Abstract
Coxsackievirus B3 (CVB3) is one of the most prevalent pathogens of viral myocarditis, which may persist chronically and progress to dilated cardiomyopathy. We previously demonstrated a critical role of the ubiquitin-proteasome system (UPS) in the regulation of coxsackievirus replication in mouse cardiomyocytes. In the present study, we extend our interest to an in vivo animal model to examine the regulation and role of the UPS in CVB3-induced murine myocarditis. Male myocarditis-susceptible A/J mice at age 4–5 wk were randomized to four groups: sham infection + vehicle (n = 10), sham infection + proteasome inhibitor (n = 10), virus + vehicle (n = 20), and virus + proteasome inhibitor (n = 20). Proteasome inhibitor was administered subcutaneously once a day for 3 days. Mice were killed on day 9 after infection, and infected hearts were harvested for Western blot analysis, plaque assay, immunostaining, and histological examination. We showed that CVB3 infection led to an accumulation of ubiquitin conjugates at 9 days after infection. Protein levels of ubiquitin-activating enzyme E1A/E1B, ubiquitin-conjugating enzyme UBCH7, as well as deubiquitinating enzyme UCHL1 were markedly increased in CVB3-infected mice compared with sham infection. However, there was no significant alteration in proteasome activities at 9 days after infection. Immunohistochemical staining revealed that increased expression of E1A/E1B was mainly localized to virus-damaged cells. Finally, we showed that application of a proteasome inhibitor significantly reduced CVB3-induced myocardial damage. This observation reveals a novel mechanism of coxsackieviral pathogenesis, and suggests that the UPS may be an attractive therapeutic target against coxsackievirus-induced myocarditis.
Keywords: myocarditis, myocardial injury, proteasome inhibitor, ubiquitin-proteasome system
coxsackievirus b3 (CVB3), a small, nonenveloped, positive-strand RNA enterovirus in the family Picornaviridae, is one of the primary causative agents of viral myocarditis, which leads to dilated cardiomyopathy with sudden, unexpected death in children and adolescents or end-stage congestive heart failure in adults (4, 6, 10, 20, 22). Although the pathogenesis of CVB3 infection has been studied for decades, it has only recently been recognized that many variables, including viral genome structure, host genetic background, and age and immune status of the host, interact with each other to determine the initiation, occurrence, and progression of viral myocarditis (24, 33).
The ubiquitin-proteasome system (UPS) is a major intracellular pathway for extralysosomal protein degradation, with over 80% of all cellular proteins being recycled through this pathway (3, 8, 25). Covalent attachment of ubiquitin to the protein substrates, a process called ubiquitination, is catalyzed by a cascade of enzymatic reactions involving ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3) (27, 37). The substrate specificity in the process of ubiquitination is thought to be conferred by the E3s. Several rounds of ubiquitination result in the formation of a polyubiquitinated target protein that is rapidly detected and degraded by the 26S proteasome, and ubiquitin is recycled by the action of deubiquitinating enzymes (DUBs). The 26S proteasome is a large multimeric protease, consisting of a 20S catalytic core and two 19S regulatory complexes (26). In addition to disposal of damaged, misfolded, or unnecessary proteins, the UPS is increasingly recognized as a key modulator in the regulation of a variety of fundamental cellular processes, including cell cycle regulation, apoptosis, antigen processing, signal transduction, transcriptional regulation, and DNA repair (3, 8, 25). Abnormalities of this system have been associated with several human diseases, including cancer, inflammation, neurodegenerative diseases, and cardiovascular diseases (9, 11, 28, 35).
Recent studies have also revealed a pivotal role of the UPS in viral infectivity. It has been demonstrated that the UPS can be utilized or manipulated by various viruses, including CVB3, to achieve successful viral infection (7, 18, 19, 21, 30, 31, 43). We previously showed (19) that coxsackievirus infection facilitates ubiquitin-dependent proteolysis of cyclin D1, which is linked to CVB3-induced cell growth arrest. It was also reported that CVB3 infection stimulates glycogen synthetase kinase 3β activity, which contributes to virus-induced cytopathic effect and apoptosis through ubiquitin-mediated degradation of β-catenin (43). Importantly, it was demonstrated recently that treatment of cardiomyocytes with proteasome inhibitors markedly reduces CVB3 replication through suppression of viral RNA transcription and protein synthesis (18). Moreover, pyrrolidine dithiocarbamate and curcumin have been found to potently inhibit CVB3 replication, likely through selective inhibition of host protein degradation (31, 32). Despite the demonstrated importance of the UPS in the life cycle of CVB3 replication, the expression and regulation of the UPS in viral myocarditis and the direct role of proteasome dysregulation in viral myocarditis have yet to be determined.
In the present report, we extend our study to an in vivo mouse model. We found that CVB3 infection led to an accumulation of ubiquitin conjugates without significant alteration in core proteasome activities. We further showed that protein levels of ubiquitin-activating enzyme E1A/E1B and ubiquitin-conjugating enzyme UBCH7 were markedly increased in CVB3-infected hearts, contributing to increased protein ubiquitination in CVB3-infected hearts. We finally showed that treatment with a proteasome inhibitor attenuated acute-phase myocarditis in mice. Our studies reveal a novel mechanism of coxsackievirus pathogenesis and suggest that manipulation of the UPS may provide a therapeutic option against viral myocarditis.
