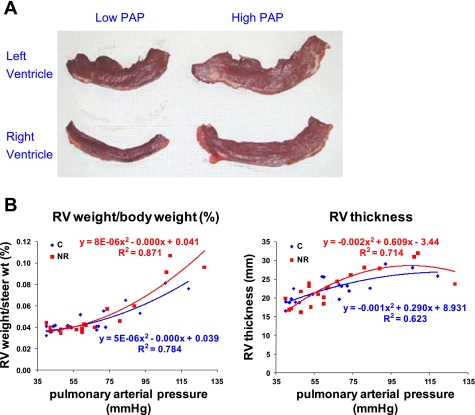
During maternal diet restriction, a 4.2% increase in body weight was observed for the Control cows, whereas Restricted cows lost 7.1% of their initial body weight. Steer weight was not affected by maternal undernutrition (Table 1). Neither total heart weight nor RV or LV weights were affected by maternal undernutrition. Measured PAP of steers ranged from 40 to 128 mmHg at 15 mo of age across the dietary treatment. The RV of steer hearts showed hypertrophy in response to increased PAP, regardless of maternal nutritional treatment (Fig. 1A). The RV hypertrophy index [RV/(LV + septum)] (27) increased from 0.325 to 0.864 as PAP score increased. The RV weight was positively correlated with PAP (r2 = 0.76; P < 0.05) when the RV weight was corrected by hot carcass weight, regardless of dietary treatment (Fig. 1B). The RV thickness was also positively correlated with PAP (r2 = 0.53; P < 0.05).
Table 1.
Carcass, heart weight, and pulmonary blood pressures of steers from control-fed and nutrient-restricted cows during early pregnancy
| Control | Restricted | |
|---|---|---|
| Hot carcass weight, kg | 297.1±6.18 | 299.2±6.07 |
| Total heart weight, kg | 2.542±0.09 | 2.575±0.12 |
| Total heart weight/body weight, % | 0.390±0.03 | 0.390±0.04 |
| Left ventricular weight, kg | 0.860±0.02 | 0.869±0.02 |
| Right ventricular weight, kg | 0.507±0.04 | 0.540±0.05 |
| Pulmonary diastolic blood pressure, mmHg | 39.3±3.96 | 39.8±4.05 |
| Pulmonary systolic blood pressure, mmHg | 100.5±6.17 | 101±5.69 |
Values are means ± SE.
Fig. 1.
Right (RV) and left ventricle thickness of the steers from low-PAP (pulmonary artery pressure) or high-PAP values. A: RV from high-PAP value is thicker than RV from low-PAP values. B: RV weight (r2 = 0.76; P < 0.05) and thickness (r2 = 0.53; P < 0.05) were significantly increased with increasing PAP. C, control-fed group; NR, nutrient-restricted group.
Bovine Gene Chip Screening
PAP.
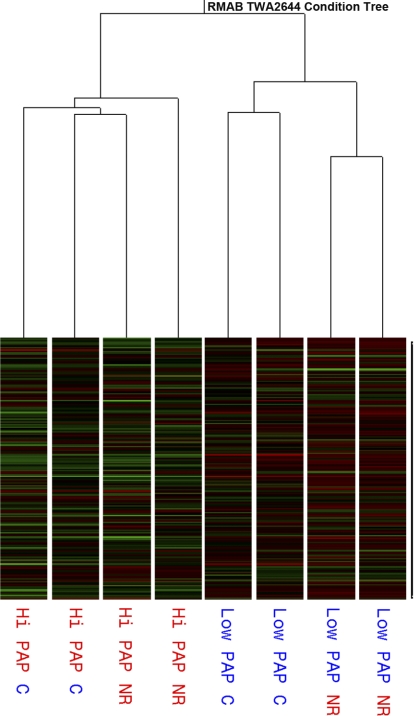
To identify the genes that were differentially expressed in the hypertrophied heart, Affymetrix bovine gene chips were screened using low PAP RV (2 from Control, 2 from Restricted) and high PAP RV (2 from Control, 2 from Restricted) (Fig. 2). The RV of high-PAP steers from Control cows had 177 differentially expressed genes (P < 0.05; >1.5-fold change) compared with low-PAP steers from Control cows. A detailed list of differentially expressed genes (top 20) in high vs. low PAP in steer RV from Control cows is given in Table 2. Activation peptide and pancreas cationic trypsinogen appear to be the genes that were most affected by high PAP (14.5-fold upregulated). α-Actin was upregulated 6.4-fold on average for the high-PAP RV. Monoamine oxidase B, FOS, and argininosuccinate synthetase gene expression were downregulated more than fourfold in high-PAP steer RV. Two genes that are similar to Sus scrofa neuron-activated orphan receptor-1α and Homo sapiens integrin-β1 binding protein were also significantly (P < 0.05) downregulated. Pathway analysis of differentially expressed genes by Kyoto Encyclopedia of Genes and Genomes (KEGG) that are affected by high PAP in steer RV from Control cows are listed in Table 3. Genes involved in the arginine and proline metabolism-related pathway (3 up- and 2 downregulated), the mitogen-activated protein kinase (MAPK) pathway (4 up- and 1 downregulated), and the fatty acids metabolism-related pathways (6 downregulated) were the most affected.
