Abstract
This study determines that vascular smooth muscle cell (VSMC) signaling through extracellular signal-regulated kinase (ERK) 1/2-mitogen-activated protein (MAP) kinase, αvβ3-integrin, and transforming growth factor (TGF)-β1 dictates collagen type I network induction in mesenteric resistance arteries (MRA) from Type 1 diabetic (streptozotocin) or hypertensive (HT; ANG II) mice. Isolated MRA were subjected to a pressure-passive-diameter relationship. To delineate cell types and mechanisms, cultured VSMC were prepared from MRA and stimulated with ANG II (100 nM) and high glucose (HG, 22 mM). Pressure-passive-diameter relationship reduction was associated with increased collagen type I deposition in MRA from HT and diabetic mice compared with control. Treatment of HT and diabetic mice with neutralizing TGF-β1 antibody reduced MRA stiffness and collagen type I deposition. Cultured VSMC stimulated with HG or ANG II for 5 min increased ERK1/2-MAP kinase phosphorylation, whereas a 48-h stimulation induced latent TGF-β1, αvβ3-integrin, and collagen type 1 release in the conditioned media. TGF-β1 bioactivity and Smad2 phosphorylation were αvβ3-integrin-dependent, since β3-integrin antibody and αvβ3-integrin inhibitor (SB-223245, 10 μM) significantly prevented TGF-β1 bioactivity and Smad2 phosphorylation. Pretreatment of VSMC with ERK1/2-MAP kinase inhibitor (U-0126, 1 μM) reduced αvβ3-integrin, TGF-β1, and collagen type 1 content. Additionally, αvβ3-integrin antibody, SB-223245, TGF-β1-small-intefering RNA (siRNA), and Smad2-siRNA (40 nM) prevented collagen type I network formation in response to ANG II and HG. Together, these data provide evidence that resistance artery fibrosis in Type 1 diabetes and hypertension is a consequence of abnormal collagen type I release by VSMC and involves ERK1/2, αvβ3-integrin, and TGF-β1 signaling. This pathway could be a potential target for overcoming small artery complications in diabetes and hypertension.
Keywords: hypertension, Type 1 diabetes, extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase, transforming growth factor-β1, vascular smooth muscle cell, resistance artery
resistance arteries are able to respond to physiological and pathophysiological stimuli to maintain adequate perfusion according to tissue metabolic demand. Vasomotor control allows rapid adaptation of lumen diameter, whereas changes in the structural properties of the vessel wall constitute an active process that occurs in response to sustained alteration of hemodynamic (pressure and flow) or neurohumoral (ANG II, bradykinine, glucose) factors (16, 17, 31, 32). Hyperglycemia (high glucose, HG) and ANG II are key contributors to the development of microvascular complications associated with structural wall remodeling (28, 30, 34, 39), including hypertrophy and hyperplasia of VSMC, protein synthesis, and abnormal extracellular matrix (ECM) accumulation (i.e., collagen). Importantly, the interaction between VSMC and ECM represents a dynamic system in which changes in the composition and/or spatial organization of ECM in Type 1 diabetes and/or hypertension may lead to microvascular complications. The increase of collagen wall content, a hallmark of fibrosis, is partially responsible for artery stiffness increase and subsequently vasculopathy with perturbation of tissue perfusion. However, the cell type and molecular mechanisms involved in microvascular abnormal collagen type I deposition in hypertension and Type 1 diabetes remain to be elucidated, especially in the resistance artery.
It has been shown that transforming growth factor-β1 (TGF-β1, a family related cytokines) plays a role in cell growth, differentiation, apoptosis, inflammatory processes, and gene expression (18). TGF-β1 has profibrotic properties and induces the expression of many matrix proteins, including collagen (11). It has been reported that TGF-β (1) can upregulate l-arginine transport and direct its metabolism to polyamines, and l-proline may lead to arterial remodeling at sites of vascular damage (8). Regulation of appropriate levels of TGF-β1 activity is critical since too much TGF-β1 results in fibrosis and immune suppression, whereas deficiencies result in rampant inflammation, epithelial hyperplasia, and defective wound healing (18). One of the major downstream targets signaling to TGF-β1 is Smad2 (11, 18, 19), considered as a marker of TGF-β1 bioactivity. The importance of TGF-β1 in the development of myocardial fibrosis is well established. Thus, in the transition from hypertrophy to heart failure, there is an increase of TGF-β1 expression associated with abnormal accumulation of ECM components (25). There are ample data to implicate TGF-β1 in the fibrotic changes occurring in chronic diabetes and hypertension (2, 15, 28). However, the role of TGF-β1 in the control of ECM (i.e., collagen type 1) of resistance artery smooth muscle cell is unknown. It is crucial to mention that data from large arteries cannot be extrapolated to resistance arteries because of differences in function and structure between the two arteries (38).
Integrins are transmembrane, heterodimeric, noncovalently bound glycoprotein complexes consisting of α- and β-chains, are widely distributed in various tissues, and mediate diverse biological functions, including cell-cell and cell-matrix interactions, cell polarity, cell migration, and angiogenesis (42). It has been shown that TGF-β1 latency-associated peptide (LAP) contains RGD motifs, and αvβ3-integrin binding to TGF-β1 LAP is RGD dependent (33). Recently, it has been shown that the assembly of collagen type I is dependent on α2β1-integrin (20). Despite the knowledge in integrins in cardiovascular pathophysiology and cancer, their role as a key mechanism in the control of collagen content in hypertension and Type 1 diabetes remains to be determined, especially in resistance artery.
