sickle cell pathophysiology comprises a complex interplay of episodic vasoocclusive events, ischemia-reperfusion injury, overproduction of reactive oxygen species (ROS), inflammation, endothelial activation, and hemolysis, all somehow driven by a single amino acid substitution in the β-globin chain of hemoglobin. Hemolysis and oxidative stress act synergistically to promote vascular dysfunction in sickle cell disease (SCD). As a result of chronic hemolysis, levels of free plasma hemoglobin are increased at baseline and nitric oxide (NO) bioavailability is diminished, producing endothelial dysfunction that has been linked to chronic vasculopathic complications of SCD such as pulmonary hypertension, cutaneous leg ulceration, priapism, and sudden death (14, 18, 19, 22, 23, 25).
NO regulates vasorelaxation and also possesses antioxidant, antiadhesive, and antithrombotic properties (33). NO is produced from the substrate l-arginine by endothelial nitric oxide synthase (eNOS) and mediates vasorelaxation through a paracrine action on vascular smooth muscle cells underlying the endothelium. Endothelial dysfunction, characterized by impaired vascular responsiveness resulting from decreased NO bioavailability, is associated with atherosclerosis, diabetes mellitus, hypertension, hypercholesterolemia, smoking, and obesity, illustrating the central importance of NO in the physiological regulation of vasomotor activity (3, 6).
Unlike coronary artery disease and its risk factors, which are associated with an impaired production of NO, SCD and other hemolytic diseases are characterized by a primary resistance to the action of NO (10, 13, 25, 29). The NO resistance state observed in SCD is multifaceted, with at least two major mechanisms contributing to impaired NO homeostasis: 1) scavenging of NO by cell-free plasma hemoglobin, and 2) oxidant stress due to the generation of ROS by both enzymatic and nonenzymatic pathways (11, 13, 19, 25). Hemolysis “unpackages” the red blood cell (RBC), releasing free hemoglobin into the plasma. No longer compartmentalized by the intact cell membrane, cell-free plasma hemoglobin rapidly reacts with and scavenges endothelial NO. Hemolysis further impairs NO bioavailability through the release of arginase from the RBC, which competes with NO synthase (NOS) for the substrate arginine. Arginase I levels and activity correlate with measures of intravascular hemolysis in patients with SCD, and notably the lowest ratios of arginine to ornithine are associated with pulmonary hypertension and prospective mortality (19, 20). The depletion of arginine leads to the functional uncoupling of NOS, whereby superoxide is preferentially formed over NO, amplifying the conditions of oxidant stress (33).
In their study, Kaul and colleagues (16) examine the mechanism of sickle cell vasculopathy in a transgenic mouse model of severe SCD and find robust correlations between in vivo NO resistance, measured by vasodilatory response to topical application of the NO donor sodium nitroprusside (SNP), hemolytic rate, and ROS generation. They present further evidence that arginine supplementation improves vascular function by ameliorating hemolysis, oxidant stress, and the NO resistance state.
The assessment of vascular reactivity in the sickle cell transgenic mouse revealed significantly blunted responses to both acetylcholine (ACh) and SNP, as well as blunted changes in mean arterial pressure (MAP) in response to NG-nitro-l-arginine methyl ester (l-NAME), consistent with a global impairment in the NO axis and, more specifically, a resistance to NO vasodilatory activity (evidenced by the lack of response to an exogenous NO donor) (13, 15). The authors confirm their previous findings of compensatory increases in eNOS and cyclooxygenase-2 protein expression in the sickle cell transgenic mouse, as well as increased baseline arteriolar vasodilation (15), indicating a potential compensatory upregulation of non-NO-dependent vasodilators (prostacyclin) in response to diminished NO bioavailability.
Interestingly, arginine treatment in the sickle cell mouse reversed the NO resistance, such that responses to ACh and SNP were augmented following arginine treatment, with increased MAP in response to l-NAME, indicating an increased basal and stimulated NO bioavailability.
