Abstract
Arachidonic acid (AA) metabolites from the 15-lipoxygenase-1 (15-LO-1) pathway, trihydroxyeicosatrienoic acids (THETAs) and hydroxy-epoxyeicosatrienoic acids (HEETAs), are endothelium-derived hyperpolarizing factors (EDHFs) and relax rabbit arteries. Rabbit vascular 15-LO-1 expression, THETA and HEETA synthesis, and nitric oxide and prostaglandin-independent relaxations to acetylcholine (ACh) and AA decreased with age (neonates to 16-wk-old). We characterized age-dependent ACh-hypotensive responses in vivo in 1-, 4-, 8-, and 16-wk-old rabbits and the contribution of THETAs and HEETAs to these responses. In anesthetized rabbits, blood pressure responses to ACh (4–4,000 ng/kg) were determined in the presence of vehicle or various inhibitors. ACh responses decreased with age (P > 0.001). In the absence or presence of Nω-nitro-l-arginine methyl ester (l-NAME) and indomethacin (Indo), maximum responses in 1 (−54.7 ± 7.4 and −37.9 ± 3.9%)- and 4 (−48.8 ± 2.4 and −35.5 ± 7.8%)-wk-old rabbits were higher than 8 (−30.0 ± 2.8 and −26.6 ± 4.4%)- and 16 (−36.7 ± 3.5 and −27.3 ± 10%)-wk-old rabbits. A lipoxygenase inhibitor, BW755C, reduced THETA and HEETA synthesis in mesenteric arteries. In the presence of Indo and Nω-nitro-l-arginine, ACh relaxations were reduced by BW755C to a greater extent in the mesenteric arteries from the younger rabbits. In 4-wk-old rabbits treated with l-NAME and Indo, the maximum ACh hypotension was reduced by the potassium channel inhibitors apamin and charybdotoxin to −6.9 ± 0.9%, by apamin alone to −19.5 ± 1.4%, and by BW755C to −18.8 ± 3.5%. The present study indicates that the age-related decrease in ACh-induced hypotension is mediated by the decreased synthesis of the 15-LO-1 metabolites THETAs and HEETAs.
Keywords: endothelium-derived hyperpolarizing factor, arachidonic acid, vasodilation, mean arterial pressure
the vascular endothelium releases dilators including nitric oxide (NO), prostaglandins (PG), and endothelium-derived hyperpolarizing factors (EDHF; Ref. 7). Agonists, such as acetylcholine (ACh), induce release of these mediators from the endothelium and thus relax arteries. In the presence of endothelial NO synthase (eNOS) and cyclooxygenase (COX) inhibitors, EDHFs contribute to ACh-induced relaxations. This has been documented in rabbits (22), rats (17, 26), dogs (21), and humans (16). EDHFs compensate for the impaired NO release and activity in eNOS knockout mice (3), eNOS and COX double knockout mice (10), and hypertensive patients (30).
Several compounds have been described as EDHFs including arachidonic acid (AA) metabolites of cytochrome 450 (CYP450; Refs. 4, 11) and 15-lipoxygenase-1 (15-LO-1; Refs. 24, 25) and the potassium (K) ion (9, 36). AA metabolites of 15-LO-1, 11,12,15-trihydroxyeicosatrienoic acid (THETA) and 15-hydroxy-11,12-epoxyeicosatrienoic acid (HEETA), are EDHFs (6) and mediate the ACh-induced relaxations in rabbit arteries in the presence of NO- and PG-synthesis inhibitors Nω-nitro-l-arginine (l-NNA) and indomethacin (Indo; Refs. 2, 33). These relaxations are inhibited by high extracellular potassium concentrations (6) and the small-conductance, calcium-dependent potassium (SKCa) channel inhibitor apamin (Apa; Refs. 13, 36). In rabbit mesenteric arteries, THETA and HEETA open SKCa channels and act synergistically with a charybdotoxin [CTX; an intermediate conductance calcium-dependent potassium (IKCa) channel inhibitor]-sensitive pathway to cause relaxations (36).
Vascular endothelial 15-LO-1 is important (33) and sufficient (2) to relax rabbit arteries by synthesizing THETA and HEETA from AA (6). We (32) recently reported that the expression of 15-LO-1 decreases with age in aortas and mesenteric arteries from neonates, 1-, 4-, 8-, and 16-wk-old rabbits. As a result, the synthesis of THETAs and HEETAs decreases with age. NO- and PG-independent ACh and AA relaxations were reduced with age in isolated arteries from these rabbits. On the other hand, contractile responses to potassium and phenylepherine and relaxations to sodium nitroprusside were unaltered by age. This implies that 15-LO-1 activity and the synthesis of THETAs and HEETAs contribute to regulation of vascular tone. However, the findings in the isolated arteries cannot be generalized for all the arteries since NO, PGs, and EDHFs may have different effects on different vascular beds (14, 20). For example, THETA is more active in mesenteric arteries than in aortas of rabbits (36). Also, 15-hydroxyeicosatetraenoic acid (15-HETE) is inactive in the aorta (12) but contracts pulmonary arteries (37) of rabbits. The precursor of the THETAs, HEETAs, and 15-HETE, 15-hydroperoxyeicosatetraenoic acid, evokes relaxations at lower concentrations but contractions at higher concentrations in aorta and pulmonary arteries (19, 34). The vascular activity of the HEETAs is in need of further investigation. In addition, unknown compensatory mechanisms may occur to negate the age-related decrease in vascular relaxations. Therefore, to corroborate our in vitro findings, we characterized the ACh responses, the role of EDHFs, and the contribution of THETAs and HEETAs in vivo in 1-, 4-, 8-, and 16-wk-old rabbits.
