Abstract
Vascular superoxide anion (O2•−) levels are increased in DOCA-salt hypertensive rats. We hypothesized that the endothelin (ET)-1-induced generation of ROS in the aorta and resistance arteries of DOCA-salt rats originates partly from xanthine oxidase (XO) and mitochondria. Accordingly, we blocked XO and the mitochondrial oxidative phosphorylation chain to investigate their contribution to ROS production in mesenteric resistance arteries and the aorta from DOCA-salt rats. Systolic blood pressure rose in DOCA-salt rats and was reduced after 3 wk by apocynin [NAD(P)H oxidase inhibitor and/or radical scavenger], allopurinol (XO inhibitor), bosentan (ETA/B receptor antagonist), BMS-182874 (BMS; ETA receptor antagonist), and hydralazine. Plasma uric acid levels in DOCA-salt rats were similar to control unilaterally nephrectomized (UniNx) rats, reduced with allopurinol and bosentan, and increased with BMS. Levels of thiobarbituric acid-reacting substances were increased in DOCA-salt rats versus UniNx rats, and BMS, bosentan, and hydralazine prevented their increase. Dihydroethidium staining showed reduced O2•− production in mesenteric arteries and the aorta from BMS- and bosentan-treated DOCA-salt rats compared with untreated DOCA-salt rats. Increased O2•− derived from XO was reduced or prevented by all treatments in mesenteric arteries, whereas bosentan and BMS had no effect on aortas from DOCA-salt rats. O2•− generation decreased with in situ treatment by tenoyltrifluoroacetone and CCCP, inhibitors of mitochondrial electron transport complexes II and IV, respectively, whereas rotenone (mitochondrial complex I inhibitor) had no effect. Our findings demonstrate the involvement of ETA receptor-modulated O2•− derived from XO and from mitochondrial oxidative enzymes in arteries from DOCA-salt rats.
Keywords: reactive oxygen species, endothelin-1, blood vessels, resistance arteries, deoxycorticosterone diacetate
vascular disease is characterized by the increased generation of ROS, specifically superoxide anion (O2•−), and is observed in hypertension, atherosclerosis, and the response to injury (44). Vascular O2•− production in angiotensin II-induced hypertension is generated by NAD(P)H oxidase (28, 44, 45). There is evidence of a contribution of NAD(P)H oxidase to O2•− production in a low-renin endothelin (ET)-1-dependent hypertensive model, the DOCA-salt hypertensive rat (41, 44). This model of experimental hypertension is characterized by the overexpression of ET-1 in resistance arteries (21, 22) and an elevation of O2•− levels in the vascular wall (43). Although the relationship between ETA and ETB receptors and NAD(P)H oxidase has been highlighted by several studies (2, 19, 23, 24), inhibition of NAD(P)H oxidase activity only partially affected O2•− generation in DOCA-salt rats (4), which may reflect controversies in apocynin specificity for NAD(P)H oxidase. Treatment with an ETA antagonist abolished O2•− generation (23, 24), suggesting the possible contribution of additional sources of O2•− other than NAD(P)H oxidase in this model. Xanthine oxidase (XO) and mitochondria produce O2•− and may contribute to vascular dysfunction in hypertension (10) and are thus candidates for an additional source of generation of O2•− in DOCA-salt rats.
The inhibition of nitric oxide (NO)-dependent function in endothelial cells that contain bound XO indicates that cell-bound XO can impair vascular cell function and produce O2•− in a sequestered microenvironment (11). XO is responsible for increases in ROS production in aortic endothelial cells in response to oscillatory shear stress (26), and treatment with the XO inhibitor allopurinol inhibited neointimal hyperplasia and improved vascular function in diabetic rats (15). XO induces hypertrophic responses in human vascular smooth muscle cells (VSMCs) (27), and its expression is upregulated in atherosclerotic plaques (32). Under normal conditions, the mitochondrial electron transport chain (METC) is a major source of superoxide, converting up to 5% of molecular O2 to O2•−. Because of its subcellular localization, the mitochondrial enzyme Mn-SOD is considered the first line of defence against oxidative stress (11). The importance of O2•− generation in the effects of ET-1 has been shown by gene transfer of Mn-SOD to carotid arteries in DOCA-salt rats, which results in reduced O2•− production in response to ET-1 (24). Similarly, ET-1 stimulated p38 MAPK, JNK, and ERK5 through mitochondria-dependent ROS generation in human VSMCs (45). Flow-induced dilation and H2O2 formation in coronary resistance arteries resulted from O2•− generated from mitochondrial respiration (25). Finally, damage to mitochondrial DNA leading to mitochondrial dysfunction has been reported in atherosclerosis and hypertension (36).