In this study, we will determine the cellular type and mechanisms involved in collagen type 1 abnormal deposition in resistance artery in hypertension and Type 1 diabetes with an emphasis on extracellular signal-regulated kinase (ERK) 1/2 mitogen-activated protein (MAP) kinase, αvβ3-integrin, and TGF-β1 by using pressurized microvessels and primary cultured vascular smooth muscle cell (VSMC) isolated from resistance arteries.
METHODS
All mice used in this study are adult of the C57BL6/j genetic background.
Induction of Type 1 diabetes.
The streptozotocin (STZ)-Na-citrate solution was prepared immediately before injection so as to avoid degradation of the STZ. Mice were fasted 4 h before STZ injection, placed in the isoflurane drop jar, and anesthetized, and STZ solution (150 mg/kg) was then injected. Mice were supplied with 10% sucrose water, if necessary, to avoid sudden hypoglycemia postinjection. Blood glucose concentration was determined 2 days before and after to assess the success of STZ injection. This protocol is widely approved by different laboratories (5, 9, 36) and the Juvenile Diabetes Research Foundation International. In this study, mice were used after 4 wk of Type 1 diabetes.
Induction of hypertension.
Mice were placed in the isoflurane drop jar and anesthetized. ANG II (200 ng·kg−1·day−1) (13, 45) was then delivered for 4 wk using a subcutaneous miniosmotic pump (Alzet osmotic pumps, model 2004, debit rate 0.25 μl/h) placed in the back under the skin. Blood pressure reaches plateau after 2 wk. Therefore, mice are considered hypertensive after 2 wk.
Blood pressure measurement.
Mice were placed in the isoflurane drop jar and anesthetized. A small catheter connected to a pressure transducer (www.livingsys.com) was then introduced in the carotid to measure blood pressure of each mouse. Mice were then subjected to a 15-min equilibration period before mean arterial pressure was measured. These studies conformed to the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Louisana State University Institutional Animal Care and Use Committee.
TGF-β1 antibody injection.
Hypertensive and Type 1 diabetic mice were subjected to intraperitoneal injection of specific TGF-β1 antibody, (24) in a volume of 240 μl, once a day for 1 wk.
Mechanical properties of mesenteric resistance artery.
The mesenteric resistance artery (MRA) from each mouse was isolated and mounted in an arteriograph in physiological salt solutions warmed to 37°C and equilibrated at 50 mmHg intraluminal pressure under no-flow conditions for 30 min. Maximal passive diameter was determined at each intraluminal pressure (25, 50, 75, 100 and 125 mmHg) by administration of sodium nitroprusside (nitric oxide donor, 100 μM) in Ca2+-free solution supplemented with 10 mM EGTA. Internal and external diameters and wall thickness were measured. Resistance artery compliance and remodeling index were determined based on a previous study (12).
Primary cultured smooth muscle cells from mouse MRA.
MRA from the last bifurcation to intestine were pooled from 8–10 mice to isolate primary cultured VSMC. Usually these resistance arteries have a diameter <100 μm measured with an arteriograph system, as previously published (22, 23). In this study, primary cultured VSMC were used from passage 3 to 8. Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). The mesenteric artery was excised and placed in serum-free DMEM. Next, with the use of a microscope, resistance arteries were cleaning from the connective tissue, isolated, placed on ice, minced, and then transferred to a flask containing Hanks' balanced salt solution supplemented with 175 U/ml of collagenase II (Worthington). The flask was placed in a CO2 incubator for 2 h, and then 0.25 mg/ml of elastase was added for an additional 45 min. The digestion was stopped by the addition of 5 ml 10% FBS-DMEM. The cells were collected by centrifugation at 1,500 g for 10 min, and the suspension was differentially plated for 20 min in 10% FBS-DMEM to remove fibroblasts. Cells suspension was then replated in 10% FBS-DMEM and allowed to reach confluency. Contamination with endothelial cells was assessed with Western blot analysis using Von Willebrand Factor antibody. For all experiments, VSMC at passages 3–8 were grown to 80% confluency in 10% FBS-DMEM with low glucose (5 mM) and then growth arrested for 48 h in serum-free DMEM before stimulation with ANG II or HG (d-glucose, 22 mM). Because of the potential osmolarity changes with d-glucose, we performed another set of experiment using VSMC cultured in high osmolarity condition using l-glucose.
Immunoprecipitation and Western blot analysis.
Cultured VSMC or resistance arteries were sonicated in lysis buffer (20 mM Tris·HCl, pH 7.5, 5 mM EGTA, 150 mM NaCl, 20 mM glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1% Triton X-100, 0.1% Tween 20, 1 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM N-tosyl-l-phenylalanine chloromethyl ketone, and 0.5 mmol/l N-p-tosyl-l-lysine chloromethyl ketone). Extracts were incubated on ice for 15 min and then centrifuged (12,000 g for 15 min at 4°C). The detergent-soluble supernatant fractions were retained, and protein concentrations in samples were determined using a Pierce protein assay. Cell conditioned media and plasma were immunoprecipitated with specific antibodies (3–5 μg) overnight and then subjected to immunoblotting with the same or different antibody (1:1,000 dilution usually used). Cells and tissue lysate were subjected to Western blot analysis.