MATERIALS AND METHODS
Rabbits and mesenteric arteries.
Animal protocols were approved by the Animal Care Committee of the Medical College of Wisconsin, and the procedures were performed in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (1996). New Zealand White rabbits (Kuiper Rabbit Ranch) 4, 8, and 16 wk of age were fed normal rabbit chow. One-week-old rabbits were on mother's milk until the time of use. For in vitro studies, mesenteric arteries were dissected from rabbits as described previously (2).
Metabolism of [14C]AA.
Arterial sections were dissected and maintained at 4°C in HEPES buffer (mM): 10 HEPES, 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, and 6 glucose, pH 7.4. The sections were cut into 2- to 3-mm rings and placed into 5 ml HEPES buffer. Rings were incubated at 37°C with Indo (10−5 M; Sigma, St. Louis, MO) or Indo and BW755C (10−4 M; Burroughs Wellcome, Sandwich, England) for 10 min, and then [14C]AA (0.5 μCi, 10−7 M) was added. After 5 min, A23187 (10−5 M; Sigma) was added. After 15 min, the reaction was stopped with ethanol (15% final concentration) and the samples were extracted using Bond Elute octadecylsilyl columns (25). The extracts were analyzed by reverse phase HPLC using solvent system I and a Nucleosil C-18 (5 μm, 4.6 × 250 mm) column. System I consisted of a 40-min linear gradient (flow rate = 1 ml/min) from 50% solvent B (acetonitrile with 0.1% glacial acetic acid) in solvent A (deionized water) to 100% solvent B. The column effluent was collected in 0.2 ml fractions, and the radioactivity was determined.
Isometric tension in mesenteric arteries.
Arterial rings were suspended in a 6 ml of tissue bath with Krebs solution of the following composition (mM): 119 NaCl, 4.7 KCl, 1.8 KH2PO4, 1.17 MgSO4, 25 NaHCO3, 2.5 CaCl2, 0.026 EDTA, and 5.5 glucose bubbled with 95% O2-5% CO2 at 37°C. Isometric tension was measured with force-displacement transducers (Danish Myo Technology) and recorded with a Macintosh computer and MacLab software. The vessels were gradually adjusted to 1-g resting tension and allowed to equilibrate for 1 h. The vessels were then tested for the maximum response with 60 mM KCl as described previously (6). The rings were treated with Indo (10−5 M) and l-NNA (Sigma; 3 × 10−5 M) for 10 min and then contracted by phenylephrine (∼l0−7 M) to 50–60% of the maximal KCl contraction. In addition to Indo and l-NNA, rings were also treated with either BW755C (10−4 M) or CTX (2 × 10−7 M) or both. Cumulative concentrations of ACh (Sigma; 10−9-10−5 M) were added to the bath, and changes in isomeric tension were measured. Vasorelaxation was expressed as a percentage of maximum precontraction.
Mean arterial pressure and measurement of ACh responses.
New Zealand white rabbits of 4, 8, and 16 wk of age were anesthetized with 20 mg/kg pentobarbital intravenously through the marginal ear vein. Neonate rabbits (1-wk-old) were anesthetized with 20 mg/kg pentobarbital intraperitoneally. Anesthesia was immediately followed by endotracheal intubation and artificial ventilation. A catheter was placed into the right carotid artery and connected to a pressure transducer (model MS-01699, AD Instruments), and the mean arterial pressure (MAP) was recorded into Mac-Lab Chart 7.0 software with PowerLab amplifier and A/D converters. A catheter was placed in the right jugular vein for drug administration. After these procedures, rabbits were given 30 min for MAP to stabilize. ACh (0.4–4,000 ng/kg iv) was administered, and the change in MAP was recorded for each ACh dose. For measuring PG- and NO-independent ACh responses, rabbits were given Indo (6 mg/kg ip) and N-nitro-l-arginine methyl ester (l-NAME; 20 mg/kg and 5 mg·kg−1·h−1 iv). After 30 min, ACh responses were recorded. In the second group of rabbits, Indo, l-NAME plus the SKCa channel inhibitor Apa (50 μg/kg iv; Sigma) or Indo, l-NAME, Apa, and the IKCa channel inhibitor charybdotoxin (CTX; 50 μg/kg iv; Sigma; Ref. 29) were given and the responses to ACh were measured. In the third group of animals, the effects of 15-LO inhibition were determined. BW755C (30 mg/kg iv) was administered with Indo and l-NAME, and the ACh responses were measured after 30 min. All the drugs except Indo were dissolved in normal saline (0.9% w/v NaCl) and were injected in a volume of 0.1 ml followed by 0.1 ml of saline. Indo was suspended in normal saline for intraperitoneal injection.
Data interpretation and statistical analysis.
The decrease in MAP to each dose of ACh was measured as the percent decrease from the basal values. Relaxations in isolated mesenteric arteries were measured as a decrease in tension upon cumulative concentrations of ACh. The experimental data were expressed as means ± SE. A repeated-measures two-way ANOVA was performed to analyze responses to each ACh dose in rabbits or in isolated arteries in an age group, followed by Bonferroni's posttest. Weight, MAP, and heart rate (HR) values among the four groups were analyzed by repeated-measures two-way ANOVA. The effect of inhibitors on MAP and HR in an age group was analyzed by unpaired student t-test. Values were considered significant at P < 0.05 or smaller.
RESULTS
Basal hemodynamics.
