Abstract
Cold increases cutaneous vasoconstriction by unmasking the contractile activity of α2C-adrenoceptors (α2C-ARs) in vascular smooth muscle cells (VSMCs), which is mediated by the cold-induced mobilization of α2C-ARs from the transGolgi to the cell surface. The expression of α2C-ARs in human cutaneous VSMCs is under dual regulation by cyclic AMP: gene transcription is inhibited by cyclic AMP acting through protein kinase A but is increased by cyclic AMP acting through the exchange protein directly activated by cyclic AMP (EPAC) and the GTP-binding protein Rap1. Experiments were performed to further characterize the Rap1 signaling pathway. Forskolin (10 μM), the selective EPAC activator, 8-pCPT-2′-O-Me-cyclic AMP (CMC; 100 μM), or a constitutively active mutant of Rap1 (Rap1CA) increased the activity of c-Jun NH2-terminal kinase (JNK) in human cutaneous VSMCs. This was associated with the increased phosphorylation of c-Jun and activation of an activator protein (AP)-1 reporter construct, which were inhibited by the JNK inhibitor SP600125 (3 μM). Rap1CA increased the activity of an α2C-AR promoter-reporter construct, which was inhibited by SP600125 (3 μM) or by the mutation of an AP-1 binding site in the α2C-AR promoter. Furthermore, forskolin (10 μM) or CMC (100 μM) increased the expression of the α2C-AR protein, and these effects were inhibited by SP600125 (3 μM). Therefore, cyclic AMP increases the expression of α2C-ARs in cutaneous VSMCs by activating a novel Rap1 signaling pathway, mediated by the activation of JNK, AP-1, and the subsequent transcriptional activation of the α2C-AR gene. By increasing the expression of cold-responsive α2C-ARs, this pathway may contribute to enhanced cold-induced vasoconstriction in the cutaneous circulation, including Raynaud's phenomenon.
Keywords: Raynaud's phenomenon, activator protein-1, c-Jun NH2-terminal kinase, transcription, adenosine 5′-monophosphate
cold-induced cutaneous vasoconstriction, which is a protective physiological response to reduce heat loss, results from increased sympathetic nerve activity and a direct sensitizing effect of cold to amplify constriction to the sympathetic neurotransmitter norepinephrine (36). This latter effect is mediated by a selective cold-induced increase in the reactivity of α2-adrenoceptors (α2-ARs) located on cutaneous vascular smooth muscle cells (VSMCs) (10, 11, 13). Although α2-ARs comprise three subtypes (α2A, α2B, and α2C) (28), only α2C-ARs appear to be regulated by cold (6, 20). Moderate cooling stimulates the redistribution of α2C-ARs from the transGolgi, where they are normally retained, to the cell surface, where they can respond to agonist stimulation (2, 3, 20). The direct sensitizing effect of cold on cutaneous VSMCs is increased in individuals with Raynaud's phenomenon and scleroderma, resulting in cold-induced vasospasm that is prevented by α2-AR blockade (14).
The expression of α2C-ARs is under dual regulation by cyclic AMP: gene transcription is inhibited by cyclic AMP acting through protein kinase A (PKA) but is selectively increased by cyclic AMP acting through the exchange protein directly activated by cAMP (EPAC) and Rap (7, 31). The latter pathway predominates in cutaneous VSMCs, and elevations in cyclic AMP, e.g., in response to serum stimulation, forskolin, β-AR stimulation, or cholera toxin, are associated with a dramatic and selective increase in α2C-AR mRNA expression (7, 8). The goal of the present study was to identify the signaling mechanisms responsible for this novel transcriptional response. In other cell types, Rap1 plays a prominent role in activating the extracellular signal-regulated kinase (ERK) signaling pathway, which contrasts with the inhibitory influence of PKA (5, 27, 35, 39). Therefore, our initial hypothesis was that the ERK signaling pathway might contribute to the Rap1-induced transcriptional activation of α2C-ARs in VSMCs.
METHODS
Materials.
8-(4-Chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate or 8-pCPT-2′-O-Me-cAMP (CMC) was from Alexis (www.axxora.com), forskolin was from Sigma-Aldrich (www.sigmaaldrich.com), and H89 [N-[2-((p-Bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide], SP600125 (Anthra[1,9-cd]pyrazol-6(2H)-one 1,9-pyrazoloanthrone), and U0126 (1,4-Diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene) were from Calbiochem (www.emdbiosciences.com). CMC was dissolved in media, whereas forskolin was dissolved in DMSO (final concentration, 1 μl/ml). The PKA inhibitor H89, the c-Jun NH2-terminal kinase (JNK) inhibitor SP600125, and the MEK inhibitor U0126 were dissolved in DMSO (final concentration, 0.2 μl/ml) and used at concentrations that act selectively to inhibit their respective enzyme systems (7, 12, 16, 34). When the effects of these inhibitors were analyzed, DMSO (0.2 μl/ml) was added to the control cells. In separate preliminary experiments, we demonstrated that DMSO (≤1 μl/ml) had no effect on the relevant assays. Unless stated otherwise, when inhibitors were used, they were incubated with the cells for 30 min before and then during the subsequent administration of the agonist.
The activator protein (AP)-1-luciferase reporter plasmid was from Stratagene (www.stratagene.com), MKK7 was a kind gift from Dr. Roger Davis (Howard Hughes Medical Institute, University of Massachusetts), the α2C-AR promoter-reporter plasmid (−1,915/+5, relative to the transcription start site +1) was a kind gift from Dr. Herve Paris (7, 8), and pRL-CMV [Renilla luciferase gene driven by cytomegalovirus (CMV) promoter/enhancer] was purchased from Promega (www.promega.com). Constitutively active Rap 1A (Rap1CA) GTPase [amino acid number 63 glutamine (Q) mutated to glutamic acid (E); Rap 1A-63E] was kindly provided by L. A. Quilliam (Indiana University School of Medicine) (26).