TGF-β1 bioactivity.
TGF-β1 bioactivation in the conditioned media was assessed using an ELISA kit for active TGF-β1, according to the manufacturer's protocols (R&D Systems, Minneapolis, MN). Briefly, to assay the level of active TGF-β1, 50 μl of sample or TGF-β1 standard was mixed with 50 μl of diluents. Each sample was added to an individual well of the assay plate. After incubation for 2 h at room temperature, the plate was washed five times, and 100 μl of TGF-β1 conjugate solution were added to each well followed by incubation for 2 h at room temperature. The wells were washed five times, and 100 μl of substrate solution were added to each well. After 30 min, 100 μl of stop solution were added to each well, and the absorbency at 450 nm was determined.
Immunohistochemistry.
Confluent cultured VSMC on cover slips was used for collagen type I staining using specific antibodies at 1:100 dilution. A negative control without collagen type 1 antibody or with secondary only was processed simultaneously. The staining was visualized by use of secondary antibodies coupled to Alexa-488 or Alexa 568 (1:300; Molecular Probes) and a fluorescent microscope.
VSMC transfection with small-interfering RNA-TGF-β1 and Smad2.
Transfection was performed according to the manufacturer's protocol (Ambion). Confluent cultured VSMC with serum-free DMEM and without antibiotics were subjected to lipofectamine 2000 mixed with DMEM without antibiotic and small-interfering RNA (siRNA)-TGF-β1 or siRNA-Smad2 (40 nM) for 6 h at 37°C. After incubation time, VSMC were washed with sterile PBS and incubated with DMEM 10% serum. The next days, VSMC were starved with serum-free DMEM for 48 h and stimulated with HG (22 mM) or ANG II (100 nM) for 48 h. Labeled and nontargeting siRNA were used as control.
Statistic analysis.
Results are expressed as means ± SE, where n is the number of mice used in this study. Significance of the differences between groups was determined by one repeated or two-factor ANOVA, where appropriate. Differences were considered significant at P < 0.05.
RESULTS
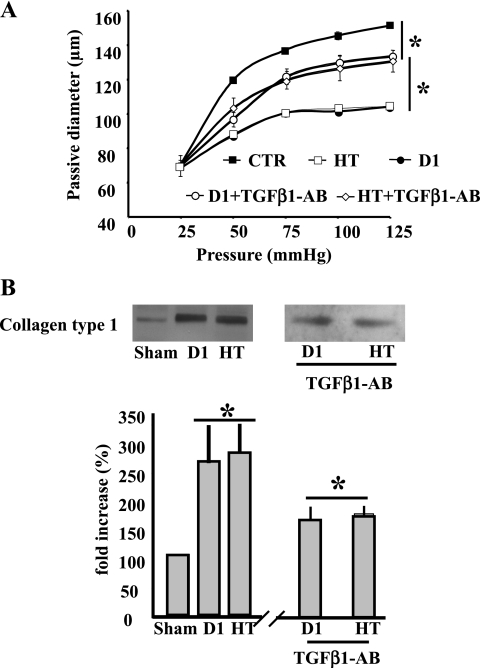
To assess the efficiency of hypertension and Type 1 diabetes, blood glucose and blood pressure were measured in mice subjected to STZ and ANG II, respectively. Animals infused with ANG II presented elevated blood pressure compared with the sham group (120 ± 5.1 vs. 95 ± 4 mmHg, P < 0.05), and mice injected with STZ showed an increased blood glucose level compared with the sham group (112 ± 0.9 vs. 449 ± 19 mg/l, P < 0.05). To determine the mechanical properties of microvessels from Type 1 diabetic and hypertensive mice, we mounted freshly isolated MRA in an arteriograph and determined the pressure (25–125 mmHg)-passive diameter relationship that is dependent of wall structure. Measurement revealed a significant reduction in the passive-diameter relationship (Fig. 1A) of MRA from hypertensive or diabetic mice, indicating an increase in stiffness. These vascular mechanical property modifications were associated with abnormal collagen type I accumulation assessed with Western blot analysis in MRA from Type 1 diabetic or hypertensive mice compared with control. Resistance artery stiffness and collagen type I deposition were reduced significantly in MRA from hypertensive or Type 1 diabetic mice treated with neutralizing TGF-β1 antibody (Fig. 1, A and B).
Fig. 1.
A: pressure-passive diameter relationship of mesenteric resistance arteries from control (sham), hypertensive (HT), Type 1 diabetic (D1), HT + transforming growth factor (TGF)-β1 antibody (TGF-β1-AB), and D1 + TGF-β1 antibody (TGF-β1-AB) mice. *P < 0.05 statistically significant differences between sham vs. HT; sham vs. D1, HT vs. HT + TGF-β1-AB; and D1 vs. D1 + TGF-β1-AB. B: Western blot analysis and cumulative data showing the increased collagen type 1 of mesenteric resistance arteries from control (sham), hypertensive (HT), Type 1 diabetic (D1), HT + TGF-β1-AB, and D1 + TGF-β1-AB mice. *P < 0.05 statistically significant differences between: sham vs. HT; sham vs. D1; HT vs. HT + TGF-β1-AB; and D1 vs. D1 + TGF-β1-AB; n = 7 mice for each experiments.