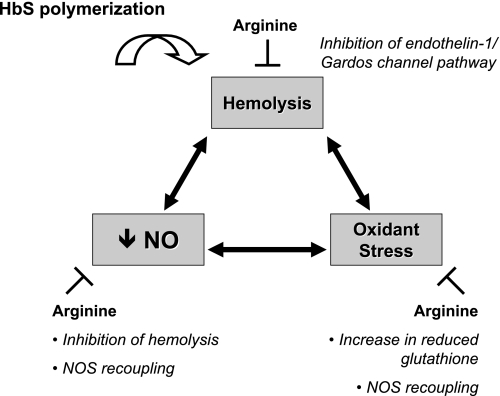
This study demonstrates striking correlations between hemolytic rate and markers of oxidant stress in the transgenic sickle cell mouse, including novel findings of tight associations between plasma hemoglobin and both tyrosine nitration and blunted SNP responsiveness. Arginine supplementation led to improved vascular responsiveness to both endothelium-dependent and -independent vasodilation, suggesting that arginine was directly correcting the primary NO resistance state. This treatment effect was associated with a 50% reduction in plasma hemoglobin and a significant decrease in tyrosine nitration, suggesting both reduced hemolysis and oxidant stress, respectively. These findings illuminate the complex interactions of hemolysis and oxidant stress in potentiating vascular dysfunction (Fig. 1).
Fig. 1.
A vicious cycle of hemolysis, nitric oxide (NO) resistance, and oxidant stress, with interruption by arginine repletion. In sickle cell disease, hemoglobin S (HbS) leads to red blood cell (RBC) hemolysis, decreased NO bioavailability, and oxidant stress. Intravascular hemolysis releases cell-free hemoglobin into the plasma compartment, contributing directly to both impaired NO bioavailability and oxidant stress. Hemolysis alters NO homeostasis through scavenging of NO by cell-free hemoglobin and consumption of arginine by arginase released from hemolyzed RBCs. Hemolysis drives oxidant stress through free hemoglobin-mediated peroxidase, autooxidation, and Fenton chemistries, producing nitrogen dioxide and tyrosine nitration. NO resistance is aggravated by enzymatic (xanthine oxidase and NADPH oxidase) production of superoxide, which scavenges NO. Oxidant stress perpetuates the cycle by rendering RBCs more susceptible to damage and hemolysis. Remarkably, arginine supplementation appears to target this triad of pathology by increasing NO formation, reducing hemolysis, and reducing oxidant stress. NOS, NO synthase.
The observation of an association between tyrosine nitration and blunted vasodilatory responses to NO donors has led to the hypothesis that superoxide formed by xanthine oxidase or NADPH oxidase is reacting with NO to form peroxynitrite, which in turn is nitrating tyrosine residues. Thus the nitrotyrosines are considered footprints for superoxide formation and superoxide-dependent NO scavenging. There is evidence suggesting that this mechanism could contribute to NO resistance in SCD; in the sickle cell mouse, xanthine oxidase is upregulated (1) and endothelial NAPDH oxidase is implicated in endothelial dysfunction of the cerebral microcirculation (32).
However, data from the NO biochemistry field suggests that the major pathway to protein nitration in vivo is via heme-mediated peroxidase chemistry (9, 24, 31). Any heme capable of Fenton-type chemistry can exert peroxidase chemistry, which in the presence of nitrite will generate nitrogen dioxide and nitrate tyrosine residues. Indeed, the myeloperoxidase knockout mouse exhibits significant reductions in protein nitration in vivo (5, 35). We would therefore propose that the high correlation between plasma hemoglobin and protein nitration in the current study by Kaul and colleagues (16) indicates that plasma hemoglobin may be driving the protein nitration rather than the superoxide-NO reaction, which forms peroxynitrite.
We would further argue that the role of hemolysis and plasma hemoglobin in fueling oxidant stress is underappreciated, creating a “chicken or the egg” dilemma as to whether oxidant stress (which leads to RBC damage and subsequent hemolysis) or hemolysis (which releases heme and free iron, powerful catalysts of ROS generation) is central to sustaining the vicious cycle of damage incited by hemoglobin S polymerization (Fig. 1). Although the association does not indicate causality, we propose the results of the current study are more consistent with hemolysis driving ROS formation and protein nitration. Intravascular hemolysis generates free heme and redox active metals, which participate in peroxidase chemistry (leading to lipid peroxidation), Fenton-type chemistry, and autooxidation chemistry (31). Thus these free heme and redox metals can mediate protein nitration under a greater range of conditions than peroxynitrite. Moreover, the uptake of plasma free heme or heme released by methemoglobin into endothelial cells promotes cellular damage, increasing the susceptibility to oxidant damage, and may directly activate xanthine oxidase and NADPH oxidase (2). Further evidence lending support to a predominant role of hemolysis is the increased heme oxygenase-1 (HO-1) expression in transgenic sickle cell mice (4), a finding confirmed in this study. The liberation of free heme by hemolysis induces the expression of HO-1, which scavenges the heme, thereby preventing its participation in redox reactions (8). This compensatory response to the increased heme burden degrades heme into iron, which is scavenged by ferritin, and carbon monoxide and biliverdin, which exhibit antioxidative properties of their own. Rather than serving as a marker of non-NO vasodilatory activity, we suggest HO-1 better reflects the extent of hemolysis. The decrease in plasma hemoglobin and the associated decrease in HO-1 expression following treatment with arginine suggest a direct effect of arginine on reducing the hemolytic rate.