MATERIALS AND METHODS
Cell culture and viral infection in vitro.
The HL-1 cell line, a murine cardiac muscle cell line established from an AT-1 mouse atrial cardiomyocyte tumor lineage, was a generous gift from Dr. William C. Claycomb (Louisiana State University Medical Center, New Orleans, LA). Cells were plated onto flasks as described previously (18) and maintained in Claycomb medium from JRH Biosciences (Lenexa, KS) supplemented with 10% fetal bovine serum, 100 μg/ml penicillin-streptomycin, 0.1 mM norepinephrine (Sigma) in ascorbic acid, and 2 mM l-glutamine (Life Technologies).
HL-1 cells were preincubated with different concentrations of proteasome inhibitor MLN353 (mol mass 382.26 kDa; Millennium Pharmaceuticals) for 30 min. Cells were then infected at a multiplicity of infection of 100 with CVB3 (Nancy strain) or sham infected with phosphate-buffered saline (PBS) for 1 h, washed with PBS, and placed in Claycomb medium containing fresh inhibitor.
Viral infection and proteasome inhibitor treatment in vivo.
Myocarditis-susceptible A/J mice were obtained from Jackson Laboratories (Bar Harbor, ME). A total of 60 male A/J mice at age 4–5 wk were randomized to four groups: sham infection + vehicle (n = 10), sham infection + MLN353 (n = 10), virus + vehicle (n = 20), and virus + MLN353 (n = 20). Mice were either infected intraperitoneally with 105 plaque-forming units of CVB3 or sham infected with PBS. Virus- or sham-infected mice were administered the proteasome inhibitor MLN353 subcutaneously (0.02 mg/kg once a day for 3 days, i.e., 1 day before virus infection and 3 and 6 days after infection) or vehicle (PBS). Mice were killed on day 9 after infection, and infected hearts were harvested for further analysis. All procedures were approved by the Animal Care Committee at the University of British Columbia.
Plaque assay.
Virus titers in cell supernatant or mouse heart were measured by an agar overlay plaque assay as previously described (18). In brief, cell supernatant or heart homogenates were serially diluted and overlaid on a monolayer of HeLa cells. After 1 h of incubation, medium was removed and complete DMEM containing 0.75% agar was overlaid. Three days after infection, cells were fixed with Carnoy's fixative (25% acetic acid, 75% ethanol) and then stained with 1% crystal violet. Viral titers were determined as plaque-forming units per milliliter.
Protein extraction and Western blot analysis.
The frozen heart tissues collected from survival mice were ground under liquid nitrogen to a fine powder and then suspended in lysis buffer containing (in mM) 10 HEPES (pH 7.4), 50 Na4P2O7, 50 NaF, 50 NaCl, 5 EDTA, 5 EGTA, 2 Na3VO4, and 1 phenylmethylsulfonyl fluoride, with 0.1% Triton X-100 and 10 μg/ml leupeptin.
Western blotting was performed as previously described (18). Briefly, equal amounts of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (GE Healthcare). The membrane was blocked with 5% nonfat dry milk solution containing 0.1% Tween 20 for 1 h. Afterwards, the membrane was probed for 1 h with the primary antibody, followed by incubation for 1 h with horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were visualized with a enhanced chemiluminescence detection system (GE Healthcare) according to the manufacturer's protocol. For detection of protein ubiquitination, membrane was heat activated by autoclaving at 121°C for 35 min before blocking to enhance antigenic site recognition.
The monoclonal anti-β-actin and anti-GAPDH antibodies were purchased from Sigma. The monoclonal anti-VP1 antibody was obtained from DakoCytomation. The polyclonal anti-E1A/E1B and anti-ubiquitin were from Calbiochem. The polyclonal anti-UbcH7 was obtained from Chemicon. The polyclonal anti-UCHL1 was purchased from Abgent, and the polyclonal anti-E6-AP was obtained from Santa Cruz Biotechnology.
Histological grading and immunohistochemistry.
Midventricular portions of heart specimens were formalin fixed and paraffin embedded, and 4-μm sections were cut and stained with hematoxylin and eosin (H & E). Sections were graded blindly by an experienced pathologist for the severity of myocarditis based on myocardial lesion area, cellular vacuolization, calcification, necrosis, and inflammatory infiltration as previously described (41, 42), with the following scales: 0, no or questionable presence; 1, limited focal distribution; 2–3, intermediate severity; and 4–5, coalescent and extensive foci over the entirety of the transversely sectioned ventricular tissue.
Sections were also submitted for immunohistochemical staining. Briefly, sections were dewaxed and rehydrated, followed by antigen unmasking by heating. After blocking, sections were incubated with primary antibody (anti-E1A/E1B) overnight at 4°C and then secondary antibody for 30 min at room temperature, followed by incubation with avidin-biotin complex and diaminobenzidine reagents (Cell Signaling) and hematoxylin staining. Sections were then dehydrated and mounted with coverslips.
Proteasome activity assay.
Fresh heart homogenates, prepared as described above but in the absence of protease inhibitors, were used to measure proteasome activity as previously described (18). Briefly, 10 μg of heart homogenates was added to an assay buffer [mM: 20 Tris·HCl (pH 8.0), 1 ATP, and 2 MgCl2]. The mixture was placed at room temperature for 10 min and then incubated with 75 μM synthetic fluorogenic substrate Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (SLLVY-AMC, Calbiochem) at 30°C for 1 h. The fluorescent product AMC in the supernatant was measured at a 465-nm emission wavelength with a fluorometer.