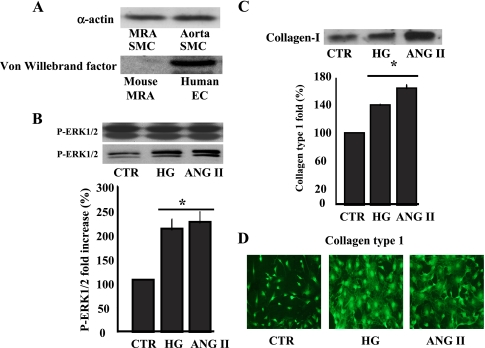
We have established a primary cultured VSMC prepared from mouse MRA with diameter <100 μm. We performed Western blot analysis and immunocytochemistry and found that primary cultured VSMC expresses α-actin and not are contaminated with endothelial cells assessed with Von Willebrand factor specific antibody (Fig. 2A). Under the process of isolating VSMC, cells were differentially plated for 20 min in 10% FBS-DMEM to remove fibroblast contamination.
Fig. 2.
A: Western blot analysis showing the expression of α-actin in primary cultured vascular smooth muscle cells (VSMC) prepared from mice mesenteric resistance arteries and aorta (top) and Von Willebrand factor (bottom); data are representative of n = 5. B: Western blot analysis and cumulative data of phosphorylated extracellular signal-regulated kinase (ERK) 1/2 mitogen-activated protein (MAP) kinase in response to acute stimulation of VSMC with ANG II (100 nM) or high glucose (HG, 22 mM). *P < 0.05, statistically significant differences between control (CTR) vs. ANG II and CTR vs. HG; n = 6 for each experiment. C: immunoprecipitation/Western blot analysis and cumulative data of collagen type 1 in the conditioned media of cultured VSMC stimulated for 48 h with ANG II or HG. *P < 0.05, CTR vs. ANG II and CTR vs. HG; n = 6. D: immunostaining showing collagen type 1 network formation on VSMC, growth on cover slip, stimulated with ANG II or HG for 48 h. Data are representative of n = 5.
There are ample studies showing increased fibrosis (collagen abnormal deposition) in different organs such heart, kidney, and aorta in hypertension or diabetes, leading to enhance vascular stiffness. To determine the contribution of VSMC and the mechanisms involved in the regulation of collagen content in resistance artery, isolated primary cultured VSMC from MRA were treated with HG or ANG II for 5 min (to determine early signaling events) and 48 h (to ascertain late signaling events). Thus ANG II (100 nM) or HG (22 mM) significantly increased ERK1/2 MAP kinase phosphorylation in VSMC after 5 min stimulation (Fig. 2B). Total ERK1/2 MAP kinase was similar in all groups, indicating the equal loading between the samples (Fig. 2B).
VSMC stimulated for 48 h with HG (22 mM) or ANG II (100 nM) showed increased collagen type 1 release in the conditioned media assessed with immunoprecipitation and Western blot analysis (Fig. 2C). IgG was used as a negative control for immunoprecipitation and revealed no band (data not shown). To strengthen our approach, VSMC growth on the cover slip showed increased collagen type 1 network staining, determined by immunocytochemistry, in response to HG and ANG II compared with control (Fig. 2D).
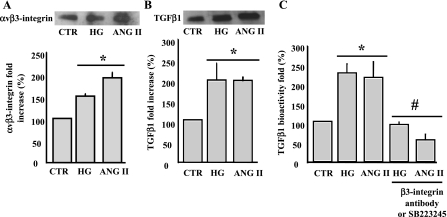
Integrins are present in different isoforms and are involved in cardiovascular physiopathology. The application of ANG II (100 nM) or HG (22 mM) for 48 h induced a significant increase in αvβ3-integrin shedding from VSMC, measured in the conditioned media by immunoprecipitation and Western blot analysis (Fig. 3A).
Fig. 3.
A: immunoprecipitation (IP)/Western blot analysis and cumulative data showing αvβ3-integrin shedding from VSMC surface in response to ANG II or HG for 48 h measured in the conditioned media. *P < 0.05, statistically significant differences between CTR vs. ANG II and CTR vs. HG; n = 6. B: IP/Western blot analysis and cumulative data showing the release of TGF-β1 measured in the conditioned media of VSMC stimulated with ANG II or HG for 48 h. *P < 0.05 statistically significant differences between CTR vs. ANG II and CTR vs. HG; n = 6. C: TGF-β1 bioactivity measured in the conditioned media of VSMC stimulated with ANG II or HG for 48 h. P < 0.05, statistically significant differences between CTR vs. ANG II and CTR vs. HG (*) and between ANG II or HG vs. ANG II + β3-integrin antibody, ANG II + SB-223245, HG + β3-integrin antibody, or HG + SB-223245 (#); n = 6 for each experiment.
