Abstract
The catecholamine release-inhibitory catestatin [Cts; human chromogranin (Cg) A352-372, bovine CgA344-364] is a vasoreactive and anti-hypertensive peptide derived from CgA. Using the isolated avascular frog heart as a bioassay, in which the interactions between the endocardial endothelium and the subjacent myocardium can be studied without the confounding effects of the vascular endothelium, we tested the direct cardiotropic effects of bovine Cts and its interaction with β-adrenergic (isoproterenol, ISO) and endothelin-1 (ET-1) signaling. Cts dose-dependently decreased stroke volume and stroke work, with a threshold concentration of 11 nM, approaching the in vivo level of the peptide. Cts reduced contractility by inhibiting phosphorylation of phospholamban (PLN). Furthermore, the Cts effect was abolished by pretreatment with either nitric oxide synthase (NG-monomethyl-l-arginine) or guanylate cyclase (ODQ) inhibitors, or an ETB receptor (ETBR) antagonist (BQ-788). Cts also noncompetitively inhibited the positive inotropic action of ISO. In addition, Cts inhibited the positive inotropic effect of ET-1, mediated by ETA receptors, and did not alter the negative inotropic ET-1 influence mediated by ETBR. Cts action through ETBR was further suggested when, in the presence of BQ-788, Cts failed to inhibit the positive inotropism of both ISO and ET-1 stimulation and PLN phosphorylation. We concluded that the cardiotropic actions of Cts, including the β-adrenergic and ET-1 antagonistic effects, support a novel role of this peptide as an autocrine-paracrine modulator of cardiac function, particularly when the stressed heart becomes a preferential target of both adrenergic and ET-1 stimuli.
Keywords: chromogranin A, myocardial contractility, inotropic agents, avascular heart, endocardial endothelium
chromogranin a (CgA), an acidic secretory proprotein, is the chromogranin/secretogranin protein family's index member (24, 53, 57). CgA proteolytic processing gives rise to several peptides of biological importance, including such counterregulatory agents as the dysglycemic hormone pancreastatin (52), vasodilator vasostatin 1 (VS-1) (2), and catecholamine release-inhibitory peptide catestatin (Cts; human CgA352-372, bovine CgA344-364) (35–40). Plasma CgA levels and epinephrine levels are increased in patients with essential hypertension or heart failure (15), whereas Cts plasma levels are significantly lower (44), suggesting that Cts has an inhibitory effect on chromaffin cells in vivo. Consistent with human studies, genetic ablation of the CgA gene (Chga) results in high blood pressure in mice (34), and the high blood pressure in Chga−/− mice can be rescued either by pretreatment with Cts or introduction of the human CgA gene (CHGA+/+) in the mice Chga−/− background by bacterial artificial chromosome (BAC) transgenic technology. Furthermore, low Cts levels predict augmented adrenergic responses to stressors (44), suggesting that a reduction in Cts may increase the risk of hypertension.
It has recently been reported that human recombinant CgA (10–100 nmol/l) stimulates the release of endothelin (ET; by ∼4-fold) from human vein endothelial cells, suggesting interaction between the sympathochromaffin system and the endothelium (29).
We have shown previously that the NH2-terminal CgA fragment VS-1 exerts profound myocardiosuppressive and antiadrenergic action in frog, eel, and rat hearts (54, 17, 25, 16). Unlike in eel (25) and rat (20), in frog, the VS-1-induced negative inotropism appears independent from the endocardial endothelium (EE) and the nitric oxide (NO)-cGMP pathway, indicating species- and tissue-specific targets and signaling mechanisms (17). Of note, in the eel and frog hearts, the VS-1-induced negative inotropism is abolished by Ca2+ and K+ channel antagonists and inhibitors of cytoskeletal reorganization (42). Similarly, involvements of either Ca2+ and K+ channels or cytoskeleton rearrangement appear also involved in VS-induced changes detected in vascular preparations (1, 8) or pulmonary and coronary arterial endothelial cells (5), respectively. These findings imply that the highly conserved NH2-terminal VS-1 domain of CgA modulates cardiovascular functions at least in three classes of vertebrates. Interestingly, another remarkable vasoreactive (26) and antihypertensive (34) peptide, Cts, is present in the COOH-terminal domain of CgA, but nothing is known regarding the direct cardiac actions of Cts. We hypothesized that this fragment could also act as a cardioactive agent modulating heart performance both under basal and stimulated conditions. If our hypothesis proves to be correct, then CgA would further epitomize that the processing of more than one peptide from a single prohormone rather than from two precursors may be homeostatically advantageous provided both peptides regulate the same physiological response.
Therefore, in the present study, we investigated Cts' direct cardiotropic action and the possible EE-NO-cGMP pathway involvement, as well as Cts' counteracting actions against both β-adrenergic and ET-1-elicited stimulations. We used the isolated working frog heart, which allowed a hemodynamic evaluation independent from coronary reactivity and extrinsic neuronal and endocrine influences. In fact, in the hearts of homeotherms (e.g., mammals), the coronary vascular endothelium and EE act in concert to modulate humorally myocardial activity, making EE contribution in the paracrine regulation of myocardial function difficult to define (10). In contrast, in the avascular frog heart, EE is the only barrier between the superfusing blood and the subjacent myocardial microenvironment and is therefore a unique model to analyze its autocrine/paracrine role in the transduction of blood-borne endoluminal chemical stimuli that can target the myocardium (21).
On the whole, the data suggest a new role of Cts in cardiac physiology, particularly under conditions of heightened neuroendocrine activation and consequent myocardial damage.
MATERIALS AND METHODS
Isolated and Perfused Working Heart Preparation
Frog hearts were isolated from both male and female specimens of Rana esculenta (weighing 16 ± 0.8 g) and connected to a perfusion apparatus as previously described (51). Animal maintenance and experimental procedures approved by the Italian University Minister were in accordance with the Guide for the Use and Care of Laboratory Animals (European Communities Council Directive 1986). Efforts were made to minimize animal suffering and reduce the number of species used. Experiments were performed at room temperature (18–20°C). A Grass S44 stimulator was used to electrically stimulate and pace heart preparations with single pulses of 20 V for 0.1 s. The stimulation rate was identical to that of the control, unpaced rate. The hearts were perfused with saline that was equilibrated with air and was composed (in mM) of 115 NaCl, 2.5 KCl, 1.0 CaCl2, 2.15 Na2HPO4, 0.85 NaH2PO4, and 5.6 glucose. The buffer pH was adjusted to 7.30–7.35 by the addition of Na2HPO4.