For each experiment, 6–11 animals/group were used. Table 1 shows the ages, weights, and basal hemodynamic measurements. Basal HR and MAP were measured after the 30-min stabilization period. The weight and MAP of the rabbits increased significantly (P < 0.01) with age (Table 1). The HR increased between 1 and 4 wk of age but did not change further in older rabbits (Table 1).
Table 1.
Weights, MAP, and HR in groups of rabbits at different ages. Effects of l-NAME, Indo, and ACh
| Age, wk | n | Weight*, kg |
Resting Conditions (no treatment) |
Indo + l-NAME Treatment
|
HR (beats/min) in Presence of 4,000 ng/kg ACh
|
|||
|---|---|---|---|---|---|---|---|---|
| MAP*, mmHg | HR, beats/min | MAP, mmHg | HR, beats/min | No Treatment | Indo + l-NAME | |||
| 1 | 6 | 0.093±0.01 | 18.1±1.8 | 190±7‡ | 31.7±7.0† | 186±5‡ | 188±2‡ | 196±13‡ |
| 4 | 11 | 0.527±0.02 | 46.1±10.7 | 282±18 | 68.9±10.9† | 281±18 | 270±12 | 272±14 |
| 8 | 7 | 1.41±0.1 | 83.4±4.0 | 247±36 | 81.0±6.2 | 249±34 | 262±14 | 250±20 |
| 16 | 7 | 2.33±0.2 | 102.3±5.8 | 264±14 | 105.5±6.2 | 261±10 | 258±20 | 248±19 |
Each value represents mean ± SE. l-NAME, Nω-nitro-l-arginine methyl ester; MAP, mean arterial pressure; HR, heart rate; Indo, indomethacin; ACh, acetylcholine.
P < 0.0001, statistically significant difference among the 4 groups in a column;
P < 0.05, statistically significant difference from the corresponding MAP at the resting condition;
P < 0.001, statistically significant difference from the HR values in other age groups.
Age-related decrease in ACh-induced hypotensive responses.
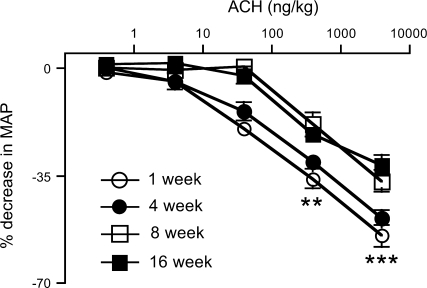
ACh caused a dose-related decrease in MAP in 1- to 16-wk-old rabbits. These ACh responses were similar for 1- and 4-wk-old rabbits (Fig. 1). The maximum decrease in MAP to ACh was significantly decreased in 8 (−30.0 ± 2.8%)- and 16 (−36.7 ± 3.5%)-wk-old rabbits when compared with 1 (−54.7 ± 7.4%)- or 4 (−48.8 ± 2.4%)-wk-old rabbits (P < 0.0001; Fig. 1). This decrease in MAP is not due to the direct effect of ACh on HR. The basal HR or HR at 4,000 ng/kg of ACh in an age group was not different (Table 1).
Fig. 1.
Effect of acetylcholine (ACh) on mean arterial pressure (MAP) in 1 (n = 6)-, 4 (n = 9)-, 8 (n = 7)-, and 16 (n = 7)-wk-old rabbits. Rabbits were anesthetized and increasing doses of ACh were injected intravenously. Decreases in MAP are expressed as percent decrease from baseline. Each value is mean ± SE. MAP changes to ACh in 1- and 4-wk-old rabbits are significantly different from 8- and 16-wk-old rabbits (**P < 0.001; ***P < 0.0001).
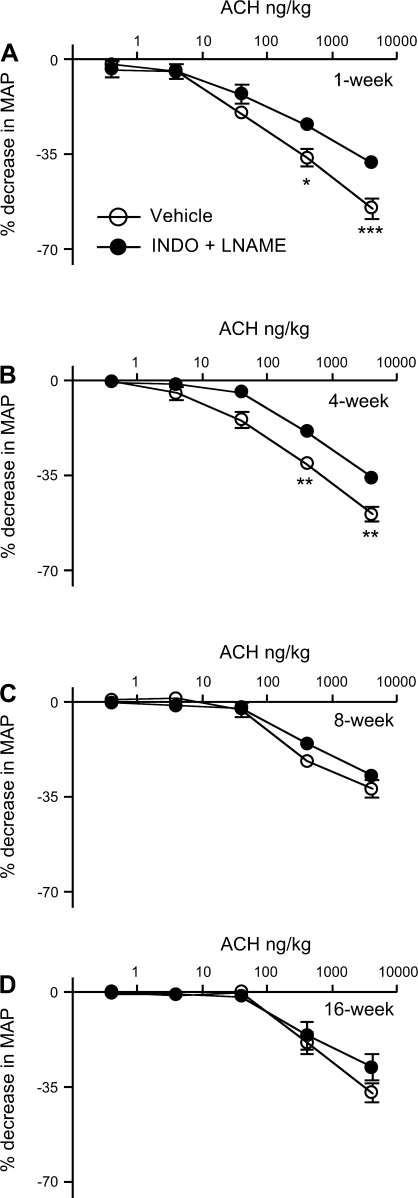
To determine the contribution of NO and PGs, rabbits were treated with Indo and l-NAME and the ACh-induced decrease in MAP was measured (Fig. 2). ACh caused dose-related decreases in MAP in the Indo- and l-NAME-treated rabbits. The responses were decreased in 1- and 4-wk-old rabbits but did not change in 8- and 16-wk-old rabbits (Fig. 2). After Indo and l-NAME treatment, basal MAP increased in 1- and 4-wk-old rabbits (Table 1) but not in 8- and 16-wk-old rabbits. The HR did not change in any age group after Indo and l-NAME treatment (Table 1). The maximum response to ACh (4,000 ng/kg) in Indo- and l-NAME-treated 1 (−37.9 ± 3.9%)- and 4 (−35.5 ± 7.8%)-wk -old rabbits was significantly greater than from that in 8 (−26.6 ± 4.4%)- and 16 (−27.3 ± 10%)-wk -old rabbits (P < 0.05).