It has been reported that HG increased TGF-β1 bioactivity through thrombospondin-1 in rat cardiac fibroblasts. (44) To determine that HG and ANG II regulate TGF-β1 expression and bioactivation, we first determined the total amount of TGF-β1 release by VSMC in the conditioned media. Accordingly, TGF-β1 release was increased in response to ANG II and HG compared with control (Fig. 3B). Using the TGF-β1 bioassay kit, we showed that the treatment of VSMC for 48 h with HG or ANG II was associated with increased TGF-β1 bioactivity (Fig. 3C). The preincubation of VSMC with β3-integrin specific antibody (1:100 dilution) or with αvβ3-integrin inhibitor (SB-223245, 1 μM) prevented the increased TGF-β1 bioactivity in response to ANG II or HG (Fig. 3C).
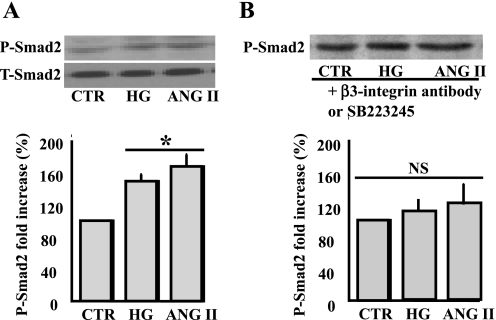
It is well known that Smad2 is a downstream target signaling to TGF-β1. Thus VSMC stimulated with sustained ANG II or HG significantly increased Smad2 phosphorylation (Fig. 4A). Total Smad2 was similar in all samples, indicating that sustained ANG II or HG did not change Smad2 expression (Fig. 4A). The preincubation of VSMC with β3-integrin antibody or SB-223245 (αvβ3-inhibitor) prevented the increased Smad2 phosphorylation in response to ANG II or HG (Fig. 4B), strengthening the role of αvβ3-integrin as an upstream molecule involved in the activation of Smad2.
Fig. 4.
A: Western blot analysis and cumulative data showing phosphorylated and total Smad2 of VSMC stimulated with ANG II or HG for 48 h. *P < 0.05, statistically significant between CTR vs. ANG II and CTR vs. HG; n = 6. B: Western blot analysis and cumulative data showing phosphorylated and total Smad2 of VSMC pretreated with β3-integrin antibody (1:100 dilution) or SB-223245 (10 μM) and stimulated with ANG II or HG for 48 h. NS, statistically no significance between CTR vs. ANG II or HG ± β3-integrin antibody or SB-223245; n = 6.
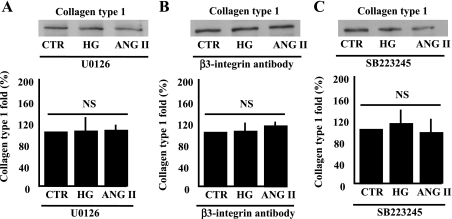
To determine the role of ERK1/2 MAP kinase, αvβ3-integrin, and TGF-β1 in the increased collagen type 1 deposition in response to ANG II or HG, pharmacological and molecular approaches were used. The pretreatment of VSMC with mitogen/extracellular signal-regulated kinase inhibitor (U-0126, 1 μM) significantly prevented the increased collagen type 1 in the conditioned media in response to ANG II and HG (Fig. 5A). Phosphorylated ERK1/2 MAP kinase was significantly inhibited, indicating the efficiency of U-0126 (data not shown). The blocking of β3-integrin by the incubation of VSMC with β3-integrin antibody (1:100 dilution) or the inhibition of αvβ3-integrin (SB-223245, 1 μM) significantly prevented the increased collagen type 1 content in response to ANG II or HG (Fig. 5, B and C).
Fig. 5.
Western blot analysis and cumulative data showing the release of collagen type 1 in the conditioned media from VSMC pretreated with mitogen-extracellular signal-regulated kinase (MEK) inhibitor (U-0126, 1 μM) and stimulated with ANG II or HG (A). NS, no statistical significance between CTR + U-0126 vs. ANG II or HG + U-0126; n = 6. B: β3-integrin specific antibody (1:100 dilution) and stimulated with ANG II or HG. NS, no statistical significance between CTR + β3-integrin antibody vs. ANG II or HG + β3-integrin antibody; n = 6. C: SB-223245 (10 μM) and stimulated with ANG II or HG. NS, no statistical significance between CTR + SB-223245 vs. ANG II or HG + SB-223245; n = 6.
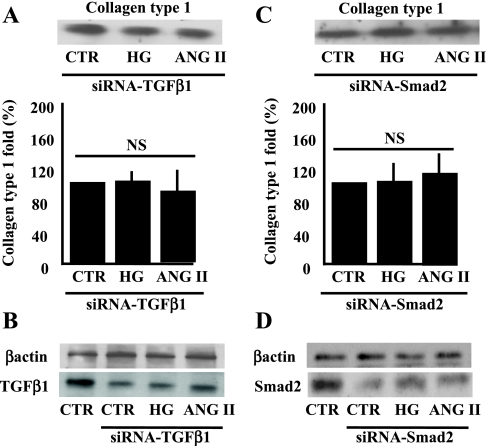
Because TGF-β1 is essential for growth, including protein synthesis and fibrosis, we investigated the impact of TGF-β1 expression knockdown in VSMC using siRNA-mediated gene silencing. Transfected VSMC with siRNA-TGF-β1 significantly prevented the increased collagen type 1 in response to ANG II or HG (Fig. 6A) and significantly decreased TGF-β1 expression (Fig. 6B), indicating the efficiency of siRNA-TGF-β1. Expression of β-actin showed similar loading between samples (Fig. 6B). As a control, VSMC were transfected with nontargeting siRNA, and collagen type 1 content upregulation was not prevented in response to ANG II or HG (data not shown).