Statistical analysis.
Results are expressed as means ± SE. Statistical analysis was performed with unpaired Student's t-test. The survival curve was plotted by the Kaplan-Meier method. The log-rank test was used to compare the survival rate between the groups over the whole time period. P values <0.05 were considered to be statistically significant.
RESULTS
CVB3 infection results in increased accumulation of protein-ubiquitin conjugates in mouse heart.
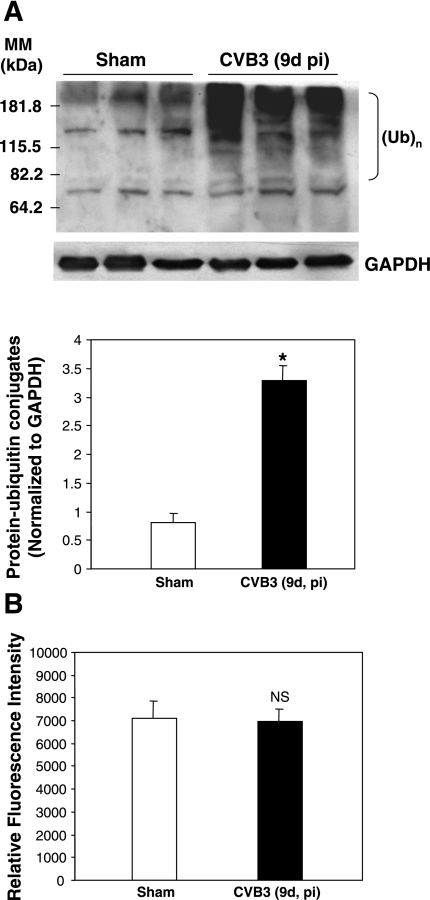
We previously demonstrated (18) that the UPS plays an important role in coxsackievirus infection in cultured cells. In the present study, we extend our interest by using an in vivo animal model to further characterize the function of the UPS in CVB3-induced myocarditis. We first examined the protein ubiquitination after CVB3 infection of myocarditis-susceptible A/J mice. We found that protein-ubiquitin conjugates were markedly increased at 9 days after infection compared with sham-infected control mice (Fig. 1).
Fig. 1.
Coxsackievirus B3 (CVB3) infection leads to an accumulation of protein-ubiquitin conjugates in mouse heart. A/J mice were infected with CVB3 [105 plaque-forming units (PFU) of Nancy stain] or phosphate-buffered saline (PBS; sham infection). At 9 days (d) after infection (pi) mice were killed and heart tissue was harvested. A: Western blot was performed to detect the ubiquitinated [(Ub)n] proteins with an anti-ubiquitin antibody. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was probed as a protein loading control. Protein levels of protein-ubiquitin conjugates [molecular mass (MM) from 82.2 to ∼230 kDa] were quantitated by densitometric analysis with the NIH ImageJ program and normalized to GAPDH expression. Data are means ± SE (sham-infected group: n = 3; CVB3 group: n = 3), and significance was determined by Student's t-test. *P < 0.05 vs. sham infection. B: heart homogenates were prepared, and proteasome activity was measured with the fluorogenic substrate Suc-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (SLLVY-AMC). Results are expressed as the amount of AMC formed by the enzymatic cleavage of substrate (means ± SE of 3 independent measurements from each animal; sham-infected group: n = 8; CVB3 group: n = 9). NS, no significant difference vs. sham infection.
Effects of CVB3 infection on protein expression of key enzymes involved in the UPS.
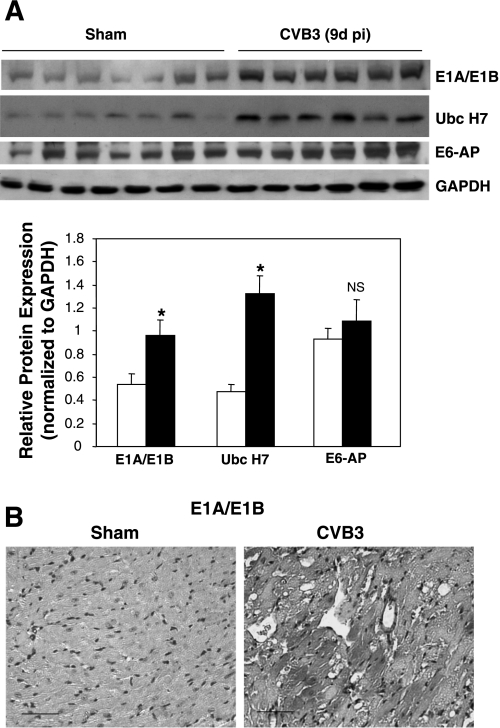
As described above, the molecular mechanism underlying regulation of the UPS can occur at two levels: 1) protein ubiquitination, which is regulated by E1, E2, and E3 ubiquitin enzymes as well as DUBs, and 2) proteasome-mediated protein degradation (3, 8). To explore the underlying mechanisms of the UPS dysregulation in the mouse heart after CVB3 infection, we examined the 20S proteasome activities in mouse heart at 9 days after infection. We found that the proteolytic activities of 20S proteasome were unchanged between CVB3-infected mouse hearts and control hearts (Fig. 1B), which was consistent with our previous results in cultured cells (18, 19). This observation suggests that the increased accumulation of ubiquitin conjugates is unlikely a result of decreased proteasome activity, prompting us to investigate whether there is a difference in the process of protein ubiquitination. We examined the protein expression of several key enzymes involved in ubiquitination, including ubiquitin-activating enzyme E1A/E1B and ubiquitin-conjugating enzyme UBCH7. We found that protein levels of both E1A/E1B and UBCH7 were significantly increased in CVB3-infected hearts compared with control hearts, suggesting that the upregulation of protein-ubiquitin conjugates was related to an increased level of these key enzymes (Fig. 2A). Immunohistochemical staining further demonstrated that increased expression of E1A/E1B was mainly localized to the vacuolated cells. Cellular vacuolization has been considered as common feature of CVB3-induced myocarditic lesion (Fig. 2B). These results indicate that CVB3 manipulates the UPS likely through upregulation of UPS-related enzymes.