Measurements and Calculations
Pressures were measured through two MP-20D pressure transducers (Micron Instruments, Simi Valley, CA) that were connected to a PowerLab data acquisition system and analyzed using Chart software (ADInstruments, Ugo Basile, Comerio, Italy). Pressures were expressed in millimeters mercury and corrected for cannula resistance. The afterload (mean aortic pressure) was calculated as 2/3 diastolic pressure +1/3 maximum pressure. Cardiac output (CO) was collected over 1 min and weighed. The CO was corrected for fluid density and expressed as volume measurements (ml/min) that were normalized to the wet body weight in kilograms. Stroke volume [SV, CO/heart rate (HR)], at constant pre- and afterload in paced hearts, was used as a measure of ventricular performance (i.e., as an index of inotropism). Ventricular stroke work (SW), an index of systolic functionality, was calculated (in mJ/g) as (afterload-preload, mmHg) × SV (ml)/ventricle weight (g).
Drugs and Chemicals
Bovine Cts (bCgA344-364) peptide was synthesized by the solid-phase method, using 9H-(f)louren-9-yl(m)eth(o)xy(c)arbonyl protection chemistry, as previously described (37). Peptides were purified to >95% homogeneity by preparative reverse-phase HPLC (RP-HPLC) on C-18 silica columns. Authenticity and purity of peptides were further verified by analytical chromatography (RP-HPLC), and electrospray-ionization or matrix-assited laser desorption/ionization mass spectrometry. Isoproterenol (ISO), ET-1 (human porcine), the ETAR inhibitor BQ-123 sodium salt [cyclo(d-Asp–Pro-d-Val-Leu-d-Trp)], the ETBR inhibitor BQ-788 sodium salt (N-cis-2,6-dimethylpiperidinocarbonyl-l-gamma-methylleucyl-d-I-methoxycorbonyltryptophanyl- d-norleucine, Na), the nonspecific nitric oxide synthase (NOS) inhibitor NG-monomethyl-l-arginine (l-NMMA), the guanylate cyclase inhibitor ODQ [1H-(1,2,4) oxadiazolo-(4,3-a)quinoxalin-1-one], and Triton X-100 (t-octylphenoxypolyethoxyethanol) were purchased from Calbiochem. ISO, BQ-123, l-NMMA and Triton X-100 were prepared as stock solutions in double-distilled water; ET-1 was prepared in acetic acid; BQ-788 was prepared in methanol; ODQ was prepared in dimethyl sulfoxide and, being light sensible, used in a darkened perfusion apparatus to prevent degradation. Dilutions were made in Ringer solution immediately before use.
Experimental Protocols
Basal conditions.
In all experiments, the minimal output pressure (diastolic afterload) was set at 29.5 mmHg, and the input pressure (preload) was regulated to obtain a CO of ∼110 ml·min−1·kg−1 (wet body wt) and are within the physiological range (51). The heart generated its own rhythm. CO, HR, and pressures were measured simultaneously during the experiments. Hearts that did not stabilize within 10–15 min from the onset of perfusion were discarded. These parameters are stable for >1 h (21). All of the experiments were carried out within this period. To analyze the inotropic effects without confounding chronotropic action of substances, the preparations were electrically paced.
Drug application.
After the 15-min control period, the hearts were perfused for 10–15 min with Ringer solution enriched with increasing concentrations of either Cts (11–110 nM), ISO (0.1–1,000 nM), or ET-1 (0.01–10 nM) to generate concentration-response curves. Repetitive exposure of each heart to a single drug concentration revealed the absence of desensitization and the recovery from drug application after washing with Ringer (data not shown). Thus concentration-response curves were generated by perfusing cardiac preparations with increasing concentrations of the drug. The effects of ET-1 (0.01–10 nM) were also analyzed, exposing each heart preparation to a single concentration of the drug.
Cardiac signaling.
Hearts were perfused with and without 110 nM Cts as before. At the end of the experiment, hearts were snap-frozen under liquid nitrogen and homogenized with 1 ml of ice-cold 0.2 M sucrose, Tris maleate (pH 7.0) buffer supplemented with 2 mM EDTA, pH 8.0, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 0.1 mM 3-isobutyl-1-methylxanthine. Sarcoplasmic reticulum (SR) membrane fractions were isolated from cytosolic proteins using methods described previously (50, 7). Protein content was determined by Bradford assay (Bio-Rad), and 100 μg of cytosolic protein were subjected to SDS-PAGE immunoblot analysis for phosphorylated (P)-extracellular signal-regulated kinase (ERK; Santa Cruz) and total ERK (Santa Cruz). For assessment of total phospholamban and phosphorylated PLN (P-PLN-Ser16) levels, 20 μg of SR membranes were subjected to electrophoresis, and the immunoblots were probed with anti-mouse PLN antibody (Affinity Bioreagents) and anti-P-PLN-Ser16.
Cts' effects on ISO stimulation.
To analyze the effects of Cts on the ISO response, Cts-stimulated hearts were perfused with Ringer containing a single concentration of Cts (33, 65, or 110 nM) plus ISO (10 nM) in the presence or absence of BQ-788 (1 μM), an antagonist of ETBR. To further investigate the antagonistic action of Cts against the positive inotropic action of ISO, the concentration-response curve of ISO (from 0.1 to 1,000 nM) was performed in the presence of single concentrations of Cts (33, 65, or 110 nM). The time exposure to each drug concentration was 10–15 min.
ET-1- or Cts-stimulated preparations.
To analyze the involvement of the EE and NOS-cGMP pathway in mediation of the effects of Cts and ET-1, hearts were perfused with ET-1 or Cts after pretreatment with Triton X-100 (0.05%), l-NMMA (NOS inhibitor, 10 μM), and ODQ (guanylate cyclase inhibitor, 10 μM).
For functional impairment of the EE, 0.1 ml of Triton X-100 (0.05%) was injected in the heart through a needle inserted in the output cannula in the ventricle, avoiding damage of the atrium (for protocol details see Ref. 21). Subsequently, variables of cardiac performance were measured after 20 min of perfusion with the saline.
To investigate the role of the ETAR and ETBR subtypes in mediation of the effects of ET-1 or Cts, cardiac preparations were pretreated with either 1 μM of BQ-123, an antagonist of the ETAR, or 1 μM of BQ-788, an antagonist of ETBR. Subsequently, the hearts were perfused with Ringer solution containing increasing concentrations of ET-1 (from 0.01 to 10 nM) or Cts (from 11 to 110 nM), with and without supplementation of BQ-123 or BQ-788. The time exposure to each drug concentration was 10–15 min.
The effects of Cts on ET-1-mediated cardiac effects.
To analyze the effects of Cts on the ET-1 response, Cts-stimulated hearts (110 nM) were perfused with increasing concentrations of ET-1 (from 0.01 to 1 nM) both in presence or absence of BQ-788. The time exposure to each drug concentration was 10–15 min.
Statistics.
All data are expressed as means ± SE. Curve fitting was accomplished in the program Kaleidagraph (Synergy Software, Reading, PA). The EC50 values of a peptide were interpolated as the concentration that achieved 50% stimulation. For analysis of phosphorylated proteins, levels were quantified using Bio-Rad QuantifyOne, and the volumes of phosphorylated proteins were divided by the levels of nonphosphorylated proteins to calculate the degree of activation. Stimulation of protein activity was expressed as fold increase over the vehicle-treated control. Multiple comparisons between groups were made using either one-way ANOVA followed by Bonferroni's post hoc test or two-way ANOVA. Statistical significance was concluded at P < 0.05. Statistics were computed with the program InStat (GraphPad Software, San Diego, CA).
