Abstract
The expression of proinflammatory cytokines increases in the hypothalamus of rats with heart failure (HF). The pathophysiological significance of this observation is unknown. We hypothesized that hypothalamic proinflammatory cytokines upregulate the activity of central neural systems that contribute to increased sympathetic nerve activity in HF, specifically, the brain renin-angiotensin system (RAS) and the hypothalamic-pituitary-adrenal (HPA) axis. Rats with HF induced by coronary ligation and sham-operated controls (SHAM) were treated for 4 wk with a continuous intracerebroventricular infusion of the cytokine synthesis inhibitor pentoxifylline (PTX, 10 μg/h) or artificial cerebrospinal fluid (VEH). In VEH-treated HF rats, compared with VEH-treated SHAM rats, the hypothalamic expression of proinflammatory cytokines was increased, along with key components of the brain RAS (renin, angiotensin-converting enzyme, angiotensin type 1 receptor) and corticotropin-releasing hormone, the central indicator of HPA axis activation, in the paraventricular nucleus (PVN) of the hypothalamus. The expression of other inflammatory/excitatory mediators (superoxide, prostaglandin E2) was also increased, along with evidence of chronic neuronal excitation in PVN. VEH-treated HF rats had higher plasma levels of norepinephrine, ANG II, interleukin (IL)-1β, and adrenocorticotropic hormone, increased left ventricular end-diastolic pressure, and increased wet lung-to-body weight ratio. With the exception of plasma IL-1β, an indicator of peripheral proinflammatory cytokine activity, all measures of neurohumoral excitation were significantly lower in HF rats treated with intracerebroventricular PTX. These findings suggest that the increase in brain proinflammatory cytokines observed in rats with ischemia-induced HF is functionally significant, contributing to neurohumoral excitation by activating brain RAS and the HPA axis.
Keywords: paraventricular nucleus of hypothalamus, inflammation, sympathetic nervous system, brain renin-angiotensin system, hypothalamic-pituitary-adrenal axis
there is increasing appreciation for the role of inflammation in heart failure (HF) (25, 45). Proinflammatory cytokines circulate in levels that correlate with the severity of HF and predict a poor prognosis (8). However, the inflammatory process is not confined to the heart and vasculature. We recently reported that the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) accumulate in the hypothalamus (27), a brain region that regulates sympathetic nerve activity and extracellular fluid volume, in a rat model of ischemia-induced HF.
Circulating cytokines have well-recognized effects on the brain, mediated by induction of cyclooxygenase-2 (COX-2) activity in the cerebral microvasculature (12, 40). Prostaglandin E2 (PGE2), a principal product of COX-2, activates the hypothalamic-pituitary-adrenal (HPA) axis (12). However, the pathophysiological significance of the increased expression of proinflammatory cytokines inside the blood-brain barrier remains obscure.
The present study tested the hypothesis that an increase in proinflammatory cytokines in the brain of HF rats contributes to upregulation of the brain renin-angiotensin system (RAS) and the HPA axis. To test this hypothesis, we inhibited cytokine synthesis in the brain with centrally infused pentoxifylline (PTX) and measured the effect on central markers of HPA axis and brain RAS activity in the paraventricular nucleus (PVN) of the hypothalamus in rats with ischemia-induced HF and sham-operated control rats.
The PVN was selected for study because: 1) ANG II, the major product of brain RAS, activates neurons in the PVN that regulate thirst, sodium appetite, and sympathetic nerve activity (26, 32); and 2) corticotropin-releasing hormone (CRH) neurons in PVN are the critical central effector neurons of the HPA axis (6). Each of these mechanisms may contribute to altered neurohumoral regulation in HF.
METHODS
Animals
Adult male Sprague-Dawley rats weighing 275–300 g were obtained from Harlan Sprague Dawley. They were housed in temperature (23 ± 2°C)- and light-controlled animal quarters and were provided with rat chow ad libitum. These studies were performed in accordance with the American Physiological Society's “Guiding Principles for Research Involving Animals and Human Beings” (1). The experimental procedures were approved by the University of Iowa Institutional Animal Care and Use Committee.
Coronary Ligation and Cannula Implantation
Fifty-six rats underwent sterile surgery under anesthesia (90 mg/kg ketamine + 7.5 mg/kg xylazine ip) to induce HF by ligating the left anterior descending coronary artery or the same surgery without ligating the vessel (SHAM), as previously described (18, 19, 24, 27). While still under anesthesia, each rat had a cannula implanted in the right lateral cerebral ventricle, using stereotaxic coordinates (38). Animals received buprenorphine (0.01 mg/kg sc) immediately after surgery and 12 h later.