Fig. 2.
Expression of ubiquitinating enzymes is upregulated in CVB3-infected mouse heart. A/J mice were infected with CVB3 or sham infected with PBS as described in Fig. 1. Nine days after infection, hearts were collected. A: Western blot was performed with anti-E1A/E1B, anti-UbcH7, anti-E6-AP, and anti-GAPDH (loading control) antibodies. Levels of expression were quantitated by densitometric analysis with the NIH ImageJ 1.37 program and normalized to GAPDH expression. Data are means ± SE (sham-infected group: n = 7; CVB3 group: n = 6). *P < 0.05 vs. sham infection; NS, no significant difference vs. sham infection. B: immunohistochemical staining for ubiquitin-activating enzyme E1A/E1B (red) was carried out as described in materials and methods. Nuclei were counterstained with hematoxylin (blue). Scale bar, 50 μm.
We also examined the expression of p53-related ubiquitin-protein ligase E6-AP (human papillomavirus E6-associated protein). However, we did not observe noticeable differences between sham- and virus-infected hearts (Fig. 2A). Since E6-AP is only one of numerous E3 ligases in the eukaryotic cell, future investigation is needed to determine whether other E3 ubiquitin ligases are dysregulated during coxsackievirus infection.
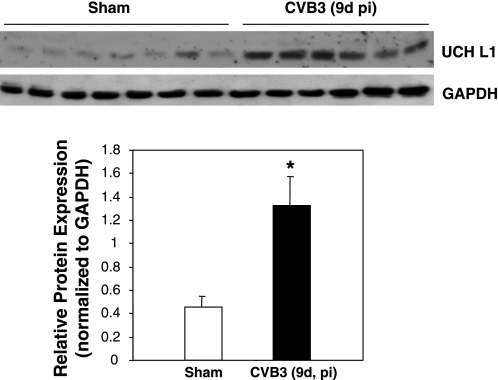
Protein ubiquitination can also be regulated by DUBs that specifically cleave ubiquitin from ubiquitin-conjugated protein substrates (38, 39). We therefore examined the protein expression of ubiquitin COOH-terminal hydrolase L1 (UCHL1), one of the DUBs primarily localized in neurons but lately found to be significantly upregulated in cardiomyocytes of dilated cardiomyopathy (36). We found that the expression level of DUB UCHL1 was significantly increased in virus-infected hearts (Fig. 3). Together, these results suggest that CVB3 infection promotes the process of protein ubiquitination via upregulating ubiquitin-activating enzyme and ubiquitin-conjugating enzyme and via increasing the expression of DUB. Our studies also suggest that enhanced protein turnover as a consequence of increased protein ubiquitination may play an important role in the pathogenesis of viral myocarditis.
Fig. 3.
Expression of deubiquitinating enzyme is increased in CVB3-infected mouse heart. A/J mice were infected and hearts collected as described in Figs. 1 and 2. Anti-ubiquitin COOH-terminal hydrolase L1 (UCHL1) antibody was used for immunoblotting of deubiquitinating enzyme UCHL1. Protein expression was quantitated and analyzed as described in Fig. 2. Data are means ± SE (sham-infected group: n = 7; CVB3 group: n = 6). *P < 0.05 vs. sham infection.
MLN353 inhibits CVB3 viral protein expression in murine cardiomyocytes.
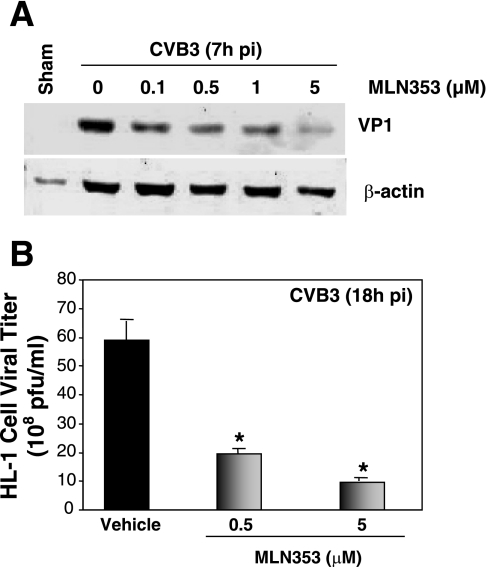
We previously showed (18) that treatment of mouse cardiomyocytes with the proteasome inhibitors MG-132 and lactacystin significantly reduced CVB3 viral RNA and protein levels and inhibited CVB3 progeny release, which suggests that proteasome inhibition may be a new therapeutic approach against viral myocarditis. To determine whether the UPS affects the pathogenesis of coxsackievirus-induced myocarditis, we decided to examine the effect of proteasome inhibition on viral replication, on host protein degradation, and on virus-mediated myocardial damage in mice. Proteasome inhibitor MLN353, suitable for administration in vivo and relatively stable under physiological conditions, was obtained from Millennium Pharmaceuticals. To verify our previous finding that proteasome inhibition blocks CVB3 replication, we first tested the effect of MLN353 on CVB3 protein expression in mouse cardiomyocytes. As shown in Fig. 4A, MLN353 inhibited CVB3 capsid protein VP1 expression in a dose-dependent manner. In addition, the titer of released virus in the supernatant was also reduced dose-dependently (Fig. 4B). These results suggest that, like other proteasome inhibitors (MG-132 and lactacystin), MLN353 also potently inhibits CVB3 replication in cardiomyocytes.