RESULTS
Basal Conditions
As previously described (21, 51), the in vitro isolated, perfused whole frog heart preparation generates cardiac hemodynamic responses that mimic those obtained in vivo. After 20 min of equilibration, the following basal recordings were obtained: SV = 1.9 ± 0.1 ml/kg, SW = 3.5 ± 0.2 mJ/g, HR = 59.6 ± 1.8 beat/min, preload = 1.6 ± 0.1 mmHg, afterload = 27.0 ± 0.3 mmHg, and CO = 112.5 ± 2.3 ml·min−1·kg−1.
Influence of Cts on Myocardial Performance
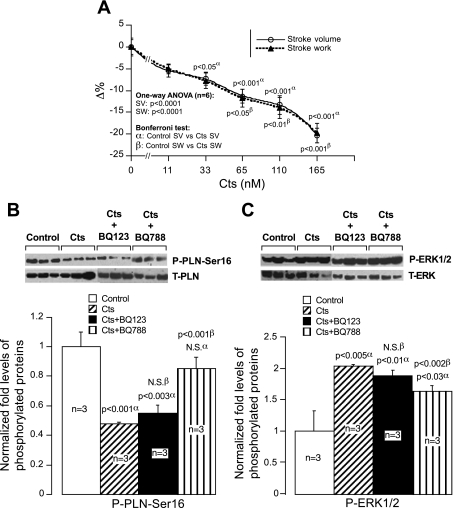
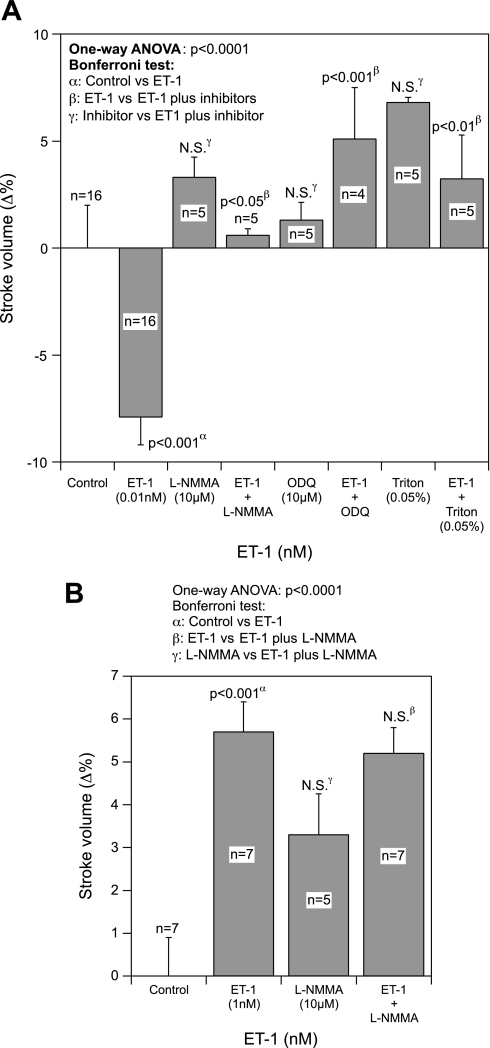
Cts concentrations ranging from 11 to 165 nM caused a concentration-dependent decrease in both SV and SW that reached a maximum reduction of ∼20% at 165 nM of Cts (Fig. 1A). A significant 7.3% decrease of SV was induced by 33 nM of Cts (Fig. 1A).
Fig. 1.
A: concentration-dependent response. Effects of Cts (11–165 nM) on stroke volume (SV) and stroke work (SW) in paced frog heart preparations. B and C: immunoblot analysis. Total and phosphorylated phospholamban (PLN; B) and extracellular signal-regulated kinase (ERK) 1/2 (C) in control and catestatin (Cts)-treated hearts both in presence and absence of BQ-123 and BQ-788. α, Comparison of control vs. treated groups; β, comparison of Cts alone vs. Cts plus inhibitors.
Signaling Mechanisms by Which Cts Modulates Cardiac Function
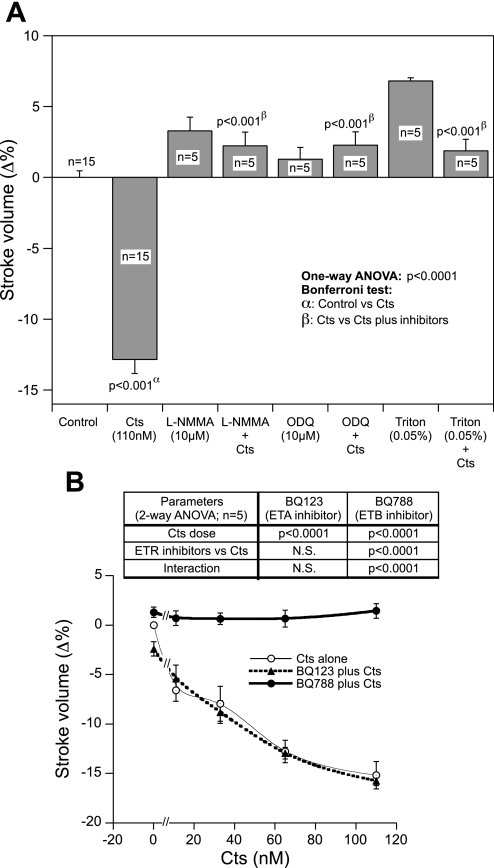
It is known that both PLN and ERK1/2 are involved in the coordination of cardiac contraction and relaxation and that they are expressed in frog heart (56, 19, 3, 4). Therefore, we checked whether Cts modulates PLN and ERK1/2. Cts inhibited phosphorylation of PLN at the protein kinase A specific site, Ser16 (by ∼50%; Fig. 1B), and augmented phosphorylation of ERK1/2 (by ∼53%; Fig. 1C). These effects were modified by BQ-788 (ETBR antagonist) but not by BQ-123 (ETAR antagonist), suggesting an involvement of ETBR in Cts signaling to cardiac function (Fig. 1, B and C). The involvement of the NO-cGMP pathway in the Cts-mediated depression of SV has been demonstrated. In fact, we found that the negative inotropic effects of Cts (110 nM) were abolished by pretreatment with either NOS (l-NMMA; 10 μM) or guanylate cyclase (ODQ; 10 μM) inhibitors (Fig. 2A). In addition, the EE integrity is necessary for the cardioinhibitory action of Cts because functional damage of the EE with Triton X-100 (0.05%) abrogated this effect (Fig. 2A). We have previously shown that l-NMMA, ODQ, and Triton X-100 alone cause significant increments in SV and SW (21, 51).
Fig. 2.