Echocardiographic Assessment of Left Ventricular Function
Echocardiography was performed under ketamine (25 mg/kg ip) sedation to assess left ventricular (LV) function as previously described (18, 19, 27). Echocardiographic data were acquired within 24 h of coronary ligation or sham operation and again toward the end of the drug treatment protocol. Images were acquired with a Sonos 5500 (Philips Medical Systems) fitted with an 8-MHz sector-array probe, which generates two-dimensional images at a rate of ∼100/s. Short- and long-axis images of the LV were analyzed. Ischemic zone (IZ) was estimated by planimetry of the region of the LV endocardial silhouette that demonstrated akinesis or dyskinesis and was expressed as a percentage of the whole (%IZ). From these measurements, %IZ, LV ejection fraction (LVEF), and LV end-diastolic volume (LVEDV), indexes of severity of congestive HF, were reported.
Drug Infusion
Within 24 h of coronary ligation or sham operation, rats were anesthetized (60 mg/kg ketamine + 5 mg/kg xylazine ip) and underwent sterile surgery to implant an osmotic minipump (Alzet) subcutaneously in the back of the neck. The minipump was connected to the lateral ventricle cannula for the continuous infusion (0.25 μl/h) of PTX (40 μg/μl) or vehicle (VEH, artificial cerebrospinal fluid) over a 4-wk treatment interval.
Study Groups
One set of HF and SHAM rats, treated with PTX or VEH, underwent anesthesia with urethane (1.5 g/kg ip) to collect cerebrospinal fluid (CSF), blood, and brain tissue for molecular studies. Hemodynamic and anatomic measures of the extent of HF were also obtained in these rats.
A second set of HF and SHAM rats, treated with PTX or VEH, was used for immunohistochemical or immunofluorescent studies. These rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and transcardially perfused with PBS and 4% paraformaldehyde. The brain was removed and fixed in 4% paraformaldehyde overnight at 4°C, and then immersed in 30% sucrose for at least 2 days.
Hemodynamic and Anatomic Measurements
A 1-mm micromanometer tipped catheter (Millar Instruments) was inserted via the right carotid artery and advanced to the aorta. Arterial pressure was measured, and the catheter was advanced in the left ventricle. Systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) were derived from the arterial pressure tracing. Left ventricular peak systolic pressure (LVPSP), left ventricular end-diastolic pressure (LVEDP) and maximum change in pressure over time (LV dP/dtmax) were derived from the LV pressure tracing.
The heart and the lungs were removed. The heart ventricles were separated, and the right ventricle was weighed. The lungs were weighed wet. Right ventricular (RV) and lung weights were expressed as a function of body weight (BW).
Molecular Studies
CSF was aspirated from the cisterna magna, and the rat was decapitated to collect trunk blood and hypothalamic tissue. Trunk blood was collected in chilled EDTA tubes. Plasma was separated and stored at −80°C. The hypothalamus was removed as previously described (16).
Enzyme-Linked Immunosorbent Assay
Plasma and tissue cytokine levels were measured using enzyme-linked immunosorbent assay (ELISA; Biosource International) techniques, as described previously (16, 20). PGE2 in CSF was measured using a high sensitivity kit (R&D Systems). Plasma norepinephrine (NE) was measured using a high sensitivity kit (Rocky Mountain Diagnostics). Plasma ACTH was measured using an ELISA kit (MD Biosciences). Plasma ANG II was measured using an EIA kit (Cayman Chemical). Minimum detectable concentrations were as follows (in pg/ml): <8.25 PGE2, 2.7 NE, 0.46 ACTH, and 1 ANG II.
Western Blot
Protein extracted from hypothalamus was used for measurement of angiotensin type 1 receptor (AT1-R) protein expression by Western blot (49). The bands were analyzed using NIH ImageJ software.
Immunohistochemical and Fluorescent Labeling Studies
The brain samples were embedded in optimum cutting temperature medium, and transverse sections were obtained from the region approximately −1.80 mm from bregma. For immunohistochemistry, 40-μm sections were obtained using a sliding microtome and stored in PBS. For immunofluorescence, 10-μm sections obtained on a cryostat were put on slides and stored at −80°C for future use.