We hypothesized that ET-1-induced XO activity and mitochondrial oxidative phosphorylation contribute to vascular ROS generation in low-renin ET-1-dependent hypertension. To test this hypothesis, we blocked, in vivo, the activity of XO, the oxidative phosphorylation chain, and ET receptors to investigate their relative contribution to O2•− production in the aorta and mesenteric resistance arteries from DOCA-salt rats.
MATERIALS AND METHODS
Animals.
Experiments were conducted in unilaterally nephrectomized (UniNx) male Sprague-Dawley rats (Charles River Laboratories, St. Constant, QC, Canada), weighing 180–200 g, treated or not with DOCA, following the recommendations of the Canadian Council on Animal Care and with the approval of the Animal Care Committee of the Clinical Research Institute of Montreal and the Lady Davis Institute. DOCA implants (800 mg/kg) were prepared by mixing DOCA in silicone rubber, resulting in a given dose of 250 mg/kg, as previously described (22). DOCA rats (n = 8 rats/treatment) received 1% saline as drinking water, whereas UniNx rats (n = 11) were given tap water. DOCA-salt-treated rats were fed a powder diet (Agribrands) containing apocynin (1.5 mmol/l, Sigma, St. Louis, MO), allopurinol (100 mg·kg−1·day−1, Sigma), bosentan (100 mg·kg−1·day−1, ETA/B antagonist, Actelion, Basel, Switzerland), or BMS-182874 (BMS; 40 mg·kg−1·day−1, ETA-selective antagonist, Bristol-Myers Squibb, New Brunswick, NJ). Hydralazine (25 mg·kg−1·day−1, Sigma) was administered in drinking water. The dosage of drugs was determined based on the exact quantity of medicated food ingested and the amount of water consumed by the rats. Systolic blood pressure (SBP) was measured by the tail-cuff method after 3 wk of treatment. Rats were killed by decapitation.
Measurement of plasma lipid peroxidation products.
Plasma thiobarbituric acid-reacting substances (TBARS) are lipid peroxidation products that are considered an expression of systemic oxidative stress and are measured as malonaldehyde (MDA) by a colorimetric method, as previously described (46). Briefly, plasma samples were incubated for 15 min at 95°C in a mixture (2% butylated hydroxytoluene, 26 mmol/l thiobarbituric acid, and 918 mmol/l trichloroacetic acid) and then centrifuged at 3,000 g for 10 min. Supernatant absorbance was measured at 535 nm. MDA standards (Sigma) were included with each assay batch, and TBARS values were expressed in micromoles per liter of MDA.
Oxidative fluorescent microtopography.
Dihydroethidium (DHE) was used to evaluate the in situ production of O2•− (24). Unfixed frozen tissue sections (10 μm) were incubated at 37°C with DHE (2 μmol/l) or vehicle for 30 min. For the study of the METC, tissue sections from DOCA-salt rats were preincubated for 20 min at room temperature with specific inhibitors prior to the simultaneous addition of ET-1 (10−7 M) and DHE. The inhibitors used were rotenone (complex I inhibitor, 5 μmol/l, Sigma), tenoyltrifluoroacetone (TTFA, complex II inhibitor, 5 μmol/l, Sigma), and CCCP (uncoupler of electron transport and complex IV inhibitor, 5 μmol/l, Sigma). Images were obtained with an Axiovert 100M Zeiss laser scanning confocal microscope (Carl Zeiss, Jena, Germany), and fluorescence was detected with a 585-nm long-pass filter. The intensity of the fluorescent signal was measured as arbitrary units and compared with that found in vessels from UniNx rats within 30 min of staining to avoid nonspecific signals.
Measurement of XO-derived O2•−.
Aortic and mesenteric artery segments originating from animals treated with inhibitors were prepared as previously described (2). The production of O2•− by the tissues was evaluated by lucigenin (5 μmol/l)-enhanced chemiluminescence in response to xanthine (100 μmol/l). Luminescence was measured every 1.8 s for 3 min in a luminometer (AutoLumat LB 953, EG&G Berthold, Bad Wildbad, Germany). Background counts obtained from vessel-free preparation were subtracted from each reading. Activity was expressed as counts per minute per milligram of dry tissue weight.
Measurement of plasma uric acid and creatinine.