Fig. 4.
Proteasome inhibitor MLN353 inhibits CVB3 replication in mouse cardiomyocytes. HL-1 cells were preincubated with various concentrations of MLN353 (as described in materials and methods) for 30 min and then infected with CVB3 (multiplicity of infection = 100) for 1 h. A: 7 h after infection, cell lysates were collected and immunoblotted with anti-VP1 and anti-β-actin (as loading control) antibodies. Data are representative of 3 independent experiments. B: 18 h after infection, medium was collected from CVB3-infected cells and virus titer was determined by plaque assay. Values are means ± SE of 3 independent experiments. *P < 0.001 vs. vehicle-treated cells.
Toxicity and survival rates after proteasome inhibitor treatment of CVB3-infected mouse.
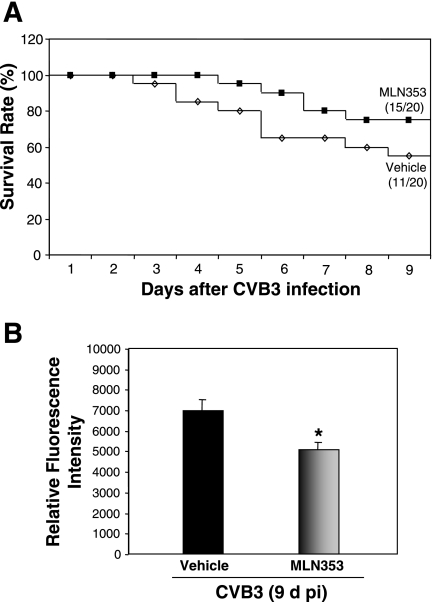
To choose a dose of proteasome inhibitor for the in vivo experiment, we performed a pilot toxicity study to test four selected doses of MLN353 (0.02, 0.06, 0.3, and 1 mg/kg) in noninfected mice. MLN353 was given at three time points, as described in materials and methods. Body weight was recorded daily after the first injection for 10 days. We found that administration of MLN353 at the doses of 0.06, 0.3, and 1 mg/kg produced toxicity after either one, two, or three injections, as judged by weight loss or lethality. However, compared with vehicle-treated, age-matched control mice, the body weight of mice treated with 0.02 mg/kg of MLN353 was not significantly different. In both groups, mouse body weight was gradually increased (10.31% vs. 9.23% increases at day 10 after vehicle or MLN353 treatment) and there was no mortality. Thus the dose of 0.02 mg/kg was chosen for the subsequent mouse experiments. In CVB3 infection studies, we further demonstrated that treatment with this dose of MLN353 significantly reduced the 20S proteasome activities in mouse heart (Fig. 5B). The survival curves over the whole time period are presented in Fig. 5A, showing that the survival rates were 50% for the vehicle group and 75% for the MLN353 group at 9 days after infection. There was no statistical difference between the two groups (P > 0.05).
Fig. 5.
MLN353 treatment reduces proteasome activity in mouse heart. A: Kaplan-Meier plot of survival curves of vehicle- and MLN353-treated mice 9 days after CVB3 infection. The numbers in parentheses at 9 days after infection are the numbers of surviving mice over the numbers of total experimental mice. P > 0.05 vs. vehicle-treated mice by log-rank test. B: A/J mice were sham infected with PBS or CVB3 infected and treated with vehicle or MLN353. At 9 days after infection, heart homogenates were prepared and proteasome activity was measured as described in Fig. 1. Results are means ± SE of 3 independent measurements from each animal (vehicle group: n = 9; MLN353 group: n = 11). *P < 0.05 vs. vehicle-treated mice at 9 days after infection.
MLN353 protects CVB3-induced murine myocarditis.
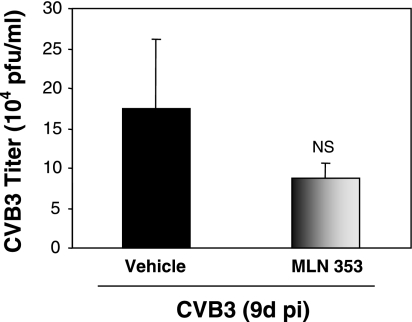
Adolescent A/J mice were infected with CVB3 in the presence or absence of MLN353. Nine days after viral inoculation, mice were killed and heart tissues were harvested. To determine whether in vivo application of MLN353 can reduce CVB3 replication in heart, we performed a plaque assay for viral titers after CVB3 infection and MLN353 treatment. As shown in Fig. 6, the reduction of CVB3 viral titer after MLN353 treatment is not significant compared with the vehicle control.
