Abstract
Triglyceride-rich lipoprotein (TGRL) lipolysis may provide a proinflammatory stimulus to endothelium. Detergent-resistant plasma membrane microdomains (lipid rafts) have a number of functions in endothelial cell inflammation. The mechanisms of TGRL lipolysis-induced endothelial cell injury were investigated by examining endothelial cell lipid rafts and production of reactive oxygen species (ROS). Lipid raft microdomains in human aortic endothelial cells were visualized by confocal microscopy with fluorescein isothiocyanate-labeled cholera toxin B as a lipid raft marker. Incubation of Atto565-labeled TGRL with lipid raft-labeled endothelial cells showed that TGRL colocalized with the lipid rafts, TGRL lipolysis caused clustering and aggregation of lipid rafts, and colocalization of TGRL remnant particles on the endothelial cells aggregated lipid rafts. Furthermore, TGRL lipolysis caused translocation of low-density lipoprotein receptor-related protein, endothelial nitric oxide synthase, and caveolin-1 from raft regions to nonraft regions of the membrane 3 h after treatment with TGRL lipolysis. TGRL lipolysis significantly increased the production of ROS in endothelial cells, and both NADPH oxidase and cytochrome P-450 inhibitors reduced production of ROS. Our studies suggest that alteration of lipid raft morphology and composition and ROS production could contribute to TGRL lipolysis-mediated endothelial cell injury.
Keywords: endothelial dysfunction, free fatty acids, oxidative stress
triglyceride-rich lipoproteins (TGRL) provide cells with lipids for use as fuels and may have proatherogenic and proinflammatory properties (28). Circulating TGRL [chylomicrons (CM), very low-density lipoproteins (VLDL), and their remnant particles] are a highly heterogeneous family of triglyceride (TG)-rich apoB-containing lipoproteins. TGRL are hydrolyzed by lipoprotein lipase (LpL), an enzyme anchored to endothelial cells causing release of smaller remnant particles and other lipolysis products, such as free fatty acids (FFA). Elevation of TGRL in the postprandial state is associated with increased endothelial cell inflammation and dysfunction and complications of atherosclerosis (45). Previous studies from our group (7, 35–37) showed that TGRL lipolysis products cause endothelial dysfunction with increased endothelial layer permeability. Clinically, elevation of the postprandial TG level is an independent predictor of coronary heart disease (CHD) (16, 49, 50).
An increased plasma TG concentration has been shown to be associated with impaired endothelial function in vitro (25) and in vivo (23, 24). Data from our laboratory and others indicate that TGRL lipolysis has significant biological effects on endothelial cell plasma membranes. Specifically, increased endothelial layer permeability was found in TGRL lipolysis perfused rat arteries (36). Furthermore, recent data show that TGRL lipolysis-mediated increases in permeability result from active rearrangement and dissolution of the junctional barrier in human aortic endothelial cells (HAEC), as well as induction of the apoptotic cascade (7). However, the mechanism of TGRL lipolysis-induced endothelial cell dysfunction is not clear.
FFA released during TGRL lipolysis may contribute to TGRL lipolysis-induced endothelial cell dysfunction. FFA have been shown to play an important role in endothelial dysfunction (42), and Hennig et al. (13–15) have shown that exposure of cultured endothelial cells to oleic acid decreases endothelial barrier function expressed as increased transfer of both albumin and LDL across the endothelium. Furthermore, hypertriglyceridemia can lead to endothelial cell dysfunction associated with increased superoxide anion production and a subsequent decrease in nitric oxide (NO) bioavailability. Recently, Halle et al. (11) showed that palmitic acid and oleic acid decreased endothelial nitric oxide synthase (eNOS) activity, whereas linolenic acid did not influence eNOS activity. These results demonstrate that elevation of FFA may contribute to endothelial dysfunction and emphasize that FFA affect the endothelium differently. However, these studies were limited to investigation of one or two kinds of FFA, and the results were not consistent. It is not clear whether TGRL lipolysis affects eNOS activity and superoxide anion production. Furthermore, the exact mechanisms of how fatty acids affect eNOS activity and endothelial function have not been elucidated.
Lipid rafts are dynamic, detergent-resistant plasma membrane microdomains that are highly enriched in cholesterol and sphingolipids (40). These domains appear to facilitate interactions among the protein and lipid component of the signal pathway and participate in cell signaling processes (40). The fatty acid side chains of the phospholipids present in lipid rafts tend to be more highly saturated than those in the surrounding membrane (31, 40). These lipid microdomains sequester proteins that mediate signal transduction in a variety of cell types, including endothelial cells and myocytes (40). As a result, lipid rafts play an important role in cell signal transduction, endocytosis, cholesterol trafficking, and membrane skeleton integrity (20). A number of studies have demonstrated that lipid rafts differ with regard to shape, size, localization, protein, and/or lipid composition based on their functional state (38). In such studies, the biophysical and biochemical microenvironments of the rafts influence the function of the raft proteins.