The plasma uric acid level was evaluated by a quantitative colorimetric assay using the quantichrom uric acid assay kit as described by the manufacturer (BioAssay Systems). The creatinine level was determined by Astra 8 Automatic Analyser Systems (Beckman Instruments, Fullerton, CA) using the Jaffe rate colorimetric method in which the rate of alkaline picrate with or without creatinine complex formation is measured.
Liver XO activity.
Liver XO activity was determined based on hepatic uric acid synthesis in response to xanthine. The rat liver was first extracted and concentrated as previously described (16). Then, 50 μl of liver extract were incubated in 50 mM potassium phosphate buffer (pH 7.5) containing 0.3 mM EDTA and 300 μM xanthine for 15 min at room temperature in a spectrophotometer (Smartspec Plus, Bio-Rad). XO activity was calculated according to the following formula: Unit activity/ml = [(ΔA290 nm/min test − ΔA290 nm/min blank) (3) (df)]/(12.2) (0.1), where A290 nm is the absorbance at 290 nm; 3 is the total volume of the assay (in ml); df is the dilution factor; 12.2 is the extinction coefficient of uric acid at 290 nm (in mM−1·cm−1); and 0.1 is the volume of tissue extract (in ml).
Western blot analysis.
The whole aorta and mesenteric artery were cleaned of fat, frozen in liquid nitrogen, and stored at −80°C until use. The vessels were homogenized in lysis buffer (20 mM MOPS, 1% Triton, 4% SDS, 10% glycerol, 5.5 mM leupeptin, 5.5 mM pepstatin, 200 KIU aprotinin, 1 mM Na3VO4, 10 mM NaF, 100 mM ZnCl2, 20 mM b-glycerophosphate, and 20 mM PMSF), kept on ice for 15 min, and centrifuged at 10,000 rpm for 15 min at 4°C. The supernatant was collected, and the protein concentration was determined using the Bio-Rad protein assay. Western blot analysis was performed on polyvinylidene difluoride membranes as previously described (1). Rabbit anti-ETA antibody or rabbit anti-ETB antibody (1:500) (Alomone Labs, Jerusalem, Israel) and rabbit anti-XO antibody (1:500) (Labvision, Fremont, CA) were used to detect protein expression. The signal was scanned by Chemicon (Bio-Rad) and quantified by the QuantityOne program. One UniNx rat sample from the aorta was set at 100%, and further UniNx and DOCA-salt samples were compared with this 100% sample. Results were expressed as percentages of UniNx values in the aorta.
Statistical analysis.
Results are expressed as means ± SE. Differences among groups were tested by one-way ANOVA followed by the Newman-Keuls post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Body weight, SBP, and heart and aorta weight.
Body weight, SBP, and heart and aorta weight were measured after 3 wk of treatment. In DOCA-salt rats, SBP was significantly increased and body weight was significantly reduced (P < 0.001) compared with UniNx rats (Table 1). All treatments significantly reduced but did not normalize SBP. The weight of the heart and aorta increased with DOCA-salt treatment. Heart weight did not change, whereas aorta weight was reduced by BMS but not by the other treatments.
Table 1.
Systolic blood pressure, heart and aorta weight, and biological parameters
| UniNx | DOCA-Salt |
DOCA-Salt |
|||||
|---|---|---|---|---|---|---|---|
| Apocynin | Allopurinol | Bosentan | BMS-182874 | Hydralazine | |||
| Body weight, g | 330±12 | 225±16* | 243±6‡ | 264±30† | 265±7‡ | 239±29 | 245±43 |
| Systolic blood pressure, mmHg | 117±5 | 207±17* | 146±7‡ | 161±6‡ | 166±23‡ | 172±10‡ | 129±4‡ |
| Heart weight/tibia length, mg/mm | 35.8±2.1 | 45.4±2.0* | 46.8±1.6 | 42.4±1.9 | 47.9±1.5 | 50.1±0.6 | 42.3±2.5 |
| Aorta weight/tibia length, mg/mm | 0.95±0.02 | 1.29±0.06* | 1.23±0.05 | 1.38±0.03 | 1.31±0.07 | 1.16±0.02‡ | 1.22±0.06 |
| Plasma uric acid, mg/dl | 3.7±0.1 | 4.1±0.3 | 4.6±0.2 | 3.3±0.2† | 3.2±0.2† | 8.3±0.5‡ | 3.5±0.1 |
| Plasma creatinine, μmol/l | 25.5±2.8 | 35.2±3.1* | 23.0±1.6† | 23.2±3.4† | 35.3±4.3 | 41.7±5.3 | 20.0±2.6† |
Values are expressed as means ± SE. UniNx, unilaterally nephrectomized rats; DOCA-salt, DOCA-salt hypertensive rats.