Fig. 2.
Effects of nitric oxide synthase and cyclooxygenase inhibition on the MAP responses to ACh in 1 (A; n = 5)-, 4 (B; n = 7)-, 8 (C; n = 5)-, and 16 (D; n = 5)-wk-old rabbits. Rabbits were treated with vehicle or Nω-nitro-l-arginine methyl ester (l-NAME; 20 mg/kg; 5 mg·kg−1·h−1) and indomethacin (Indo; 6 mg/kg). After 30 min, ACh responses were determined. Decreases in MAP are expressed as percent decrease from baseline. Each value is mean ± SE (*P < 0.05; **P < 0.001; ***P < 0.0001). All symbols are as defined in A.
Contribution of 15-LO-1 metabolites.
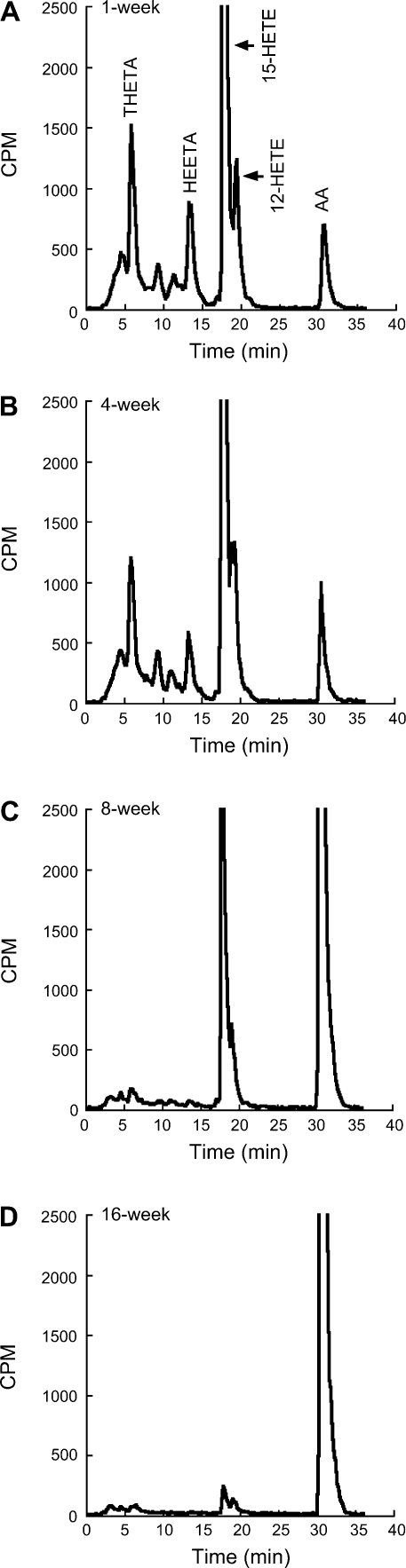
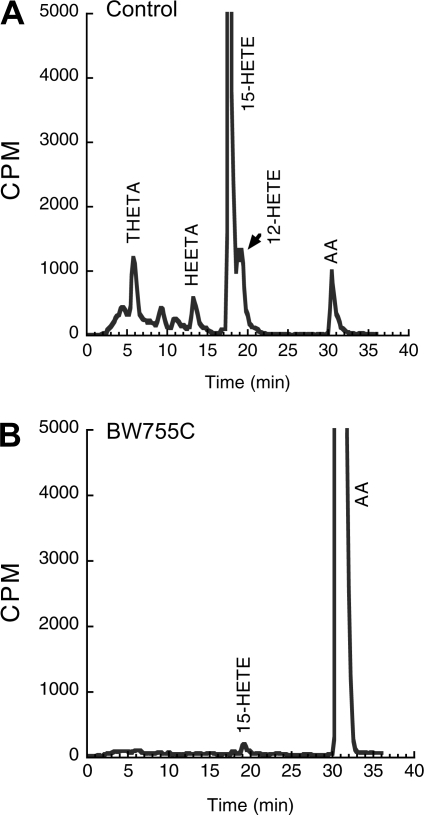
The mesenteric arterial rings metabolize [14C]AA to radioactive products that comigrate with 15-HETE, 12-HETE, THETA, and HEETA (Fig. 3, A–D). As we have previously shown (31), the synthesis of these metabolites decreased with age. The synthesis of 15-LO-1 metabolites was greatest in mesenteric arteries of 1-wk-old rabbits and was almost absent in 16-wk-old rabbits. The effect of BW755C on the metabolism of [14C]AA was determined in the isolated mesenteric arteries of a 4-wk-old rabbit. BW755C reduced the synthesis of 15-HETE, 12-HETE, THETA, and HEETA (Fig. 4). Similar results with BW755C were obtained in aortic rings (data not shown).
Fig. 3.