Compared with the intensive study of LDL-cholesterol and lipid rafts, there is less known of the effects of TGRL and their lipolysis on lipid rafts. Fatty acids have been reported to incorporate into lipids of the cytoplasmic leaflet in rafts and change the lipid composition of rafts (43). Cells incubated with polyunsaturated fatty acids show replacement of saturated fatty acids with unsaturated ones in acylated proteins, causing these proteins to dissociate from rafts (46). Li et al. (21) demonstrated that dietary polyunsaturated fatty acids remodel mouse T-cell lipid rafts. Furthermore, a detrimental effect of fatty acids on T-cell lipid rafts, which inhibits Jurkat cells proliferation and IL-2 receptor α expression on the surface of T cells has been reported (21). While cholesterol and LDL have been associated with abnormal NO metabolism and endothelial dysfunction as a result of change of lipid rafts (2), it is not clear whether TGRL and their lipolysis also affect lipid rafts, and whether there are any consequences in cell signaling or endothelial dysfunction.
Endothelial injury in hypertriglyceridemia is caused, at least in part, by high concentrations of lipolysis products, but the mechanism of this effect is still not clear. In the present study, we sought to understand the mechanisms of TGRL and their lipolysis products in the pathogenesis of endothelial injury associated with endothelial cell membrane microdomains.
MATERIALS AND METHODS
Cell culture.
HAEC were purchased from Cascade Bioscience (Winchester, MA) and cultured in medium 200 (Cascade Bioscience) supplemented with low-serum growth supplement and penicillin, streptomycin, and amphotericin B at 37°C in a humidified atmosphere of 5% CO2. HAEC between passages 4–6 were grown in T-75 flasks or four-well lab-Tek glass chamber slides (Nunc, Rochester, NY) until confluence.
Lipoprotein isolation and characterization.
Postprandial blood samples were obtained from healthy volunteers 3.5 h after a moderately high-fat meal, which is the period of peak elevation in plasma triglyceride concentrations in normal individuals. TGRL isolated from these healthy volunteers during this time frame, and to which endothelial cells are exposed, will primarily consist of VLDL and VLDL remnant particles and to a significantly lesser extent CM and CM remnant particles (7). All procedures were conducted under a protocol approved by the Human Subject Review Committee at the University of California Davis, and all volunteers gave informed consent. TGRL were isolated from human plasma at a density of <1.0063 g/ml by aspiration with a narrow-bore pipette after 18 h centrifugation at 40,000 rpm in a SW41 Ti swinging bucket rotor (Beckman Coulter, Sunnyvale, CA) held at 14°C within a Beckman L8-70M ultracentrifuge (7). The top fraction (TGRL) was collected and exhaustively dialyzed in Spectrapor membrane tubing (mol wt cutoff 3,500; Spectrum Medical Industries, Los Angeles, CA) at 4°C overnight against a saline solution containing 0.01% EDTA. The purity of TGRL was confirmed by analytical sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie staining and lipid analysis. SDS electrophoresis showed the presence of apoB48 and apoB100 in TGRL. Plasma TG and cholesterol levels were determined enzymatically with kits (Sigma, St. Louis, MO).
Lipoprotein labeling and treatments.
The isolated lipoprotein subfractions were concentrated and subsequently analyzed by modified Lowry assay (Sigma) (22). Samples containing 1 mg of protein (at the concentration of 2 mg/ml) were labeled by Atto565 NHS ester with an excitation wavelength of 561 nm and emission at 585 nm (Sigma). NHS ester readily reacts with ɛ-amino groups of lysines of the amine terminus, forming a chemically stable amide bond between the dye and the protein. The labeled protein was separated from unreacted dye by P-10 column (Amersham Bioscience, Piscataway, NJ). The conjugates were stored at 4°C, protected from light, and used within 3 days.
HAEC were treated with serum-free medium (untreated cells) as a control, LpL only (2 U/ml), or TGRL (456 μmol/l TG) with or without LpL (2 U/ml). For LpL-dependent TGRL lipolysis, LpL was preincubated with TGRL for 30 min at 37°C before cell treatment.
Localization of lipid rafts and TGRL by confocal microscopy.
For the localization studies, HAEC were plated at a density of 5 × 105 cells/ml in four-well lab-Tek glass chamber slides (Nunc). HAEC were treated with Atto565-labeled TGRL with or without LpL for 20 min at 37°C. After incubation with labeled lipoproteins, cells were washed twice with PBS, followed by incubation in cell culture medium for 30 min. HAEC were then incubated for 15 min at room temperature with a lipid raft marker, FITC-labeled cholera toxin B subunits (FITC-CTB; 5 μg/ml), which is known to bind to the pentasaccharides of the plasma membrane ganglioside Gm1. The cells were rinsed three times with PBS and visualized in a LSM 5 PASCAL laser scanning microscope (Axiovert 200M/LSM 510, Carl Zeiss). For visualization of lipid rafts, imaging was performed with a fluorescence microscope at FITC excitation of 494-nm and 518-nm emission filters. TGRL were visualized with an Atto filter at excitation of 561 nm and emission at 585 nm.
Isolation of lipid rafts from HAEC.
On reaching confluence, HAEC (2 × 107) were treated with TGRL with or without LpL for 3 h at 37°C. Control cells were left untreated. At the end of incubation, cells were removed from culture dishes with a trypsin-EDTA solution followed by a trypsin neutralizer and pelleted by centrifugation. Lipid rafts were prepared according to the method of Prinetti et al. (32). Briefly, the cell pellet was rinsed twice with PBS and sonicated three times for 20 s in 1 ml of Tris-buffered saline (TBS; 25 mM Tris·HCl, pH 7.4, 150 mM NaCl) containing 1% (vol/vol) Triton X-100, 5 mM EDTA, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 10 mM phenylmethylsulfonyl fluoride, and 5 mM DTT. The cells were lysed in TBS-Triton X-100 buffer for 30 min on ice. The homogenized cell lysates were mixed with 2 ml of 60% OptiPrep gradient (Sigma), and a discontinuous 5–30% OptiPrep gradient was layered on the top of the cell lysates. The resulting gradient was subjected to ultracentrifugation at 40,000 rpm for 16–20 h at 4°C with a Beckman SW41 Ti rotor. After centrifugation, 12 density fractions of 840 μl each were collected from top to bottom; density fractions were either used immediately for Western blotting or snap-frozen in dry ice and stored at −80°C until use.