Antibodies to ERK1/2 (p44/42 mitogen-activated protein kinase) and phosphorylated ERK (Thr202/Tyr204), JNK and phosphorylated JNK (Thr183/Tyr185), and c-Jun and phosphorylated c-Jun (Ser73) were obtained from Cell Signaling Technologies (www.cellsignal.com). Antibody to total filamin was from US Biological (www.usbio.net), phosphorylated filamin-2 (Ser2113) was from Biosource (www.invitrogen.com), for calponin was from Sigma (www.sigmaaldrich.com), and for B-Raf was from Upstate (www.upstate.com). The custom antibody to α2C-AR was generated and characterized as previously described (2, 7, 8). Primary antibodies were used at a dilution of 1:1,000, apart from total filamin, which was used at a dilution of 1:500.
Cell culture.
Cutaneous VSMCs were cultured from human small cutaneous arteries and used between passages 9 and 12, as previously described (2, 7, 8). The expression and regulation of α2C-ARs in these VSMCs is similar between passages 4 and 12 (8). Although cultured VSMCs are phenotypically distinct from those present in the blood vessel media, they provide a useful model to assess relevant signaling processes. Indeed, an analysis of cultured VSMCs from cutaneous arteries has provided relevant insight into mechanisms regulating α2C-AR expression and function in cutaneous blood vessels (2, 3, 8, 9). VSMCs were grown in Ham's Growth medium (DMEM, F12; 50:50) supplemented with 10% heat-inactivated FBS, l-glutamine, and an antibiotic/antimycotic cocktail. Cells were made quiescent in 0% or 0.5% serum for 72 h before the start of experiments. When the effect of the antagonists was analyzed, they were administered 30 min before an agonist and remained in contact with the cells during the agonist exposure.
Western blot analysis.
Unless stated otherwise, cells were washed twice with phosphate-buffered saline and scraped into lysis buffer containing 2% SDS and 60 mM Tris (pH 6.8). After sonication, the lysate was centrifuged at 5,000 g for 10 min and the supernatant was analyzed for protein concentration (bicinchoninic acid; Pierce, Rockford, IL). Analysis of α2C-AR expression was performed using membrane preparations; VSMCs were suspended in homogenization buffer of (in mM) 50 Tris (pH 7.5), 1 EDTA, and 2 MgCl2 containing antiproteases [15.7 μg/ml each of chymostatin, antipain, pepstatin; 57.7 μg/ml leupeptin; and 250 μg/ml 4-(2-aminoethyl)benzenesulfonyl fluoride] and homogenized on ice with a glass homogenizer using 30 strokes followed by centrifugation (500 g, 4°C, 10 min). The resulting supernatant was then spun at 40,000 g (1 h, 4°C) in an ultracentrifuge, the pellet was resuspended in homogenization buffer, and the protein concentration was determined. Equal protein amounts (20 to 40 μg, depending on primary antibody) were separated using 10% SDS-PAGE, transferred to polyvinylidene difluoride membrane (Millipore), and incubated with the relevant primary antibody for 60 min at room temperature. After extensive washing, the membranes were incubated with the relevant secondary antibody (goat anti-rabbit or anti-mouse, 1:2,000 dilution; 1 h, room temperature). Blots were then developed using enhanced chemiluminescence and quantified using ImageQuant TL (Amersham Biosciences, www5.gelifesciences.com). For α2C-ARs, results from VSMCs were compared with those of human embryonic kidney 293 cells that had been transiently transfected with control plasmid (pcDNA3) or plasmid expressing α2C-ARs, as previously described (8).
Mutagenesis.
Site-directed mutagenesis was performed using mutant oligonucleotide primers and double-stranded DNA using the QuickChange XL site-directed mutagenesis kit (Stratagene, www.stratagene.com) following manufacturer recommendations. The wild-type AP-1 site (ATGATTCAT, −346/−338, relative to the transcription start site) in the α2C-AR promoter-luciferase reporter construct was mutated to ACTGTTTGT. The mutated construct (mutant) was characterized and confirmed by DNA sequencing.
Transient transfections.
Cells were transiently transfected by nucleofection with the Amaxa Nucleofector (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer's instructions. Transfection was optimized to achieve an approximate 80% transfection efficiency, with minimal toxicity, achieved with a nucleofection of 400,000 cells with 4 μg of nucleic acids (total amount of transfected DNA was kept constant by using appropriate empty plasmids). With the use of promoter-reporter constructs, pRL-CMV (Renilla luciferase driven by CMV immediate early enhancer/promoter; Promega) was used as an internal control to normalize the firefly luciferase units (7, 8). Cotransfections were therefore performed using 3.4 μg of firefly luciferase reporter plasmid (α2C-AR promoter or AP-1 promoter), 0.001 μg of pRL-CMV, and 0.6 μg of MKK7, Rap1CA, or pcDNA3 (for a total of 4 μg). After transfection, cells were allowed to recover overnight in Ham's growth medium. After aspiration of the growth medium, cells were washed thoroughly and maintained in serum-free media for 72 h before experimentation. When the effects of pharmacological inhibitors were analyzed, they were included in the culture media for the final 24 h. For luciferase analysis, cells were washed, lysed in luciferase lysis buffer (Promega), snap frozen, and then thawed at room temperature. Cell lysates were then spun down at 9,300 g for 10 min, and luciferase activity in the supernatant was determined.
Statistical analyses.
