Abstract
We have reported that eucapnic intermittent hypoxia (E-IH) causes systemic hypertension, elevates plasma endothelin 1 (ET-1) levels, and augments vascular reactivity to ET-1 and that a nonspecific ET-1 receptor antagonist acutely lowers blood pressure in E-IH-exposed rats. However, the effect of chronic ET-1 receptor inhibition has not been evaluated, and the ET receptor subtype mediating the vascular effects has not been established. We hypothesized that E-IH causes systemic hypertension through the increased ET-1 activation of vascular ET type A (ETA) receptors. We found that mean arterial pressure (MAP) increased after 14 days of 7 h/day E-IH exposure (109 ± 2 to 137 ± 4 mmHg; P < 0.005) but did not change in sham-exposed rats. The ETA receptor antagonist BQ-123 (10 to 1,000 nmol/kg iv) acutely decreased MAP dose dependently in conscious E-IH but not sham rats, and continuous infusion of BQ-123 (100 nmol·kg−1·day−1 sc for 14 days) prevented E-IH-induced increases in MAP. ET-1-induced constriction was augmented in small mesenteric arteries from rats exposed 14 days to E-IH compared with those from sham rats. Constriction was blocked by the ETA receptor antagonist BQ-123 (10 μM) but not by the ET type B (ETB) receptor antagonist BQ-788 (100 μM). ETA receptor mRNA content was greater in renal medulla and coronary arteries from E-IH rats. ETB receptor mRNA was not different in any tissues examined, whereas ET-1 mRNA was increased in the heart and in the renal medulla. Thus augmented ET-1-dependent vasoconstriction via vascular ETA receptors appears to elevate blood pressure in E-IH-exposed rats.
Keywords: sleep apnea, BQ-123, BQ-788, mitochondrial ribonucleic acid
obstructive sleep apnea is characterized by repeated upper airway obstruction during sleep and affects between 5% and 20% of the population (32). This syndrome of sleep-disordered breathing leads to episodic hypoxia and sleep deprivation. Recent epidemiological studies reveal that sleep apnea and other forms of sleep-disordered breathing increase the risk for hypertension and associated metabolic disorders (24). However, the mechanisms whereby sleep apnea and the associated eucapnic intermittent hypoxia (E-IH) cause systemic hypertension remain incompletely understood. Recent studies show that patients with sleep apnea have increased circulating levels of endothelin (ET)-1 (29, 42), a potent vasoconstrictor and mitogenic peptide implicated in the development of many forms of arterial hypertension (1). Furthermore, the synthesis of ET-1 is increased by hypoxia, shear stress, oxidative stress, and catecholamines, which are elevated in E-IH-exposed rats and sleep apnea (42, 47). We have previously described that both blood pressure and plasma ET-1 levels are increased after 12 to 14 days of E-IH exposure in rats and that a nonselective ET-1 receptor antagonist acutely lowers blood pressure in E-IH rats to control levels but does not affect blood pressure in sham rats (31). Thus increased circulating ET-1 appears to be one important underlying cause of hypertension in this rat model.
ET-1 elicits its effects by activating one of two receptor subtypes on target cells. Vascular endothelial cells appear to express primarily endothelin type B (ETB) receptors, and the activation of these ETB receptors increases intercellular [Ca2+], resulting in increased nitric oxide production and vasodilation (44). Arterial vascular smooth muscle cells express both endothelin type A (ETA) and ETB receptors (16). Activation of these receptors also increases intracellular [Ca2+], leading to the contraction of the smooth muscle and vasoconstriction (49). ETA receptors appear to be more important for arterial constriction, whereas ETB receptors contribute to venous constriction, ET-1 clearance, and endothelium-dependent dilation (48). Therefore, ET-1-induced increases in vascular tone may be due to changes in the type, location, or the number of receptors expressed. For example, rats with fructose-induced hypertension have increased vascular ETA receptor mRNA compared with that of control rats and demonstrate a fall in blood pressure when ETA receptors are blocked (30). Mice with genetic disruption of the ETB receptor have significantly higher blood pressure despite similar plasma levels of ET-1 compared with those of the controls (44), whereas the blockade of ETB receptors in normal rats causes a salt-dependent increase in mean arterial pressure (MAP) (50). ETB receptors also participate in the plasma clearance of ET-1, mainly in the lung (19). Thus decreased ETB receptor expression in the lungs could contribute to increased plasma levels of ET-1 as observed in E-IH.
Thus both increased ETA receptor expression and the loss of ETB receptors contribute to a variety of forms of hypertension. The regulation of ETA and ETB receptor transcription is not completely defined although the promoter regions for both genes have been sequenced (7, 59). Both promoters have several response elements in common including activator protein (AP)-1, glucocorticoid response elements, and CCAAT/enhancer-binding protein (58), all of which can be activated during pressure-induced stretch and/or environmental stress. Therefore, E-IH may directly or indirectly regulate the expression of ET-1 receptors to contribute to E-IH-induced hypertension.