Immunohistochemistry
A general avidin-biotin-peroxidase complex procedure was used (23). A double-staining protocol (23) was used to identify chronically activated PVN neurons as determined by fra-like (Fra-LI) activity (Santa Cruz Biotechnology) that were also positive for CRH (Phoenix Pharmaceuticals) or angiotensin-converting enzyme (ACE; Santa Cruz Biotechnology). Images were captured at ×10 magnification using a Diaphot 300 microscope (Nikon), and threshold intensity values for each section were set to allow for most of the positively labeled cells to be visualized. For each animal, labeled neurons within the borders of PVN bilaterally were counted manually in two representative 40-μm transverse sections at about −1.80 mm from bregma (15, 27), and an average value was reported. NIH ImageJ software was used to confirm the manual cell counts and to quantify the intensity of COX-2 expression in the PVN.
Fluorescent Labeling
Sections were air-dried for 60 min at room temperature, washed in PBS for 5 min at room temperature, and then treated in 0.2% Triton X-100 for 15 min at room temperature. Sections were treated in 50 mM glycine for 15 min and then incubated with 5% normal goat serum for 30 min at room temperature and washed in PBS three times for 5 min. Sections were incubated with primary antibody for COX-2 (Cayman), the perivascular cell marker ED2 (Serotec), ACE (Santa Cruz Biotechnology), renin (Fitzgerald), or the AT1-R (Abcam) for 24 h at 4°C and washed in PBS three times for 5 min at room temperature. Sections were incubated with the corresponding fluorescent secondary antibody for 60 min at room temperature and washed in PBS three times for 5 min at room temperature. Some sections were incubated with To-Pro-3 for 10 min at room temperature. Sections were washed in PBS three times for 5 min at room temperature. Slides were mounted and viewed on a laser confocal microscope.
Superoxide generation was determined by fluorescent-labeled dihydroethidium (DHE; Molecular Probes). DHE staining was performed as previously described (11, 34, 47). Fluorescence intensity of DHE in PVN was visualized and measured using laser confocal microscopy (34, 47).
Statistical Analysis
All data are expressed as means ± SE. The significance of differences between mean values was analyzed by a two-way ANOVA followed by a post hoc Tukey test. A probability value of P < 0.05 was considered to be statistically significant.
RESULTS
Echocardiography
At baseline, within 24 h of coronary artery ligation or sham operation, rats assigned to treatment with PTX and VEH were well matched with regard to echocardiographically defined LV function (Table 1). LVEF was significantly reduced, and LVEDV and the LVEDV-to-mass ratio were significantly increased, in rats with ischemic injury assigned to PTX or VEH compared with sham-operated rats assigned to those same treatments. At baseline, however, there were no differences in LVEF, LVEDV, LVEDV/mass ratio or %IZ among rats with ischemic injury assigned to PTX vs. VEH treatment.
Table 1.
Echocardiographic measurements
| Measurements at | HF + PTX (n = 12) | HF + VEH (n = 12) | SHAM + PTX (n = 12) | SHAM + VEH (n = 12) |
|---|---|---|---|---|
| Baseline | ||||
| LVEDV, ml | 0.73±0.09* | 0.69±0.08* | 0.35±0.04 | 0.36±0.05 |
| LVEDV/mass | 1.05±0.06* | 1.09±0.08* | 0.54±0.06 | 0.56±0.05 |
| LVEF | 0.35±0.04* | 0.37±0.05* | 0.82±0.05 | 0.84±0.06 |
| IZ, % | 50±3 | 48±2 | ||
| 4 Wk | ||||
| LVEDV, ml | 1.33±0.08*‡ | 1.42±0.07*‡ | 0.34±0.04 | 0.37±0.04 |
| LVEDV/mass | 1.63±0.10*‡ | 1.77±0.13*‡ | 0.53±0.05 | 0.58±0.05 |
| LVEF | 0.29±0.02*† | 0.22±0.03*‡ | 0.86±0.06 | 0.81±0.05 |
| IZ, % | 48±3 | 52±4 |
Values are means ± SE; n, no. of rats. PTX, pentoxifylline; VEH, vehicle; SHAM, sham-operated control; HF, heart failure; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; IZ, ischemic zone.
P < 0.05 vs. SHAM,
HF + PTX vs. HF + VEH,
and 4 wk vs. baseline value.
At 4 wk, LVEDV and LVEDV/mass ratio were significantly higher than the 24-h baseline values in both the PTX-treated and VEH-treated HF rats, and LVEF was significantly lower in the VEH-treated HF rats (Table 1). At 4 wk, LVEF was higher in the PTX-treated HF rats compared with the VEH-treated HF rats. There were no significant differences in LVEDV, LVEDV/mass ratio, or %IZ between the PTX-treated and VEH-treated HF rats (Table 1).