Statistical evaluation of the data was performed by Student's t-test for either paired or unpaired observations. When more than two means were compared, either a one-way ANOVA with Dunnett's post hoc test or a two-way ANOVA with Tukey-Kramer's post hoc test (GraphPad InStat3 Software; San Diego, CA) was used. Data are presented as means ± SE, where n equals the number of different cell culture experiments.
RESULTS
ERK signaling in cutaneous VSMCs.
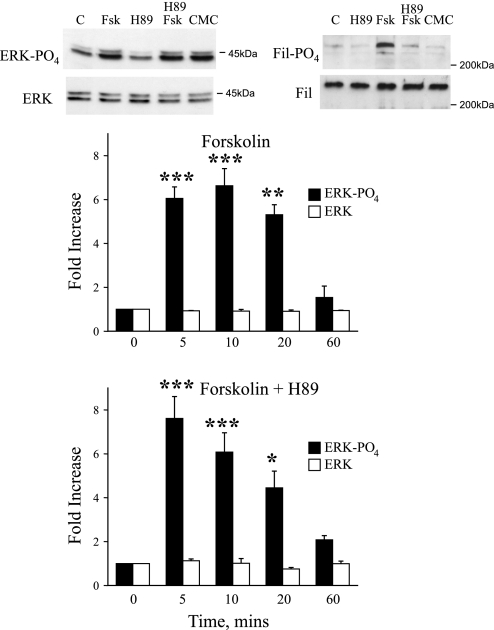
In non-VSMCs, a major signaling role of Rap1 is the activation of the Raf/MEK/ERK pathway (5, 27, 35, 39). Although cyclic AMP signaling through PKA can inhibit this pathway by phosphorylating C-Raf (33, 38), Rap1 can activate MEK/ERK signaling by stimulating B-Raf (5, 27, 35, 39). In cutaneous VSMCs, forskolin (10 μmol/l) increased the phosphorylation of ERK1/2, which peaked at 10 min and had declined to basal levels by 60 min (Fig. 1). The forskolin-induced increase in ERK phosphorylation was not altered by the PKA inhibitor H89 (2 μmol/l) at any time point (Fig. 1). We previously demonstrated that H89 (2 μmol/l) abolished the forskolin-induced phosphorylation of the PKA substrate cyclic AMP response element blinding protein (CREB) (7). In the present study, forskolin (10 μmol/l; 10 min) also stimulated the phosphorylation of the PKA substrate filamin [PO4-filamin increased by 6.4 ± 0.3-fold compared with that of untreated cells, n = 3, P < 0.001; whereas total filamin remained unchanged at 0.9 ± 0.1-fold, n = 3, P = not significant (NS)]. This effect was abolished by H89 (2 μmol/l; after forskolin plus H89, PO4-filamin was 1.7 ± 0.5-fold of untreated cells, n = 3, P < 0.001 compared with forskolin alone and P = NS compared with untreated cells; whereas total filamin remained unchanged at 0.9 ± 0.1-fold, n = 3, P = NS; Fig. 1). The selective EPAC activator CMC (100 μmol/l; 10 min) had no effect on the phosphorylation of filamin (PO4-filamin remained unchanged at 1.0 ± 0.2-fold, n = 4, P = NS, as did total filamin at 0.8 ± 0.1-fold, n = 4, P = NS; Fig. 1). In contrast, CMC (100 μmol/l) increased the phosphorylation of ERK1/2, with a similar intensity and time course to that observed with forkolin (10 μmol/l; Fig. 1). For example, after 10 min, CMC increased PO4-ERK by 8.6 ± 0.9-fold (n = 3), whereas forskolin increased PO4-ERK by 6.6 ± 0.8-fold (n = 4; total ERK remained unchanged at 0.9 ± 0.3 and 0.9 ± 0.1-fold, n = 3 and 4, respectively). Transfection of cutaneous VSMCs with Rap1CA increased the phosphorylation of ERK (PO4-ERK increased by 2.6 ± 0.4-fold, P < 0.05, n = 3; whereas total ERK remained unchanged at 1.0 ± 0.1, P = NS, n = 3).
Fig. 1.
Effect of forskolin (FSK; 10 μmol/l), in the absence (top bars) and presence (bottom bars) of the PKA inhibitor H89 (2 μmol/l), on ERK1/2 phosphorylation in human cultured cutaneous vascular smooth muscle cells (VSMCs). Cells were harvested 5, 10, 20, and 60 min after addition of FSK, and cell lysates were assessed for total and phosphorylated ERK using Western blot analysis. Top: representative blots demonstrating effects of FSK (10 μmol/l; in the presence and absence of H89 at 2 μmol/l) or 8-pCPT-2′-O-Me-cyclic AMP (CMC; 100 μmol/l) on levels of phospho-ERK1/2 or total ERK1/2 (left) and on levels of phospho-filamin (Fil) or total Fil (right) 10 min after administration of the agonists. When present, H89 was administered 30 min before and during exposure of the cells to FSK. FSK or CMC increased phosphorylation of ERK1/2 that was sustained for at least 20 min but had returned to basal levels by 60 min. H89 did not influence the response to FSK at any time point. The results are expressed as fold increase from time 0 and are presented as means ± SE for n = 4 different cell culture experiments. *Significantly different from control; *P < 0.05; **P < 0.01; ***P < 0.001. C, control.
JNK signaling in cutaneous VSMCs.