P < 0.001, DOCA-salt vs. UniNx rats;
P < 0.05, treated vs. DOCA-salt rats;
P < 0.001, treated vs. DOCA-salt rats (all by one-way ANOVA followed by the Newman-Keuls post hoc test).
Lipid peroxidation.
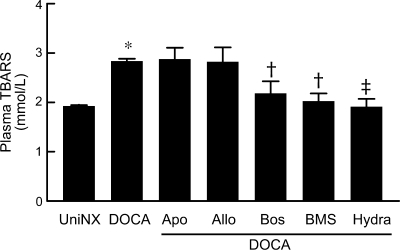
Lipid peroxidation, a marker of systemic oxidative stress measured by plasma TBARS as MDA, was significantly higher in DOCA-salt rats (P < 0.001) compared with UniNx rats (Fig. 1). Apocynin and allopurinol did not reduce TBARS in DOCA-salt rats, whereas bosentan, BMS, and hydralazine completely prevented their increase.
Fig. 1.
Graph showing plasma lipid peroxidation levels in DOCA-salt rats treated with or without redox enzyme inhibitors or endothelin (ET) receptor antagonists. n = 8 animals/group. TBARS, thiobarbituric acid-reactive substances; Apo, apocynin; Allo, allopurinol; Bos, bosentan; BMS, BMS-182874; Hyd, hydralazine. Results are means ± SE. *P < 0.001 vs. unilaterally nephrectomized (UniNx) rats; †P < 0.01 or ‡P < 0.001 vs. DOCA-salt rats.
ET receptor-induced O2•− production.
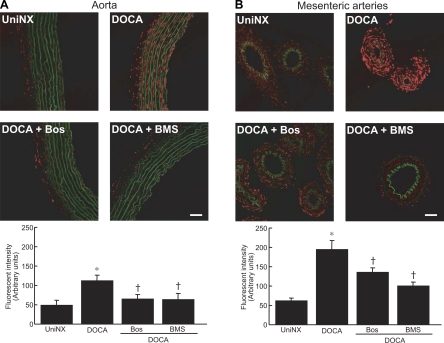
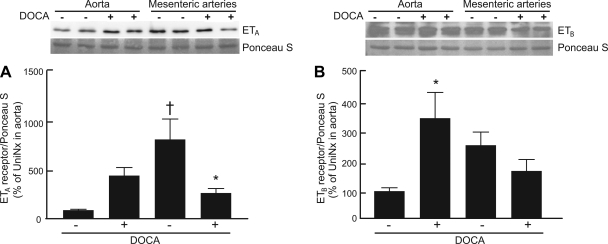
Aorta and mesenteric resistance artery O2•− generation were investigated by oxidative fluorescent microtopography. O2•− generation was increased to a greater degree in media of mesenteric arteries than in the aorta (Fig. 2, A and B) from DOCA-salt rats compared with UniNx rats. Dual blockade of ETA and ETB receptors with bosentan or selective ETA receptor blockade with BMS decreased the production of O2•− compared with untreated DOCA-salt rats in both vascular tissues. However, BMS had greater effects on mesenteric arteries than bosentan. To correlate differences in O2•− production observed in both vasculatures with the abundance of ET receptors, ET receptor expression was measured by Western blot analysis. The ETB protein level was higher in DOCA-salt rats in the aorta compared with mesenteric arteries (Fig. 3B). ETA expression was decreased in mesenteric arteries from DOCA-salt rats (P < 0.05) and was found to be less expressed in the aorta from UniNx rats than in DOCA-salt rats (P < 0.01).
Fig. 2.
Representative fluorescent confocal micrographs showing in situ O2•− detection in the aorta (A) and mesenteric arteries (B) from UniNx and DOCA-salt rats treated with or without Bos and BMS. Red fluorescence represents O2•− production, and green fluorescence represents the autofluorescence of elastin fibers. Scale bar = 50 μm. Data are representative of 4 separate experiments. Results are means ± SE. *P < 0.001 vs. UniNx rats; †P < 0.01 vs. DOCA-salt rats.
Fig. 3.
Graphs showing ETA receptor (A) and ETB receptor (B) expression in the aorta and mesenteric arteries from UniNx and DOCA-salt rats. Results are means ± SE. n = 5 animals/group. *P < 0.05 vs. UniNx counterparts; †P < 0.01 vs. aorta counterparts.
Vascular XO-derived O2•−.