We recently reported that the increased vasoconstrictor sensitivity of mesenteric arteries to ET-1 is accompanied by the increased expression of the ETA receptor protein without any change in the ETB receptor protein (4). Thus E-IH appears to alter ET receptor protein levels in this particular vascular bed, and we utilized our rat model of E-IH to further investigate the role of ETA and ETB receptor activation and expression in E-IH-induced hypertension. Studies were designed to test the hypothesis that E-IH causes systemic hypertension through the increased ET-1 activation of vascular ETA receptors. These studies examined the effect of the chronic and acute administration of the specific ETA receptor antagonist BQ-123 on arterial blood pressure and analyzed ET-1, ETA receptor, and ETB receptor mRNA in arteries, hearts, lungs, and kidneys from animals exposed to either E-IH or sham conditions. Finally, receptor antagonists were used to define the receptor subtype mediating ET-1-induced constriction in small mesenteric resistance arteries.
METHODS
Animal Preparation
Male Sprague-Dawley rats (250–300 g; Harlan, Houston, TX) were prepared for E-IH exposure as described previously (31). Briefly, animals used for acute antagonist studies were instrumented with arterial and venous catheters surgically implanted via the femoral vessels. For the chronic antagonist studies, telemetry devices for recording arterial pressure were implanted into the abdominal aorta through the femoral artery with the body of the telemeter fixed in the peritoneal cavity. Two other groups of rats without catheters were exposed to E-IH or sham cycling for 14 days. In these rats, systolic blood pressure was recorded using a tail cuff on day 1 and day 14 to confirm the effect of the E-IH protocol on blood pressure. This exposure protocol (90 s of N2, CO2 influx until inspired air contained 5% O2 and 5% CO2 followed by a 90-s cage flush with room air) causes eucapnic hypoxia as recently reported (54). These rats were euthanized on day 14, and tissues were collected for the analysis of mRNA and contractile studies. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine and conform to National Institutes of Health guidelines for animal use.
ETA Antagonist Studies
To evaluate the contribution of ETA receptor activation to acute blood pressure control, rats were exposed to either E-IH or sham conditions for 14 days and then given either an ETA receptor antagonist (BQ-123; 0.1, 1, 10, 100, 1,000, and 3,000 nmol/kg) or equal volumes of vehicle (saline) as an intravenous bolus on day 15 before E-IH exposure. MAP and heart rate were recorded before, during, and after treatment. For the chronic studies, BQ-123 was infused subcutaneously via an osmotic minipump for 14 days (100 nmol·kg−1·day−1), and MAP was recorded 24 h/day using telemetry devices (DSI, model C-40).
Mesenteric Arteriole Constrictor Studies
Small mesenteric artery segments were dissected from the vascular arcade, cannulated, and pressurized as described previously (4). Arterial segments secured at both ends onto glass cannulae in a vessel chamber (Living Systems) were slowly pressurized to 60 mmHg with physiological salt solution (PSS; in mmol/l) of 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, and 5.5 glucose using a servo-controlled peristaltic pump (Living Systems). Pressurized segments were superfused with oxygenated PSS at 37°C (21% O2, 5% CO2, and balance N2). The endothelium was disrupted in some arteries by passing air through the lumen, and only arteries where the acetylcholine-mediated vasodilation of constricted arteries was eradicated were considered to be endothelium disabled. After baseline internal diameter was determined, the arteries were exposed for 10 min to the ETA receptor blocker BQ-123 (0.1 or 1.0 μM), the ETB receptor blocker BQ-788 (1 μM), or vehicle. After inhibitor incubation, the arteries were exposed to increasing concentrations of ET-1 (10−10 to 10−8 mol/l) with the antagonist continuously present in the superfusion media. Each artery was used for only one concentration response curve and after the ET-1 exposure was superfused with Ca2+-free PSS to cause complete dilation and demonstrate that the constriction was caused by active tone. Only data from arteries dilating 100% or greater to Ca2+-free PSS were used.
mRNA Expression
The thoracic aorta, mesenteric artery cascade, renal medulla, renal cortex, left ventricle of the heart, and the upper right lobe of the lung were collected and placed in RNAlater (Ambion) from pentobarbital sodium-anesthetized rats (150 mg/kg). Total RNA was isolated using a RNeasy kit (Qiagen) and used to synthesize cDNA by reverse transcription using a high-capacity reverse transcription kit (Applied Biosystems). PreproET-1, ETA receptor, and ETB receptor mRNA levels were evaluated using TaqMan gene expression assays (Applied Biosystems). PCR was performed using the Applied Biosystems 7500 Fast Real-Time PCR System. The comparative critical threshold (CT) method (2−ΔΔCT) for the relative quantification of gene expression was used (38) with 18S mRNA as the reference gene. The average of sham was used as the calibrator.