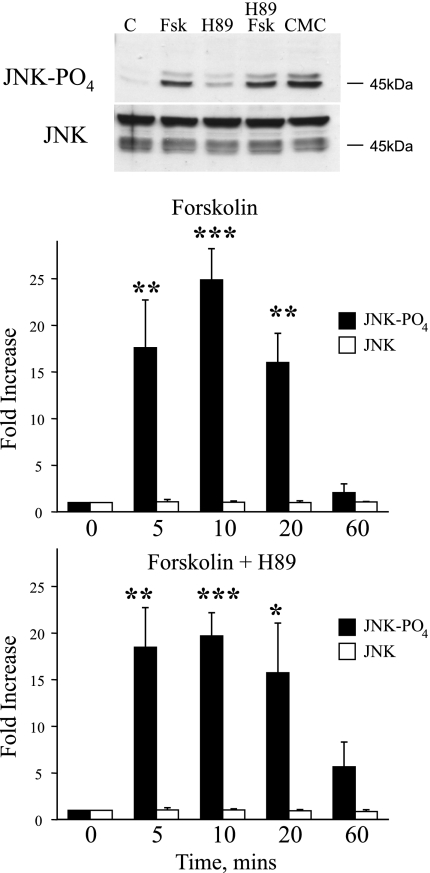
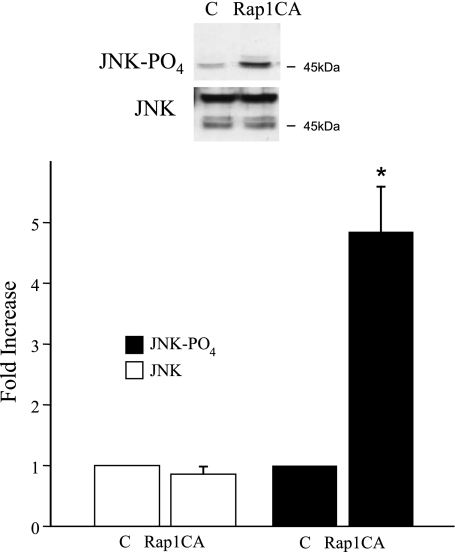
Forskolin (10 μmol/l) increased the phosphorylation of JNK, which had peaked by 10 min and had declined to basal levels by 60 min (Fig. 2). The forskolin-induced increase in JNK phosphorylation was not affected by the PKA inhibitor H89 (2 μmol/l; Fig. 2). The selective EPAC activator CMC (100 μmol/l) also increased the phosphorylation of JNK with a similar intensity and time course to that observed with forkolin (10 μmol/l; Fig. 2). For example, after 10 min, CMC increased PO4-JNK by 42.0 ± 6.5-fold (n = 3), whereas forskolin increased PO4-JNK by 24.9 ± 3.4-fold (n = 4; total JNK remained unchanged at 1.2 ± 0.1 and 1.0 ± 0.1-fold, respectively). Transfection of cutaneous VSMCs with Rap1CA increased the phosphorylation of JNK (Fig. 3).
Fig. 2.
Effect of FSK (10 μmol/l), in the absence (top bars) and presence (bottom bars) of the PKA inhibitor H89 (2 μmol/l) on JNK phosphorylation in human cultured cutaneous VSMCs. Cells were harvested 5, 10, 20, and 60 min after addition of FSK, and cell lysates were assessed for total and phosphorylated JNK using Western blot analysis. Top: representative blot demonstrating effect of FSK (10 μmol/l), in the presence and absence of H89 (2 μmol/l), or CMC (100 μmol/l) on levels of phospho-JNK or total JNK 10 min after administration of the agonists. When present, H89 was administered 30 min before and during exposure of the cells to FSK. FSK or CMC increased phosphorylation of JNK that was sustained for at least 20 min but had returned to basal levels by 60 min. H89 did not influence the response to FSK at any time point. The results are expressed as fold increase from time 0 and are presented as means ± SE for n = 4 different cell culture experiments. *Significantly different from control; *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 3.
Effect of a constitutively active mutant of Rap1 (Rap1CA) on JNK phosphorylation in human cultured cutaneous VSMCs. Cells were transfected with plasmids for Rap1CA or the control plasmid (pcDNA3, C) as described in methods. Cells were harvested 72 h later, and cell lysates were assessed for total and phosphorylated JNK using Western blot analysis. Top: representative blot demonstrating effect of Rap1CA on levels of phospho-JNK or total JNK. Rap1CA increased phosphorylation of JNK. The results are expressed as fold increases compared with control and are presented as means ± SE for n = 3 different cell culture experiments. *P < 0.05, significantly different from control.
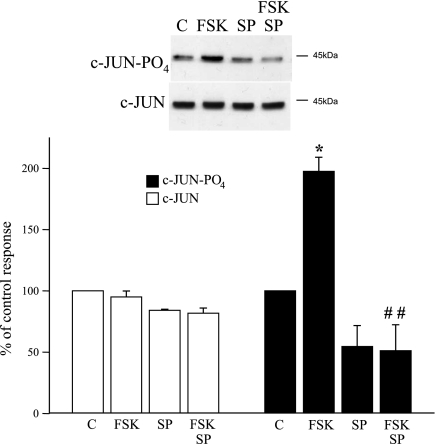
Consistent with the activation of JNK, forskolin (10 μmol/l) increased the phosphorylation of c-Jun (Fig. 4), a component of the AP-1 transcription factor. The increase in c-Jun phosphorylation was abolished by the JNK inhibitor SP600125 (3 μmol/l; Fig. 4).
Fig. 4.
Effect of FSK (10 μmol/l), in the absence and presence of the JNK inhibitor SP600125 (SP; 3 μmol/l), on c-Jun phosphorylation in human cultured cutaneous VSMCs. Cells were harvested 20 min after addition of FSK, and cell lysates were assessed for total and phosphorylated c-Jun using Western blot analysis. Top: representative blot demonstrating effect of FSK, in the presence and absence of SP, on levels of phospho-c-Jun or total c-Jun. When present, SP was administered 30 min before and during exposure of the cells to FSK. FSK increased phosphorylation of c-Jun, which was inhibited by SP. The results are expressed as percent changes in the control response and are presented as means ± SE for n = 3 different cell culture experiments. *Significantly different from control, P < 0.05; ##significantly different from effect of FSK alone, P < 0.01.