A: nitric oxide (NO)-cGMP pathway. The effects of 110 nM Cts on SV before and after treatment with either the nitric oxide synthase (NOS) inhibitor [NG-monomethyl-l-arginine (l-NMMA), 10 μM], guanylate cyclase inhibitor (ODQ, 10 μM), or Triton X-100 (0.05%). B: involvement of endothelin A (ETAR) and B (ETBR) receptor. Concentration-dependent effects of Cts (11–110 nM) after treatment with the ETAR (BQ-123, 1 μM) or ETBR antagonists (BQ-788; 1 μM) on SV in paced frog heart preparations.
The role of the ETR subtypes in the Cts response (11, 33, 65, and 110 nM) was also analyzed. Although the pretreatment with ETAR inhibitor (BQ-123; 1 μM) failed to modify the negative inotropism of Cts at all concentration tested, BQ-788 (1 μM), the ETBR antagonist, completely blocked this response (Fig. 2B). At the concentration tested, ET receptor antagonists alone did not induce significant changes on SV (1.08 ± 0.66 for BQ-788 and −2.37 ± 1.15 for BQ-123).
Cts Modulation of ISO-Induced Cardiac Changes
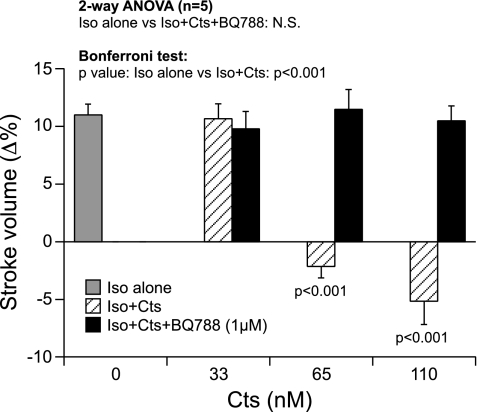
Cardiac frog preparations exposed to ISO (10 nM) confirmed the classical positive inotropic response (21, 49) as reflected by significant increments in SV (by 10.3%; Fig. 3). Although the low dose of Cts (33 nM) failed to inhibit the ISO-induced increase in SV, Cts at higher concentrations of 65 and 110 nM completely abolished the ISO-mediated increase of SV (Fig. 3). Cts failed to inhibit the ISO-induced response in the presence of ETBR antagonist (Fig. 3).
Fig. 3.
Effects of isoproterenol (ISO, 10 nM) on SV before and after treatment with Cts (33, 65, and 110 nM) or Cts plus BQ-788 (1 μM) in paced frog heart preparations.
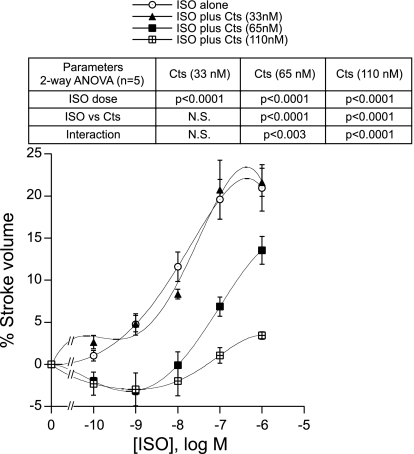
The antagonistic effect of Cts (33, 65, and 110 nM) toward the ISO (from 0.1 to 1,000 nM)-mediated increase in cardiac function was investigated. ISO alone caused a concentration-dependent increment in SV (Fig. 4). The analysis of the percentage of variations of SV permitted deduction of EC50 values with ISO alone or with ISO plus a single concentration of Cts (33, 65, or 110 nM). The EC50 values (in log M) were as follows: ISO alone 7.71 ± 0.15 (r2 = 0.98), ISO plus 33, 65, and 110 nM Cts −7.98 ± 0.04 (r2 = 0.99), −6.97 ± 0.04 (r2 = 0.99) and −7.3 ± 0.07 (r2 = 0.99), respectively. Two-way ANOVA revealed significant interactions between ISO and Cts peptides [ISO vs. ISO + Cts (65 nM): P < 0.02; ISO vs. ISO + Cts (110 nM): P < 0.016]. Because increasing concentrations of ISO failed to overcome the antagonistic effects of Cts, these effects are considered as a noncompetitive antagonism (Fig. 4).
Fig. 4.
Concentration-response curves of ISO alone (from 10−10 to 10−6 M) and ISO + a single concentration of Cts (33, 65, or 110 nM) on SV in paced frog heart preparations. The EC50 values were as follows (in log M): ISO alone, −7.71 ± 0.15 (r2 = 0.98); ISO + Cts (33, 65, or 110 nM), −7.98 ± 0.04 (r2 = 0.99), −6.97 ± 0.04 (r2 = 0.99), and −7.3 ± 0.07 (r2 = 0.99), respectively.
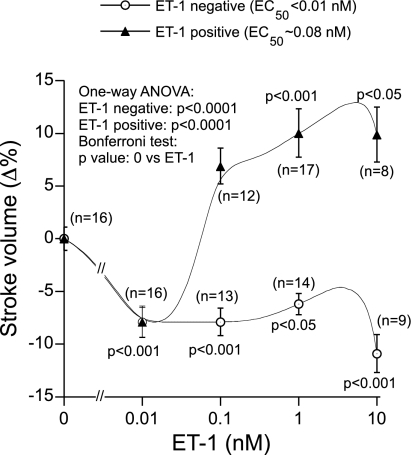
Concentration-Dependent Effects of ET-1 in the Frog Heart
Exposure of the isolated heart to ET-1 alone caused a dose-dependent biphasic effect on cardiac function. ET-1 exerted both negative and positive inotropic effects as documented by changes in SV (Fig. 5). Although the low dose of ET-1 (0.01 nM) always caused a negative inotropic effect (∼8%), the higher concentrations of ET-1 (0.1–10 nM) triggered either the negative (50% preparations; by −7.9 to −11%) or positive (50% preparations; by ∼7 to ∼10%) inotropic effects. Pretreatment with l-NMMA (10 μM) or ODQ (10 μM) completely abolished the ET-1 (0.01 nM)-induced negative inotropic effects (Fig. 6A). Negative inotropic effects of a low dose of ET-1 (0.01 nM) were also abolished by the damage of the EE with Triton X-100 (0.05%) (Fig. 6A). In contrast, the positive inotropic effects caused by higher doses of ET-1 (1 nM) remained unaffected by pretreatment with l-NMMA (Fig. 6B). Hence, we did not pursue the effects of ODQ and Triton X-100 on the positive inotropic effects of ET-1.
Fig. 5.
Negative and positive effects of ET-1 (0.01–10 nM) on SV in paced frog heart preparations.
Fig. 6.
A: effects of ET-1 (0.01 nM) on SV before and after treatment with either the NOS inhibitor (l-NMMA, 10 μM), guanylate cyclase inhibitor (ODQ, 10 μM), or Triton X-100 (0.05%). B: effects of ET-1 (1 nM) on SV before and after treatment with l-NMMA (10 μM).