Hypothalamic Expression of Proinflammatory Cytokines
Hypothalamic levels of TNF-α and IL-1β were higher in HF compared with SHAM rats (Fig. 1A). Hypothalamic levels of TNF-α and IL-1β were lower in the PTX-treated HF rats compared with VEH-treated HF rats and did not differ significantly from the levels in VEH-treated SHAM rats. Intracerebroventricular PTX had no effect on proinflammatory cytokine levels in hypothalamus of SHAM rats.
Fig. 1.
Group data showing the effect of treatment with intracerebroventricular (ICV) PTX on hypothalamic tissue levels of proinflammatory cytokines and on neuronal activity in paraventricular nucleus (PVN) of hypothalamus in rats with ischemia-induced heart failure (HF). A: tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) increased in hypothalamus of vehicle (VEH)-treated HF rats compared with VEH-treated sham-operated (SHAM) rats. TNF-α and IL-1β levels were less in pentoxifylline (PTX)-treated than VEH-treated HF rats. B: Fra-LI activity, an indicator of chronic neuronal excitation, increased in the PVN of VEH-treated HF rats compared with VEH-treated SHAM. Fra-LI activity was less in the PTX-treated than VEH-treated HF rats. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
Indicators of Hypothalamic Excitation
Fra-LI activity.
Fra-LI activity was higher in the PVN of VEH-treated HF rats compared with VEH-treated SHAM rats. PTX-treated HF rats had fewer Fra-LI positive PVN neurons than VEH-treated HF rats (Fig. 1B).
COX-2 and PGE2.
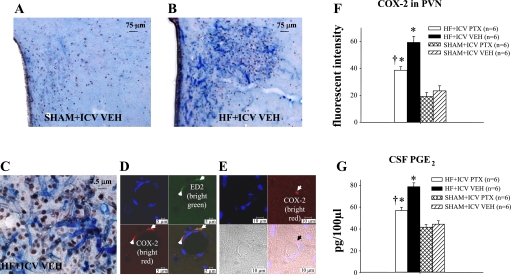
There was more intense staining for COX-2 in the PVN region in VEH-treated HF rats than VEH-treated SHAM rats (Fig. 2, A and B). COX-2 activity was most prominent in the microvasculature penetrating the PVN (Fig. 2C). Confocal microscopy localized COX-2 expression to perivascular (ED2-positive) cells (Fig. 2, D and E) rather than endothelial cells in the microvasculature of PVN, as previously reported (27, 46). The intensity of COX-2 immunofluorescence in PVN was significantly attenuated in the PTX-treated HF rats compared with the VEH-treated HF rats (Fig. 2F). PGE2, a marker of COX-2 activity, was higher in the CSF of VEH-treated HF than VEH-treated SHAM rats (Fig. 2G). The PTX-treated HF rats had lower CSF PGE2 levels than the VEH-treated HF rats but higher levels than the VEH-treated SHAM rats.
Fig. 2.
Expression of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) in the brain. Double-labeling immunohistochemistry showing COX-2 (blue) and Fra-LI activity (black dots), an indicator of chronic neuronal excitation, in a coronal section of the PVN of a SHAM rat (A) and a HF rat (B). C: high-power view of the section shown in B, demonstrating COX-2 activity in the microvasculature penetrating the PVN, closely associated with Fra-LI positive PVN neurons. D: confocal images demonstrating triple immunostaining for nuclei (blue), the perivascular cell marker ED2 (bright green), and COX-2 (bright red) in the PVN. The yellow color in the combined image confirms dual labeling for ED2 and COX-2. E: confocal images showing endothelial cells (blue) and COX-2 (bright red), with an apparent localization of COX-2 in perivascular rather than endothelial cells in the microvasculature of PVN. F: group data showing effect of ICV PTX on COX-2 fluorescent intensity in PVN of HF and SHAM rats. COX-2 immunofluorescence was reduced in PTX-treated HF rats. G: group data showing cerebrospinal fluid (CSF) levels of PGE2, a physiological indicator of COX-2 activity, increase in VEH-treated HF rats compared with VEH-treated SHAM. The CSF PGE2 level is lower in the PTX-treated rats. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
HPA axis.
Immunohistochemistry revealed that more Fra-LI positive PVN neurons were also CRH-positive in VEH-treated HF than VEH-treated SHAM rats (Fig. 3, A–D). There were fewer CRH- and Fra-LI-positive PVN neurons in PTX-treated than VEH-treated HF rats but more than in VEH-treated SHAM rats. Plasma ACTH levels correlated with the numbers of CRH-positive PVN neurons (Fig. 3E).
Fig. 3.