AP-1 and Rap1-mediated induction of α2C-ARs.
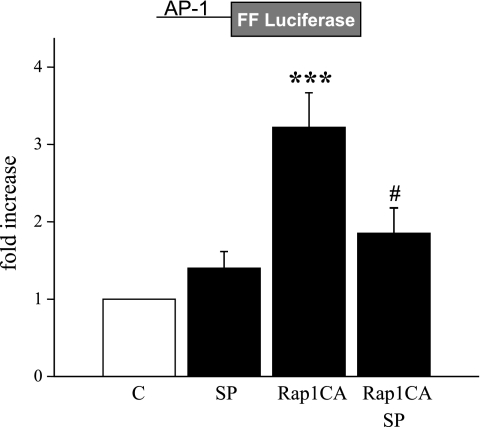
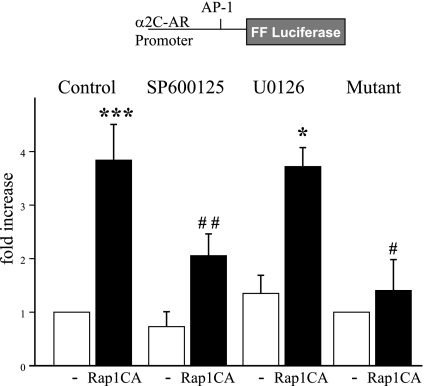
Transfection of cutaneous VSMCs with Rap1CA caused the activation of an AP-1 reporter construct, and this effect was inhibited by SP600125 (3 μmol/l; Fig. 5). Rap1CA also increased the transcriptional activity of an α2C-AR promoter-reporter construct that was inhibited by the JNK inhibitor SP600125 (3 μmol/l) but not by the MEK inhibitor U0126 (10 μmol/l; Fig. 6). Transfection of cutaneous VSMCs with MKK7, an upstream activator of JNK, also increased α2C-AR promoter activity (2.25 ± 0.23-fold increase, n = 5, P < 0.01).
Fig. 5.
Effect of Rap1CA on the activity of an activator protein (AP)-1 reporter construct. Cells were transfected with the reporter construct, an internal renilla control construct, and either Rap1CA or the control plasmid (pcDNA3, included in C and SP groups) as described in methods. Cells were harvested 72 h later, and cell lysates were assessed for luciferase activity. Rap1CA increased the activity of the AP-1 reporter, which was inhibited by SP. The results are expressed as fold increase in the firefly (FF)/Renilla fluorescent signal from the control level and are presented as means ± SE for n = 4 different cell culture experiments. ***Significantly different from control, P < 0.001; #significantly different from effect of Rap1 in the absence of SP, P < 0.05.
Fig. 6.
Analysis of the effects of Rap1CA on the activity of the α2C-adrenoceptor (AR) promoter. Cells were transfected with an α2C-AR reporter construct [wild-type (WT) or AP-1 binding site mutant], an internal renilla control construct, and either Rap1CA or the control plasmid (pcDNA3, -) as described in methods. Rap-1CA increased the activity of the WT α2C-AR promoter, and this effect was significantly reduced by the JNK inhibitor SP (3 μmol/l) but not by the MEK inhibitor U0126 (10 μmol/l). Rap-1CA did not alter the activity of the α2C-AR promoter following mutation of the AP-1 binding site. The results are expressed as fold increases in the FF/Renilla fluorescent signal from the control level and are presented as means ± SE for n = 3 to 5 different cell culture experiments. *Significantly different from untreated pcDNA3 control; *P < 0.05; ***P < 0.001; #significantly different from effect of Rap1CA on untreated WT α2C-AR promoter; #P < 0.05; ##P < 0.01.
The α2C-AR promoter contains an AP-1 consensus binding site located at −346/−338 (relative to the transcription start site) (32). Mutation of this site from ATGATTCAT to ACTGTTTGT prevented the increase in α2C-AR transcription in response to Rap1CA (Fig. 6).
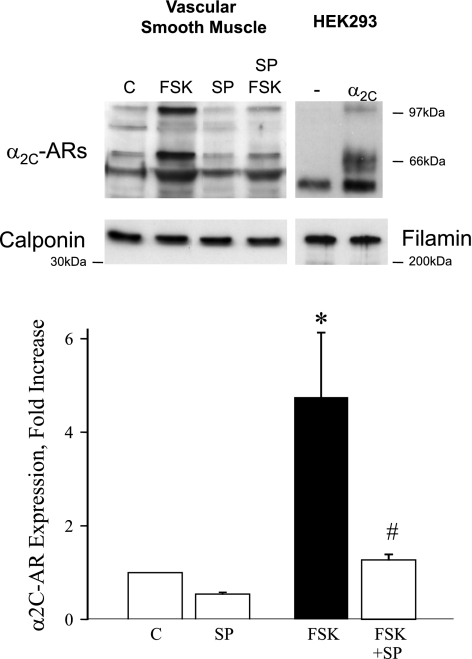
Forskolin (10 μmol/l) or CMC (100 μmol/l; 16 h) increased the expression of the α2C-AR protein in cutaneous VSMCs, which was inhibited by SP600125 (3 μmol/l). Forskolin (10 μmol/l; 16 h) increased the receptor expression by 4.7 ± 1.4-fold (n = 3, P < 0.05) under control conditions but had no effect in the presence of SP600125 (3 μmol/l; 1.2 ± 0.1-fold, P = NS; Fig. 7). Likewise, CMC (100 μmol/l; 16 h) increased the receptor expression by 2.2 ± 0.1-fold (n = 3, P < 0.001) under control conditions but had no effect in the presence of SP600125 (3 μmol/l; 0.6 ± 0.1-fold; P = NS).