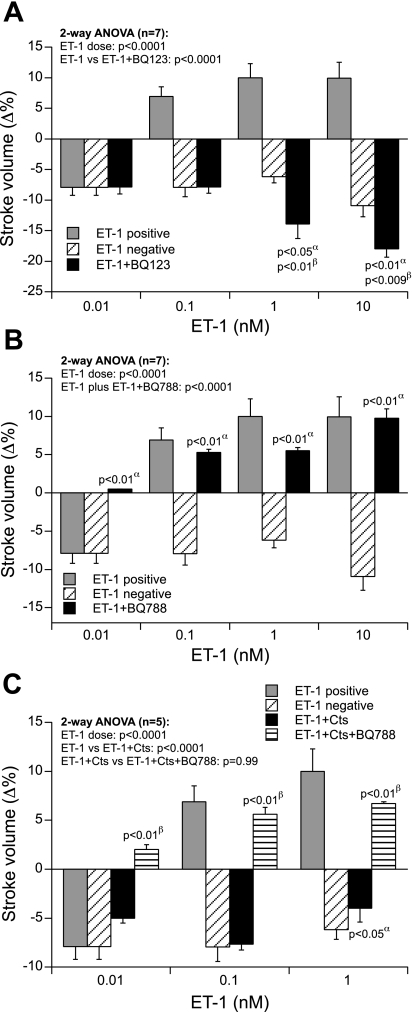
Chemical blockade of the ETAR by BQ-123 caused complete inhibition of the positive inotropic effects of ET-1 (0.1–10 nM) (Fig. 7A). After chemical inhibition of ETAR, ET-1 exerted only the negative inotropic effects through the unopposed ETBR (Fig. 7A). In contrast, the chemical inhibition of ETBR by BQ-788 inhibited the negative inotropic effects of ET-1 at all doses tested. In the absence of ETBR, ET-1 induced only the positive inotropic effects (0.1–10 nM) through the unopposed ETAR (Fig. 7B).
Fig. 7.
A: concentration-dependent effects of ET-1 (0.01–10 nM) on SV in paced frog heart preparations after treatment with ETAR antagonist (BQ-123, 1 μM). α, Comparison of ET-1 negative vs. ET-1 + BQ-123; β, comparison of ET-1 positive vs. ET-1 + BQ-123. B: ETBR antagonist (BQ-788, 1 μM). α, Comparison of ET-1 negative vs. ET-1 + BQ-788. C: Cts (110 nM) + BQ-788. α, Comparison of ET-1 negative vs. ET-1 + Cts; β, comparison of ET-1 + Cts vs. ET-1 + Cts + BQ-788.
Cts-ET-1 Interaction
Cts (110 nM) completely abolished the positive inotropic effects of ET-1 on SV (0.1–1 nM) but did not modulate ET-1's negative inotropic effects on SV, indicating that ET-1 and Cts act through a convergent signaling pathway to exert the latter effect (Fig. 7C). Interestingly, Cts failed to abolish ET-1's positive inotropic effects in the presence of the ETBR antagonist BQ-788 (Fig. 7C). These findings point out that Cts activates the ETBR to antagonize the positive inotropic effects of ET-1 on SV (Fig. 7C).
DISCUSSION
Cts and Basal Mechanical Performance
It is well established that Cts acts as an endogenous noncompetitive antagonist of nicotine-evoked catecholamine secretion from rat PC-12 or bovine chromaffin cells (36–39). Cts was also shown to act as a potent in vivo vasodilator by stimulating histamine release through H1 receptor activation (26). Furthermore, clinical and human studies indicated that Cts may function as an antihypertensive agent (44), whereas, on the contrary, nothing is known about its direct effects on myocardial physiology.
We used the avascular frog heart, which is exclusively supplied from the luminal (lacunae) blood, to investigate Cts' direct myocardiotropic action in the absence of coronary reactivity. At the same time, this natural heart model is ideally suited for analyzing the distinct paracrine role of the EE in the signal transduction of blood-borne chemical stimuli (exogenous Cts) targeting the heart. The isolated and perfused heart, working at physiological loads, showed a cardiac performance stable for >1 h, as indicated by typical time course experiments (21). With this preparation, we demonstrated that Cts dose-dependently modulates the basal mechanical performance of the isolated working frog heart, acting as a direct cardiodepressing peptide. Because preliminary experiments revealed the absence of desensitization, concentration-response curves were generated.
We recently demonstrated that peptides derived from the NH2 terminus of CgA (VS-1-related fragments) exert notable cardiosuppressive action on the isolated rat, frog, and eel hearts (17, 25, 16). It is interesting to note that amphipathic VS-1 and amphiphilic Cts peptides, representing the NH2- and COOH-terminal domains of CgA, respectively, exhibit analogous negative contractile responses at similar concentrations, reaching a maximum (by ∼20%) reduction of SV and SW. Despite the analogous cardiosuppressive effects of both VS-1 and Cts, only Cts was able to rescue Chga null mice from high blood pressure (34). Thus these results further document that CgA, as being the precursor of cardioactive peptides, modulates the mechanical performance of the heart under nonstimulated conditions. In the present study, Cts causes marked reduction in SV with an EC50 ∼50 nM. In normotensive individuals, circulating Cts (∼1.5–2 nmol/l) is inversely proportional to intact CgA (∼5–10 nmol/l) (44). Because CgA and CgA-derived fragments are expressed in rat heart tissue extracts (23) and in human ventricular myocardium (46), the local concentration of Cts in the myocardium may reach 50 nM and regulate cardiac function in an autocrine/paracrine manner.
Signaling Mechanisms of Cts Modulation
Intracellular Ca2+ handling is the central coordinator of cardiac contraction and relaxation. PLN is a reversible inhibitor of sarco(endo)plasmic reticulum (SERCA) 2's Ca2+ affinity and cardiac contractility. Studies in genetically altered mouse models have demonstrated that the levels of phosphorylated PLN are critical in modulating basal Ca2+ handling and contractility. The net result is increased SR Ca2+ release via ryanodine receptor 2 and enhanced SR Ca2+ uptake by the SR Ca2+ pump (SERCA2a), resulting in larger intracellular Ca2+ transients (22). Furthermore, phosphorylation of ERKs is implicated in mediating the cardiac contractile response (12). In the isolated perfused frog heart, ERKs are reported to be activated in response to pressure overload (3) or in response to α1- and β-adrenoceptor stimulation (4). Therefore, the interaction between Cts and PLN and/or ERK1/2 may be of potential interest in relation to further work on Cts-induced changes of cardiac contractility.