Gel electrophoresis and Western blotting.
To characterize lipid rafts, caveolin-1, an essential structural component of caveolae that is a subset of the lipid raft, was used as a lipid raft marker and quantified by Western blotting. Gradient fractions (25 μl) were mixed with 25 μl of Laemmli denaturing sample buffer. Equal volumes of each density fraction were loaded on to each lane of 4–15% polyacrylamide gels. After electrophoresis, separated proteins were transferred to nitrocellulose membranes, which then were incubated in blocking buffer BLOTTO (TBS containing 0.1% Tween 20 and 5% nonfat dry milk) for 1 h, followed by incubation with rabbit anti-caveolin-1 (molecular mass 22 kDa, 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Membranes were washed four times with TBS containing 0.1% Tween 20 and subsequently incubated in BLOTTO with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:2,000; Amersham Bioscience) at room temperature for 1 h. Membranes were washed again four times with TBS containing 0.1% Tween 20 before incubation with ECL plus Western blotting detection reagents (Amersham) and then exposed to ECL hyperfilm (Amersham). The identities of the bands visualized in the Western blots were confirmed by comparison with the molecular mass standard and positive controls provided by the manufacturer of the antibody. Gradient fractions 5–7 corresponded to lipid raft-rich membrane.
To analyze the effect of lipolysis products on lipid raft-associated proteins, raft fractions were pooled and protein was determined by the modified Lowry assay (22). Protein (20 μg) from raft fraction protein and nonraft fraction protein (supernatant) was loaded onto and separated by 4–15% SDS-PAGE. The procedure for blotting was the same as above, with primary antibodies mouse anti-eNOS (molecular mass 144 kDa, 1:750), rabbit anti-caveolin-1 (molecular mass 22 kDa, 1:1,000), rabbit LDL receptor-related protein (LRP) (molecular mass 504 kDa, 1:1,000), and horseradish peroxidase-conjugated secondary antibodies to either mouse (eNOS) or rabbit (caveolin-1, LRP). Densitometric analyses were performed with UN-SCAN-IT gel software (Silk Scientific).
Lipid analysis.
TG and phospholipid concentrations were determined enzymatically with kits (WAKO, Richmond, VA). The amount of total cholesterol in the lipid raft fraction and cell lysate and FFA in TGRL and TGRL lipolysis were determined by the Nutritional Assessment Lab (Davis, CA).
Measurement of reactive oxygen species.
Reactive oxygen species (ROS) generation was determined based on the oxidation of 2′,7′-dichlorofluorescein diacetate (DCFDA, Molecular Probes) by superoxide and hydrogen peroxide, forming the highly fluorescent derivative dichlorofluorescein (DCF) (5). Confluent HAEC (104 cells/well) in 96-well plates were preincubated with the fluorescence probe DCFDA (10 μM) for 30 min. After the cells were washed in PBS twice, they were incubated with TGRL with or without LpL for 2 h. After removal of medium from wells, cells were washed three times in PBS, followed by measurement of fluorescence intensity at 485-nm excitation and 538-nm emission spectra with a fluorescence microplate reader (FUJIFLIM FLA 5100). Furthermore, the effects of inhibitors for NADPH oxidase and CYP 2C9, an isoform of cytochrome P-450, on TGRL + LpL-induced ROS generation were determined with apocynin (NADPH oxidase inhibitor, 100 μM; Ref. 12), diphenyleneiodonium (DPI; NADPH oxidase inhibitor, 50 μM; Ref. 48), or sulfaphenazole (SP; cytochrome P-450 inhibitor, 10 μM; Ref. 44) (Sigma). The doses of these inhibitors were based on those reported in previous studies that effectively blocked enzyme activity. 4β-Phorbol 12-myristate 13-acetate (10 μM; Sigma) was used to stimulate oxidative activity as a positive control.
Measurement of intracellular superoxide anions.
Intracellular superoxide anions in HAEC were measured by dihydroethidium (DHE, Molecular Probes), a cell-permeant dye that is oxidized by superoxide anions to yield the fluorescent ethidium cation (3, 4). Confluent HAEC (104 cells/well) in 96-well plates were stained with DHE (10 μg/ml) for 15 min at 37°C. After washing, the cells were incubated with TGRL with or without LpL for 2 h at 37°C. DHE fluorescence was quantified with a fluorescence microplate reader (FUJIFLIM FLA 5100) set with excitation at 392 nm and emission at 410 nm. H2O2 (50 μM) was used as a positive control.
Statistical analysis.
All experiments were performed in triplicate in at least three independent trials. Values are expressed as means ± SE unless otherwise noted. Comparisons among treatments were performed with one-way ANOVA. Differences in mean values were tested by a least-squares means procedure. P < 0.05 was considered statistically significant.
RESULTS
Localization of lipid rafts in HAEC.