Fig. 2.
Affymetrix bovine gene chip screening of RV. RV from low PAP (steer RVs from C and NR cows) and high PAP (steer RVs from C and NR cows) showed 177 genes were differentially expressed between low-PAP and high-PAP RVs, regardless of diet treatment of cows. Steer RVs from NR vs. C cows in low PAP also showed 41 differentially expressed genes.
Table 2.
Genes that were up- or downregulated (top 20) in steer right ventricle with pulmonary hypertension from control-fed cows
| NCBI Gene Name | Common Name | Identity (%) | Fold Change |
|---|---|---|---|
| D38507 | Activation peptide and pancreas cationic trypsinogen | ↑ 14.5 | |
| NM_174225 | Actin, α1 skeletal muscle | ↑ 6.4 | |
| CB468334 | Macaca mulatta ubiquitin carboxylesterase | 690/699 (98%) | ↑ 6.4 |
| CK848882 | Macaca mulatta histidine acid phosphatase | 724/778 (93%) | ↑ 5.4 |
| CK973728 | Bos taurus follistatin-related protein | 604/609 (99%) | ↑ 5.1 |
| BF603325 | Bos taurus heatshock protein α6 | 267/267 (100%) | ↑ 5.0 |
| NM_174669 | Serine protease inhibitor clade E | ↑ 3.9 | |
| NM_174383 | Lysyl oxidase-like 1 | ↑ 3.6 | |
| NM_178318 | Biglycan | ↑ 3.0 | |
| NM_174196 | Thrombospondin | ↑ 2.1 | |
| CB427040 | Sus scrofa neuron-derived orphan receptor-1α | 305/327 (93%) 205/219 (93%) | ↓ 6.1 |
| CK775534 | Monoamine oxidase B | ↓ 5.9 | |
| CK773116 | Fos | ↓ 5.6 | |
| CK847787 | Homo sapiens integrin-β1 binding protein | 505/559 (90%) | ↓ 5.1 |
| NM_174743 | Spondin 1, extracellular protein | ↓ 4.4 | |
| NM_173892 | Argininosuccinate synthetase | ↓ 4.3 | |
| NM_173980 | Adipose differentiation related protein | ↓ 3.9 | |
| CK769749 | Dual specificity phosphatase 10 isoform a | ↓ 3.8 | |
| NM_174118 | Myocilin, trabecular meshwork inducible glucocorticoid response | ↓ 3.1 | |
| NM_174308 | Endothelin receptor type A | ↓ 2.5 |
NCBI, National Center for Biotechnology Information; ↑, upregulated; ↓, downregulated. P < 0.05.
Table 3.