Fig. 6.
A: Western blot analysis and cumulative data showing the release of collagen type 1 in the conditioned media from VSMC transfected with small-interfering RNA (siRNA)-TGF-β1 (40 nM) and stimulated with ANG II or HG. NS, no statistical significance between CTR + siRNA-TGF-β1 vs. ANG II or HG + siRNA-TGF-β1; n = 4. B: Western blot analysis showing the expression of TGF-β1 and α-actin in VSMC transfected with siRNA-TGF-β1, n = 4. C: Western blot analysis and cumulative data showing the release of collagen type 1 in the conditioned media from VSMC transfected with siRNA-Smad2 (40 nM) and stimulated with ANG II or HG. NS, no statistical significance between CTR + siRNA-Smad2 vs. ANG II or HG + siRNA-Smad2; n = 4. D: Western blot analysis showing the expression of TGF-β1 and β-actin of VSMC transfected with siRNA-Smad2; n = 4.
To determine whether Smad2 (down stream target signaling to TGF-β1) is a key element in the regulation of collagen type 1 content, we performed transfection of VSMC with siRNA-Smad2 and found that increased collagen type 1 content in response to ANG II or HG was prevented (Fig. 6C) and was associated with decreased Smad2 expression (Fig. 6D). β-Actin expression showed similar loading between all samples (Fig. 6D).
The use of l-glucose did not affect any signaling target activated by d-glucose, indicating the absence of nonspecific effect that could be related to HG.
DISCUSSION
The present study demonstrates abnormal deposition of collagen type I in resistance artery from hypertensive and Type 1 diabetic mice. The treatment of hypertensive and Type 1 diabetic mice with neutralizing TGF-β1 antibody significantly reduced the content of collagen type I and subsequently resistance artery stiffness. ANG II and HG induced an increase in collagen type 1 formation of primary cultured VSMC prepared from resistance arteries, that is, ERK1/2 MAP kinase, αvβ3-integrin shedding, and TGF-β1 pathway dependent. In addition, αvβ3-integrin is a key element in TGF-β1 bioactivity, leading to Smad2 pathway activation and subsequently collagen type 1 content deposition.
Resistance arteries play a crucial role in the regulation of blood pressure, local blood flow, and tissue perfusion. To accomplish these functions, resistance arteries are highly dependent on the capability of SMC to contract and relax in an integrated ECM. It has been shown that hypertension (ANG II-dependent) and diabetes (hyperglycemia) are associated with SMC dysfunction, artery wall structural remodeling changes, and stiffness increase responsible for microvascular complications (7, 32, 37). The important feature of vascular disease is abnormal contraction/relaxation, protein synthesis and release, proliferation, migration, hypertrophy of VSMC, and abnormal ECM deposition (ECM, collagen abnormal accumulation) a key factor of fibrosis, stiffness, and vasculopathy. However, the mechanism underlying fibrosis, related to abnormal deposition of collagen type 1, remains to be elucidated, especially in resistance arteries.
The primary importance of collagen type 1 can be inferred by its presence in almost all human tissues and by the nonviability of embryos deficient in this ECM protein (4). Collagen type 1 exerts its roles as a loading-bearing structure and regulator of cell function only after it has polymerized to a network. Indeed, it has been reported that collagen polymerization can be regulated by soluble agonists of cell function and is under close cellular control, which is dynamically integrated with fibronectin assembly (20).
Interestingly, arterial stiffness is an additional independent risk factor for cardiovascular and metabolic diseases, and strategies aimed at lowering arterial stiffness may be effective in reducing cardiovascular risk. To determine the molecular mechanisms involved in abnormal collagen type 1 deposition in hypertension and Type 1 diabetes, we established a primary cultured VSMC prepared from MRA with diameter <100 μm and subjected to acute and sustained stimulation with ANG II or HG. Acute stimulation of VSMC with ANG II and HG induced a similar increase in ERK1/2 MAP kinase phosphorylation. These data are largely supported by studies conducted in vitro (26, 27, 29, 41), indicating that ANG II and HG can rapidly activate a common early signaling event that coordinates intermediate downstream signaling pathways. Sustained stimulation of VSMC with ANG II and HG induced an increase in αvβ3-integrin shedding from cell surface, TGF-β1, and collagen type 1 upregulation measured in the conditioned media using immunoprecipitation and Western blot analysis. Our data are strongly supported by immunostaining of collagen type 1 network formation on confluent VSMC stimulated with ANG II or HG. The inhibition of ERK1/2 MAP kinase significantly prevented the upregulation of collagen type 1 in response to sustained ANG II or HG, indicating a link between ERK1/2 MAP kinase as an early event and collagen type 1 content regulation. Our data are supported by a recent study showing that hypoxia and platelet-derived growth factor-BB induced synthesis of soluble collagen type 1 via ERK1/2 and p38 MAP kinase. (14) Similarly, it has been shown that inhibition of the ERK1/2 pathway can attenuate ANG II-induced growth and collagen deposition in hypertensive rats (40). The concept that early event ERK1/2 MAP kinase can coordinate intermediate signaling leading to collagen type 1 upregulation is supported by different studies. For instance, Dzau's (3) group reported that a 24-h suppression of the cell cycle and thus VSMC proliferation was sufficient to significantly block neointima formation in carotid artery observed at 14 days.