Fig. 7.
Effect of FSK (10 μmol/l, 16 h) on the protein expression of α2C-ARs in human cultured cutaneous VSMCs, determined by Western blot analysis. FSK increased α2C-AR expression in the absence but not in the presence of the JNK inhibitor SP. The results are expressed as fold increases in α2C-AR expression from control unstimulated cells and are presented as means ± SE for n = 3. *Significantly different from control, P < 0.05; #significantly different from effect of FSK alone, P < 0.05. Top: representative Western blot illustrating the effect of FSK (in the absence and presence of SP) on α2C-AR expression in VSMCs. α2C-ARs comprise multiple molecular mass species, which is also reflected in the representative blot for human embryonic kidney (HEK)293 cells that were transiently transfected with control plasmid (-) or plasmid expressing α2C-ARs. Prominent α2C-AR-specific bands of ∼70 kDa and ∼100 kDa are expressed in HEK293 and VSMCs. The ∼70 kDa species is the form retained in the transGolgi and translocated in response to cold exposure (Refs. 2, 3, and 20). It has been the species that we have focused on in previous studies and was the species quantified in the bar graph. Similar results were obtained if the quantification was extended to the other receptor species. The physiological relevance of the other α2C-AR species has not been determined. Western blot for calponin (vascular smooth muscle) or Fil (HEK293) were performed on the same cell lysates as those used for α2C-AR blots.
DISCUSSION
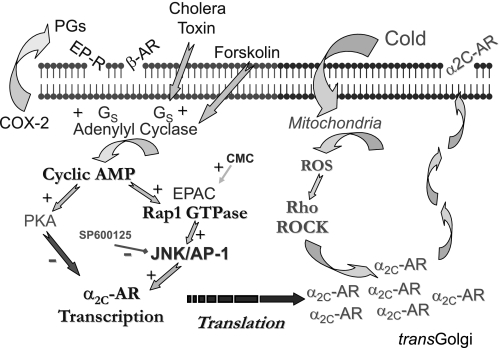
Cold-induced cutaneous vasoconstriction is mediated in part by an increased ability of α2C-ARs to initiate VSMC contraction. In mouse cutaneous arteries, constriction to α2-AR stimulation at warm temperatures is mediated by α2A-ARs, with no apparent contribution from α2C-ARs, whereas during cold exposure, the augmented α2-AR response is mediated entirely by α2C-ARs (6, 19). Cold sensitivity of α2C-ARs is not mediated by the increased expression of the receptors but rather by the cold-induced mobilization of α2C-ARs from the transGolgi network to the cell surface where they can respond to stimulation (2, 3, 6, 20). In the present study, we demonstrate that the expression of α2C-ARs in cutaneous VSMCs can be increased by a novel cyclic AMP/Rap1/JNK/AP-1 signaling pathway, which increases α2C-AR gene transcription (Fig. 8). By increasing the available pool of α2C-ARs for cold-induced mobilization, activation of this novel signaling pathway may contribute to increased cold-induced vasoconstriction, e.g., in individuals with Raynaud's phenomenon and scleroderma.
Fig. 8.
Summary of the novel signaling mechanisms underlying regulation of α2C-ARs in cutaneous VSMCs. Several stimuli including the β-AR agonist isoproterenol, estrogen, cholera toxin, FSK, and serum, which increases expression of cyclooxygenase (COX)-2 and generation of prostanoids, increase α2C-AR expression by acting through cyclic AMP-dependent activation of exchange protein directly activated by cAMP (EPAC) and Rap, leading to an increase in receptor transcription (Refs. 7 and 8). In contrast with the positive regulation by EPAC/Rap, the cyclic AMP-dependent protein kinase PKA suppresses α2C-AR transcription (Refs. 7, 8, and 31). Results of the present study indicate that the response to Rap1 is mediated by JNK and the AP-1 transcription factor. α2C-ARs are normally retained in the transGolgi network. However, cold exposure stimulates translocation of the receptors to the cell surface where they can respond to agonist stimulation and contribute to cold-induced vasoconstriction (Refs. 2, 3, and 20). This effect is mediated by cold-induced activation of Rho and Rho kinase (ROCK), which is in turn mediated by a cold-induced increase in mitochondrial reactive oxygen species (ROS). Increased expression of α2C-ARs may increase the pool of receptor available for cold-induced mobilization and contribute to enhanced cold-induced constriction (Ref. 9). EP-R, prostaglandin receptor.