Data Analysis and Statistics
Data are reported as means ± SE. Two- or three-way ANOVA with Tukey's post hoc tests were used to compare between groups and treatments. T-tests were used for single measurements in two groups. Values for n represent the number of animals used. A P value of ≤0.05 was considered statistically significant.
RESULTS
Mean Arterial Pressure and Effect of ETA Receptor Antagonist
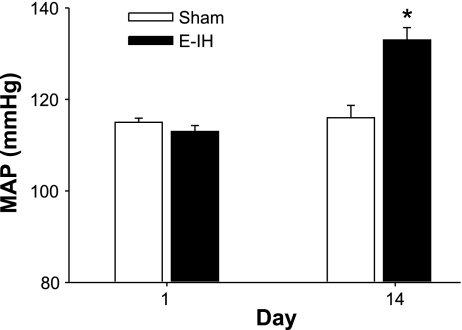
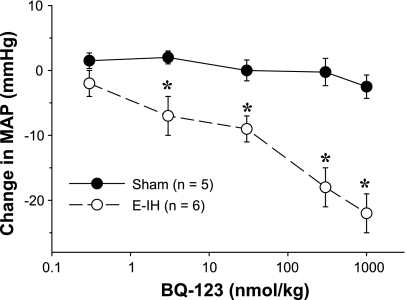
After 14 days, MAP measured with indwelling arterial catheters was elevated in E-IH compared with the sham rats (Fig. 1). This is consistent with our earlier work that measured arterial pressure daily over 12 days and documented a similar increase in MAP (31). The administration of boluses with increasing doses of the ETA receptor antagonist BQ-123 in 100 μl of phosphate-buffered saline (PBS) caused a dose-dependent decrease in MAP in the E-IH-exposed rats. In contrast, the antagonist did not affect MAP in sham rats (Fig. 2). After the administration of the highest dose of the antagonist, MAP was not different between E-IH (112 ± 5 mmHg) and sham (110 ± 2 mmHg) rats. The administration of PBS alone did not affect blood pressure in either group (data not shown).
Fig. 1.
Mean arterial pressure (MAP) on day 1 and day 14. MAP was recorded via an indwelling arterial catheter before the start of eucapnic intermittent hypoxia (E-IH) exposure on days 1 and 14. Blood pressure was significantly elevated in the E-IH rats on day 14 (*) compared with that of both day 1 E-IH values and day 14 control animals for P ≤ 0.05 (n = 8 for each group).
Fig. 2.
MAP changes to BQ-123 administration. BQ-123 in phosphate-buffered saline was administered as a bolus dose (10, 30, 100, 300, and 1,000 nml/kg) via the venous catheter. MAP was recorded continuously, and each dose was administered 10 min apart in increasing order. The endothelin type A (ETA) receptor antagonist lowered MAP in E-IH but not sham rats, and the decrease was both significant (*P ≤ 0.05) and dose dependent. At the highest dose of BQ-123 (1,000 nmol/kg), MAP was not different between sham and E-IH rats.
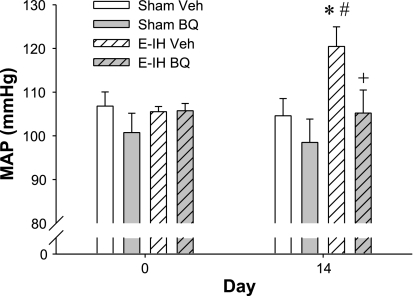
The chronic administration of BQ-123 (100 nmol·kg−1·day−1 sc) prevented the E-IH-induced increase in MAP recorded with telemetry (Fig. 3) but did not affect MAP in the sham rats and did not affect heart rate in either group (data not shown). The administration of the vehicle (PBS) did not affect MAP in either group.
Fig. 3.
Effect of chronic BQ-123 (BQ) administration on blood pressure. MAP was recorded using indwelling telemetery devices. The 24-h average on day 14 was significantly elevated in the E-IH vehicle (Veh) group compared with the sham Veh (*) and in the E-IH Veh group on day 0 (#). MAP in the E-IH group treated with BQ-123 was different from the E-IH Veh group (+) but not from the sham groups.
ET-1 Vasoconstriction
ETA receptor antagonist.
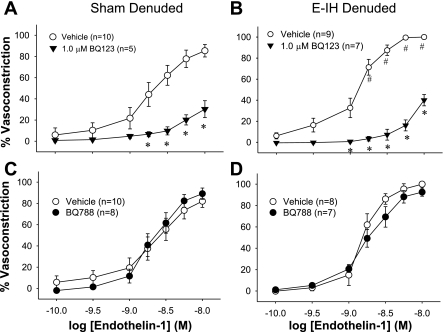
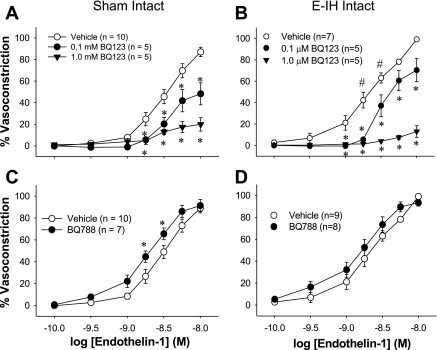
ET-1 was more potent at eliciting constriction in E-IH than in sham arteries, as in our previous studies (4). The ETA receptor antagonist (BQ-123) inhibited ET-1 constriction in both denuded (Fig. 4) and intact (Fig. 5) mesenteric arteries from sham and E-IH rats (Fig. 4). The inhibition was concentration dependent and similar in the two groups of arteries (Table 1).