Laser scanning confocal microscopy was used to examine the localization of lipid rafts in the plasma membrane of HAEC. FITC-CTB is known to bind specifically to the pentasaccharides of the plasma membrane ganglioside Gm1, a raft lipid marker (30). The confocal microscopy image (Fig. 1) reveals the distribution of punctate lipid rafts (light blue color) in the plasma membrane of HAEC. Lipid rafts are more concentrated in the perinuclear region of cells, corresponding to domains enriched with gangliosides. These imaging experiments demonstrated that lipid rafts are heterogeneous in terms of their size, localization, and distribution.
Fig. 1.
Localization of lipid rafts in human aortic endothelial cells (HAEC). HAEC were incubated with FITC-cholera toxin B (FITC-CTB) and visualized with laser scanning confocal microscopy. Twenty slices (scan speed 30 s/slice) from top to bottom of the cells were viewed under the Z-stack mode. Lipid rafts were shown as punctate areas of fluorescence in HAEC of the confocal laser image (×40 Z stack; top: slice 6; bottom: slice 15). Example lipid rafts are indicated with an asterisk.
TGRL association with HAEC lipid rafts.
We visualized TGRL interactions with lipid rafts of HAEC using confocal microscopy. First, we performed a series of time-response experiments. Cells were incubated with Atto565-labeled TGRL at a triglyceride concentration of 456 μmol/l (40 mg/dl) for 5 min, 20 min or 4 h. No signal was found at 5 min of incubation, and the TGRL images were not significantly different between the 20-min and 4-h incubations. As a result, a 20-min incubation time was chosen for the visualization study.
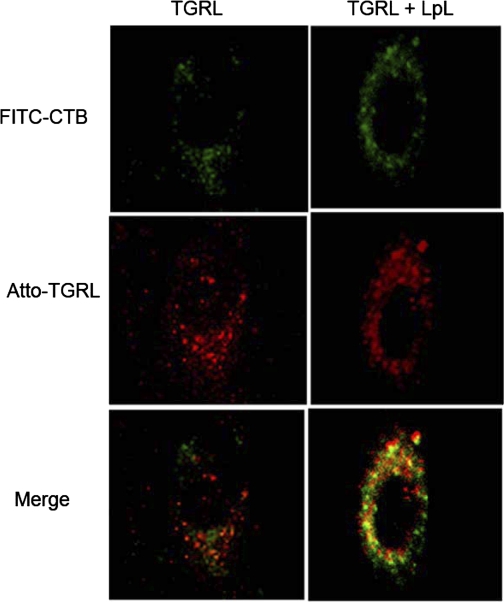
Next, we directly visualized TGRL distribution on HAEC after 20-min incubation by confocal microscopy with an Atto565 excitation filter at 561 nm and an emission filter at 585 nm. Figure 2 shows the TGRL localization (red color) on HAEC. Round, punctate areas of fluorescence, which represent TGRL particles, are distributed in the perinuclear area, and especially at the apical region. TGRL had a distribution pattern similar to that of lipid rafts.
Fig. 2.
Effect of triglyceride-rich lipoprotein (TGRL) lipolysis products on lipid rafts of HAEC. HAEC were incubated with Atto565-labeled TGRL with or without lipoprotein lipase (LpL) for 20 min at 37°C, followed by incubation with FITC-CTB, and visualized with laser scanning confocal microscopy. Typical confocal images (×63) of HAEC treated with TGRL without and with LpL are shown. Colocalization of TGRL and lipid rafts is shown in merged panels. Green represents lipid rafts; red represents TGRL; orange/yellow represents colocalization of TGRL and lipid rafts.
Employing this double fluorescence imaging technique with living HAEC, we further directly visualized TGRL interaction with lipid rafts on HAEC. After Atto565-labeled TGRL incubation with HAEC, lipid rafts in HAEC were labeled with FITC-CTB in phenol-free culture medium. Colocalization of TGRL and FITC-CTB (lipid rafts) on HAEC was performed with confocal microscopy (Fig. 2). The TGRL colocalized with lipid rafts in the perinuclear region of the cells was demonstrated by orange color in the confocal image. This is the first direct visualization of TGRL interacting with membrane microdomains in living HAEC.
TGRL lipolysis products increase aggregation of lipid rafts in HAEC.
HAEC were treated with labeled TGRL in the presence or absence of LpL for 20 min, followed by confocal fluorescence imaging of lipid rafts in HAEC. Distribution patterns of TGRL and lipid rafts on HAEC were observed by laser scanning confocal microscopy and are shown in Fig. 2. Treatment of TGRL with LpL for 20 min before incubation with cells resulted in increased TGRL fluorescent signal (Fig. 2). Atto NHS esters readily react with amino groups of proteins, in particular the ɛ-amino groups of lysine. Compared with apoC and apoE, there are >10-fold more lysines on apoB than apoC or apoE. Therefore, most Atto labeled apoB rather than apoC or apoE. The enhanced fluorescent signal found in TGRL lipolysis-treated HAEC compared with TGRL-treated cells indicates that more TGRL remnants were associated with endothelial cells. Labeling of living cells with FITC-CTB revealed multiple areas of lipid raft aggregation in the plasma membrane of TGRL + LpL-treated cells (Fig. 2). Moreover, there was more colocalization of TGRL remnant particles and lipid rafts in the lipolysis-treated group compared with the TGRL-only group. These results show increased remnant accumulation, lipid raft clustering, and colocalization of remnants and lipid rafts on endothelial cells when treated with TGRL lipolysis products.