Fig. 6.
Effect of proteasome inhibition on CVB3 viral titer in mice. A/J mice were CVB3 infected with vehicle or MLN353 treatment. Heart tissues were collected at 9 days after infection, and heart homogenates were used for plaque assay (means ± SE; vehicle group: n = 8, MLN353 group: n = 11). NS, no significant difference vs. sham infection.
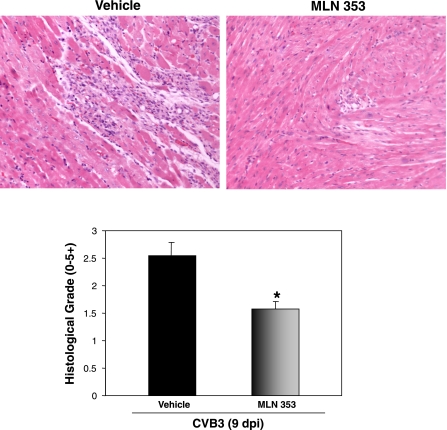
We finally examined the histological changes after MLN353 treatment. H & E staining (Fig. 7, top) showed that the extent of myocarditis, especially inflammation, induced by CVB3 infection was significantly decreased by treatment with MLN353 compared with the vehicle controls. The corresponding histological grades of the extent of myocarditis are shown in Fig. 7, bottom. These results strongly suggest that MLN353 protects myocarditis induced by CVB3 in mice. This is the first in vivo evidence demonstrating the effect of the proteasome inhibitor in CVB3-induced myocarditis.
Fig. 7.
MLN353 treatment attenuates CVB3-induced myocardial injury in mice. A/J mice were CVB3 infected in the presence of vehicle or MLN353. At 9 days after infection, heart tissue from both vehicle and MLN353 groups were collected and hematoxylin and eosin stained (top) and the extent of myocarditis was histologically graded (bottom) based on the intensity and character of injury and inflammatory infiltration as described in materials and methods. Results are means ± SE (vehicle group: n = 11; MLN353 group: n = 15). *P < 0.05 vs. vehicle-treated mice at 9 days after infection.
DISCUSSION
Virus infectivity and host responses determine the severity of viral myocarditis: CVB3 directly injures the infected myocardium, and the extent of such injury determines the severity of late-stage organ dysfunction (23). Host responses to viral infection may reflect a host defense mechanism. However, improper host protein regulation, such as aberrant host protein degradation, may cause further tissue damage (14). In the present study, we examined the expression and regulation of the UPS in CVB3-induced murine hearts and explored the direct role of proteasome inhibition in the pathogenesis of coxsackieviral myocarditis. We found that protein-ubiquitin conjugates are abnormally accumulated in CVB3-infected hearts. We further demonstrated that increased accumulation of ubiquitin conjugates is attributed to augmented protein ubiquitination, but not to decreased proteasome proteolytic activities. Finally, we showed that application of a proteasome inhibitor attenuates CVB3-induced myocardial injury. Our study reveals a novel mechanism of coxsackievirus infection and suggests that the UPS may be an attractive therapeutic target against coxsackievirus-induced myocarditis.
The level of protein-ubiquitin conjugates is determined by a balance between the rates of protein conjugation and degradation (3, 8). A breakdown in this balance will lead to abnormal expression of ubiquitin conjugates. The finding in this study that proteolytic activities of the proteasome are unchanged after CVB3 infection of mouse heart is consistent with our previous observation in cultured cells (18), indicating that the proteasome is unlikely a direct target of the virus. These results also exclude a possible contribution of proteasome dysfunction in the pathogenesis of viral myocarditis. As alluded to above, three classes of enzymes, known as E1, E2, and E3, catalyze the conjugation reaction of ubiquitin to the protein substrates (27). In the present study, we found that CVB3 infection induces the expression of ubiquitin enzymes E1A/E1B and UBCH7, suggesting that increased protein ubiquitination may be a factor resulting in the aberrant accumulation of ubiquitin conjugates. Although the protein level of p53-related E3 ligase E6-AP is not different after virus infection, we cannot rule out the influence of CVB3 infection on other E3 ligases since more than 500 E3s are predicted based on the human genome (27). It is known that protein ubiquitination can also be regulated by DUBs (39). In eukaryotes, ubiquitin is generated in the cells only by proteolysis of polyubiquitin chains or ubiquitin fused to carboxyl extension proteins (12). Protein deubiquitination has been reported to play a critical role in the supply of free ubiquitin to the cells for protein ubiquitination. In this study, we demonstrated that CVB3 infection increases UCHL1 expression, suggesting that enhanced protein deubiquitination appears also to be a cause of the increased accumulation of protein conjugates, because UCHL1 may increase protein ubiquitination by providing the available pool of free ubiquitin.