Pathways analyses of genes that were differentially expressed in high pulmonary arterial pressure of steers from control-fed cows
| Pathway | Total | Up | Down |
|---|---|---|---|
| Arginine and proline metabolism | 5 | 3 | 2 |
| MAPK signaling pathway | 5 | 4 | 1 |
| Butanoate metabolism | 3 | 0 | 3 |
| Complement and coagulation cascades | 3 | 1 | 2 |
| Cytokine-cytokine receptor interaction | 3 | 1 | 2 |
| Fatty acid elongation in mitochondria | 3 | 0 | 3 |
| Fatty acid metabolism | 3 | 0 | 3 |
| Purine metabolism | 3 | 1 | 2 |
| TGF-β signaling pathway | 3 | 1 | 2 |
| Tryptophan metabolism | 3 | 0 | 3 |
| Valine, leucine, and isoleucine degradation | 3 | 0 | 3 |
TGF-β, transforming growth factor-β.
Maternal undernutrition.
Although maternal undernutrition did not affect LV and RV weight in steers, screening of the bovine gene chips also revealed that 41 genes were differentially expressed (>2-fold) in the RV of steers from Restricted cows compared with RV of steers from Control cows within the low-PAP group (Table 4). KEGG pathway analysis (Table 5) indicates that these genes are involved in adipocytokine signaling, cell communication, the insulin signaling pathway, and the arginine and proline pathway. Interestingly, there were seven genes that were differentially expressed in between high-PAP and maternal undernutrition groups (Table 6). Both high-PAP score and maternal undernutrition induced upregulation of α-actin and thrombospondin, which is known to be increased in cardiac hypertrophy.
Table 4.
List of genes that were differentially expressed in steer right ventricle from nutrient-restricted cows during early to midgestation compared with steer right ventricle from control-fed cows
| NCBI Name | Common Name | Identity (%) | Fold Change |
|---|---|---|---|
| CK846010 | Pentraxin-related protein | ↑ 5.5 | |
| CK778731 | Interferon-related developmental regulator | 667/670 (99%) | ↑ 3.8 |
| NM_177945 | Bos taurus peroxisome proliferators-activated receptor-γ coactivator-1α | ↑ 3.2 | |
| NM_174144 | PIM-1 | ↑ 3.0 | |
| NM_174255 | Actin, α1 skeletal muscle | ↑ 2.9 | |
| NM_174231 | Adrenergic-β2 receptor | ↑ 2.9 | |
| NM_174196 | Thrombospondin | ↑ 2.2 | |
| NM_174659 | Solute carrier family 25 member 5 | ↑ 2.2 | |
| NM_173888 | Adrenomedullin | ↑ 2.0 | |
| CK773116 | FOS | ↓ 3.6 | |
| CK848705 | Homo sapiens leucine rich repeat containing 17 isoform 1 variant | 578/642 (90%) 81/84 (96%) | ↓ 3.1 |
| AB052747 | Vascular cell adhesion molecule-1 6D variant lacking D7 | ↓ 2.9 | |
| NM_173980 | Adipose differentiation-related protein | ↓ 2.3 | |
| NM_174557 | Insulin-like growth factor-binding protein 4 | ↓ 2.2 | |
| CK769749 | Bos taurus dual specificity phosphatase 10 isoform a | 687/687 (100%) 62/62 (100%) | ↓ 2.1 |
P < 0.05.
Table 5.
Pathway analyses of genes that were differentially expressed in steer right ventricle from nutrient-restricted cows during early to midgestation compared with steer right ventricle from control-fed cows
| Pathway | Total | Up | Down |
|---|---|---|---|
| Adipocytokine signaling pathway | 2 | 2 | 0 |
| Cell communication | 2 | 2 | 0 |
| Insulin signaling pathway | 2 | 2 | 0 |
| Arginine and praline metabolism | 1 | 1 | 0 |
| MAPK signaling pathway | 1 | 0 | 1 |
| Oxidative phosphorylation | 1 | 1 | 0 |
| Pyrimidine metabolism | 1 | 1 | 0 |
| T-cell receptor signaling pathway | 1 | 0 | 1 |
Table 6.