Metabolism of [14C]arachidonic acid (AA) by mesenteric arteries of 1 (A)-, 4 (B)-, 8 (C)-, and 16 (D)-wk-old rabbits. Mesenteric arterial rings with intact endothelium were incubated with [14C]AA in the presence of Indo (10−5 M). Media were extracted, and eicosanoids were resolved by HPLC. The production of 14C metabolites is normalized by tissue weight. Migration times of known standards are shown above chromatogram in A. THETA, 11,12,15-trihydroxyeicosatrienoic acid; HEETA, 15-hydroxy-11,12-epoxyeicosatrienoic acid; HETE, hydroxyeicosatetraenoic acid.
Fig. 4.
Effect of BW755C on [14C]AA metabolism in rabbit mesenteric arteries. Mesenteric arterial rings from 4-wk-old rabbits were incubated with [14C]AA in the presence of Indomethacin (10−5 M) without (A) and with (B) BW755C (10−4 M). Media were removed and extracted, and the metabolites were resolved by HPLC. Migration times of known standards are indicated above chromatograms. Data represent 3 sets of experiments on arteries from different rabbits.
Contribution of EDHFs.
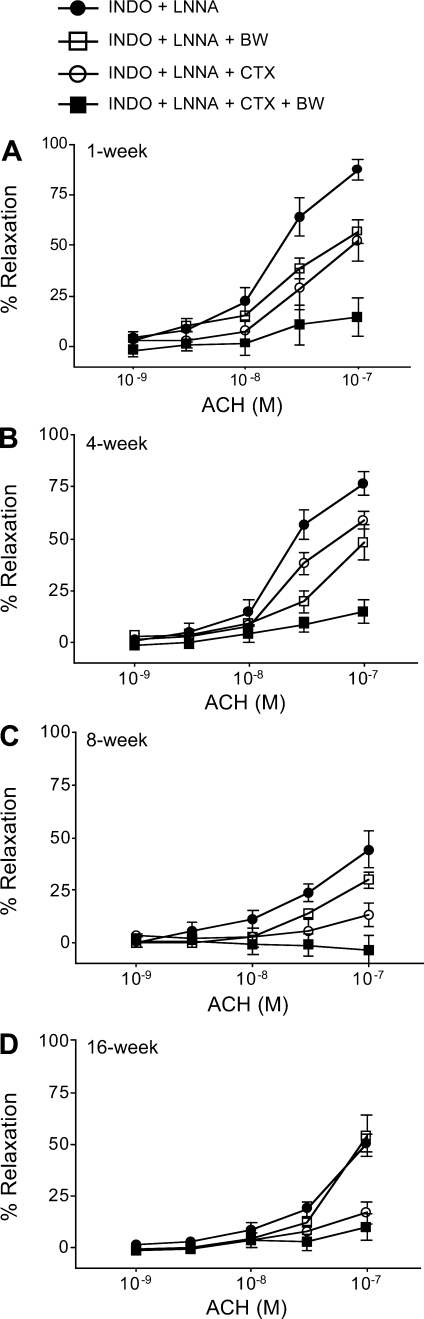
NO- and PG-independent maximum ACh relaxations were reduced with age in mesenteric arteries from 1 (87.3 ± 5%)-, 4 (76.6 ± 5%)-, 8 (48.2 ± 8%)-, and 16 (50.3 ± 4%)-wk-old rabbits (Fig. 5, A–D). BW755C reduced these relaxations to a greater extent in the arteries from 1 (56.7 ± 5%)- and 4 (48.2 ± 8%)-wk-old rabbits compared with the arteries from 8 (29.8 ± 3%)- and 16 (53.7 ± 9%)-wk-old rabbits. Since the relaxations to ACh are contributed by a CTX-sensitive pathway and the 15-LO pathway (36), we also tested the relaxations in presence of CTX alone or CTX and BW755C. CTX alone reduced the relaxations to a greater extent in the arteries from 8 (13.4 ± 5%)- and 16 (17.0 ± 5%)-wk-old rabbits compared with 1 (54.2 ± 9%)- and 4 (37.3 ± 8%)-wk-old rabbits. Finally, the relaxations were completely inhibited by the combination of CTX and BW755C in the arteries from all the age groups.
Fig. 5.
Effects of the lipoxygenase (LO) inhibitor BW755C (BW) and the IKCa channel inhibitor charybdotoxin (CTX) on the vascular activity in rabbit mesenteric arteries. Arterial rings (n = 8–12) were pretreated with Indo (10−5 M) and Nω-nitro-l-arginine (l-NNA; 3 × 10−5 M) with vehicle, or BW755C (10−4 M), or CTX (2 × 10−7 M), or both CTX and BW755C; they were precontracted with phenylephrine (10−7 to 10−6 M), and relaxations to cumulative concentrations of ACh were determined. Data are expressed as percent relaxations, and each value represents mean ± SE. All symbols are as defined in A.
ACh-induced MAP decrease was measured in the presence of Apa and CTX in l-NAME- and Indo-treated 4-wk-old rabbits (Fig. 6A). Four-week-old rabbits were used as representative for the study as they showed an intermediate responses between 1- and 16-wk-old rabbits. In these rabbits, the maximum ACh responses were inhibited by treatment with Apa and CTX from −38.8 ± 3.4 to −6.9 ± 0.9%. These NO- and PG-independent ACh responses (max: −35.0 ± 4.1%) were also significantly inhibited by treatment with Apa alone (max: −19.5 ± 1.4%) depicting the role of SKCa channels (Fig. 6B). In these Indo- and l-NAME-treated rabbits, administration of Apa and CTX or Apa alone increased basal MAP from 46.1 ± 10.7 to 99.6 ± 8.9 and 87.33 ± 6.5 mmHg, respectively. HR did not differ with Apa and CTX treatment (262 ± 23 beats/min) or with Apa alone (256 ± 16) when compared with basal (282 ± 18 beats/min) or with Indo and l-NAME treatment (281 ± 18 beats/min).