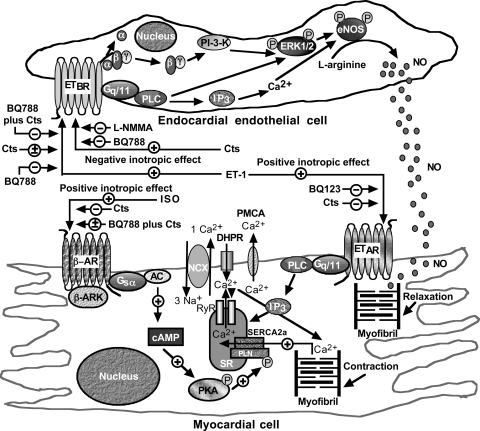
Here we demonstrate that Cts directly inhibits PLN-Ser16 and augments P-ERK1/2 levels. It may be postulated that the Cts-mediated increment in P-ERK1/2 levels led to the stimulation of endothelial NOS (eNOS) with consequent production of NO (Fig. 8). In fact, a recent work (45) suggests that ERK1/2 directly activates the NO signal transduction pathway. Another study (58) suggested an interaction between ETBR and ERK at the level of the caveolae. At present, the receptor that mediates the effects of Cts on the heart is unknown. However, we postulate that Cts mediates its effects through a G protein-coupled ETBR (see Fig. 8 for putative mechanisms of action). This hypothesis is supported by our results in which the inhibitory and stimulatory effects of Cts on PLN and ERK1/2, respectively, appear mediated by ETBR, but not by ETAR, receptors.
Fig. 8.
Schematic diagram showing the putative ET-1, ISO, or Cts signaling in endothelial and myocardial cells. AC, adenylate cyclase; β-AR, β-adrenergic receptor; β-ARK, β-adrenergic receptor kinase; DHPR, dihydropyridine receptor; eNOS, endothelial NOS; IP3, inositol trisphosphate; NCX, Na+/Ca2+ exchangers; PKA, protein kinase A; PI 3-K, phosphoinositide 3-kinase; PLC, phospholipase C; PMCA, plasma membrane Ca2+-ATPases; RyR, ryanodine receptor; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPases; SR, sarcoplasmic reticulum; +, stimulation; −, inhibition; ±, no effect.
In the frog heart, we have previously demonstrated the presence of a constitutive NOS (eNOS) located within EE, the only barrier between the cardiac lumen and the subjacent myocardium (51). The EE is able to modulate ventricular performance under both basal (i.e., nonstimulated) (51) and chemically (i.e., cholinergic)-stimulated conditions (21) through a NO-cGMP signaling pathway. In the present study, we found that Cts-mediated negative inotropism is abolished by pretreatment with NOS or guanylate cyclase inhibitors, consistent with Cts-induced stimulation of the NO-cGMP pathway. In addition, the results obtained with Triton X-100, which made EE dysfunctional (10, 21), implicated an EE-mediated mechanism in Cts signaling transduction. Notably, this contrasts with the VS-1-induced negative inotropism reported in the frog heart, which, unlike the pattern demonstrated in eel and rat hearts (25, 13), involved neither the EE nor the G protein nor the NO-cGMP-protein kinase G mechanism (17). Therefore, the signal transduction mechanisms underlying the cardiosuppressive action of the two CgA-derived peptides are not identical. As discussed later, the question remains open as to how and why an amphipathic peptide VS-1 and an amphiphilic peptide Cts, representing NH2- and COOH-terminal domains of CgA, respectively, utilize divergent signaling pathways to converge on their effects on cardiosuppression. The analogous effects of VS-1 and Cts on cardiosuppression raise the intriguing question regarding the biological significance of such apparently redundant molecular strategy. Further studies will clarify whether, behind this similarity, the two peptides may subserve subtly different functions, e.g., summation and synergism, or distinct spatiotemporal compartmentation (cell- and tissue-specific proteolytic processing and release).
Cts and ISO Signaling
Loewi (32) initially described adrenergic regulation of cardiac contractility in the denervated frog heart using Accellerans-Stoff or Sympathin, which was later identified as epinephrine (31). It has long been known that epinephrine is the predominant neurotransmitter in the frog heart, and in frog plasma under resting conditions it is five times more concentrated than norepinephrine; the latter, however, may increase considerably under stress, following release from the adrenal medulla (6). In excessive amounts, these two endogenous catecholamines are well known to be cardiotoxic for both amphibian and mammalian (including human) hearts, since they induce dose-dependent myocardial lesions. For example, exogenous ISO has been frequently used to produce myocardial necrosis both in frog (14) and mammalian hearts (47).
In a previous study, it has been established that ISO induces positive inotropic responses in frog heart via β-adrenergic receptors (49). In this work, the complete inhibition of ISO (10 nM)-induced positive inotropic effects by Cts indicates that Cts is acting as an antiadrenergic peptide, and Cts signaling pathways dominate over the adrenergic one. The costimulation experiments with both Cts and ISO in the presence of BQ-788 reinforce the hypothesis that Cts mediates its dominant antiadrenergic action through the activation of the ETBR. Because the concentration-dependent positive inotropism of ISO failed to overcome the inhibitory effects of Cts on positive response, we concluded that the Cts inhibition of ISO is noncompetitive. Interestingly, the Cts-mediated inhibition of nicotine-evoked catecholamine secretion is also noncompetitive (39, 36, 33). Similar to Cts, analogous antiadrenergic actions of several CgA NH2-terminal fragments have been demonstrated in frog, eel, and rat hearts (17, 25, 16). Tota and coworkers (54) also reported that the homologous frog CgA peptides (CgA4-16 and CgA47-66) are able to counteract the positive inotropism exerted by ISO in the frog heart. Thus, in addition to the pharmacological implications of our data, it is possible to hypothesize that the autocrine-paracrine role of Cts, as well as of the other CgA-derived peptides, may play a physiological role acting as a natural protective mechanism against excessive adrenergic stimulation.
ET-1-Cts Interaction
ETs are essential physiological regulators of normal cardiac and vascular functions, and an excessive generation or activity of ETs has been linked to several cardiovascular pathologies, including hypertension (9). The recent finding that human CHGA gene influences endothelial ET-1 secretion in vivo suggests an interaction between the sympatochromaffin system and the endothelium (29).
In the present study, we have demonstrated that ET-1 induces negative inotropism at lower concentrations (0.01 nM). In contrast, higher concentrations of ET-1 (0.1–10 nM) induced both positive (50% of hearts) and negative inotropism (50% of hearts). In mammals, either positive or negative inotropic responses of ET-1 have been reported in cardiac preparations of different species and experimental models (cell, tissue, organ). For example, in whole cardiac preparations, the effects of ET are often complicated by concomitant influences of coronary perfusion (9). By using the avascular frog heart, we analyzed the direct myocardial effects of ET-1 independently from the coronary-induced changes occurring in coronary-supplied hearts. The findings that l-NMMA, ODQ, and Triton X-100 completely abolished the negative inotropic effects of ET-1 indicate that ET-1 utilizes the functionally intact EE and the NO-cGMP pathway to exert its negative inotropic effects.