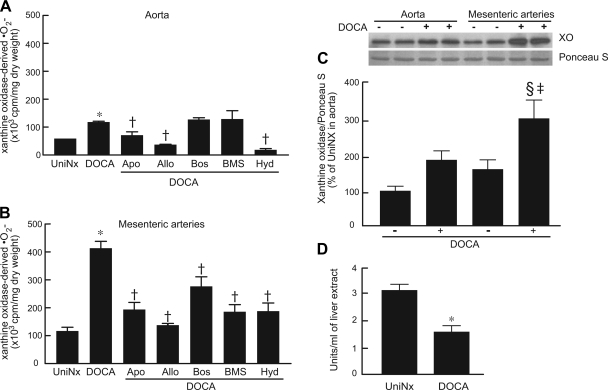
Aortic XO-derived O2•− was significantly increased in DOCA-salt rats compared with UniNx rats (P < 0.001; Fig. 4A). Apocynin, allopurinol, and hydralazine prevented the activation of aortic XO, whereas bosentan and BMS had no effect. Mesenteric artery production of O2•− by XO, which was higher than that of the aorta, was significantly higher in DOCA-salt rats compared with UniNx rats (P < 0.001). This increase was prevented by all the treatments used (Fig. 4B). Western blot analysis of XO showed greater protein expression in mesenteric arteries from DOCA-salt rats compared with UniNx rats from both vasculatures (P < 0.01), but no significant differences were observed between UniNx and DOCA-salt rats within the aorta (Fig. 4C).
Fig. 4.
Graphs showing the effect of redox enzyme inhibitors and ET receptor antagonists on xanthine oxidase (XO)-derived O2•− in the aorta (A) and mesenteric arteries (B), expression levels of XO in the aorta and mesenteric arteries (C), and activity of liver XO (D). Results are means ± SE. n = 5–8 rats/group. *P < 0.001 vs. UniNx rats; †P < 0.001 vs. DOCA-salt rats; ‡P < 0.05 vs. aorta counterparts; §P < 0.05 vs. UniNx counterparts.
Plasma uric acid concentration, creatinine concentration, and XO activity.
To further evaluate the involvement of XO in the DOCA-salt model, plasma uric acid was measured (Table 1). There were no significant differences of plasma uric acid levels between UniNx and DOCA-salt rats. Uric acid levels were significantly reduced (P < 0.05) after treatment of DOCA-salt rats with allopurinol and bosentan, whereas uric acid increased with BMS treatment. Plasma creatinine in DOCA-salt rats was reduced by apocynin, allopurinol, and hydralazine. XO activity was reduced in the liver of DOCA-salt rats compared with UniNx rats (Fig. 4D).
Role of mitochondrial oxidative phosphorylation in vascular O2•− generation.
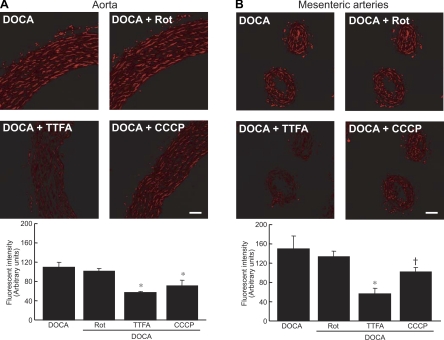
Rotenone treatment did not affect aortic O2•− production (Fig. 5A). TTFA-treated aortic rings exhibited a 50% reduction in fluorescence, whereas CCCP treatment decreased the signal by 25%. Mesenteric artery rings from DOCA-salt rats treated with rotenone showed significant differences of fluorescence compared with untreated rings from DOCA-salt rats (Fig. 5B). TTFA reduced the signal by 70%, whereas CCCP-treated rings showed a 30% reduction in O2•− production.
Fig. 5.
Representative fluorescent confocal micrographs showing in situ O2•− detection in aortic rings (A) and mesenteric arteries (B) from DOCA-salt rats treated with rotenone (Rot), tenoyltrifluoroacetone (TTFA), and CCCP. Red fluorescence represents O2•− production. Scale bar = 50 μm. Data are representative of 4 experiments. Results are means ± SE. *P < 0.001 or †P < 0.01 vs. DOCA-salt rats.