Genes that are differentially expressed in common by maternal undernutrition and pulmonary hypertension in steers
| Common Name | GenBank No. | PAP Fold Change* | Undernutrition Fold Change† | Frequency |
|---|---|---|---|---|
| ACTA1 | NM_174225 | 5.0 | 2.6 | 4 |
| CB464568 | 0.26 | 2.46 | 1 | |
| CB427040 | 0.17 | 4.97 | 1 | |
| CK769749 | 0.26 | 0.47 | 1 | |
| AW437468 | 0.20 | 0.33 | 1 | |
| ADFP | NM_173980 | 0.26 | 0.44 | 1 |
| FOS | CK773116 | 0.18 | 0.28 | 1 |
| THBS | NM_174196 | 2.11 | 2.24 | 1 |
Steers with high and low pulmonary arterial pressure (PAP) from control-fed cows.
Steers from Control and Restricted cows are from normal pulmonary arterial steers.
RT-PCR
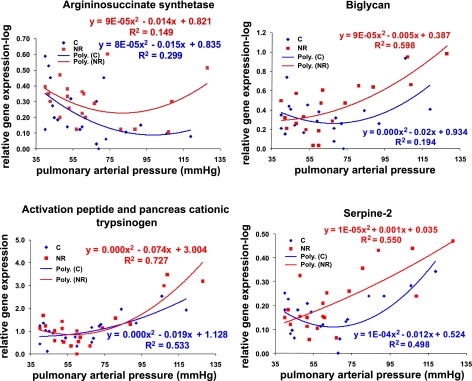
RT-PCR confirmation of a few selected genes was performed in each individual RV sample. RT-PCR analyses revealed that upregulation of these genes was greater in fold change compared with fold change determined by gene chip screening. Interestingly, confirmation of mRNAs in individual RV mRNA revealed a different mRNA expression response to increased PAP in steer RV from Restricted cows compared with steer RV from Control cows (Fig. 3). For example, the activation peptide and pancreas cationic trypsinogen mRNA was upregulated (14.5-fold) in high-PAP steers compared with low-PAP steers. However, confirmation of gene expression in RT-PCR activation peptide and pancreas cationic trypsinogen was greater in high-PAP steers compared with low-PAP steers. This was also observed in other genes, such as biglycan and serine-threonine protease inhibitor.
Fig. 3.
Real-time confirmation of selected genes that were up- or downregulated in high PAP in RV. Top left: arginino-succinate synthetase. Top right: activation peptide and pancreas cationic trypsinogen. Bottom left: biglycan. Bottom right: serine threonine protease inhibitor.
Gene expression with increased PAP score in control or restricted diets revealed different expression patterns. RT-PCR confirmation of activation peptide and pancreas cationic trypsinogen showed that activation peptide and pancreas cationic trypsinogen mRNA expression was increased (P < 0.05) in a quadratic manner as PAP score increased. However, response of activation peptide and pancreas cationic trypsinogen mRNA expression to increased PAP score was greater (P < 0.05) in steers from Restricted cows compared with those steers from Control cows (Fig. 3). Biglycan mRNA expression in steer RV from Control and Restricted cows was also upregulated (P < 0.05) in a quadratic manner as PAP score increased. Serpine-2 also increased (P < 0.05) with elevated PAP score. The responses of biglycan and serpine-2 were more significant in steer RV from Restricted cows than in steer RV from Control cows.
DISCUSSION
PAP
Pulmonary hypertension, the result of elevated pressures in the pulmonary artery, is caused primarily by a shortage of oxygen. Because pulmonary hypertension is a common symptom for brisket disease or high mountain disease, PAP measures have been shown to be a successful indicator of susceptibility to brisket disease (43). Generally, cattle with PAP > 50 mmHg are considered at higher risk. In the present study, RV weight did not increase until PAP reached ∼50 (Restricted) to 60 mmHg (Control) (Fig. 1). This is different from what is commonly suggested as a safe level of PAP at 40 mmHg. As RV weight increases, the thickness of RV also increased. However, as PAP approaches 60 mmHg, the ratio of RV thickness to RV weight started to decrease. These data are interpreted to mean that steers with heavier and thicker RV are experiencing eccentric hypertrophy (increase in the volume of the RV cavity). This eccentric hypertrophy is common in RV hypertrophy with pulmonary hypertension.