ANG II and HG induced an increase in αvβ3-integrin shedding and TGF-β1 release (late event signaling) measured in the conditioned media. TGF-β1 is normally expressed in a latent form that requires activation to be functional (10). Thus inhibition of αvβ3-integrin or pretreatment of VSMC with β3-integrin antibody significantly prevented the increased bioactivity of TGF-β1, indicating that αvβ3-integrin is a key element in TGF-β1 bioactivity. Our data are in agreement with a previous study showing TGF-β bioactivity is dependent on αvβ6-integrin, αvβ8-integrin, and probably α2β1-integrin (6, 10, 20). On the other hand, it has been reported that HG increased TGF-β1 bioactivity through thrombospondin-1 in rat cardiac fibroblasts (44). This divergence with the latest data could be related to cell type and the vessel bed.
Smad is a downstream target signaling to TGF-β1 (35). To determine that TGF-β1 effect is through Smad2, VSMC were stimulated with ANG II or HG, and Smad2 phosphorylation was assessed. ANG II and HG significantly increased Smad2 phosphorylation, and no effect was observed on total Smad2, indicating that ANG II and HG targeted Smad2 phosphorylation level rather than gene expression. To establish the link between TGF-β1, Smad2, and collagen content, VSMC were transfected with siRNA-TGF-β1 or siRNA-Smad2. The siRNA-TGF-β1 or siRNA-Smad2 significantly prevented the upregulation of collagen type 1 deposition in response to ANG II or HG, indicating that the TGF-β1 effect is through Smad2. The specificity and efficiency of siRNA transfection were determined using nontargeting siRNA, which did not prevent the upregulation of collagen type 1 content in response to ANG II or HG. Together, these data strongly indicate a common pathway and link between ANG II/HG, αvβ3-integrin, TGF-β1, Smad2, and collagen type I content regulation.
The treatment of hypertensive and Type 1 diabetic mice with neutralizing TGF-β1 specific antibody for 1 wk significantly reduced the abnormal deposition of collagen type I in resistance arteries compared with resistance arteries from nontreated hypertensive and Type 1 diabetic mice. Our results are in agreement with studies showing that Autocrine TGF-β1 signaling in VSMC is required for ANG II-induced fibrogenic responses in cerebral vessels in vivo (43), in human VSMC in response to HG (21), and in animal VSMC (from large arteries) through arginine transport and direction of its metabolism to polyamines and l-proline.(8) Moreover, in Type 1 diabetes, it has been shown that TGF-β1 is important in the formation of cardiac fibrosis (1).
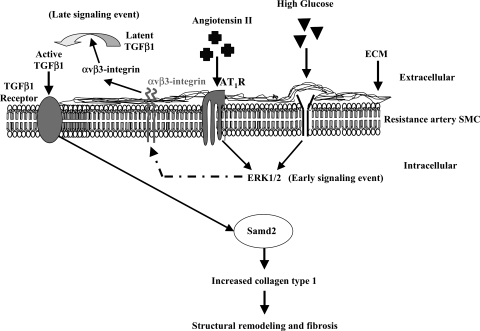
This study provides insight into the poorly understood cellular and molecular mechanisms that underlie resistance artery fibrosis resulting from abnormal collagen type 1 deposition. Despite the plethora of drugs available, hypertension and diabetes are adequately controlled in only about one-third of patients. Hormones and vasoactive peptides, like lead actors in a drama, have garnered much attention in the context of the pathogenesis and vasculopathy in hypertension and diabetes. The common pathway (ERK1/2 as early, and αvβ3-integrin/TGF-β1-Smad2 as late signaling events) (Fig. 7) is a very interesting observation and brought to center stage as a functional integrin and cytokine “αvβ3-integrin and TGF-β1” in the control of collagen type 1 content. This offers a new direction for the investigation of the mechanism of vasculopathy in hypertension and Type 1 diabetes, and ultimately for treatment.
Fig. 7.
Model illustrating the mechanism leading to the abnormal collagen type 1 deposition in resistance artery wall from hypertensive and Type 1 diabetic mice.
Understanding the structural vascular alterations linked to abnormal accumulation of ECM, especially collagen type 1 content, and the mechanisms whereby generated may offer important insight that may help in the development of therapies contributing to the correction of vasculature initiated end-organ damage in cardiovascular and metabolic diseases.
GRANTS
This work was supported by the American Heart Association, National Scientist Development Grant 0430278N (K. Matrougui), Tulane Center of Biomedical Research Excellence in Hypertension and Renal Biology (2P20RR-017659-06; L. Navar and K. Matrougui), and National Heart, Lung, and Blood Institute Grant HL-072889 (A. H. Boulares).