Fig. 6.
Effects of potassium channel inhibitors and the LO-inhibitor BW755C on the MAP response to ACh in l-NAME- and Indo-treated 4-wk-old (n = 6) rabbits. Rabbits were treated with l-NAME (20 mg/kg; 5 mg·kg−1·h−1) and Indo (6 mg/kg) for 30 min, and ACh responses were determined. Rabbits were then injected with (A) apamin (Apa; 50 μg/kg) and CTX (50 μg/kg), Apa (B; 50 μg/kg), or BW755C (C; 20 mg/kg) and, after 30 min the ACh responses were repeated. Decreases in MAP are expressed as percent decrease from baseline. Each value represents mean ± SE (**P < 0.001; ***P < 0.0001).
The effect of BW755C on the ACh-induced decrease in MAP was measured in 4-wk-old rabbits. In Indo- and l-NAME-treated 4-wk-old rabbits, the maximum MAP decrease to ACh was reduced by BW755C from −34.25 ± 1.9% to a maximum of −18.8 ± 3.5% (Fig. 6C). Administration of BW755C to these rabbits did not alter basal MAP (49.9 ± 3.8 mmHg) or HR (258 ± 15 mmHg) compared with untreated animals.
DISCUSSION
Previously, we demonstrated that 15-LO-1 expression, THETA and HEETA synthesis, and ACh and AA relaxation in vitro decreased with age (neonates to 16-wk-old) in rabbits (32). We tested whether these changes occur in vivo by measuring ACh-induced hypotensive responses in anesthetized rabbits at 1–16 wk of age. Although the age of the rabbit used in the present study reflects maturation, we referred to the effect as age dependent.
Basal MAP and HR were measured in the rabbits after pentobarbital anesthesia. Resting MAP increased with age, but HR remained the same. This may imply that there is an age-related increase in peripheral vascular resistance (PVR). ACh (0.4–4,000 ng/kg) caused dose-dependent, transient decreases in MAP without affecting the HR. Thus this decrease in MAP results from decreased vascular resistance and not secondary to the effect of ACh on cardiac muscarinic receptors. Similar doses of ACh lower MAP in rabbits, rats, and humans (8, 16, 27). These dose-dependent decreases in MAP after ACh are also unrelated to the basal MAP, as previously reported in rats and rabbits (8, 18, 35). The decrease in MAP to ACh was similar in 1- and 4-wk-old rabbits and was greater than those in and 8- and 16-wk-old rabbits. These responses corroborate our in vitro data (32) that the AA and ACh relaxations in isolated aortas were higher in 1- and 4-wk-old rabbits than in 8- and 16-wk-old rabbits. Also, 15-LO-1 expression and THETA and HEETA synthesis were similar in arteries of 1- and 4-wk- old rabbits but higher than those in arteries of 8- and 16-wk-old rabbits. The reduction in the relaxation responses to ACh and AA in the arteries of older rabbits is probably not due to a reduced sensitivity of the arteries towards THETA and HEETA but due to their reduced synthesis. We have reported that an increased synthesis of THETA and HEETA by increasing 15-LO-1 expression in the mesenteric arteries of 8-wk-old rabbits was sufficient to increase ACh relaxations compared with the normal arteries (1). Similar results were obtained in preliminary studies in aortas from 16-wk-old rabbits (unpublished data). Therefore, the sensitivity of the arteries towards THETA and HEETA does not appear to change with age (8- and 16-wk-old), but the reduced synthesis of THETA and HEETA in arteries from older animals accounts for the reduced relaxations.
To measure the role of EDHFs in mediating these ACh-induced decreases in MAP, we treated rabbits with Indo and l-NAME. We used doses that have been shown to produce maximal inhibition of COX and eNOS, respectively (5, 23, 28). After NOS and COX inhibition, MAP increased in 1- and 4-wk-old rabbits but did not change in 8- and 16-wk-old rabbits. This suggests that the contribution of NO and PG was greater in younger rabbits (1 and 4 wk) but decreased in older rabbits (8 and 16 wk). A similar dose of l-NAME did not change the systemic blood pressure in older rabbits weighing 2 kg (28). COX inhibition by ibuprofen only increased blood pressure transiently for 10 min in rabbits of similar age and weight (22). Similarly, an age-related reduction in NO-mediated relaxation has been reported in mesenteric and pulmonary arteries from neonate to 2-wk-old piglets (15). Therefore, our results and the reported studies indicate that the role of NO and PG are more prominent in the regulation of the MAP of younger rabbits. We measured ACh-induced decrease in MAP in the Indo- and l-NAME-treated rabbits. With l-NAME and Indo treatment, the maximum decrease in MAP to ACh was reduced in 1- and 4-wk-old rabbits compared with untreated rabbits but did not change significantly in 8- and 16-wk-old rabbits. This once again implies that the effect of NO and PGs was reduced with age in 1–16 wk old rabbits. These reductions in the ACh-induced decrease in MAP and the effects of Indo and l-NAME could not be due to the increased metabolism of ACh, Indo, or l-NAME, because a similar profile was obtained in vitro in isolated aortas of the rabbits (1–16 wk old; Ref. 32).