In this model, arginine therapy reduces hemolysis and oxidant stress and normalizes the responsiveness to NO. This observation should be further explored to better elucidate the primary mechanism of action in targeting these tightly linked processes. Does arginine inhibit oxidant stress through increased erythrocytic glutathione and glutamine (an intraerthyrocytic antioxidant) and NOS recoupling (by restoring arginine availability), thereby reducing hemolysis secondary to (reduced) free oxygen radical-induced damage (13, 21)? Or, is the primary effect of arginine the result of decreased hemolysis via the inhibition of the endothelin-1/Gardos channel pathway, thus decreasing free heme and iron and removing the catalyst for lipid peroxidation, nitration, and autooxidation (27, 28)? Will dissecting these interrelationships allow us to better design combinations of therapies that effectively disrupt the cycle of hemolysis and oxidant injury?
Previous studies of arginine supplementation in experimental animal models and patients with SCD have produced variable results. Increased NO bioavailability after the BERK sickle mouse was treated with l-arginine was associated with the reduction in lipid peroxidation and augmented antioxidant activity (7). This treatment was also found to reduce RBC density via a NO-dependent downregulation of the Gardos channel (likely via an intermediate effect of endothelin receptors on RBCs) (26). Arginine treatment of patients with SCD increases plasma NO metabolite levels and acutely reduces pulmonary artery pressures (20). Recently, the enthusiasm for the clinical application of arginine for patients with SCD has been tempered by the results of a multicenter, blinded, phase II clinical trial. Presented in abstract form, arginine supplementation in pediatric patients with SCD at 0.05 or 0.1 g·kg−1·day−1 failed to raise blood arginine levels or show significant changes in laboratory indexes of clinical benefit (30). However, the doses of arginine were notably lower than the typical dosing in the cardiovascular field, and the lack of measurable increase in blood arginine levels suggests that a pharmacological dose of arginine was not achieved. An assessment of efficacy cannot be made with a homeopathic dosing regimen. Questions remain as to whether a higher dose of arginine might have been more effective or whether a lack of effect is due to a lesser degree of arginine deficiency in a pediatric population. Based on the findings of this study by Kaul and colleagues (16), we would propose targeting arginine therapy to patients with SCD with hyperhemolysis and elevated endothelin-1 levels, as these patients may potentially derive the most therapeutic benefit.
Further insight into the cause-and-effect relationship between hemolytic rate and oxidant stress is applicable to our understanding of the mechanisms driving other hemolytic disease states. The data suggest a role for hemolysis in the pathogenesis of endothelial dysfunction in many of these diseases; for example, pulmonary hypertension is a common complication of several chronic and hereditary hemoglobinopathies, including SCD, thalassemia major and intermedia, paroxysmal nocturnal hemoglobinuria, hereditary spherocytosis, and microangiopathic hemolytic anemia (17). Low NO bioavailability has been implicated in the development of experimental cerebral malaria (12). In addition to decreased NO bioavailability, malaria and SCD share several features, including arginine depletion, endothelial dysfunction, increased expression of adhesion molecules, and microvascular occlusion, suggesting the possibility of a common mechanism of disease. A clinical study reported last year demonstrated the improved reactive hyperemia responses in individuals with moderately severe malaria and endothelial dysfunction following treatment with parenteral arginine, illustrating another example of a potential role for decreased arginine in impaired NO bioavailability in human disease (34).