Effects of TGRL lipolysis products on composition of HAEC lipid rafts.
To investigate how TGRL lipolysis products affect the composition of lipid rafts, we conducted experiments to study the biochemical changes of lipid rafts in HAEC. First, detergent-resistant microdomains were isolated from HAEC by extraction with cold 1% Triton X-100, followed by density gradient sedimentation. A total of 12 fractions were collected and analyzed for total protein content (modified Lowry assay), cholesterol level (colorimetric method), and the raft marker caveolin-1 (Western blot). Caveolin is the major structural protein in caveolae, specialized domains of the plasma membrane. Several tissue-specific isoforms were found, whereas caveolin-1 is prominent in the vascular endothelium (8). Combined caveolin-1, cholesterol, and protein analyses confirmed that fractions 5–7 represented the detergent-resistant microdomains (lipid rafts). We used fractions 5–7 to conduct the following experiment.
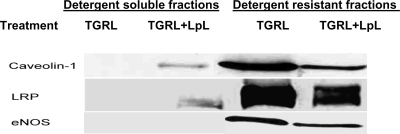
To determine whether the membrane localization of eNOS, LRP, and caveolin-1 were altered by TGRL lipolysis products, HAEC were grown to confluence before treatment with TGRL (456 μmol/l TG) or TGRL + LpL (2 U/ml) for 3 h. Cells were lysed in hypotonic buffer, and lipid raft-enriched membranes were prepared as described in materials and methods. Western blot analyses for caveolin-1, eNOS, and LRP were conducted in density gradients from HAEC. A large amount of caveolin-1 was found in low-density fractions 5–7; no caveolin-1 was found in the lower-density fractions 1–4. Both eNOS and LRP were found in fractions 5–7, demonstrating that these proteins localize to lipid raft fractions. Compared with TGRL treatment, HAEC treated with TGRL + LpL (Fig. 3) showed substantial changes in the amount of caveolin-1, eNOS, and LRP associated with the lipid raft fraction and the nonraft fraction (low-density fraction), although no change was noted in the amount of these proteins in the total cell lysate. After treatment with TGRL lipolysis, significant amounts of caveolin-1 and LRP were found in the nonraft low-density fraction. Lipolysis product treatment of HAEC significantly decreased caveolin-1, as determined by densitometric analyses (UN-SCAN-IT gel software, Silk Scientific) (875,352 ± 67,523 vs. 303,284 ± 15,934 pix), LRP (1,661,483 ± 10,023 vs. 1,070,035 ± 21,936 pix), and eNOS (404,933 ± 36,524 vs. 251,984 ± 25,934 pix) associated with lipid raft-enriched membranes by 65.6%, 35.6%, and 37.8% (P < 0.05), respectively, compared with TGRL treatment. These findings indicate that lipolysis products caused raft-associated protein redistribution in the cell membrane.
Fig. 3.
TGRL lipolysis products alter the distribution of caveolin-1, LDL receptor-related protein (LRP), and endothelial nitric oxide synthase (eNOS) in HAEC. HAEC were treated with TGRL (456 μmol/l triglyceride) with or without LpL (2 U/ml) for 3 h at 37°C. Cells then were lysed, and lipid raft-enriched membranes were prepared by OptiPrep gradient density analysis. Western blot analyses for caveolin-1, LRP, and eNOS were performed on 20-μg protein fractions representing detergent-soluble fractions (fractions 1–4) and lipid raft-enriched membranes (fractions 5–7). The result shown corresponds to a representative experiment of 3 independent assays.
In addition, we investigated whether TGRL lipolysis altered the cholesterol concentration in the lipid raft-enriched regions of the membrane. Lipid raft fractions were isolated, and the cholesterol level was determined by the colorimetric method. Lipolysis decreased the amount of cholesterol associated with rafts (6.72 ± 0.75 μg/107 cells, 9.8% decrease) compared with TGRL only (7.45 ± 0.57 μg/107 cells), but there was no statistical difference. However, a significant decrease of the total amount of cholesterol in the cell lysate was detected in lipolysis-treated HAEC (8.90 ± 0.50 μg/107 cells, 31.5% decrease) compared with TGRL-treated cells (13 ± 0.89 μg/107 cells; P < 0.05). Data show the means ± SE of three independent analyses.
Lipid analysis of TGRL and TGRL lipolysis products.
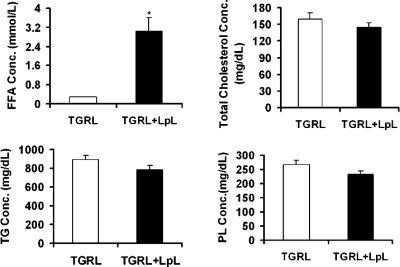
To explore the mechanism by which TGRL lipolysis affects endothelial cell lipid rafts, we compared the lipid composition of freshly isolated postprandial TGRL and TGRL lipolysis by enzymatic assay. After incubation with LpL for 30 min, there was a slight decrease in the concentrations of TG, phospholipid, and total cholesterol compared with TGRL treatment only, which was not statistically significant. However, there was a significant increase in FFA concentration (10-fold; P < 0.001; Fig. 4) in TGRL lipolysis products.
Fig. 4.