The mechanisms leading to the increased expression of E1A/E1B, UBCH7, and UCHL1 are unclear. Experimental and clinical studies have indicated a pivotal role of myocardial inflammation in the development and progression of viral myocarditis. Increased release of proinflammatory cytokines has been implicated as contributing to the pathogenesis of this disease (5). Recent studies have suggested that cytokines are important modulators for protein degradation through the regulation of protein ubiquitination and degradation/deconjugation (15–17). Thus we speculate that CVB3 infection leads to increased inflammatory cytokine release, which stimulates protein ubiquitination by upregulation of ubiquitin enzymes. Moreover, the fact shown in this study that increased expression of E1A/E1B appears to localize to virus-damaged, noninflammatory cells, together with our previous observation that CVB3 infection of cultured murine cardiomyocytes is unable to induce E1A/E1B expression (data not shown), suggests that this enzyme may be influenced by inflammatory cytokines in a paracrine or autocrine mechanism.
Following the observation that 26S proteasome is a primary component of the protein degradation pathway of the cell, proteasome inhibitors with low molecular weight have been widely used in basic research and in the clinical trials of many diseases. Bortezomib (also known as Velcade or PS-341), developed by Millennium Pharmaceuticals, is a dipeptidyl boronic acid that potently inhibits 26S proteasome activity in a specific and reversible manner. This chemical has been shown to have significant antitumor activity as a single agent and in combination with other cytotoxic drugs (1, 2). Other proteasome inhibitors, such as MG-132 and lactacystin, have been used frequently in basic research. We previously reported (18) that these two agents can reduce CVB3 replication in murine cardiomyocytes. In the present study, we used a new proteasome inhibitor, MLN353, which has a structure similar to that of Bortezomib and is more soluble and suitable for animal study than MG-132 and lactacystin, to investigate the effect of proteasome inhibitor on CVB3-induced myocarditis.
Through their ability to block the activation of NF-κB, proteasome inhibitors have been shown to dramatically reduce the production of multiple inflammatory mediators and leukocyte adhesion molecules, which play a crucial role in many diseases (34). Viral myocarditis is an inflammatory disease of the heart. It was classically considered that infiltrating immune cells play a critical role in the host defense mechanism by clearing the invaded viruses. However, accumulating evidence suggests that an inappropriate immune response may also lead to tissue damage (14). It has been shown that depletion of T lymphocytes results in a reduction in mortality and a decrease in the inflammatory infiltrate following CVB3 infection (40). Transfer of mononuclear cells from mice infected with CVB3 or from patients with myocarditis into genetically identical or immunodeficient mice, respectively, exacerbates myocardial damage (13, 29). In this study, we showed that treatment with MLN353 attenuates the severity of myocarditis, especially inflammation, suggesting that the UPS plays an important role in the pathogenesis of viral myocarditis.
Although it was demonstrated in vitro that MLN353 treatment reduces viral replication in cardiomyocytes, animal study showed no direct antiviral effect of this inhibitor. As stated above, early host immune response plays a critical role in clearance of virus. It is speculated that, after treatment with proteasome inhibitor, a balance between a direct inhibition of viral replication and a suppression of host immune response determines the final virus load inside the heart. It is believed that an appropriately regulated immune system is crucial in virus clearance and in the regulation of myocardial damage. However, we still cannot rule out the possibility that the observation of no effect on virus titers may also be due to a suboptimal efficacy of the dose of MLN353 administered and/or the experimental model and timing employed in the present study.
In summary, we demonstrate that protein-ubiquitin conjugates are aberrantly accumulated in CVB3-infected hearts, which is attributed to increased protein ubiquitination/deubiquitination. We further demonstrate that proteasome inhibition attenuates myocardial injury induced by coxsackievirus infection. Our data suggest that the UPS may be an attractive therapeutic target for virus-induced myocarditis. Further investigation to identify more specific targets of the UPS by CVB3 infection [e.g., the ubiquitin ligase(s) involved in the process of specific protein ubiquitination] will allow for even more precise targeting of drug therapy.
GRANTS
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) (H. Luo), the Heart and Stroke Foundation of British Columbia and Yukon (H. Luo), and the Canada Foundation for Innovation (H. Luo). G. Gao is the recipient of a Doctoral Traineeship from the Michael Smith Foundation for Health Research (MSFHR) and the Heart and Stroke Foundation. X. Si is supported by a CIHR IMPACT Post-Doctoral Fellowship and a CIHR Michael Smith Post-Doctoral Fellowship. H. Luo is a New Investigator of the CIHR/St. Paul's Hospital Foundation Award and a MSFHR Scholar.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams J Development of the proteasome inhibitor PS-341. Oncologist 7: 9–16, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Adams J, Behnke M, Chen S, Cruickshank AA, Dick LR, Grenier L, Klunder JM, Ma YT, Plamondon L, Stein RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett 8: 333–338, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J 17: 7151–7160, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dec GW, Palacios IF, Fallon JT, Aretz HT, Mills J, Lee DC, Johnson RA. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med 312: 885–890, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Fairweather D, Rose NR. Inflammatory heart disease: a role for cytokines. Lupus 14: 646–651, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Feldman AM, McNamara D. Myocarditis. N Engl J Med 343: 1388–1398, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Gao G, Luo H. The ubiquitin-proteasome pathway in viral infections. Can J Physiol Pharmacol 84: 5–14, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Golab J, Bauer TM, Daniel V, Naujokat C. Role of the ubiquitin-proteasome pathway in the diagnosis of human diseases. Clin Chim Acta 340: 27–40, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Grist NR, Reid D. Organisms in myocarditis/endocarditis viruses. J Infect 34: 155, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J, Ciechanover A, Lerman LO, Lerman A. The ubiquitin-proteasome system in cardiovascular diseases—a hypothesis extended. Cardiovasc Res 61: 11–21, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 67: 425–479, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Huber SA, Lodge PA. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol 116: 21–29, 1984. [PMC free article] [PubMed] [Google Scholar]