Although generally associated with the activation of PKA, cyclic AMP can regulate other important components of cellular signaling, including Rap1, a member of the Ras family of small GTP-binding proteins (5, 27, 33, 35, 37–39). Activation of Rap1 by cyclic AMP is achieved by the binding of cyclic AMP to EPAC proteins, which function as G protein exchange factors for Rap1. The role of Rap1 in VSMCs remains relatively unexplored. We demonstrated that the activation of Rap1 by an elevation in cyclic AMP was responsible for the transcriptional activation and increased expression of α2C-ARs in cutaneous VSMCs in response to serum stimulation of these cells (7). Interestingly, although PKA can suppress the transcriptional activity of the α2C-AR gene, this role appears to be silenced in these VSMCs and the excitatory role of Rap1 predominates (7) (Fig. 8). In other cell types, a major signaling role of Rap1 appears to be the regulation of the Ras/Raf/MEK/ERK signaling pathway (5, 27, 35, 37, 39). Cyclic AMP signaling through PKA can inhibit this pathway by phosphorylating C-Raf (33, 38). Although the cyclic AMP-dependent activation of Rap1 may also contribute to C-Raf inhibition, Rap1 can activate MEK/ERK signaling by stimulating B-Raf activity (5, 27, 35, 39). B-Raf has higher activity toward MEK than C-Raf and may be the preferred physiological activator (27). Indeed, Rap1 activation leads to sustained ERK activation or acts synergistically with Ras-dependent signaling to convert transient to sustained ERK stimulation (1, 4, 15, 22, 25, 27, 29, 39). The expression of B-Raf in cells is therefore thought to determine whether cyclic AMP will activate (Rap1) or inhibit (PKA) ERK signaling (5, 27, 35, 39). In previous analyses of VSMCs, cyclic AMP was reported to reduce signaling through the Raf/MEK/ERK pathway (21, 23, 30). This likely reflects a low expression of B-Raf (24) and a predominance of a PKA-dependent inhibitory signaling in these large artery, noncutaneous VSMCs. Indeed, the expression of B-Raf in the cutaneous VSMCs used in the present study was 6.6-fold higher than its expression in human aortic VSMCs (data not shown). Interestingly, although serum and cyclic AMP elevation increased the expression of α2C-ARs in cutaneous VSMCs, this does not occur in aortic VSMCs (7, 8). Elevation in cyclic AMP in response to forskolin caused a powerful and prolonged activation of ERK signaling in these cutaneous VSMCs. This response was not affected by the PKA inhibitor H89 (2 μmol/l), a concentration that abolished the forskolin-induced phosphorylation of the PKA substrates filamin and CREB in these cells (7). ERK activation was also observed using the selective EPAC activator CMC. Therefore, these results confirm findings from non-VSMCs that Rap activity can initiate signaling through the ERK pathway. However, the Rap1-dependent activation of α2C-AR transcription was unaffected by the inhibition of ERK signaling using the MEK inhibitor U0126. This is consistent with our previous unpublished observations that the cyclic AMP-dependent induction of α2C-ARs is unaffected by the MEK inhibitor PD-98059 (3 to 30 μmol/l). These results therefore suggested that cyclic AMP and Rap1 utilize an alternate signaling pathway in cutaneous VSMCs to increase transcription of α2C-ARs.
Forskolin also increased the phosphorylation of JNK, with a time course that was similar to ERK activation. This response was also not influenced by the PKA inhibitor H89 (2 μmol/l) and was mimicked by CMC. Although a previous report suggested that EPACs could act independently of Rap to activate JNK (18), Rap1 can activate JNK signaling indirectly through a src- and Rac-dependent pathway (17). In the present study, Rap1CA increased the phosphorylation of JNK in cutaneous VSMCs. Therefore, Rap1 can initiate signaling through JNK in these cells. A major signaling pathway activated by JNK is the phosphorylation of c-Jun and assembly of the AP-1 transcription factor. In the present study, JNK phosphorylation was associated with the increased phosphorylation of c-Jun, which was inhibited by the JNK inhibitor SP600125. Likewise, Rap1CA caused the activation of an AP-1 promoter-reporter construct that was also inhibited by SP600125. A potential role for JNK in α2C-AR transcription was evident from the increased activity of an α2C-AR promoter-reporter construct following the transfection of VSMCs with MKK7, an upstream kinase for JNK activation. Indeed, the Rap1CA-induced activation of the α2C-AR promoter-reporter construct was inhibited by the JNK inhibitor SP600125. Schaak et al. (32) identified an AP-1 binding site in the promoter region of the human α2C-AR (−346/−338, relative to transcription start site), although its functional activity was never assessed. Mutation of this site prevented the Rap1CA-induced activation of α2C-AR promoter-reporter construct and the increase in transcription, confirming that the transcriptional activation was mediated by AP-1 signaling. The Rap1-mediated increase in α2C-AR transcriptional activity was paralleled by the increased protein expression of the α2C-AR. Incubation of cutaneous VSMCs with forskolin or the selective EPAC activator CMC increased α2C-AR protein expression that was significantly reduced by the JNK inhibitor SP600125.
α2C-ARs in cutaneous arteries are functionally silent under normal conditions (6, 19), and constriction to α2-AR stimulation in mouse tail arteries is mediated exclusively by α2A-ARs at warm temperatures (6). However, in response to cold exposure, these receptors are no longer silent and α2C-ARs mediate the cold-induced increase in vasoconstriction to α2-AR activation (6). The cold-induced functional rescue of α2C-ARs results from a cold-induced translocation of α2C-ARs from the transGolgi to the cell surface (2, 3, 20) (Fig. 8). When the VSMC expression of α2C-ARs is increased, the constrictor activity of α2-ARs is unaffected at warm temperatures but dramatically increased during exposure to cold, increasing cold-induced constriction in cutaneous arteries (9). This suggests that the increased expression of α2C-ARs augments the pool of receptors available for cold-induced mobilization to the cell surface, resulting in a selective increase in the cold-induced amplification of sympathetic constriction. The present study has identified a novel cyclic AMP/Rap1/JNK/AP-1 signaling pathway in cutaneous vascular smooth muscle that enables the increased expression of α2C-ARs (Fig. 8). Activation of this pathway in response to physiological stimuli or following vascular injury may contribute to the increased cold-induced vasoconstriction in the cutaneous circulation.