Quantification of the lipid composition from TGRL and TGRL lipolysis treatments. Human postprandial TGRL was isolated by density gradient ultracentrifugation. Before the lipid assay, TGRL was incubated with LpL (2 U/ml) for 30 min at 37°C. Each lipid fraction was quantified by enzymatic assay. Data show the means ± SE of 3 independent analyses. *P < 0.01. FFA, free fatty acids; TG, triglyceride; PL, phospholipid.
TGRL lipolysis products induce ROS generation.
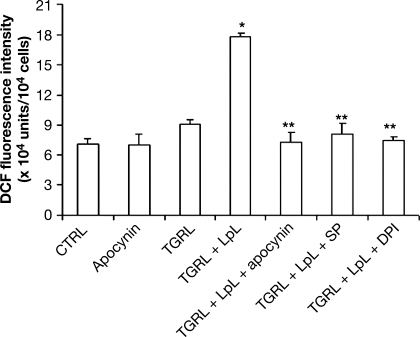
The concentration of ROS in HAEC was assessed by DCFDA oxidation. Pretreatment of HAEC with freshly isolated postprandial TGRL lipolysis for 2 h significantly increased ROS generation compared with TGRL only (P < 0.05; Fig. 5). In a preliminary time-dependent experiment, HAEC were incubated with TGRL lipolysis for 30 min, 60 min, 90 min, 2 h, and 3 h and ROS were measured. There was a maximum release of ROS during 90 min to 2 h, so we used 2-h incubation in our study. To determine whether TGRL-induced ROS generation was caused by increased NADPH oxidase, HAEC were treated with TGRL + LpL with or without coincubation with different NADPH oxidase and CYP2C9 inhibitors; ROS production significantly increased in HAEC treated with TGRL lipolysis, and ROS production was inhibited by apocynin (100 μM), SP (10 μM), or DPI (50 μM) (P < 0.01). To identify which ROS was induced by TGRL lipolysis products, we also used DHE as a fluorescent probe to specifically measure intracellular superoxide anions in HAEC. In accord with our results from the DCFDA fluorescence assay, TGRL lipolysis showed a significant increase in superoxide anion production within HAEC (3.8 × 104 vs. 3.2 × 104 units/104 cells, P = 0.03).
Fig. 5.
Effects of TGRL and TGRL lipolysis products on reactive oxygen species (ROS) production in HAEC. HAEC were preincubated for with fluorescence probe 2′,7′-dichlorofluorescein diacetate (DCFDA, 10 μM) or dihydroethidium (DHE, 10 μg/ml), followed by incubation with TGRL (456 μmol/l triglyceride) without or with LpL (2 U/ml) and NADPH oxidase and CYPC2C9 inhibitors apocynin (100 μM), diphenyleneiodonium (DPI, 50 μM), or sulfaphenazole (SP, 10 μM) for 2 h. The florescence intensity of cells was measured with a fluorescence microplate reader. Florescence distribution of DCFDA or DHE oxidation was expressed as fluorescence units. *P < 0.05 compared with TGRL-treated cells; **P < 0.05 compared with TGRL + LpL-treated cells. DCF, dichlorofluorescein.
DISCUSSION
Our study showed that TGRL lipolysis products have a number of actions on endothelial cells in culture including lipid raft aggregation, activation of NADPH oxidase with production of ROS, and colocalization of TGRL remnant particles with plasma membrane lipid rafts. These new observations add to our fundamental understanding of how TGRL induce vascular inflammation and atherosclerotic cardiovascular disease.
Using confocal fluorescence microscopic analysis, we were able to visualize the interactions of lipid rafts and TGRL in living HAEC. Currently, lipid raft microdomains can be morphologically identified by electron microscopy (33) and various fluorescence methods (10, 17, 18, 39). Compared with these techniques, our approach using a combination of fluorescence double labeling and confocal microscopic approaches allows us to visualize proteins colocalizing with lipid rafts and other morphologically featureless microdomains within living cells (30) and to monitor the dynamics of the proteins and lipids. This approach provides direct observation of the interaction between lipoproteins and specific membrane domains and does not require highly specialized equipment or technical expertise. Therefore, dual fluorescent labeling of lipid rafts and proteins (e.g., lipoproteins) has the potential to determine plasma membrane organization and the spatial dynamics of regulated signaling and membrane trafficking events associated with the cell surface.
Our localization studies indicate that TGRL remnant particles colocalize with plasma membrane lipid rafts. LRP is a multifunctional receptor that mediates uptake of TGRL and their remnant particles. Western blot showed that LRP was associated with lipid raft fractions before lipolysis, and treatment with TGRL lipolysis products caused LRP to move from detergent-resistant membrane fractions to low-density, detergent-soluble membrane fractions. This result demonstrates that LRP is dynamically associated with lipid rafts, which is consistent with a recent study (1). LRP could be exported from rafts to the detergent-soluble fractions, such as clathrin-coated pits where endocytosis occurs. Additional time course experiments are needed to determine when LRP moves from rafts to the detergent-soluble fractions and whether this occurs after LRP has bound TGRL remnant particles.