Cardiac hypertrophy during adaptation to increased stress could be either adaptive (exercise-induced hypertrophy) or maladaptive (pressure or volume overload-induced hypertrophy) and lead to heart failure. There are distinct differences in markers for adaptive and maladaptive hypertrophy, as well as distinct signaling pathways (8, 19). Pathological cardiac hypertrophy is often characterized by upregulation of fetal genes, such as atrial natriuretic peptide, β-myosin heavy chain, and skeletal α-actin (9, 26, 37). Thrombospondin expression was also increased in the early stage of hypertrophied hearts that are prone to fail in rats (38). In the present study, maladaptive cardiac hypertrophy markers α-actin and thrombospondin were upregulated in RV from high PAP. Strikingly, these markers were also upregulated in steer RV with low PAP from Restricted cows (Table 4).
Although there are conflicting results, differential signal transduction pathways exist between physiological and pathological cardiac hypertrophy (26). It has been shown that the Ca2+/calmodulin pathway or MAPK pathway induces pathological hypertrophy (1, 42). The MAPK/ERK1/2 pathway induces cardiac hypertrophy through activation of the Raf-1-MAPKK-ERK (MAPK) cascade (7). In our study, four of five genes that are involved in the MAPK pathway were upregulated. Upregulation of MAPK pathway components suggests that steers with these changes in gene expression experience irreversible pathological cardiac hypertrophy with increased PAP (26).
Undernutrition
Maternal undernutrition during the first half of gestation is detrimental to fetal and placental growth and development in mammalian species (17, 41). In a previous study, 50% of global maternal undernutrition caused compensatory growth of the fetal heart, while total fetal weight was lighter than in the Control group at day 78 of gestation in ewes (17, 41). Although sheep are identified as hyporeactive to pulmonary hypertension in high altitude (40), systemic blood pressure was higher in lambs from undernourished ewes compared with lambs from control-fed ewes (39). Although systemic blood pressure of steers is not available from the present study, bovine gene chip screening of RV tissue from steers of Restricted cows revealed that genes differentially expressed in the RV from high-PAP steers were greater (P < 0.05) as pulmonary blood pressure increased, compared with steer RV from Control cows.
Many studies have tried to identify markers that allow the detection of indexes of inflammation during myocardial infarction or heart failure (14, 22, 25). C-reactive protein belongs to the pentraxin family. Pentraxin-related protein is elevated in acute myocardial infarction and disappears in damaged myocytes in humans (31). Although increased serum level of C-reactive protein is nonspecific to tissue injury, C-reactive protein may assist in early recognition of myocardial infarction (11). Elevated level of the pentraxin-related protein was also observed in humans with Type 2 diabetes mellitus (32). These studies support the concept that upregulation of pentraxin-related protein mRNA expression in steers from undernourished cows may predisposed them to these diseases.
The heart requires high-capacity mitochondrial ATP production to meet its energy demands. The heart obtains its energy from fatty acid oxidation in mitochondria (28, 36). Cardiac hypertrophy caused by volume overload (13) and pressure overload (2) is associated with an increase in glycolysis and decrease in fatty acid utilization (35). The shift of the main fuel of the heart from fatty acid to glucose is due to downregulation of fatty acid oxidation (3). Pathway analysis of differentially expressed genes in RV of steers from Control or Restricted cows (Table 5) revealed that adipocytokine and insulin signaling pathways were affected by maternal undernutrition. Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) controls mitochondrial biogenesis in conjunction with peroxisome proliferator-activated receptors and induces mitochondrial fatty acid oxidation cycle enzyme expression. Upregulation of PGC-1α results in uncontrolled mitochondrial proliferation in cardiac myocytes and induced the loss of sarcomeric structure and dilated cardiomyopathy (23). Altered glucose/fatty acid metabolism induced by upregulation of PGC-1α even in normal heart from undernourished cows suggests that the energy metabolism was shifted to that of the hypertrophied heart. Adipose differentiation-related protein (also known as ADFP) is distributed in various cells and regulates cellular uptake and storage of long-chain fatty acids (6, 18). Downregulation of adipose differentiation-related protein implies the reduced uptake and storage of lipids for the energy source in the RV. The alteration of energy metabolism in steer RV from undernourished cows in the present study is similar to metabolic changes in response to cardiovascular disease in other animals, including humans.