Immunohistochemistry for corticotropin-releasing hormone (CRH) expression in the PVN of hypothalamus. A: double labeling for CRH (red) and Fra-LI activity (black dots), an indicator of chronic neuronal excitation, in a coronal section of the PVN of a HF rat. B: high-power view of the section shown in A demonstrating CRH labeling of the cytoplasm of a Fra-LI positive PVN neuron. C: effect of ICV PTX treatment on CRH expression and Fra-LI activity in PVN of a HF rat. D: group data showing effects of ICV PTX on numbers of PVN neurons positive for Fra-LI activity that also express CRH in HF and SHAM rats. E: group data showing effects of ICV PTX on plasma levels of ACTH in HF and SHAM rats. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
RAS.
Immunofluorescent studies revealed increased expression of renin (Fig. 4, A–C) and ACE (Fig. 4, D–F) in the PVN of VEH-treated HF vs. VEH-treated SHAM rats. The expression of renin and ACE was significantly less in PTX-treated HF rats compared with VEH-treated HF rats. Immunohistochemistry revealed that ACE expression was increased among Fra-LI-positive PVN neurons in VEH-treated HF rats compared with VEH-treated SHAM rats and was significantly less in the PTX-treated HF rats (Fig. 5). Expression of the AT1-R was also increased in PVN of VEH-treated HF rats compared with VEH-treated SHAM rats, as demonstrated by immunofluorescent studies (Fig. 6, A and C) and by Western blot analysis (Fig. 6D). Both indexes of upregulated AT1-R activity were reduced in PTX-treated HF rats (Fig. 6, B–D).
Fig. 4.
Immunofluorescence for renin and angiotensin-converting enzyme (ACE) in the parvocellular region of the PVN of hypothalamus. A: coronal section of the PVN of a VEH-treated HF rat showing immunofluorescence for renin (red) and neuronal nuclei (blue). Side panel shows magnified image. B: coronal section of the PVN of a PTX-treated HF rat showing reduced immunofluorescence for renin. C: group data showing effect of ICV PTX on fluorescent intensity of renin in PVN of HF and SHAM rats. D: coronal section of the PVN of a VEH-treated HF rat showing immunofluorescence for ACE (red) and neuronal nuclei (blue). Side panel shows magnified image. E: coronal section of the PVN of a PTX-treated HF rat showing reduced immunofluorescence for ACE. F: group data showing effect of ICV PTX on fluorescent intensity of ACE in PVN of HF and SHAM rats. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
Fig. 5.
Immunohistochemistry for ACE expression in the PVN of hypothalamus. A: immunohistochemical double-labeling for ACE (red) and Fra-LI activity (black dots), an indicator of chronic neuronal excitation, in a coronal section of the PVN of a HF rat. B: high-power image taken from the section shown in A demonstrating ACE labeling of the cytoplasm of a Fra-LI-positive PVN neuron. C: effect of ICV PTX treatment on ACE expression in Fra-LI-positive PVN neurons of a HF rat. D: group data showing effects of ICV PTX on numbers of Fra-LI-positive neurons also expressing ACE in PVN of HF and SHAM rats. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
Fig. 6.
Angiotensin type 1 receptor (AT1-R) expression in the parvocellular region of the PVN of hypothalamus. A: immunofluorescence for AT1-R (red) and neuronal nuclei (blue) in a coronal section of the PVN of a VEH-treated HF rat. Side panel shows magnified image. B: immunofluorescence for AT1-R (red) and neuronal nuclei (blue) in a coronal section of the PVN of a PTX-treated HF rat. C: group data showing effect of ICV PTX on fluorescent intensity of AT1-R in PVN of HF and SHAM rats. D: group data showing AT1-R protein in the hypothalamus of ICV PTX- and VEH-treated HF and SHAM rats. Representative Western blots are shown above the bar graph. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
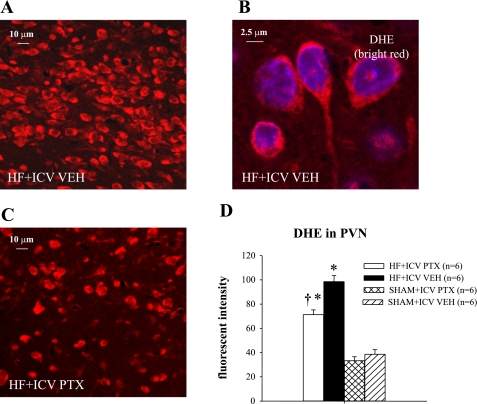
Superoxide.
Immunofluorescence revealed increased superoxide in PVN neurons of VEH-treated HF rats, as determined by fluorescent-labeled DHE. DHE fluorescence in PVN was less in the PTX-treated than VEH-treated HF rats (Fig. 7) but more than in the VEH-treated SHAM rats.