- 14.Knowlton KU, Badorff C. The immune system in viral myocarditis: maintaining the balance. Circ Res 85: 559–561, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19: 362–370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YP, Lecker SH, Chen Y, Waddell ID, Goldberg AL, Reid MB. TNF-alpha increases ubiquitin-conjugating activity in skeletal muscle by up-regulating UbcH2/E220k. FASEB J 17: 1048–1057, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Llovera M, Carbo N, Lopez-Soriano J, Garcia-Martinez C, Busquets S, Alvarez B, Agell N, Costelli P, Lopez-Soriano FJ, Celada A, Argiles JM. Different cytokines modulate ubiquitin gene expression in rat skeletal muscle. Cancer Lett 133: 83–87, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Luo H, Zhang J, Cheung C, Suarez A, McManus BM, Yang D. Proteasome inhibition reduces coxsackievirus B3 replication in murine cardiomyocytes. Am J Pathol 163: 381–385, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo H, Zhang J, Dastvan F, Yanagawa B, Reidy MA, Zhang HM, Yang D, Wilson JE, McManus BM. Ubiquitin-dependent proteolysis of cyclin D1 is associated with coxsackievirus-induced cell growth arrest. J Virol 77: 1–9, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martino TA, Liu P, Sole MJ. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ Res 74: 182–188, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Masucci MG Epstein-Barr virus oncogenesis and the ubiquitin-proteasome system. Oncogene 23: 2107–2115, 2004. [DOI] [PubMed] [Google Scholar]
- 22.McManus BM, Chow LH, Radio SJ, Tracy SM, Beck MA, Chapman NM, Klingel K, Kandolf R. Progress and challenges in the pathological diagnosis of myocarditis. Eur Heart J 12, Suppl D: 18–21, 1991. [DOI] [PubMed] [Google Scholar]
- 23.McManus BM, Chow LH, Wilson JE, Anderson DR, Gulizia JM, Gauntt CJ, Klingel KE, Beisel KW, Kandolf R. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol 68: 159–169, 1993. [DOI] [PubMed] [Google Scholar]
- 24.McManus BM, Yanagawa B, Rezai N, Luo H, Taylor L, Zhang M, Yuan J, Buckley J, Triche T, Schreiner G, Yang D. Genetic determinants of coxsackievirus B3 pathogenesis. Ann NY Acad Sci 975: 169–179, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Myung J, Kim KB, Crews CM. The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev 21: 245–273, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naujokat C, Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest 82: 965–980, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Pickart CM Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz AL, Ciechanover A. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu Rev Med 50: 57–74, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Schwimmbeck PL, Badorff C, Schultheiss HP, Strauer BE. Transfer of human myocarditis into severe combined immunodeficiency mice. Circ Res 75: 156–164, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Shackelford J, Pagano JS. Tumor viruses and cell signaling pathways: deubiquitination versus ubiquitination. Mol Cell Biol 24: 5089–5093, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, Suarez A, Morgan A, Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol 79: 8014–8023, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simms MG, Walley KR. Activated macrophages decrease rat cardiac myocyte contractility: importance of ICAM-1-dependent adhesion. Am J Physiol Heart Circ Physiol 277: H253–H260, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Tam PE Coxsackievirus myocarditis: interplay between virus and host in the pathogenesis of heart disease. Viral Immunol 19: 133–146, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T. Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie 83: 351–356, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Vu PK, Sakamoto KM. Ubiquitin-mediated proteolysis and human disease. Mol Genet Metab 71: 261–266, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Weekes J, Morrison K, Mullen A, Wait R, Barton P, Dunn MJ. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics 3: 208–216, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Weissman AM Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2: 169–178, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson KD Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin Cell Dev Biol 11: 141–148, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Wing SS Deubiquitinating enzymes—the importance of driving in reverse along the ubiquitin-proteasome pathway. Int J Biochem Cell Biol 35: 590–605, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Woodruff JF, Woodruff JJ. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol 113: 1726–1734, 1974. [PubMed] [Google Scholar]
- 41.Yanagawa B, Spiller OB, Choy J, Luo H, Cheung P, Zhang HM, Goodfellow IG, Evans DJ, Suarez A, Yang D, McManus BM. Coxsackievirus B3-associated myocardial pathology and viral load reduced by recombinant soluble human decay-accelerating factor in mice. Lab Invest 83: 75–85, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Yanagawa B, Spiller OB, Proctor DG, Choy J, Luo H, Zhang HM, Suarez A, Yang D, McManus BM. Soluble recombinant coxsackievirus and adenovirus receptor abrogates coxsackievirus B3-mediated pancreatitis and myocarditis in mice. J Infect Dis 189: 1431–1439, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Yuan J, Zhang J, Wong BW, Si X, Wong J, Yang D, Luo H. Inhibition of glycogen synthase kinase 3beta suppresses coxsackievirus-induced cytopathic effect and apoptosis via stabilization of beta-catenin. Cell Death Differ 12: 1097–1106, 2005. [DOI] [PubMed] [Google Scholar]