DISCUSSION
The present study provides the first evidence that XO and mitochondrial sources are involved in vascular O2•− production in a low-renin ET-1-dependent hypertension model, the DOCA-salt hypertensive rat. We showed that 1) the production of O2•− by XO is greater in mesenteric resistance arteries than in the aorta in DOCA-salt rats, 2) ETA receptors stimulate vascular XO to induce vascular oxidative stress, and 3) mitochondrial oxidative phosphorylation complexes can contribute to ET-1-induced vascular O2•− production. Dual blockade of ET receptors demonstrated a possible antagonism of ETB on ETA receptor function, since bosentan treatment resulted in the partial inhibition of O2•− generation derived from XO, in contrast to ETA receptor-selective blockade.
NAD(P)H oxidase has been suggested to be the most important ROS source in the vascular wall of DOCA-salt rats (2, 23, 26, 36, 42). Low-grade inflammation occurs in the vascular wall of DOCA-salt rats (14) and may exert a key regulatory role on XO gene expression (4). XO contributes to vascular endothelial dysfunction by inhibiting NO-dependent cGMP production in VSMCs (14), which underlies in part the impairment in endothelium-dependent vasorelaxation found in DOCA-salt rats (33). Thus, XO is a potential candidate as an important source of vascular O2•− in DOCA-salt rats. Xanthine oxidoreductase reduces O2 to produce O2•−, whereas xanthine dehydrogenase reduces NAD+ to produce NADH. The conversion of xanthine oxidoreductase into XO, which transforms hypoxanthine to xanthine and xanthine to uric acid, is controlled by growth factors, cellular pH, or O2 availability (9). Levels of plasma uric acid were affected by blocking XO or ETA and ETB receptors, independently of BP reduction and in the absence of significant differences of plasma uric acid levels between control and DOCA-salt rats. Allopurinol is capable of reducing tissue injury without measurably affecting XO activity (12), which may suggest that allopurinol could exert protective effects through other mechanisms such as free radical scavenging actions. The ETA-selective antagonist BMS increased uric acid by 180% compared with DOCA-salt rats. This probably results from increased ET-1 binding to renal tubular ETB receptors with the associated stimulation of natriuresis and diuresis that is accompanied by the decreased secretion of uric acid and consequently increased uric acid concentration in plasma (39). Increased ET-1-mediated oxidative stress through ETA receptors has been reported in mesenteric resistance (34) and carotid arteries (24), aortas (5), and veins (23) of DOCA-salt rats. The role of ETB receptors in ROS generation (15) is more controversial. ETA/B blockade reduced O2•− production in mesenteric arteries to a lesser extent than of ETA-selective blockade, which could suggest that ETB receptors may antagonize the function of ETA receptors.
Our results suggest, for the first time, that although XO activity is lower in DOCA-salt hypertensive rats in the liver, where it can be measured, it is involved in vascular ROS production in this experimental hypertensive rodent model. Discrepancies with other studies (5, 23, 24) may be related to the tissue-specific O2•− generation produced through XO that is much higher in small resistance arteries than in conduit vessels, which can be attributed to the increased XO expression in mesenteric arteries shown in the present study. Our results extend a previous proposal of the existence of a loop regulation of redox enzymes (43). By not only inhibiting NAD(P)H oxidase but also by scavenging radicals such as hydroxyl radicals, peroxynitrite, and O2•− with apocynin, XO is affected in both the aorta and mesenteric arteries, suggesting that O2•− produced by XO may be dependent on radicals generated by other sources of oxidative stress. Furthermore, we showed that ET receptors, particularly ETA receptors, are involved in the stimulation of XO-derived O2•− from resistance arteries. Although the protein levels of ET receptors do not explain the difference of XO-derived O2•− between UniNx and DOCA-salt rats in mesenteric arteries, these differences might reflect the affinity of the receptors for the peptide. In addition, we showed that ETA receptors are more highly expressed in mesenteric arteries than in the aorta, which could explain the differences observed in both tissues of UniNx rats. ETA receptors may have a direct effect on XO or an indirect effect via other redox-sensitive system (34). ET-1 content is higher in mesenteric arteries than in the aorta in DOCA-salt rats (47), which could explain the fact that XO-derived O2•− in the aorta was not affected by ET receptor blockade. Interestingly, XO has been shown to regulate the ET-1 promoter (16). Hypothetically, this transcriptional regulation could suggest a feedback loop and support our observation that O2•− originating from XO is regulated by the ET system.