To determine the role of 15-LO-1, we used BW755C, a lipoxygenase inhibitor. BW755C reduced THETA and HEETA synthesis from AA in mesenteric arteries and aortas. BW755C inhibited the synthesis of 12-HETE, 15-HETE, THETA, and HEETA. These results are consistent with previous reports (24) of inhibition of 15-LO-1 by BW755C.
Since small resistance arteries contribute to PVR and thus towards MAP, we determined the contribution of THETA and HEETA to ACh relaxations in small mesenteric arteries. NO- and PG-independent ACh relaxations in the mesenteric arteries from 1-, 4-, 8-, and 16-wk-old rabbits decreased with age. This suggests that the EDHF response is greater in arteries from the younger rabbits. BW755C reduced these relaxations to a greater extent in arteries from younger rabbits compared with the older rabbits, suggesting a greater role of THETA and HEETA in younger rabbits. The inhibition of the ACh relaxations by BW755C was less in the arteries from 8- and 16-wk-old rabbits due to the minimal synthesis of THETA and HEETA in these arteries. CTX treatment also inhibited the relaxations partially in arteries from 1- and 4-wk-old rabbits. In rabbit mesenteric arteries, ACh induces relaxations via two different pathways: a CTX-sensitive pathway and a Apa-sensitive pathway (36). THETA and HEETA hyperpolatize the smooth muscle cells by opening the Apa-sensitive SKCa channels (13). Therefore, treating the mesenteric arteries with CTX alone or BW755C alone inhibited the relaxations only partially. On the other hand, CTX inhibited the ACh relaxations almost completely in the arteries from the older rabbits, implying that CTX pathway prevails in these arteries presumably because THETA and HEETA synthesis is reduced. Finally, the combinations of CTX and BW755C inhibited the relaxations completely in the arteries from all age groups, suggesting the role of both pathways. The relaxation studies suggest that both the THETA and HEETA pathways and CTX-sensitive pathway are important in the arteries from younger (1- to 4-wk-old) rabbits, while only the CTX-sensitive pathway is prevalent in the arteries from older rabbits. Therefore, these results also provided insight into the role of 15-LO-1 in regulating PVR and therefore MAP in younger rabbits.
To confirm the EDHF activity in vivo, we measured the effect of ACh on MAP in the presence of Apa and CTX in 4-wk-old rabbits. The 4-wk age group represents an intermediate age group in terms of 15-LO-1 expression, THETA and HEETA synthesis, and ACh relaxations and ACh hypotension (31). In Indo- and l-NAME-treated 4-wk-old rabbits, Apa and CTX increased MAP, implying the role of EDHFs in regulating vasodilation and PVR. These doses of Apa and CTX reduced the NO- and PG-independent ACh responses in rabbits in vivo (29). We found that Apa and CTX inhibited the ACh-induced decrease in MAP in Indo- and l-NAME-treated 4-wk-old rabbits. These findings indicated that the non-NO, non-PG hypotensive responses to ACh are mediated by EDHF. In Indo- and l-NAME-treated rabbits, Apa alone inhibited the maximal decrease in MAP to ACh. This suggest a role of an EDHF that activates SKCa channels. It is noteworthy that Apa alone increased the basal MAP transiently for 5 min and then returned to the basal MAP (data not shown). The transitory effect could be related to a compensatory mechanism that returned the increased MAP to the basal levels. THETA hyperpolarizes smooth muscle cells by opening the Apa-sensitive, SKCa channels in rabbit vascular smooth muscle (13, 36). Therefore, we measured the ACh-induced decrease in MAP in Indo- and l-NAME-treated rabbits in the presence of the 15-LO inhibitor BW755C. The NO- and PG-independent ACh relaxations in vitro were also reduced by BW755C, indicating a role of THETA as an EDHF. Like the results obtained from Apa treatment in l-NAME- and Indo-treated rabbits, the basal MAP was not altered after BW755C treatment but the ACh-induced decreases in MAP were reduced. BW755C did not completely block these responses to ACh. The inhibition by BW755C was similar to the inhibition by Apa. BW755C inhibits both LO and COX and reduces the synthesis of HETEs, THETAs, HEETAs, and PGs. The ACh hypotensions, ACh relaxations, and MAP were determined in rabbits that were pretreated with Indo to inhibit PG synthesis. Thus the PG component of ACh responses was inhibited, so the effect of BW755C can be attributed to LO inhibition.
The present in vivo studies confirm our in vitro findings that in the absence of NO and PG there is a decrease in EDHF activity with age. These studies also demonstrate that 15-LO-1 metabolites of AA, specifically THETA and HEETA, act as EDHFs in vivo and thus regulate blood pressure independently of NO and PGs. In conclusion, our results suggest that the age-related decline in ACh or AA relaxations of rabbit arteries is due to the decreased synthesis of vasoactive THETAs and HEETAs.
GRANTS
The studies were supported by a grant from the National Heart, Lung, and Blood Institute (HL-37981). N. T. Aggarwal is supported by a predoctoral fellowship from the American Heart Association, Greater Midwest Affiliate.