The ET-1 actions occur via G protein-coupled ETAR and ETBR subtypes. Although vascular smooth muscle cells, cardiomyocytes, fibroblasts, hepatocytes, and neurons express ETAR, ETBR are mainly expressed in endothelial and vascular smooth muscle cells. ET-1 binding to ETAR activates phospholipase C (PLC), leading to the generation of inositol trisphosphate, which in turn stimulates intracellular calcium release (43) (Fig. 8). Stimulation of ETAR also activates the mitogen-activated protein kinase cascade (41). ETBR are coupled to a Gq/11 protein. Activation of ETBR recruits pathway-dependent PLC, Na+/H+ exchanger, adenylate and guanylate cyclases (18), in addition to intracellular Ca2+ and Ca2+ entry (48) (Fig. 8). It has recently been shown that ET-1 binding to the ETBR causes eNOS phosphorylation and NO synthesis (30). In our preparations, the chemical blockade of ETAR by BQ-123 completely inhibited ET-1-induced positive inotropic effects, suggesting that ET-1 utilizes the ETAR to exert its positive inotropic effects. However, BQ-123 failed to inhibit the negative inotropic effect of ET-1 at a concentration of 0.01 nM, indicating that ET-1, at this concentration, acts exclusively through the ETBR. Interestingly, since after chemical blockade of ETAR, ET-1 exerted only negative inotropism, we suggest that ET-1 action via ETBR could now act unopposed without the influence of the ETAR. Furthermore, inhibition of the ETBR by BQ-788 completely inhibited the negative (0.01–10 nM) inotropic effects of ET-1 that appear mediated by NO and an intact EE. Of note, our results agree with a recent study (28) that demonstrated, in rabbit papillary muscles, that higher concentrations (10−9 M) of ET-1 produced a sustained positive inotropic effect, whereas the negative inotropic effect was observed only when ET-1 was given in the presence of an ETAR antagonist. In addition, the same study demonstrated that negative inotropism induced by ETBR stimulation is EE and NO dependent.
In the frog heart, Cts blocked ET-1's positive, but not the negative, inotropic effects. Because BQ-788, but not BQ-123, completely abolished Cts' negative inotropic effects, we can conclude that Cts acts through ETBR (Fig. 8). It is interesting to note that, while Cts acts through ETBR to exert its negative inotropic effect, it completely abolished ET-1's positive inotropic effect mediated via ETAR. This indicates that activation of ETBR by Cts dominates over the activation of ETAR by ET-1. Cts action through ETBR was reinforced when Cts failed to inhibit ET-1-induced positive inotropism in the presence of BQ-788. The demonstration that Cts is able to counteract the stimulatory influence of ET-1 may represent another potentially beneficial attribute of this CgA-derived peptide.
Significance of Two Cardioactive Peptides in one Precursor
The analogous cardiosuppressive effects of VS-1 and Cts raise the question regarding the biological significance of two peptides processed from one precursor. Further studies will clarify whether, behind such apparently redundant molecular strategy, there is an advantage of having two peptides regulating the same physiological response. Because of coordinate actions, they may act on overlapping or different sites, subserving subtly different functions, e.g., summation and synergism, or distinct spatiotemporal compartmentation (cell- and tissue-specific proteolytic processing and release). With specific reference to cardiovascular prohormones, an example is provided by the two peptide hormones processed from the pro-atrial natriuretic peptide (ANP) precursor, i.e., vessel dilator and ANP (55), both showing vasodilatory, diuretic, and natriuretic properties.
Conclusions
The avascular frog heart, used as bioassay to investigate the role of EE-dependent transduction of the ET-1 and Cts endoluminal stimuli, has contributed to reveal the importance of this tissue in mediating the interaction between the sympatochromaffin system and the myocardium. Should this mechanism be experimentally extended to the mammalian heart, it could contribute to account for the antihypertensive effects of Cts. In conclusion, the present study suggests a novel paracrine/autocrine role of Cts in the frog heart, since Cts depresses cardiac function and inhibits both β-adrenergic and ET-1-elicited stimuli. These Cts' cardiotropic features could be homeostatically relevant, particularly under stress conditions, when the heart becomes a preferential target of both adrenergic and endothelin stimuli, thus emphasizing the concept of “zero steady-state error” homeostasis proposed for other CgA-derived peptides (27).
GRANTS
This work was supported by the Ministero dell'lstruzione e dell'Università of Italy (60%: B. Tota, A. Gattuso, R. Mazza) and the “Dottorato di Biologia Animale” University of Calabria-Italy (S. F. Barbieri). S. K. Mahata was supported by grants from the Dept. of Veterans Affairs and the National Institutes of Health (R01 DA-011311 and P01 HL-58120).
Acknowledgments
We are grateful to Laura Jean Carbonaro for revising the text.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aardal S, Helle KB. The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept 41: 9–18, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol 5: 405–412, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Aggeli IK, Gaitanaki C, Lazou A, Beis I. Activation of multiple MAPK pathways (ERKs, JNKs, p38-MAPK) by diverse stimuli in the amphibian heart. Mol Cell Biochem 221: 63–69, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Aggeli IK, Gaitanaki C, Lazou A, Beis I. Alpha(1)- and beta-adrenoceptor stimulation differentially activate p38-MAPK and atrial natriuretic peptide production in the perfused amphibian heart. J Exp Biol 205: 2387–2397, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Blois A, Srebro B, Mandalà M, Corti A, Helle KB, Serck-Hanssen G. The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regul Pept 135: 78–84, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois P, Dupont W, Vaillant R. Estimation du taux des catecholamines circulantes chez la Grenouille a l'aide d'une technique de dosage radio-enzymatique. C R Acad Sci Paris 287: 1149–1152, 1978. [PubMed] [Google Scholar]
- 7.Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjøs OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology 145: 24–35, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Brekke JF, Osol GJ, Helle KB. N-terminal chromogranin-derived peptides as dilators of bovine coronary resistance arteries. Regul Pept 105: 93–100, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther 111: 508–531, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Brutsaert DL, Meulemans AL, Sipido KR, Sys SU. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circ Res 62: 358–366,1988. [DOI] [PubMed] [Google Scholar]
- 11.Brutsaert DL Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83: 59–115, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res 91: 776–781, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Cappello S, Angelone T, Tota B, Pagliaro P, Penna C, Rastaldo R, Corti A, Losano G, Cerra MC. Human recombinant chromogranin A-derived vasostatin-1 mimics preconditioning via an adenosine/nitric oxide signalling mechanism. Am J Physiol Heart Circ Physiol 293: H719–H727, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Carlsten A, Poupa O, Winell S. Cardiac lesions in the frog induced by adrenaline. Acta Pharmac Toxic 47: 359–364, 1980. [DOI] [PubMed] [Google Scholar]
- 15.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J 23: 967–974, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Cerra MC, De Iuri L, Angelone T, Corti A, Tota B. Recombinant N-terminal fragments of chromogranin-A modulate cardiac function of the Langendorff-perfused rat heart. Basic Res Cardiol 101: 43–52, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Corti A, Mannarino C, Mazza R, Angelone T, Longhi R, Tota B. Chromogranin A N-terminal fragments vasostatin-1 and the synthetic CGA 7–57 peptide act as cardiostatins on the isolated working frog heart. Gen Comp Endocrinol 136: 217–224, 2004. [DOI] [PubMed] [Google Scholar]