PAP and Maternal Undernutrition Relationships
A significant amount of evidence suggests that undernutrition during early pregnancy predisposes animals to diseases/metabolic disorders, such as cardiac disease, hypertension, obesity, and diabetes in mammals (4, 12, 16, 21). Increased glycolysis in the steer heart from undernourished cows may also reflect the typical changes of glucose metabolism in insulin-resistant animals from undernourished mothers (5, 10, 24, 30). From our study, gene expression, as well as pathway analysis of differentially expressed genes in maternal undernutrition, exhibits maladaptive gene expression profiles without cardiac hypertrophy.
Insults to the fetus during early pregnancy may manifest the permanent programming of genes that could be detrimental to the health of offspring. These genes may not be differentially expressed in normal conditions. However, exposure to stress (i.e., nutritional or oxidative stress) may induce differential gene expression in the fetus as well as in the adult. In low-PAP score steers, maternal undernutrition induced differential gene expression by maternal dietary treatment. In steers from undernourished cows, there was a difference in gene expression that was also shared with the high-PAP score steers. However, there was no effect of maternal diet treatment on gene expression in high-PAP score steers. This could be due to the higher effect of RV hypertrophy than dietary effect.
In summary, several genes associated with maladaptive cardiac hypertrophy were shown to be differentially expressed in the RV of steers under high-PAP conditions, which was further perturbed by maternal undernutrition during fetal development. For example, α-actin and thrombospondin are cardiac hypertrophy markers that were upregulated in RV from high-PAP steers. These markers were also upregulated in steer RV with low PAP and in response to maternal undernutrition. Several members of the MAPK pathway, in addition to regulators of metabolism, mitochondrial biogenesis, and uptake of long-chain fatty acids, also were upregulated in RV under increased PAP. Study of RV gene expression in the RV of the steers with PAP score, where RV weight and/or thickness starts to increase (i.e., early stage of RV hypertrophy), will clarify which genes are responsible for the initiation of RV hypertrophy. These studies may also help in understanding why some animals are predisposed to cardiac problems at high altitude.
GRANTS
This project was supported by the United States Department of Agriculture-National Research Initiative Grant 2003-35206-12814 and National Institutes of Health Institutional Development Award Networks of Biomedical Research Excellence Grant P20RR16474-04.
Acknowledgments
The authors thank Dr. Tim Holt (Department of Clinical Sciences, Colorado State University) and Dr. Steven Paisley (Department of Animal Science, University of Wyoming) for collecting pulmonary artery pressure measurements and Dr. Warrie Means (Department of Animal Science, University of Wyoming) for help when processing steers and collecting hearts.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280: 574–577, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol Heart Circ Physiol 267: H742–H750, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med 10: 238–245, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ Fetal origins of coronary heart disease. BMJ 311: 171–174, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassett NS, Oliver MH, Breier BH, Gluckman PD. The effect of maternal starvation on plasma insulin-like growth factor I concentrations in the late gestation ovine fetus. Pediatr Res 27: 401–404, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263, 1997. [PubMed] [Google Scholar]
- 7.Brede M, Roell W, Ritter O, Wiesmann F, Jahns R, Haase A, Fleischmann BK, Hein L. Cardiac hypertrophy is associated with decreased eNOS expression in angiotensin AT2 receptor-deficient mice. Hypertension 42: 1177–1182, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Calderone A, Murphy RJ, Lavoie J, Colombo F, Beliveau L. TGF-beta(1) and prepro-ANP mRNAs are differentially regulated in exercise-induced cardiac hypertrophy. J Appl Physiol 91: 771–776, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Carreno JE, Apablaza F, Ocaranza MP, Jalil JE. [Cardiac hypertrophy: molecular and cellular events]. Rev Esp Cardiol 59: 473–486, 2006. [PubMed] [Google Scholar]
- 10.Crescenzo R, Samec S, Antic V, Rohner-Jeanrenaud F, Seydoux J, Montani JP, Dulloo AG. A role for suppressed thermogenesis favoring catch-up fat in the pathophysiology of catch-up growth. Diabetes 52: 1090–1097, 2003. [DOI] [PubMed] [Google Scholar]