One of the important observations in the present study is that TGRL lipolysis products cause lipid raft clustering or aggregation. Our study shows that lipid rafts randomly distribute in the cell surface membrane and perinuclear region, whereas stimulation by TGRL lipolysis products results in the formation of multiple “nonpolarized” CTB aggregates. The clustering or aggregation of cell membrane lipid rafts in response to different stimuli exerts an important signaling action of many cell types (6, 41). It has been shown that clustering of rafts forms membrane platforms, which serve to recruit various signaling molecules such as sphingomyelinase, ceramide, and CD95, resulting in activation of signaling pathways (6, 9). Zhang et al. (47) demonstrated that lipid raft clustering on the HAEC plasma membrane increased NADPH oxidase, which is a major enzymatic source of superoxide in vascular endothelial cells. Using tumor necrosis factor-α and Fas ligand to stimulate the lipid raft clustering on the HAEC, the authors showed that the clustered rafts contained high NADPH oxidase in vascular endothelial cells. This study indicates that lipid raft clustering may play a role in redox transmembrane signaling.
ROS play an important role in the development of vascular disease, including atherosclerosis, hypertension, and restenosis after angioplasty. Excessive ROS generation has been associated with endothelial dysfunction and accelerated atherogenesis (27). ROS can induce oxidative modification of LDL, expression of adhesion molecules, such as intercellular adhesion molecule-1 and vascular cell adhesion molecule (VCAM)-1, and receptors for oxidized lipid particles, as well as the activation of transcriptional factors (26), which in turn facilitate inflammatory cell recruitment as well as lipid deposition in the intimal layer (29). There are various sources of ROS, such as mitochondrial respiration, purine metabolism and the xanthine oxidase pathway, the NOS pathway, and the NADPH oxidase pathway (27); however, the main source of ROS production in vascular endothelial cells is the NADPH oxidase pathway. Our study shows that TGRL lipolysis induces ROS generation, and we also confirm that superoxide anion, an important ROS, is generated from endothelial cells as a result of TGRL lipolysis product effects on endothelial cells. TGRL lipolysis-induced ROS can be blocked by NADPH oxidase inhibitors DPI and apocynin. DPI is an inhibitor of flavin-containing oxidases, including NADPH oxidase. The vascular NADPH oxidase consists of at least five subunits, which assemble membrane-bound cytochrome b558, p22phox, and gp91phox and are important for electron transport and the reduction of molecular oxygen to O2−. Apocynin acts by interfering with NADPH subunit assembly in the membrane and is therefore a more specific inhibitor than DPI. Taken together, the inhibition of O2− production induced by TGRL lipolysis indicates that TGRL lipolysis-induced oxidative stress is mediated by the NADPH oxidase pathway.
Recent evidence indicates that oxidized LDL (oxLDL) could act as a proatherogenic stimulus by activating NADPH oxidase, thus promoting the production of superoxide anion, which in turn has a positive feedback effect on further oxLDL production (34). Our study shows that TGRL lipolysis products, which cause lipid raft clustering, significantly increase the production of ROS in endothelial cells, and NADPH oxidase inhibitors suppress the lipolysis-induced ROS generation. This result demonstrates that TGRL lipolysis products induce NADPH oxidase activity. Furthermore, a recent study (47) demonstrated that the clustering of rafts results in the activation of NADPH oxidase and consequent endothelial dysfunction. That study and our study indicate that the formation of redox signaling platforms on endothelial cell membranes may contribute to endothelial injury and that lipid raft clustering may serve as a common mechanism for transmembrane signaling, which mediates the actions of these agonists.
We propose that TGRL lipolysis products stimulate lipid raft clustering, which provides a platform for NADPH oxidase and increases enzyme activity (47) and thereby stimulates the production of ROS. As a result, excessive ROS disrupts lipid rafts and affects raft function (e.g., eNOS), which may contribute to endothelial injury. Furthermore, TGRL lipolysis products induce increased accumulation of TGRL remnant particles in the plasma membrane in association with lipid rafts. The mechanism for the association of the TGRL remnant particle and the lipid raft is not known.
The purpose of this study was to investigate whether postprandial TGRL lipolysis affects the endothelial cell lipid raft and ROS production. Thus we incubated freshly isolated human postprandial TGRL with LpL to generate a mixture of lipolysis products, which is a setting similar to physiological TGRL lipolysis mediated by LpL that the vascular endothelium would encounter after a meal. LpL, an enzyme anchored to endothelial cells, primarily hydrolyzes triglycerides in TGRL. LpL can have either pro- or anti-inflammatory effects dependent on the cytokine stimulus (19) and dose (51). We previously showed (7, 35–37) that LpL had no direct effects on endothelial cells when we used a small amount of LpL to minimize any direct LpL effect. The present preliminary study also showed that there was no significant effect of LpL alone on HAEC compared with the control. Our study demonstrated that postprandial TGRL lipolysis mediated by LpL affects endothelial cell lipid raft and ROS production. In future studies, we will further explore which lipid fraction and which specific lipids (e.g., fatty acids) will cause raft and ROS changes by separating remnant particles, FFA, phospholipids, monoglycerides, and diglycerides from the TGRL lipolysis mixture.
GRANTS
The study was supported by National Heart, Lung, and Blood Institute Grants HL-78615 and HL-55665 and the Richard A. and Nora Eccles Harrison Endowed Chair in Diabetes Research.