Fig. 4.
Effect of ET receptor antagonists on ET-1 constriction in endothelium-inactivated arteries. Cumulative addition of ET-1 caused a concentration-dependent constriction of endothelium-inactivated mesenteric arteries from both sham and E-IH rats. BQ-123 inhibited constriction in arteries from sham rats (A) and those from E-IH rats (B) in a concentration-dependent manner (*different from vehicle for P < 0.05). BQ-788 did not inhibit constriction (C and D). #Significant difference from sham for P < 0.05.
Fig. 5.
Effect of ET receptor antagonists on ET-1 constriction. Cumulative addition of ET-1 caused a concentration-dependent constriction of endothelium-intact mesenteric arteries from both sham and E-IH rats. BQ-123 inhibited constriction in arteries from sham rats (A) and those from E-IH rats (B) in a concentration-dependent manner (*different from vehicle for P < 0.05). BQ-788 increased constriction slightly in sham but not E-IH arteries (C and D). #Significant difference from sham for P < 0.05.
Table 1.
Effect of endothelin antagonists on EC50
| Treatment | Sham | E-IH | ||
|---|---|---|---|---|
| Intact mesenteric arteries EC50, nmol/l ± SE | ||||
| Vehicle | 3.1±0.4 | 2.0±0.2 | ||
| BQ-123, 0.1 μM | 13.05±0.5* | 5.5±0.1* | ||
| BQ-123, 1 μM | 146.9±1.5* | 144.3±1.6 | ||
| BQ-788, 1 μM | 2.1±0.4* | 1.5±0.3 | ||
| Endothelium-inactivated mesenteric arteries EC50, nmol/l ± SE | ||||
| Vehicle | 2.8±0.2 | 1.2±0.1 | ||
| BQ-123, 1 μM | 21.9±1.2* | 12.4±1.4* | ||
| BQ-788, 1 μM | 1.9±0.3 | 1.8±0.1 | ||
Values are means ± SE. E-IH, eucapnic intermittent hypoxia.
Significantly different from vehicle.
ETB receptor antagonist.
The ETB receptor antagonist (BQ-788) did not affect ET-1 constriction in endothelium-inactivated arteries from either sham or E-IH rats (Fig. 4) but augmented constriction significantly in intact arteries from sham arteries (Fig. 5). The combination of BQ-123 and BQ-788 was similar to BQ-123 alone in denuded arteries (data not shown).
Endothelin System mRNA
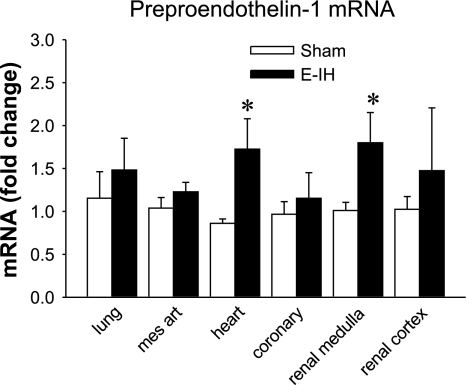
Preproendothelin-1 mRNA was present in all of the tissues evaluated (Fig. 6). In the left ventricle of the heart and the renal medulla, normalized ET-1 mRNA expression was significantly greater in the E-IH than in the sham tissues. In the other tissues examined, there was no significant difference in ET-1 mRNA expression between groups, but it tended to be elevated in all E-IH tissues.
Fig. 6.
Preproendothelin-1 mRNA. ET-1 mRNA expression was calculated as fold change from sham normalized to 18S mRNA. *Significantly different from controls for P ≤ 0.05 (n = 5). Mes art, mesenteric arteries.
ETA Receptor mRNA
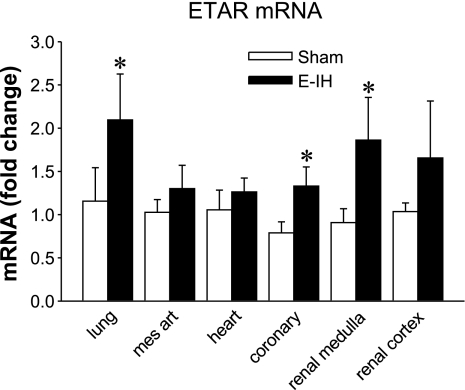
ETA receptor mRNA was present in all of the tissues evaluated (Fig. 7). In coronary arteries, the lung, and renal medulla homogenates, normalized ETA receptor mRNA expression was significantly greater in E-IH than in sham tissues. There were no significant differences in normalized ETA receptor mRNA expression in other tissues although levels again tended to be higher in the E-IH tissues.