Fig. 7.
Superoxide expression in the parvocellular region of the PVN of hypothalamus. A: immunofluorescence for superoxide in PVN of a HF rat indicated by dihydroethidium (DHE) staining (red). B: high-power image taken from the section shown in A. C: DHE labeling in the PVN of a PTX-treated HF rat. D: group data showing effect of ICV PTX on fluorescent intensity of DHE in PVN of HF and SHAM rats. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
Humoral Indicators of HF
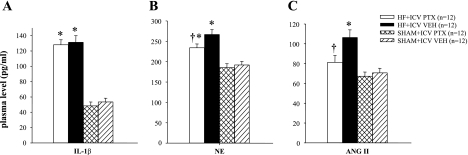
Humoral indicators of HF paralleled the hypothalamic findings. Plasma NE, ANG II, and IL-1β levels were all higher in HF than in SHAM rats (Fig. 8). The plasma NE and ANG II levels were lower in PTX-treated than VEH-treated HF rats, but plasma IL-1β level was unaffected by intracerebroventricular PTX (Fig. 8).
Fig. 8.
Humoral indicators of HF. Plasma levels of IL-1β (A), norepinephrine (NE; B) and ANG II (C) in HF and SHAM rats treated for 4 wk with ICV PTX or VEH. NE and ANG II were lower in HF rats treated with ICV PTX, but IL-1β was unaffected. P < 0.05 vs. SHAM + VEH (*) and HF + PTX vs. HF + VEH (†).
Functional/Anatomical Indicators of HF
Compared with the VEH-treated SHAM rats, the VEH-treated HF rats had significantly higher LVEDP, RV/BW, and lung/BW ratios and significantly lower LV dP/dtmax (Table 2). SBP and LVPSP were also significantly lower in the VEH-treated HF than the VEH-treated SHAM rats. PTX-treated HF rats had significantly higher LV dP/dtmax and lower LVEDP and lung/BW ratio than the VEH-treated HF rats, but these values were all still significantly different from VEH-treated SHAM rats. SBP and LVPSP were not affected. There were no significant differences in DBP or HR across the treatment groups. In this small sampling, PTX treatment appeared to improve survival [PTX-treated HF: 80.0% (12/15); VEH-treated HF: 70.6% (12/17)] over the 4-wk interval between the first and second echocardiograms, but some animals did not survive the second echocardiography session.
Table 2.
Hemodynamic and anatomic measurements
| Measurements at 4 Wk | HF + PTX (n = 6) | HF + VEH (n = 6) | SHAM + PTX (n = 6) | SHAM + VEH (n = 6) |
|---|---|---|---|---|
| BW, g | 360±10 | 355±8 | 371±12 | 369±11 |
| RV/BW, mg/g | 1.08±0.12* | 1.12±0.14* | 0.64±0.06 | 0.68±0.10 |
| Lung/BW, mg/g | 7.58±0.36*† | 10.64±0.48* | 4.62±0.20 | 4.81±0.23 |
| HR, beats/min | 332±7 | 342±8 | 323±11 | 325±10 |
| SBP, mmHg | 110±3* | 111±4* | 123±3 | 126±4 |
| DBP, mmHg | 87±3 | 84±4 | 87±3 | 91±3 |
| LVPSP, mmHg | 100±3* | 101±4* | 111±3 | 112±3 |
| LVEDP, mmHg | 10±2*† | 21±2* | 4±2 | 4±2 |
| LV dP/dtmax, mmHg/s | 5,893±229*† | 4,612±181* | 8,341±451 | 8,575±470 |
Values are means ± SE; n, number of rats. BW, body weight; RV, right ventricular; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVPSP, left ventricular peak systolic pressure; LVEDP, left ventricular end-diastolic pressure; LV dP/dtmax, maximum rate of change in left pressure ventricular pressure.
P < 0.05 vs. SHAM
and HF + PTX vs. HF + VEH.
DISCUSSION
This study reports three important new findings: 1) a substantial portion of the proinflammatory cytokines that appear in the brain in rats with ischemia-induced HF are synthesized inside the blood-brain barrier; 2) brain proinflammatory cytokines upregulate the activity of sympathoexcitatory and volume regulatory systems in rats with ischemia-induced HF; and 3) inhibiting brain synthesis of proinflammatory cytokines reduces hypothalamic activation and ameliorates the manifestations of HF. Because systemically administered anti-cytokine agents also lower the expression of proinflammatory cytokines in the brain (27), they have the potential to quench central inflammatory mechanisms that are difficult to approach with pharmacological interventions.