Acknowledgments
We thank Dr. Y. Chawengsub for helpful discussions and advice and G. Barg for secretarial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aggarwal NT, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension 51: 246–251, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal NT, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: role in arachidonic acid-induced relaxation. Am J Physiol Heart Circ Physiol 292: H1033–H1041, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WB, Graham RM, Jackson EK. Inhibition of hydralazine-induced renin release by indomethacin in the rat. Arch Int Pharmacodyn Ther 246: 315–323, 1980. [PubMed] [Google Scholar]
- 6.Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-Trihydroxyeicosatrienoic acid mediates ACh-induced relaxations in rabbit aorta. Am J Physiol Heart Circ Physiol 285: H2648–H2656, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization. Beyond nitric oxide and cyclic GMP. Circulation 92: 3337–3349, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Desai KM, Gopalakrishnan V, Hiebert LM, McNeill JR, Wilson TW. EDHF-mediated rapid restoration of hypotensive response to acetylcholine after chronic, but not acute, nitric oxide synthase inhibition in rats. Eur J Pharmacol 546: 120–126, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Forstermann U, Neufang B. The endothelium-dependent relaxation of rabbit aorta: effects of antioxidants and hydroxylated eicosatetraenoic acids. Br J Pharmacol 82: 765–767, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension 43: 413–419, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Gerova M, Kittova M. Systemic blood pressure response to the inhibition of two hyperpolarizing pathways: a comparison to NO-synthase inhibition. Physiol Res 55: 603–610, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales-Luis G, Fletcher AJ, Moreno L, Perez-Vizcaino F, Blanco CE, Villamor E. Nitric oxide-mediated nonadrenergic noncholinergic relaxation of piglet pulmonary arteries decreases with postnatal age. J Physiol Pharmacol 58: 45–56, 2007. [PubMed] [Google Scholar]
- 16.Halcox JP, Narayanan S, Cramer-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol Heart Circ Physiol 280: H2470–H2477, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Harada Y, Ikeda M, Murasato Y, Suzuka H, Nanri H, Hayashida Y. Integrative effects of nitric oxide and endothelium-derived hyperpolarizing factor induced by acetylcholine and bradykinin in rat hindquarter perfusion. Nitric Oxide 4: 354–362, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Laight DW, Desai KM, Anggard EE, Carrier MJ. Endothelial dysfunction accompanies a pro-oxidant, pro-diabetic challenge in the insulin resistant, obese Zucker rat in vivo. Eur J Pharmacol 402: 95–99, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda H, Miyatake K, Dahlen SE. Pharmacodynamics of 15(S)-hydroperoxyeicosatetraenoic (15-HPETE) and 15(S)-hydroxyeicosatetraenoic acid (15-HETE) in isolated arteries from guinea pig, rabbit, rat and human. J Pharmacol Exp Ther 273: 1182–1189, 1995. [PubMed] [Google Scholar]
- 20.Matz RL, Schott C, Stoclet JC, Andriantsitohaina R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol Res 49: 11–18, 2000. [PubMed] [Google Scholar]
- 21.Nishikawa Y, Stepp DW, Chilian WM. In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol Heart Circ Physiol 277: H1252–H1259, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Oliver JJ, Eppel GA, Rajapakse NW, Evans RG. Lipoxygenase and cyclo-oxygenase products in the control of regional kidney blood flow in rabbits. Clin Exp Pharmacol Physiol 30: 812–819, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Oliver JJ, Rajapakse NW, Evans RG. Effects of Indomethacin on responses of regional kidney perfusion to vasoactive agents in rabbits. Clin Exp Pharmacol Physiol 29: 873–879, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Pfister SL, Spitzbarth N, Edgemond W, Campbell WB. Vasorelaxation by an endothelium-derived metabolite of arachidonic acid. Am J Physiol Heart Circ Physiol 270: H1021–H1030, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of the 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem 273: 30879–30887, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-epoxyeicosatrienoic acid mediates the enhanced renal vasodilation to arachidonic acid in the SHR. Hypertension 42: 548–554, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Rajapakse NW, Flower RL, Eppel GA, Denton KM, Malpas SC, Evans RG. Prostaglandins and nitric oxide in regional kidney blood flow responses to renal nerve stimulation. Pflügers Arch 449: 143–149, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rajapakse NW, Oliver JJ, Evans RG. Nitric oxide in responses of regional kidney blood flow to vasoactive agents in anesthetized rabbits. J Cardiovasc Pharmacol 40: 210–219, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Sankaralingam S, Desai KM, Wilson TW. Clofibrate acutely reverses saline-induced endothelial dysfunction: role of calcium-activated potassium channels. Am J Hypertens 19: 1167–1173, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation 100: 1400–1405, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Tang X, Aggarwal N, Holmes BB, Kuhn H, Campbell WB. Age-related decrease in 15-lipoxygenase contributes to reduced vasorelaxation in rabbit aorta. Am J Physiol Heart Circ Physiol 294: H679–H687, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Tang X, Aggarwal NM, Holmes BB, Kuhn H, Campbell WB. Age-related decrease in 15-lipoxygenase contributes to reduced vasorelaxation in rabbit aorta. Am J Physiol Heart Circ Physiol 294: H679–H687, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-I is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol 26: 78–84, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Van Diest MJ, Verbeuren TJ, Herman AG. 15-lipoxygenase metabolites of arachidonic acid evoke contractions and relaxations in isolated canine arteries: role of thromboxane receptors, endothelial cells and cyclooxygenase. J Pharmacol Exp Ther 256: 194–203, 1991. [PubMed] [Google Scholar]
- 35.Weldon SM, Winquist RJ, Madwed JB. Differential effects of l-NAME on blood pressure and heart rate responses to acetylcholine and bradykinin in cynomolgus primates. J Pharmacol Exp Ther 272: 126–133, 1995. [PubMed] [Google Scholar]
- 36.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. Acetylcholine-induced relaxations of rabbit small mesenteric arteries: role of arachidonic acid metabolites and K+. Am J Physiol Heart Circ Physiol 293: H152–H159, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-HETE and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res 92: 992–1000, 2003. [DOI] [PubMed] [Google Scholar]