Fig. 7.
ETA receptor (ETAR) mRNA. ETA receptor mRNA expression was calculated as fold change from sham normalized to 18S mRNA. *Significantly different from controls for P ≤ 0.05 (n = 5).
ETB Receptor Protein
The ETB receptor protein was present in all of the tissues evaluated, but there were no significant differences in normalized ETB receptor protein levels between groups for any of the tissues examined (data not shown).
DISCUSSION
The goal of this study was to determine whether ET-1-dependent hypertension in E-IH-treated rats is caused by the increased activation of vascular ETA receptors. The major findings are: 1) ETA receptor antagonist BQ-123 acutely lowers blood pressure in E-IH but not in sham rats and prevents E-IH-induced increases in arterial pressure, 2) ET-1 constriction in mesenteric arteries is mediated through ETA receptor-dependent constriction, 3) preproendothelin-1 mRNA expression is also increased in the renal medulla and the left ventricle, 4) ETA receptor mRNA is greater in the renal medulla, lungs, and coronary arteries of E-IH compared with sham rats, and 5) ETB receptor mRNA expression is not different in tissues from E-IH and sham rats.
Together these finding suggest that increased circulating levels of ET-1 in E-IH rats come from the kidney, heart, or lungs and act on vascular and/or renal ETA receptors to cause ET-1-dependent hypertension.
Clinical studies have demonstrated that elevated circulating ET-1 contributes to the development of systemic hypertension, especially in low-renin forms of hypertension (36). In addition, patients with sleep apnea also have elevated circulating levels of ET-1 (52, 55) that correlate with the severity of apnea (52). We have previously demonstrated that rats exposed to E-IH during sleep to simulate the hypoxemia of sleep apnea have increased circulating ET-1 levels (31) and augmented constrictor responses to ET-1 (4). Therefore, these new data support the hypothesis that repeated exposure to E-IH increases ET-1-dependent vasoconstriction to contribute to the associated hypertension.
The higher levels of preproendothelin-1 mRNA observed in the renal medulla and heart from E-IH rats suggest that elevated circulating ET-1 results from increased transcription in these sites. This is in agreement with other studies of ET-1 synthesis that suggest plasma ET-1 levels are primarily regulated at the level of promoter activation (15, 28) and that the kidney and heart are primary sites of ET-1 production (17, 25). Several transcription factors have been identified that modulate ET-1 synthesis including hypoxia inducible factor (HIF) (11, 41). The increased ET-1 message in the renal medulla and the left ventricle could be caused by E-IH activation of HIF-mediated transcription. This is in agreement with studies in which a similar protocol of E-IH increased blood pressure and ET-1 synthesis in wild-type but not in HIF-1 knockout mice (45). In addition, E-IH increases oxidative stress (37), which in turn increases levels of transcription factors such as AP-1 and NF-κB (37), which also stimulate ET-1 synthesis (18, 51). Previous work in our laboratory has shown that the treatment of E-IH rats with the superoxide dismutase Tempol prevents the increase both in MAP and in circulating ET-1, suggesting oxidative stress is a primary stimulus for the increased ET-1 synthesis (56). Thus multiple stimuli may interact to increase the synthesis and release of ET-1 during IH.
Chronic elevations in circulating ET-1 cause hypertension in animal models by direct ETA receptor-mediated vasoconstriction (1), by activating the renin-angiotensin system (8), or through effects on the renal handling of sodium (27, 33) and activation of the sympathetic nervous system (26, 40). Our previous studies using this model of sleep apnea demonstrate that the hypertension is acutely reversed with a nonselective ET-1 receptor antagonist, PD-145065 (31), whereas the current study demonstrates that acute ETA receptor antagonism also reverses IH-induced hypertension but acts more potently and efficaciously than the nonselective antagonist, suggesting that the ETA receptor mediates this response. Chronic treatment with a low concentration of this same antagonist completely prevented the development of hypertension, suggesting that this mechanism is a primary cause of the hypertension and not a secondary result of the sustained increase in blood pressure. Furthermore, our results demonstrate the increased expression of ETA receptor mRNA in kidneys from E-IH-exposed rats, suggesting that the increased ETA receptor activation may be mediated by both increased circulating ET-1 (31) and by increased ETA receptor expression in some target organs. Protocols in this study did not directly address the role of changes in the receptor expression in the renal medulla, but the E-IH-induced increase suggests the renal medulla is a potential contributor to the ET-1-dependent hypertension. Therefore, increased circulating ET-1 acting on an increased number of ETA receptors appears to contribute to E-IH-induced hypertension.