The involvement of XO in DOCA-salt rats was also supported by the BP results in this study. The contribution of vascular O2•− to elevate BP depends in part on its ability to quench endogenous NO and to activate vasoconstrictor signaling mechanisms in VSMCs (42, 43). Several studies (5, 42) have shown that antioxidants reduce BP in experimental hypertensive models. Our study is the first in which DOCA-salt rats received a XO inhibitor. Data have been previously reported on the effect of XO activity on BP (20, 29). We were unable to find evidence of enhanced XO activity by measuring uric acid plasma or enzymatic activity in the liver from DOCA-salt rats. However, BP and vascular O2•− were reduced after treatment with allopurinol, demonstrating that XO is an important player in DOCA-salt hypertension. The decreased SBP did not affect lipid peroxidation in apocynin- or allopurinol-treated rats, whereas the reduction of lipid peroxidation (oxidative stress) in bosentan- and BMS-treated rats may contribute to SBP lowering (30).
In addition to redox enzyme sources, nonenzymatic O2•− production occurs in tissues. The four different complexes of the METC participate in the production of mitochondria-derived O2•− (45) that can be released into the cytoplasm by voltage-dependent anion channels (13). Complexes I and III have been found to be responsible for most of the O2•− produced in several conditions including heart, lung, and nervous system diseases (45). By blocking complex I (NADH dehydrogenase) with rotenone and complex II (succinate dehydrogenase) with TTFA, two complexes independent from each other, we identified the limiting step of the METC induced by ET-1. Our results showed that complex II appears to be critical for ROS formation in DOCA-salt rats, which is supported by previous work (31). Rotenone inhibits complex I in the proximity of the ubiquinone binding site, one of several sites available (3). The lack of effect of rotenone does not rule out the possibility that complex I can be involved in vascular ROS production. Complex IV (cytochrome c oxidase) may be important for vascular O2•− production stimulated by ET-1 in DOCA-salt rats. The present data are supported by reports in which a reduction of ET-1-induced p38 MAPK phosphorylation was found when complex II and IV were inhibited (44), indicating a role of METC in ROS-dependent signaling pathways. The central role played by mitochondria in ROS production in hypertension has been reviewed recently (6).
In summary, our data suggest, for the first time, that the ET-1/ETA receptor pathway is involved in the XO-derived O2•− detected in DOCA-salt hypertension and that ET-1 may stimulate METC to further contribute to vascular ROS formation in this model of hypertension. In addition, we have shown that XO is dependent on other redox sources, supporting the concept of ROS-triggering ROS formation (6). ET-1 plays a critical role in the pathogenesis of salt-sensitive hypertension, in part through ROS production in vascular tissues. Vascular bed localization seems to be an important factor in the sources involved in ROS production in each tissue. Because O2•− production, which contributes to vascular disease, is abrogated by inhibitors of XO and mitochondrial oxidative phosphorylation, targeting the production of O2•− dependent on XO activity and mitochondrial oxidation may represent new strategies to prevent vascular disease in hypertension.
GRANTS
This work was supported by Canadian Institutes of Health Research (CIHR) Grant 37917 (to E. L. Schiffrin), the Canada Research Chair Program of the Government of Canada, and the Canada Fund for Innovation (to E. L. Schiffrin and R. M. Touyz). E. C. Viel and K. Benkirane were supported by studentships from CIHR.
Acknowledgments
We are grateful to André Turgeon, Suzanne Diebold, and Annie Vallée for excellent technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdulnour RE, Peng X, Finigan JH, Han EJ, Hasan EJ, Birukov KG, Reddy SP, Watkins JE III, Kayyali US, Garcia JG, Tuder RM, Hassoun PM. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependant pathways. Am J Physiol Lung Cell Mol Physiol 291: L345–L353, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation 110: 2233–2240, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry 70: 200–214, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555: 589–606, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brandes RP Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45: 847–849, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension 42: 811–817, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Callera GE, Montezano AC, Touyz RM, Zorn TMT, Carvalho MHC, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor mediates altered leukocyte-endothelial cell interaction and adhesion molecules expression in DOCA-salt rats. Hypertension 43: 872–879, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Kilcoyne CM, Cannon RO, Quyyumi AA, Panza JA. Xanthine oxidase inhibition with oxipurinol improves endothelial vasodilator function in hypercholesterolemic but not in hypertensive patients. Hypertension 30: 57–63, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Godin DV, Bhimji S. Effects of allopurinol on myocardial ischemic injury induced by coronary artery ligation and reperfusion. Biochem Pharmacol 36: 2101–2107, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Houston M, Estevez A, Chumbley P, Aslan M, Marklund S, Parks DA, Freeman BA. Binding of xanthine oxidase to vascular endothelium: kinetic characterization and oxidative impairment of nitric oxide-dependent signalling. J Biol Chem 274: 4985–4994, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Inkster