Acknowledgments
SB-223245 (αvβ3-inhibitor) was kindly provided by Glaxo SmithKline Pharmaceuticals.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell'italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol 171: 777–789, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernadet-Monrozies P, Rostaing L, Kamar N, Durand D. The effect of angiotensin-converting enzyme inhibitors on the progression of chronic renal failure. Presse Med 31: 1714–1720, 2002. [PubMed] [Google Scholar]
- 3.Braun-Dullaeus RC, Mann MJ, Ziegler A, von der Leyen HE, Dzau VJ. A novel role for the cyclin-dependent kinase inhibitor p27(Kip1) in angiotensin II-stimulated vascular smooth muscle cell hypertrophy. J Clin Invest 104: 815–823, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breindl M, Harbers K, Jaenisch R. Retrovirus-induced lethal mutation in collagen I gene of mice is associated with an altered chromatin structure. Cell 38: 9–16, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54: 1829–1837, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol 166: 1883–1894, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diez J Arterial stiffness and extracellular matrix. Adv Cardiol 44: 76–95, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Transforming growth factor-beta(1) stimulates l-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation 103: 1121–1127, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Elased KM, Cool DR, Morris M. Novel mass spectrometric methods for evaluation of plasma angiotensin converting enzyme 1 and renin activity. Hypertension 46: 953–959, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest 90: 456–461, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashida T, Schnaper HW. High ambient glucose enhances sensitivity to TGF-beta1 via extracellular signal–regulated kinase and protein kinase Cdelta activities in human mesangial cells. J Am Soc Nephrol 15: 2032–2041, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Intengan HD, Thibault G, Li JS, Schiffrin EL. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats: effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation 100: 2267–2275, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res 93: 874–883, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Karakiulakis G, Papakonstantinou E, Aletras AJ, Tamm M, Roth M. Cell type-specific effect of hypoxia and platelet-derived growth factor-BB on extracellular matrix turnover and its consequences for lung remodeling. J Biol Chem 282: 908–915, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Klahr S Progression of chronic renal disease. Heart Dis 3: 205–209, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Koller A Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation 9: 277–294, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Langille BL Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol 21, Suppl 1: S11–S17, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence DA Transforming growth factor-beta: a general review. Eur Cytokine Netw 7: 363–374, 1996. [PubMed] [Google Scholar]
- 19.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int 63: 2010–2019, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Van Den Diepstraten C, D'Souza SJ, Chan BM, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol 163: 1045–1056, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Luo F, Pan K, Wu W, Chen H. High glucose upregulates connective tissue growth factor expression in human vascular smooth muscle cells (Abstract). BMC Cell Biol 8: 1, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 23: 571–579, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation 110: 3587–3593, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res 89: 930–934, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Masson S, Arosio B, Fiordaliso F, Gagliano N, Calvillo L, Santambrogio D, D'Aquila S, Vergani C, Latini R, Annoni G. Left ventricular response to beta-adrenergic stimulation in aging rats. J Gerontol A Biol Sci Med Sci 55: B35–B41, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Matrougui K, Eskildsen-Helmond YE, Fiebeler A, Henrion D, Levy BI, Tedgui A, Mulvany MJ. Angiotensin II stimulates extracellular signal-regulated kinase activity in intact pressurized rat mesenteric resistance arteries. Hypertension 36: 617–621, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Matrougui K, Tanko LB, Loufrani L, Gorny D, Levy BI, Tedgui A, Henrion D. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler Thromb Vasc Biol 21: 1288–1293, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Michel JB Renin-angiotensin system and vascular remodelling. Med Sci (Paris) 20: 409–413, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan R, Scott S, Bai W, Yerneni KK, Nadler J. Angiotensin II signaling in vascular smooth muscle cells under high glucose conditions. Hypertension 33: 378–384, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol 14: 561–566, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Plante GE Vascular response to stress in health and disease. Metabolism 51: 25–30, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Risler NR, Cruzado MC, Miatello RM. Vascular remodeling in experimental hypertension. ScientificWorldJournal 5: 959–971, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. alpha(v)beta(3) Integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J Biol Chem 279: 37726–37733, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Schiffrin EL Small artery remodeling in hypertension: can it be corrected? Am J Med Sci 322: 7–11, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther 315: 1005–1012, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Wang J, Li Y, Du Y, Arteel GE, Saari JT, Kang YJ, Cai L. Cardiac metallothionein synthesis in streptozotocin-induced diabetic mice, and its protection against diabetes-induced cardiac injury. Am J Pathol 167: 17–26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Susic D, Varagic J, Ahn J, Frohlich ED. Collagen cross-link breakers: a beginning of a new era in the treatment of cardiovascular changes associated with aging, diabetes, and hypertension. Curr Drug Targets Cardiovasc Haematol Disord 4: 97–101, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Tanko LB, Matrougui K. Can we apply results from large to small arteries? (Abstract) Circ Res 90: e68, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AA Pathophysiology of hypertension and endothelial dysfunction in patients with diabetes mellitus. Endocrinol Metab Clin North Am 30: 983–997, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Touyz RM, He G, El Mabrouk M, Schiffrin EL. p38 Map kinase regulates vascular smooth muscle cell collagen synthesis by angiotensin II in SHR but not in WKY. Hypertension 37: 574–580, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kubler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol 20: 940–948, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg 38: 11–23, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Yurovsky VV, Gerzanich V, Ivanova S, Dong Y, Tsymbalyuk O, Simard JM. Autocrine TGF-beta1 mediates angiotensin II-induced proliferative response of cerebral vessels in vivo. Am J Hypertens 20: 950–956, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun 339: 633–641, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res 95: 532–539, 2004. [DOI] [PubMed] [Google Scholar]