This is consistent with previous reports in other models of hypertension wherein the elevated expression of ETA receptors appears to lead to increased ET-1-mediated vasoconstriction (6, 30). Our data demonstrate that small mesenteric arteries from hypertensive E-IH rats have increased vasoconstrictor sensitivity to ET-1 that is blocked by an ETA receptor antagonist but not by an ETB receptor antagonist. Although vascular ETA receptor mRNA levels were not elevated, constriction was blocked only by the ETA antagonist, suggesting altered receptor signaling or the increased stability of the protein contributes to the elevated constrictor response. Both are supported by our previous observations that these arteries have a modest increase in the protein levels of the ETA receptor and that postreceptor signal transduction is altered by E-IH exposure (4, 5). Indeed, ETA receptors appear to activate PKCδ in arteries from E-IH-exposed rats but not in sham arteries, further indicating that E-IH alters the signaling cascade of this receptor in vascular smooth muscle (3). Recent studies in diabetic rats also found that despite elevated levels of ET-1, vascular sensitivity to ET-1 was increased in small but not large arteries (53). Thus it appears that the elevated synthesis of ET-1 does not always lead to impaired vascular sensitivity to the peptide.
The depressor response to ETA receptor blockade in vivo further indicates that vascular ETA receptor activation contributes more to blood pressure control in E-IH than in sham rats. The transcriptional regulation of ET receptors is less well defined than the transcriptional regulation of ET-1, but several in vivo conditions have been identified that increase ETA receptor mRNA including elevated glucocorticoids (12). E-IH acutely activates the sympathetic nervous system and increases cortisol levels (13), suggesting adrenal steroids could mediate the modest increase in ETA receptor mRNA levels. However, the steroid induction of the ETA receptor is somewhat controversial (34, 57), and other studies will be needed to decipher the stimulus for this response.
Chronic hypoxia also stimulates ET-1 synthesis (2, 14) and augments ETA receptor-mediated vasoconstriction (9, 10), suggesting that similar mechanisms may mediate the intermittent- and chronic hypoxia-induced synthesis of ET-1 and ETA receptor expression. It is of particular interest that the expression of pulmonary ETA receptors is increased in the E-IH rats, providing a potential mechanism for the pulmonary hypertension previously reported in animals exposed to IH (20).
Vascular ETB receptors may be expressed in both endothelial and smooth muscle cells to mediate endothelium-dependent vasodilation and direct vasoconstriction. We found that ETB receptor expression was not changed in any of the tissues examined. A reasonable interpretation is that the relative contribution of the ETB receptor to the overall ET-1 effect in E-IH-exposed rats is diminished given that ETA receptor expression was increased. Thus our previous observation that E-IH exposure leads to elevated circulating ET-1 levels does not appear to be due to the diminished clearance of the peptide by ETB receptors but rather due to enhanced synthesis. In addition, ETB-mediated arterial constriction does not appear to be an important contributor to systemic hypertension in this setting based on the lack of effect of the ETB receptor antagonist in the denuded arteries and the increased efficacy of the ETA receptor antagonist compared with previous reports of the nonselective antagonist (31). However, the slight augmentation of constriction in the endothelium-intact sham but not E-IH arteries by the ETB receptor blocker suggests there may also be loss of ETB receptor-mediated vasodilation. Although our hemodynamic data are consistent with the increased activation of central, renal, or vascular ETA receptors, the increased vascular expression of ETA receptors (4) coupled with our previous observation of increased vascular constrictor sensitivity to ET-1 is most consistent with at least a portion of the hypertension being dependent on the ET-1 activation of vascular ETA receptors. Therefore, we conclude that the increased ET-1 activation of vascular and/or renal ETA receptors mediates the hypertension in this model with a potential small contribution of the loss of ETB receptor-mediated dilation.
Epidemiological evidence shows a strong association between sleep apnea and hypertension, yet functional links between intermittent exposure to hypoxia and elevated arterial pressure are not clearly defined. Studies from Fletcher and coworkers (23) in rats exposed to several weeks of IH suggest that the resultant hypertension is caused by the activation of either the sympathetic nervous system (21) or the renin angiotensin system (22). However, epidemiological studies (35, 43) suggest that the activation of the sympathetic nervous system only partially explains the increased incidence of hypertension in sleep apnea patients. Rather, the activation of multiple systems during this syndrome produces a hypertension that is resistant to most antihypertensive treatments aimed at a single system (39). Our studies showing increased ET-1 levels, augmented depressor responses to ETA receptor blockade, and the elevated expression of ETA receptors support the concept that this model of hypertension requires the activation of ETA receptors. Furthermore, ETA receptor activation appears to be mediated by a combination of increased vascular receptor number, the increased synthesis of ET-1, and altered postreceptor signaling. This finding in our rat model coupled with the observation of increased circulating levels of ET-1 in sleep apnea patients (46, 52) suggests that targeting ETA receptors may be a selective and effective treatment for hypertension secondary to sleep apnea.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-58124, HL-63207 (to B. R. Walker), and HL-03852 (to N. L. Kanagy) and American Heart Association Desert Mountain Affiliate Grant-in-Aid (to N. L. Kanagy and L. D. Nelin). N. L. Kanagy is an established investigator of the American Heart Association.