ME, Cotter MA, Cameron NE. Treatment with the xanthine oxidase inhibitor, alopurinol, improves nerve and vascular function in diabetic rats. Eur J Pharmacol 561: 63–71, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Kadam RS, Iyer KR. Isolation of different animal liver xanthine oxidase containing fractions and determination of kinetic parameters for xanthine oxidase. Indian J Pharm Sci 69: 41–45, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahler J, Mendel S, Weckmuller J, Orzechowski HD, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T. Oxidative stress increases synthesis of big endothelin-1 by activation of the endothelin-1 promoter. J Mol Cell Cardiol 32: 1429–1437, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Kurz S, Hink U, Nickenig G, Borthayre AB, Harrison DG, Münzel T. Evidence for a causal role of the renin-angiotensin system in nitrate tolerance. Circulation 99: 3181–3187, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landmesser U, Spiekermann S, Preuss C, Sorrentino S, Fisher D, Mones C, Mueller M, Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol 27: 943–948, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Larivière R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension 21: 294–300, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Larivière R, Sventek P, Thibault G, Schiffrin EL. Endothelin-1 expression in blood vessels of DOCA-salt hypertensive rats treated with the combined ETA/ETB endothelin receptor antagonist bosentan. Can J Physiol Pharmacol 73: 390–398, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Watts SW, Banes AK, Galligan JJ, Fink GD, Chen AF. NADPH oxidase-derived superoxide augments endothelin-1-induced venoconstriction in mineralocorticoid hypertension. Hypertension 42: 316–321, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AL. Endothelin-1 increases vascular superoxide via endothelinA-NADPH oxidase pathway in low-renin hypertension. Circulation 107: 1053–1058, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Zhao H, Li H, Klyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. [DOI] [PubMed] [Google Scholar]
- 26.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Matesanz N, Lafuente N, Azcutia V, Martin D, Cuadrado A, Nevado J, Rodriguez-Manas L, Sanchez-Ferrer CF, Peiro C. Xanthine oxidase-derived extracellular superoxide anions stimulate activator protein 1 activity and hypertrophy in human vascular smooth muscle via c-Jun N-terminal kinase and p38 mitogen-activated protein kinases. J Hypertens 25: 609–618, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Oktyabrsky ON, Smirnova GV. Redox regulation of cellular function. Biochemistry 72: 132–145, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Ong SLH, Vickers JJ, Zhang Y, McKensie KUS, Walsh CE, Whitworth JA. Role of xanthine oxidase in dexamethasone-induced hypertension in rats. Clin Exp Pharmacol Physiol 34: 517–519, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz MC, Sanabria E, Manriquez MC, Romero JC, Juncos LA. Role of endothelin and isoprostanes in slow pressor responses to angiotensin II in humans. Hypertension 37: 505–510, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Paddenberg R, Ishaq B, Goldenberg A, Faulhammer P, Rose F, Weissman N, Braun-Dullaeus RC, Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 284: L710–L719, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Patetsios P, Song M, Shutze WP, Pappas C, Rodino W, Ramirez JA, Panetta TF. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am J Cardiol 88: 188–191, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Pu Q, Touyz RM, Schiffrin EL. Comparison of angiotensin-converting enzyme (ACE), neutral endopeptidase (NEP) and dual ACE/NEP inhibition on blood pressure and resistance arteries of deoxycorticosterone acetate-salt hypertensive rats. J Hypertens 25: 899–907, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Pu Q, Fritsch Neves M, Virdis A, Touyz RM, Schiffrin EL. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension 42: 49–55, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. J Clin Invest 98: 2572–2579, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandra A, levonen AL, Brookes PS, Ceasar E, Shiva S, Barone MC, Darley-Usamr V. Mitochondria, nitric oxide, and cardiovascular dysfunction. Free Radic Biol Med 33: 1465–1474, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Rao GN, Berk BC. Reactive oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 70: 593–599, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Reyes AJ Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther 17: 397–414, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol 234: 279–293, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Schiffrin EL Vascular endothelin in hypertension. Vasc Pharmacol 43: 19–29, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Sedeek MS, Llinas MT, Drummond H, Fortepiani L, Abram SR, Alexander BT, Reckelhoff JF, Granger JP. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension 42: 806–810, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Somers MJ, Macromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation 101: 1722–1728, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Touyz RM, Yao GY, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens 22: 1141–1149, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Turrens JF Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 40: 504–510, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Chen AF, Watts SW, Galligan JJ, Fink GD. Endothelin in the planchnic vascular bed of DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol 288: H729–H736, 2005. [DOI] [PubMed] [Google Scholar]