As this article highlights, hemolysis and oxidative stress are intricately coupled in promoting vascular dysfunction, leaving the uncertainty about the primary mechanism (i.e., the chicken or the egg). These relationships and the ameliorating role of arginine are highlighted in Fig. 1. Several potential approaches to dissect and establish the relative contributions of hemolysis versus oxidant stress to endothelial dysfunction can be proposed. Murine models deficient in components of antioxidant systems could be transplanted with bone marrow from transgenic sickle cell mice, assessing for relationships between vasoreactivity, plasma hemoglobin, and protein nitration, as was performed in Fig. 6 of the study (16). Additionally, mice could be supplemented with an excess of antioxidants and hemolysis induced at different rates, by different mechanisms (e.g., alloimmune, RBC membrane defect, sickle, or thalassemia). A reductionist approach might be to compare the effects of injecting the components released by hemolysis (free hemoglobin and RBC membrane fragments) at graded levels and compare them in mice with different levels of oxidant stress and antioxidant loading. Finally, in a spectrum of mice with different levels of oxidant stress, there can be a focus on acute versus chronic effects of hemolysis, acknowledging that HO-1 upregulation and other compensatory mechanisms can blunt some of the hemolytic effects.
In summary, Kaul and colleagues (16) verify the strong association of NO resistance in SCD with free hemoglobin and further our understanding by establishing an association of NO resistance with oxidant stress. This connection should challenge us to consider hemolysis as the driving force sustaining the polymerization-induced cycle of hemolysis, decreased NO bioavailability, and oxidant stress underlying vascular dysfunction. Short of inhibiting the hemolytic rate, the inhibition of enzymatic generation of ROS alone may fail to effectively disrupt this vicious cycle in patients with SCD.
REFERENCES
- 1.Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA 98: 15215–15220, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal 9: 2119–2137, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol 90: 40L–48L, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest 116: 808–816, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 277: 17415–17427, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med 41: 1771–1780, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res 80: 557–564, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Espey MG, Xavier S, Thomas DD, Miranda KM, Wink DA. Direct real-time evaluation of nitration with green fluorescent protein in solution and within human cells reveals the impact of nitrogen dioxide vs. peroxynitrite mechanisms. Proc Natl Acad Sci USA 99: 3481–3486, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladwin MT Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. J Clin Invest 116: 2330–2332, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, Csako G, Waclawiw MA, Panza JA, Cannon RO 3rd. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation 107: 271–278, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde HC. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med 12: 1417–1422, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood 109: 3088–3098, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev 21: 37–47, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaul DK, Liu XD, Chang HY, Nagel RL, Fabry ME. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest 114: 1136–1145, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol (May 2, 2008). doi: 10.1152/ajpheart.00162.2008. [DOI] [PMC free article] [PubMed]
- 17.Lin EE, Rodgers GP, Gladwin MT. Hemolytic anemia-associated pulmonary hypertension in sickle cell disease. Curr Hematol Rep 4: 117–125, 2005. [PubMed] [Google Scholar]
- 18.Machado RF, Kyle Mack A, Martyr S, Barnett C, Macarthur P, Sachdev V, Ernst I, Hunter LA, Coles WA, Nichols JP, Kato GJ, Gladwin MT. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol 136: 319–325, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 294: 81–90, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris CR, Morris SM Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med 168: 63–69, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, Vichinsky EP, Shigenaga M, Ames B, Kuypers FA, Klings ES. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood 111: 402–410, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolan VG, Baldwin C, Ma Q, Wyszynski DF, Amirault Y, Farrell JJ, Bisbee A, Embury SH, Farrer LA, Steinberg MH. Association of single nucleotide polymorphisms in klotho with priapism in sickle cell anaemia. Br J Haematol 128: 266–272, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood 106: 3264–3267, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeiffer S, Mayer B. Lack of tyrosine nitration by peroxynitrite generated at physiological pH. J Biol Chem 273: 27280–27285, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 8: 1383–1389, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood 99: 357–603, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Romero JR, Rivera A, Muñiz A, Nagel R, Fabry ME. Arginine diet for sickle transgenic mice reduces endothelin-1-stimulated Gardos channel activity (Abstract). Blood 110: 3399, 2007. [Google Scholar]
- 28.Romero JR, Suzuka SM, Nagel RL, Fabry ME. Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood 99: 1103–1108, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293: 1653–1662, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Styles L, Kuypers F, Kesler K, Reiss U, Lebeau P, Nagel R, Fabry M. Arginine therapy does not benefit children with sickle cell anemia—results of the CSCC Clinical Trial Consortium Multi-Institutional Study (Abstract). Blood 110: 2252, 2007. [Google Scholar]
- 31.Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink DA. Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2− reaction. Proc Natl Acad Sci USA 99: 12691–12696, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J 19: 989–991, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med 44: 1506–1528, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. Impaired nitric oxide bioavailability and l-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 204: 2693–2704, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 277: 46116–46122, 2002. [DOI] [PubMed] [Google Scholar]