Acknowledgments
We thank Michael Paffett and Laura Gonzalez Bosc for assistance in conducting the RNA studies and Laura Duling for assistance with the blood pressure studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Syst 3: 1–15, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre JI, Morrell NW, Long L, Clift P, Upton PD, Polak JM, Wilkins MR. Vascular remodeling and ET-1 expression in rat strains with different responses to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 278: L981–L987, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCδ. Am J Physiol Heart Circ Physiol 294: H920–H927, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension 45: 705–709, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Allahdadi KJ, Walker BR, Kanagy NL. ROK contribution to endothelin-mediated contraction in aorta and mesenteric arteries following intermittent hypoxia/hypercapnia in rats. Am J Physiol Heart Circ Physiol 293: H2911–H2918, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Allcock GH, Venema RC, Pollock DM. ETA receptor blockade attenuates the hypertension but not renal dysfunction in DOCA-salt rats. Am J Physiol Regul Integr Comp Physiol 275: R245–R252, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Arai H, Nakao K, Takaya K, Hosoda K, Ogawa Y, Nakanishi S, Imura H. The human endothelin-B receptor gene. Structural organization and chromosomal assignment. J Biol Chem 268: 3463–3470, 1993. [PubMed] [Google Scholar]
- 8.Ballew JR, Watts SW, Fink GD. Effects of salt intake and angiotensin II on vascular reactivity to endothelin-1. J Pharmacol Exp Ther 296: 345–350, 2001. [PubMed] [Google Scholar]
- 9.Bialecki RA, Fisher CS, Abbott BM, Barthlow HG, Caccese RG, Stow RB, Rumsey J, Rumsey W. ZD1611, an orally active endothelin-A receptor antagonist, prevents chronic hypoxia-induced pulmonary hypertension in the rat. Pulm Pharmacol Ther 12: 303–312, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Bialecki RA, Stinson-Fisher C, Murdoch W, Bertelsen D, Desiato M, Rumsey W. A novel orally active endothelin-A receptor antagonist, ZD1611, prevents chronic hypoxia-induced pulmonary hypertension in the rat (Abstract). Chest 114: 91S, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Bodi I, Bishopric NH, Discher DJ, Wu X, Webster KA. Cell-specificity and signaling pathway of endothelin-1 gene regulation by hypoxia. Cardiovasc Res 30: 975–984, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Borcsok I, Schairer HU, Sommer U, Wakley GK, Schneider U, Geiger F, Niethard FU, Ziegler R, Kasperk CH. Glucocorticoids regulate the expression of the human osteoblastic endothelin A receptor gene. J Exp Med 188: 1563–1573, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratel T, Wennlund A, Carlstrom K. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP). Respir Med 93: 1–7, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Chen SJ, Chen YF, Opgenorth TJ, Wessale JL, Meng QC, Durand J, DiCarlo VS, Oparil S. The orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling in Sprague-Dawley rats. J Cardiovasc Pharmacol 29: 713–725, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Chua CC, Hamdy RC, Chua BH. Regulation of endothelin-1 production by a thromboxane A2 mimetic in rat heart smooth muscle cells. Biochim Biophys Acta 1313: 1–5, 1996. [DOI] [PubMed] [Google Scholar]
- 16.David FL, Montezano AC, Reboucas NA, Nigro D, Fortes ZB, Carvalho MH, Tostes RC. Gender differences in vascular expression of endothelin and ETA/ETB receptors, but not in calcium handling mechanisms, in deoxycorticosterone acetate-salt hypertension. Braz J Med Biol Res 35: 1061–1068, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Deng LY, Schiffrin EL. Endothelin-1 gene expression in blood vessels and kidney of spontaneously hypertensive rats (SHR), l-NAME-treated SHR, and renovascular hypertensive rats. J Cardiovasc Pharmacol 31 Suppl 1: S380–S383, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Drunat S, Moatti N, Demuth K. Homocysteine decreases endothelin-1 expression by interfering with the AP-1 signaling pathway. Free Radic Biol Med 33: 659–668, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol 81: 1510–1515, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Fagan KA Selected contribution: pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol 90: 2502–2507, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher EC Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol 119: 189–197, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol 92: 627–633, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Foresman BH, Gwirtz PA, McMahon JP. Cardiovascular disease and obstructive sleep apnea: implications for physicians. J Am Osteopath Assoc 100: 360–369, 2000. [PubMed] [Google Scholar]