GRANTS
This study was funded by the National Institutes of Health Grants HL-080119 and OH-008531 (to N. A. Flavahan) and from the American Heart Association Great Rivers Affiliate (to M. Chotani).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Altschuler DL, Ribeiro-Neto F. Mitogenic and oncogenic properties of the small G protein Rap1b. Proc Natl Acad Sci USA 95: 7475–7479, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res 94: 1367–1374, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bouschet T, Perez V, Fernandez C, Bockaert J, Eychene A, Journot L. Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J Biol Chem 278: 4778–4785, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Caron E Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci 116: 435–440, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2C-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 278: H1075–H1083, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Chotani MA, Mitra S, Eid AH, Han SA, Flavahan NA. Distinct cyclic AMP signaling pathways differentially regulate α2C-adrenoceptor expression: role in serum induction in human arteriolar smooth muscle cells. Am J Physiol Heart Circ Physiol 288: H69–H76, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Chotani MA, Mitra S, Su BY, Flavahan S, Eid AH, Clark KR, Montague CR, Paris H, Handy DE, Flavahan NA. Regulation of α2-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 286: H59–H67, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Eid AH, Maiti K, Mitra S, Chotani MA, Flavahan S, Bailey SR, Thompson-Torgerson CS, Flavahan NA. Estrogen increases smooth muscle expression of α2C-adrenoceptors and cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 293: H1955–H1961, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Ekenvall L, Lindblad LE, Norbeck O, Etzell BM. α-Adrenoceptors and cold-induced vasoconstriction in human finger skin. Am J Physiol Heart Circ Physiol 255: H1000–H1003, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Faber JE Effect of local tissue cooling on microvascular smooth muscle and postjunctional α2-adrenoceptors. Am J Physiol Heart Circ Physiol 255: H121–H130, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Flavahan NA, Lindblad LE, Verbeuren TJ, Shepherd JT, Vanhoutte PM. Cooling and α1- and α2-adrenergic responses in cutaneous veins: role of receptor reserve. Am J Physiol Heart Circ Physiol 249: H950–H955, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR, Baer RP, Mayes MD. Blockade of vasospastic attacks by alpha2-adrenergic but not alpha1-adrenergic antagonists in idiopathic Raynaud's disease. Circulation 92: 1448–1451, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Frodin M, Peraldi P, Van Obberghen E. Cyclic AMP activates the mitogen-activated protein kinase cascade in PC12 cells. J Biol Chem 269: 6207–6214, 1994. [PubMed] [Google Scholar]
- 16.Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108: 73–81, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He JC, Gomes I, Nguyen T, Jayaram G, Ram PT, Devi LA, Iyengar R. The G alpha(o/i)-coupled cannabinoid receptor-mediated neurite outgrowth involves Rap regulation of Src and Stat3. J Biol Chem 280: 33426–33434, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hochbaum D, Tanos T, Ribeiro-Neto F, Altschuler D, Coso OA. Activation of JNK by Epac is independent of its activity as a Rap guanine nucleotide exchanger. J Biol Chem 278: 33738–33746, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Honda M, Suzuki M, Nakayama K, Ishikawa T. Role of alpha2C-adrenoceptors in the reduction of skin blood flow induced by local cooling in mice. Br J Pharmacol 152: 91–100, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of α2C-adrenoceptors from golgi to plasma membrane in transfected HEK293 cells. Mol Pharmacol 60: 1195–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Koyama H, Bornfeldt KE, Fukumoto S, Nishizawa Y. Molecular pathways of cyclic nucleotide-induced inhibition of arterial smooth muscle cell proliferation. J Cell Physiol 186: 1–10, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J Biol Chem 277: 18598–18604, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Li RC, Cindrova-Davies T, Skepper JN, Sellers LA. Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ Res 94: 759–767, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Liao DF, Duff JL, Daum G, Pelech SL, Berk BC. Angiotensin II stimulates MAP kinase kinase kinase activity in vascular smooth muscle cells. Role of Raf. Circ Res 79: 1007–1014, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Lou L, Urbani J, Ribeiro-Neto F, Altschuler DL. cAMP inhibition of Akt is mediated by activated and phosphorylated Rap1b. J Biol Chem 277: 32799–32806, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Maly FE, Quilliam LA, Dorseuil O, Der CJ, Bokoch GM. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J Biol Chem 269: 18743–18746, 1994. [PubMed] [Google Scholar]
- 27.O′Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signalling pathway. Br J Cancer 90: 283–288, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philipp M, Brede M, Hein L. Physiological significance of α2-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol 283: R287–R295, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro-Neto F, Urbani J, Lemee N, Lou L, Altschuler DL. On the mitogenic properties of Rap1b: cAMP-induced G(1)/S entry requires activated and phosphorylated Rap1b. Proc Natl Acad Sci USA 99: 5418–5423, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachinidis A, Seul C, Gouni-Berthold I, Seewald S, Ko Y, Vetter H, Fingerle J, Hoppe J. Cholera toxin treatment of vascular smooth muscle cells decreases smooth muscle alpha-actin content and abolishes the platelet-derived growth factor-BB-stimulated DNA synthesis. Br J Pharmacol 130: 1561–1570, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaak S, Cayla C, Lymperopoulos A, Flordellis C, Cussac D, Denis C, Paris H. Transcriptional down-regulation of the human alpha2C-adrenergic receptor by cAMP. Mol Pharmacol 58: 821–827, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Schaak S, Devedjian JC, Cayla C, Sender Y, Paris H. Molecular cloning, sequencing and functional study of the promoter region of the human alpha2C4-adrenergic receptor gene. Biochem J 328: 431–438, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schramm K, Niehof M, Radziwill G, Rommel C, Moelling K. Phosphorylation of c-Raf-1 by protein kinase A interferes with activation. Biochem Biophys Res Commun 201: 740–747, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, DiCorleto PE. ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res 102: 448–456, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Stork PJ Does Rap1 deserve a bad Rap? Trends Biochem Sci 28: 267–275, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Vanhoutte PM Physical factors of regulation. In: Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am Physiol Soc, 1980, sect. 2, vol. II, chapt. 16, p. 443–474.
- 37.Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol 26: 2130–2145, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262: 1065–1069, 1993. [DOI] [PubMed] [Google Scholar]
- 39.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392: 622–626, 1998. [DOI] [PubMed] [Google Scholar]