Recent literature (17, 28, 43, 50) has implicated upregulation of the brain RAS, with subsequent ANG II-driven and NADPH oxidase-mediated increases in superoxide (50) in critical presympathetic regions of the brain, as a major factor leading to increased sympathetic nerve activity in HF. ANG II has other important functions in hypothalamus that may contribute to the HF syndrome, including regulation of thirst and sodium appetite (26, 32) and activation of neurons containing CRH (36, 39, 41) and arginine vasopressin (5, 31). The present study, demonstrating that lowering hypothalamic levels of TNF-α and IL-1β without changing plasma cytokine levels is associated with reductions in brain renin, ACE, and AT1-R, strongly suggests that proinflammatory cytokines are intrinsically involved in regulating brain RAS, and thus sympathetic nerve activity and extracellular fluid volume, in rats with HF. However, a caveat to be considered is that circulating ANG II was also reduced in the HF rats treated with pentoxifylline, likely because of a reduction in sympathetic nerve activity (10). Thus we cannot exclude the possibility that decreased circulating ANG II contributed indirectly to the downregulation of brain RAS.
Activation of the HPA has received little attention in the HF literature, although it is an expected outcome of the increase in circulating proinflammatory cytokines. The hypothalamus is a well-recognized target of circulating cytokines (6, 12, 40). The present study confirms our recent observation (27) that the expression of CRH in PVN neurons (the central marker of HPA activation) is increased in rats with ischemia-induced HF and is responsive to anti-cytokine therapy. However, although the previous study (27) demonstrated an effect of lowering plasma cytokines, the present study demonstrates that a similar effect can be achieved by reducing brain cytokine synthesis.
Cytokine activation of the HPA, like cytokine activation of the brain RAS, has important implications for cardiovascular regulation. In the classic studies of Brown and Fisher (4, 14), the central administration of CRH (called corticotropin-releasing factor in those studies) elicited sympathetically mediated increases in HR and arterial pressure in addition to increases in circulating catecholamines and ACTH. Thus HPA activation may contribute substantially to neurohumoral excitation in HF. In the present study, inhibition of brain cytokine synthesis reduced both the expression of CRH among PVN neurons and plasma ACTH. Plasma NE level was also reduced, but that observation could be attributed to decreases in brain RAS, reactive oxygen species (ROS), or CRH.
The potential role of PGE2 also deserves consideration. PGE2 is both a mediator of the proinflammatory cytokine effects (12) and an independent stimulus to sympathetic drive (21, 35, 48). The latter effect may be indirect; a recent study suggests that PGE2 inhibits GABAergic neurons that normally restrain the activity of presympathetic neurons in the PVN (13). Whatever the mechanism, the central administration of PGE2 elicits a prominent sympathoexcitatory response (21, 48), mimicking the sympathetic response to systemic administration of TNF-α (48). In the present study, brain proinflammatory cytokines accounted for at least some of the increase in PGE2 in the CSF of rats with ischemia-induced HF.
We can only speculate on the mechanisms by which these central actions of the proinflammatory cytokines contribute to the peripheral manifestations of HF. The brain RAS regulates thirst, sodium appetite, and sympathetic drive to the kidney, all factors that contribute to volume regulation. Thus cytokine upregulation of the brain RAS is likely an important factor. As mentioned above, several other neuroactive factors that are closely linked to cytokine actions and are increased in the brain in HF (ROS, CRH, and PGE2) also activate the sympathetic nervous system and so may influence renal handling of sodium and water. In our model, HF rats treated with intracerebroventricular PTX to inhibit of brain proinflammatory cytokine synthesis had substantially lower LVEDP and lung/BW ratios than VEH-treated HF rats. Thus a reduction in extracellular fluid volume, likely resulting primarily from a reduction in renal sympathetic nerve activity, yielded lower cardiac preload (LVEDP). Despite this, PTX-treated HF rats had less impairment of LV dP/dtmax and LVEF compared with VEH-treated HF rats. This observation, in the absence of any significant change in the indexes of LV remodeling (LVEDV, LVEDV/mass ratio) or the extent of LV injury (%IZ), indicates that contractile function was better preserved in the nonischemic myocardium of PTX-treated HF rats. Possible explanations include reduced exposure to the myocardial depressant effects of chronic adrenergic drive (3) and reduced sympathetically mediated peripheral vasoconstriction (i.e., reduced afterload). In rats with smaller myocardial infarctions, more dramatic improvements in cardiac function have been observed after interventions that inhibit brain RAS activity and reduce sympathetic drive (22) and have been attributed to reduced cardiac remodeling. Reduced sympathetic drive may also explain the lower circulating levels of ANG II, another humoral factor that may impair cardiac function and increase afterload (33, 37), in the PTX-treated HF rats. Thus, by manipulating central cytokine synthesis in rats with ischemia-induced HF, we achieved measurable improvements in neurohumoral drive, volume status, and cardiac function.