- 25.Girchev R, Bäcker A, Markova P, Kramer HJ. Renal endothelin system and excretory function in Wistar-Kyoto and Long-Evans rats. Acta Physiol (Oxf) 186: 67–76, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Gulati A, Kumar A, Morrison S, Shahani BT. Effect of centrally administered endothelin agonists on systemic and regional blood circulation in the rat: role of sympathetic nervous system. Neuropeptides 31: 301–309, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh TJ, Lin SR, Lee YJ, Shin SJ, Lai YH, Hsu CH, Tsai JH. Increased renal medullary endothelin-1 synthesis in prehypertensive DOCA- and salt-treated rats. Am J Physiol Renal Physiol 279: F112–F121, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun 245: 894–899, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Jordan W, Reinbacher A, Cohrs S, Grunewald RW, Mayer G, Ruther E, Rodenbeck A. Obstructive sleep apnea: plasma endothelin-1 precursor but not endothelin-1 levels are elevated and decline with nasal continuous positive airway pressure. Peptides 26: 1654–1660, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Juan CC, Fang VS, Hsu YP, Huang YJ, Hsia DB, Yu PC, Kwok CF, Ho LT. Overexpression of vascular endothelin-1 and endothelin-A receptors in a fructose-induced hypertensive rat model. J Hypertens 16: 1775–1782, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 37: 511–515, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Kapur V, Strohl KP, Redline S, Iber C, O′Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 6: 49–54, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Konishi F, Okada Y, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y. Role of endothelin ETB receptors in the renal hemodynamic and excretory responses to big endothelin-1. Eur J Pharmacol 451: 177–184, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Koshino Y, Hayashi T, Matsukawa S, Asazuma K, Eguchi K, Kato H, Nakai T, Miyamori I. Dexamethasone modulates the expression of endothelin-1 and -A receptors in A7r5 vascular smooth muscle cells. J Cardiovasc Pharmacol 32: 665–672, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Krieger AC, Redeker NS. Obstructive sleep apnea syndrome: its relationship with hypertension. J Cardiovasc Nurs 17: 1–11, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med 338: 784–790, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Lavie L Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens 19: 2271–2277, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Matsumura K, Abe I, Fukuhara M, Tominaga M, Tsuchihashi T, Kobayashi K, Fujishima M. Naloxone augments sympathetic outflow induced by centrally administered endothelin in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 266: R1403–R1410, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Minchenko A, Caro J. Regulation of endothelin-1 gene expression in human microvascular endothelial cells by hypoxia and cobalt: role of hypoxia responsive element. Mol Cell Biochem 208: 53–62, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens 16: 274–280, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, Quan SF. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health study. Am J Epidemiol 154: 50–59, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Ohuchi T, Kuwaki T, Ling GY, Dewit D, Ju KH, Onodera M, Cao WH, Yanagisawa M, Kumada M. Elevation of blood pressure by genetic and pharmacological disruption of the ETB receptor in mice. Am J Physiol Regul Integr Comp Physiol 276: R1071–R1077, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens 17: 61–66, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Phillips BG, Somers VK. Neural and humoral mechanisms mediating cardiovascular responses to obstructive sleep apnea. Respir Physiol 119: 181–187, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Pollock DM Renal endothelin in hypertension. Curr Opin Nephrol Hypertens 9: 157–164, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J 9: 1196–1204, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP. Endothelin 1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells. Diabetes 49: 1561–1570, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Saarelainen S, Seppala E, Laasonen K, Hasan J. Circulating endothelin-1 in obstructive sleep apnea. Endothelium 5: 115–118, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Sachidanandam K, Harris A, Hutchinson J, Ergul A. Microvascular versus macrovascular dysfunction in type 2 diabetes: differences in contractile responses to endothelin-1. Exp Biol Med (Maywood) 231: 1016–1021, 2006. [PubMed] [Google Scholar]
- 54.Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104: 110–118, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Trakada G, Marangos M, Spiropoulos K. Mechanisms of endothelin-1 elevation in chronic obstructive pulmonary disease patients with nocturnal oxyhemoglobin desaturation. Respiration 68: 134–139, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol 293: H2971–H2976, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villeneuve A, Gignac S, Provencher PH. Glucocorticoids decrease endothelin-A- and -B-receptor expression in the kidney. J Cardiovasc Pharmacol 36: S238–S240, 2000. [PubMed] [Google Scholar]
- 58.Wagner AH, Krzesz R, Gao D, Schroeder C, Cattaruzza M, Hecker M. Decoy oligodeoxynucleotide characterization of transcription factors controlling endothelin-B receptor expression in vascular smooth muscle cells. Mol Pharmacol 58: 1333–1340, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita J, Yoshimasa T, Arai H, Hiraoka J, Takaya K, Miyamoto Y, Ogawa Y, Itoh H, Nakao K. Identification of cis-elements of the human endothelin-A receptor gene and inhibition of the gene expression by the decoy strategy. J Biol Chem 273: 15993–15999, 1998. [DOI] [PubMed] [Google Scholar]