- 11.de Beer FC, Hind CR, Fox KM, Allan RM, Maseri A, Pepys MB. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J 47: 239–243, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol 533: 561–570, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Alaoui-Talibi Z, Guendouz A, Moravec M, and Moravec J. Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-l-carnitine. Am J Physiol Heart Circ Physiol 272: H1615–H1624, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Cassani G, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation 92: 1479–1486, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85: 1285–1294, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 71: 1344S–1352S, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Han HC, Austin KJ, Nathanielsz PW, Ford SP, Nijland MJ, Hansen TR. Maternal nutrient restriction alters gene expression in the ovine fetal heart. J Physiol 558: 111–121, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 294: 309–321, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Iemitsu M, Miyauchi T, Maeda S, Sakai S, Kobayashi T, Fujii N, Miyazaki H, Matsuda M, Yamaguchi I. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol Regul Integr Comp Physiol 281: R2029–R2036, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans 27: 88–93, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 86: 217–222; discussion 121, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Latini R, Bianchi M, Correale E, Dinarello CA, Fantuzzi G, Fresco C, Maggioni AP, Mengozzi M, Romano S, Shapiro L, et al. Cytokines in acute myocardial infarction: selective increase in circulating tumor necrosis factor, its soluble receptor, and interleukin-1 receptor antagonist. J Cardiovasc Pharmacol 23: 1–6, 1994. [PubMed] [Google Scholar]
- 23.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leury BJ, Bird AR, Chandler KD, Bell AW. Glucose partitioning in the pregnant ewe: effects of undernutrition and exercise. Br J Nutr 64: 449–462, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 331: 417–424, 1994. [DOI] [PubMed] [Google Scholar]
- 26.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34: 255–262, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA 98: 8798–8803, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neely JR, Morgan HE. Carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 36: 413–459, 1974. [DOI] [PubMed] [Google Scholar]
- 29.National Research Council. Nutrient Requirements of Beef Cattle: Washington, DC: National Research Council, 2000.
- 30.Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on ovine fetal growth. J Endocrinol 173: 131–141, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, Pizzetti F, Maggioni AP, Moccetti T, Metra M, Cas LD, Ghezzi P, Sipe JD, Re G, Olivetti G, Mantovani A, Latini R. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation 102: 636–641, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327–334, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 70: 811–816, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Rhodes J Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol 98: 1092–1100, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94: 2837–2842, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266: 8162–8170, 1991. [PubMed] [Google Scholar]
- 37.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 59: 551–571, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res 95: 515–522, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Symonds ME, Gopalakrishnan G, Bispham J, Pearce S, Dandrea J, Mostyn A, Ramsay MM, Stephenson T. Maternal nutrient restriction during placental growth, programming of fetal adiposity and juvenile blood pressure control. Arch Physiol Biochem 111: 45–52, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Tucker A, McMurtry IF, Reeves JT, Alexander AF, Will DH, Grover RF. Lung vascular smooth muscle as a determinant of pulmonary hypertension at high altitude. Am J Physiol 228: 762–767, 1975. [DOI] [PubMed] [Google Scholar]
- 41.Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod 69: 133–140, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Wettschureck N, Rutten H, Zywietz A, Gehring D, Wilkie TM, Chen J, Chien KR, Offermanns S. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat Med 7: 1236–1240, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Will DH, Horrell JF, Reeves JT, Alexander AF. Influence of altitude and age on pulmonary arterial pressure in cattle. Proc Soc Exp Biol Med 150: 564–567, 1975. [DOI] [PubMed] [Google Scholar]
- 44.Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res 40: 438–443, 1996. [DOI] [PubMed] [Google Scholar]