Acknowledgments
We thank Trang Vo and Dr. Yongjin Hou for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anderson RA, Evans ML, Ellis GR, Graham J, Morris K, Jackson SK, Lewis MJ, Rees A, Frenneaux MP. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis 154: 475–483, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem 274: 32512–32519, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Budd SL, Castilho RF, Nicholls DG. Mitochondrial membrane potential and hydroethidine-monitored superoxide generation in cultured cerebellar granule cells. FEBS Lett 415: 21–24, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Carter WO, Narayanan PK, Robinson JP. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol 55: 253–258, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134: 111–116, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Cremesti AE, Goni FM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett 531: 47–53, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol 292: H2745–H2753, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem 271: 22810–22814, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem 276: 20589–20596, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Stralfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 13: 1961–1971, 1999. [PubMed] [Google Scholar]
- 11.Halle M, Eriksson P, Tornvall P. Effects of free fatty acids and a triglyceride-rich fat emulsion on endothelial nitric oxide synthase. Eur J Clin Invest 35: 154–155, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hennig B, Shasby DM, Fulton AB, Spector AA. Exposure to free fatty acid increases the transfer of albumin across cultured endothelial monolayers. Arteriosclerosis 4: 489–497, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Hennig B, Shasby DM, Spector AA. Exposure to fatty acid increases human low density lipoprotein transfer across cultured endothelial monolayers. Circ Res 57: 776–780, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Hennig B, Watkins BA. Linoleic acid and linolenic acid: effect on permeability properties of cultured endothelial cell monolayers. Am J Clin Nutr 49: 301–305, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Karpe F, Humphreys SM, Samra JS, Summers LK, Frayn KN. Clearance of lipoprotein remnant particles in adipose tissue and muscle in humans. J Lipid Res 38: 2335–2343, 1997. [PubMed] [Google Scholar]
- 17.Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J Cell Biol 142: 69–84, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell 11: 1645–1655, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kota RS, Ramana CV, Tenorio FA, Enelow RI, Rutledge JC. Differential effects of lipoprotein lipase on tumor necrosis factor-alpha and interferon-gamma-mediated gene expression in human endothelial cells. J Biol Chem 280: 31076–31084, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol 21: 193–205, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Tan L, Wang C, Li N, Li Y, Xu G, Li J. Polyunsaturated eicosapentaenoic acid changes lipid composition in lipid rafts. Eur J Nutr 45: 144–151, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 23.Lundman P, Eriksson M, Schenck-Gustafsson K, Karpe F, Tornvall P. Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease. Circulation 96: 3266–3268, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Lundman P, Eriksson MJ, Stuhlinger M, Cooke JP, Hamsten A, Tornvall P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J Am Coll Cardiol 38: 111–116, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Lundman P, Tornvall P, Nilsson L, Pernow J. A triglyceride-rich fat emulsion and free fatty acids but not very low density lipoproteins impair endothelium-dependent vasorelaxation. Atherosclerosis 159: 35–41, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation 96: 2361–2367, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Mehta JL, Rasouli N, Sinha AK, Molavi B. Oxidative stress in diabetes: a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. Int J Biochem Cell Biol 38: 794–803, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Moers A, Fenselau S, Schrezenmeir J. Chylomicrons induce E-selectin and VCAM-1 expression in endothelial cells. Exp Clin Endocrinol Diabetes 105, Suppl 2: 35–37, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Nishio E, Watanabe Y. The involvement of reactive oxygen species and arachidonic acid in alpha1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br J Pharmacol 121: 665–670, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pike LJ Lipid rafts: bringing order to chaos. J Lipid Res 44: 655–667, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Pike LJ, Han X, Chung KN, Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41: 2075–2088, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Prinetti A, Iwabuchi K, Hakomori S. Glycosphingolipid-enriched signaling domain in mouse neuroblastoma Neuro2a cells. Mechanism of ganglioside-dependent neuritogenesis. J Biol Chem 274: 20916–20924, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Prior IA, Parton RG, Hancock JF. Observing cell surface signaling domains using electron microscopy. Sci STKE 2003: PL9, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation 104: 1767–1772, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Rutledge JC, Goldberg IJ. Lipoprotein lipase (LpL) affects low density lipoprotein (LDL) flux through vascular tissue: evidence that LpL increases LDL accumulation in vascular tissue. J Lipid Res 35: 1152–1160, 1994. [PubMed] [Google Scholar]
- 36.Rutledge JC, Mullick AE, Gardner G, Goldberg IJ. Direct visualization of lipid deposition and reverse lipid transport in a perfused artery: roles of VLDL and HDL. Circ Res 86: 768–773, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Rutledge JC, Woo MM, Rezai AA, Curtiss LK, Goldberg IJ. Lipoprotein lipase increases lipoprotein binding to the artery wall and increases endothelial layer permeability by formation of lipolysis products. Circ Res 80: 819–828, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science 269: 1435–1439, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Schutz GJ, Kada G, Pastushenko VP, Schindler H. Properties of lipid microdomains in a muscle cell membrane visualized by single molecule microscopy. EMBO J 19: 892–901, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 387: 569–572, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100: 1230–1239, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Switzer KC, Fan YY, Wang N, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in Th1-polarized murine CD4+ T-cells. J Lipid Res 45: 1482–1492, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viswanathan S, Hammock BD, Newman JW, Meerarani P, Toborek M, Hennig B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr 22: 502–510, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol 79: 350–354, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem 275: 261–270, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension 47: 74–80, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol 284: H605–H612, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Zilversmit DB Atherogenesis: a postprandial phenomenon. Circulation 60: 473–485, 1979. [DOI] [PubMed] [Google Scholar]
- 50.Zilversmit DB Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem 41: 153–158, 1995. [PubMed] [Google Scholar]
- 51.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA 100: 2730–2735, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]