- 18.D'Orleans-Juste P, Labonte J, Bkaily G, Choufani S, Plante M, Honoré JC. Function of the endothelin(B) receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther 95: 221–238, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Shuba YM, Morad M. Regulation of cardiac sodium-calcium exchanger by beta-adrenergic agonists. Proc Natl Acad Sci USA 93: 5527–5532, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo MP, Levi RC, Ramella R, Brero A, Boero O, Tota B, Alloatti G. Endothelium-derived nitric oxide mediates the anti-adrenergic effect of human vasostatin-1 (CgA 1-76) in rat ventricular myocardium. Am J Physiol Heart Circ Physiol 292: H2906–H2912, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Gattuso A, Mazza R, Pellegrino D, Tota B. Endocardial endothelium mediates luminal ACh-NO signaling in isolated frog heart. Am J Physiol Heart Circ Physiol 276: H633–H641, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Ginsburg KS, Bers DM. Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol 556: 463–480, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glattard E, Angelone T, Strub JM, Corti A, Aunis D, Tota B, Metz-Boutigue MH, Goumon Y. Characterization of natural vasostatin-containing peptides in rat heart. Febs J 273: 3311–3321, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci 64: 2863–2886, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imbrogno S, Angelone T, Corti A, Adamo C, Helle KB, Tota B. Influence of vasostatins, the chromogranin A-derived peptides, on the working heart of the eel (Anguilla anguilla): negative inotropy and mechanism of action. Gen Comp Endocrinol 139: 20–28, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides 19: 1241–1248, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Koeslag JH, Saunders PT, Wessels JA. The chromogranins and the counter-regulatory hormones: do they make homeostatic sense? J Physiol 517: 643–649, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leite-Moreira AF, Bras-Silva C. Inotropic effects of ETB receptor stimulation and their modulation by endocardial endothelium, NO, and prostaglandins. Am J Physiol Heart Circ Physiol 287: H1194–H1199, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Lillie EO, Mahata M, Khandrika S, Rao F, Bundey RA, Wen G, Chen Y, Taupenot L, Smith DW, Mahata SK, Ziegler MG, Cockburn M, Schork NJ, O'Connor DT. Heredity of endothelin secretion: human twin studies reveal the influence of polymorphism at the chromogranin A locus, a novel determinant of endothelial function. Circulation 115: 2282–2291, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, Premont RT, Kontos CD, Zhu S, Rochey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med 11: 952–958, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Loewi O Quantitative und qualitative untersuchungen uber den sympaticusstoff. Pflugers Arch Gesamte Physiol 237: 504–14, 1936. [Google Scholar]
- 32.Loewi O Ubertragbarkeit der Herznervenwirkung. Pflugers Arch Gesamte Physiol 189: 239–242, 1921. [Google Scholar]
- 33.Mahapatra NR, Mahata M, Mahata SK, O'Connor DT. The chromogranin A fragment catestatin: specificity, potency and mechanism to inhibit exocytotic secretion of multiple catecholamine storage vesicle co-transmitters. J Hypertens 24: 895–904, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115: 1942–1952, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, O'Connor DT. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J Biol Chem 278: 32058–32067, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Mahata SK, Mahata M, Parmer RJ, O'Connor DT. Desensitization of catecholamine release: the novel catecholamine release-inhibitory peptide catestatin (chromogranin A344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem 274: 2920–2928, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Mahata SK, Mahata M, Wakade AR, O'Connor DT. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A344-364): Identification of amino acid residues crucial for activity. Mol Endocrinol 14: 1525–1535, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O'Connor DT. The catecholamine release-inhibitory “catestatin” fragment of chromogranin a: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol 66: 1180–1191, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Inves 100: 1623–1633, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahata SK Catestatin: the catecholamine release inhibitory peptide: a structural and functional overview. Curr Med Chem Immun Endoc Metab Agents 4: 221–234, 2004. [Google Scholar]
- 41.Marasciulo FL, Montagnani M, Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13: 1655–1665, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Mazza R, Mannarino C, Imbrogno S, Barbieri SF, Adamo C, Angelone T, Corti A, Tota B. Crucial role of cytoskeleton reorganization in the negative inotropic effect of chromogranin A-derived peptides in eel and frog hearts. Regul Pept 138: 145–151, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Muldoon LL, Rodland KD, Forsythe ML, Magun BE. Stimulation of phosphatidylinositol hydrolysis, diacylglycerol release, and gene expression in response to endothelin, a potent new agonist for fibroblasts and smooth muscle cells. J Biol Chem 264: 8529–8536, 1989. [PubMed] [Google Scholar]
- 44.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20: 1335–1345, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Philipp S, Critz SD, Cui L, Solodushko V, Cohen MV, Downey JM. Localizing extracellular signal-regulated kinase (ERK) in pharmacological preconditioning's trigger pathway. Basic Res Cardiol 101: 159–167, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 29: 1117–1127, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Rona G, Chappel CI, Balasz T, Gaudry R. Severe myocardial necrosis produced by isoproterenol in the rat. Arch Int Pharmacodyn Ther 122: 123–128, 1959. [PubMed] [Google Scholar]
- 48.Scotland R, Vallance P, Ahluwalia A. Endothelin alters the reactivity of vasa vasorum: mechanisms and implications for conduit vessel physiology and pathophysiology. Br J Pharmacol 128: 1229–1234, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skeberdis VA, Jurevicius J, Fischmeister R. Pharmacological characterization of the receptors involved in the beta-adrenoceptor-mediated stimulation of the L-type Ca2+ current in frog ventricular myocytes. Br J Pharmacol 121: 1277–1286, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slack JP, Grupp IL, Luo W, Kranias EG. Phospholamban ablation enhances relaxation in the murine soleus. Am J Physiol Cell Physiol 273: C1–C6, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Sys SU, Pellegrino D, Mazza R, Gattuso A, Andries LJ, Tota B. Endocardial endothelium in the avascular heart of the frog: morphology and role of nitric oxide. J Exp Biol 200: 3109–3118, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324: 476–478, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. New Engl J Med 348: 1134–1149, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Tota B, Mazza R, Angelone T, Nullans G, Metz-Boutigue MH, Aunis D, Helle KB. Peptides from the N-terminal domain of chromogranin A (vasostatins) exert negative inotropic effects in the isolated frog heart. Regul Pept 114: 91–99, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Vesely DL Which of the cardiac natriuretic peptides is most effective for the treatment of congestive heart failure, renal failure and cancer? Clin Exp Pharmacol Physiol 33: 169–76, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Will H, Kuttner I, Kemsies C, Vetter R, Schubert E. Comparative analysis of phospholamban phosphorylation in crude membranes of vertebrate hearts. Experientia 41: 1052–1054, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 49: 497–528, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi T, Murata Y, Fujiyoshi Y, Doi T. Regulated interaction of endothelin B receptor with caveolin-1. Eur J Biochem 270: 1816–1827, 2003. [DOI] [PubMed] [Google Scholar]