This study emphasizes the importance of central neural mechanisms in the pathophysiology of HF and suggests a novel strategy for minimizing their influence. Excitatory systems in the brain that are activated in HF (RAS, ROS, CRH, COX-2/PGE2) are not directly addressed by current pharmacological therapy for HF. However, our previous work (27) in rats with ischemia-induced HF suggests that systemically administered pentoxifylline can effectively reduce the expression of TNF-α and IL-1β in the brain as well as the periphery. Thus systemic anticytokine therapy may be an indirect but effective way to access and quench inflammatory/excitatory mechanisms in the brain, including RAS and ROS, that contribute to the HF syndrome.
Clinical trials testing the effects of the receptor binding protein etanercept, which lowers circulating TNF-α (30), and of the antibody infliximab (7), which binds to circulating and tissue TNF-α, failed to demonstrate a beneficial effect of anticytokine therapy despite promising results of smaller clinical studies. Pentoxifylline operates by a different mechanism, inhibiting the synthesis of TNF-α and other proinflammatory cytokines (2, 9). Pentoxifylline is already in clinical use in the treatment of peripheral vascular disease and has minimal side effects. In small studies, including one in patients with ischemic cardiomyopathy (42), pentoxifylline has been shown to improve cardiac function. The present study suggests that it may be time to consider a larger clinical trial with this agent.
Finally, the finding that centrally administered pentoxifylline lowers brain proinflammatory cytokines expands current understanding of cytokine physiology. The mechanism accounting for the appearance of proinflammatory cytokines in the brain has been a subject of controversy, with some suggesting that circulating cytokines cross the blood-brain barrier (44). The present study demonstrates conclusively that a large fraction of the TNF-α and IL-1β that appears in the brains of rats with HF is actually produced inside the blood-brain barrier; brain levels were substantially reduced in pentoxifylline-treated HF rats, but circulating levels were unaffected.
Two limitations of this study deserve comment. First, we did not administer PTX systemically, in the same dose that was administered centrally, to exclude a possible effect of leakage from the CSF in the circulation. Had centrally administered PTX leaked into the peripheral circulation to reduce blood-borne proinflammatory cytokines, that certainly would have affected our results. However, the dose of PTX injected centrally in this study was very small compared with peripherally effective doses and would not have been expected to have any systemic effect. Moreover, plasma IL-1β, which is reduced by peripheral administration of PTX (27) and was used in this study as an indicator of systemic proinflammatory cytokine activity in the HF rats, was unaffected by centrally administered PTX. Second, specific cell markers for neurons and glia were not employed in this study, so we cannot definitively identify the cell types labeled for renin, ACE, AT1-R, and DHE. However, close inspection of the higher-power confocal images (Figs. 4, 6, and 7) reveals that the immunofluorescent-labeled cells have the typical appearance of PVN neurons (29), not the typical morphology of microglia (29), and the staining in the immunohistochemical studies appears to be confined to the cytoplasm of Fra-LI positive PVN neurons (Figs. 3B and 5B). Thus the evidence strongly suggests that changes in brain RAS and ROS occurred in neuronal elements of the PVN.
Perspectives
The present study illustrates the global nature of inflammation and excitation in the hypothalamus of rats with ischemia-induced HF. The RAS is activated, and there is an increase in superoxide production, as is commonly reported. Concomitantly, the expression of proinflammatory cytokines is increased, COX-2 activity is increased as manifest by increased CSF PGE2, and the HPA is activated. In the PVN of hypothalamus, these inflammatory/excitatory neurochemical changes are not confined to any particular region; neurons in both presympathetic and neuroendocrine regions are excited. In this scenario, the proinflammatory cytokines are not simply markers of inflammation or downstream products of other active systems. Our data demonstrate that proinflammatory cytokines play a proactive role in the brain, stimulating or facilitating the activity of other excitatory neural systems regulating sympathetic drive and extracellular fluid volume. Removing their influence with a cytokine synthesis inhibitor quells the central neural excitation in rats with ischemia-induced HF. Interventions targeting cytokine-driven mechanisms that promote the activity of the brain RAS and the HPA axis may prove useful in the treatment of HF.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-O73986 to R. B. Felder and by the Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bienvenu J, Doche C, Gutowski MC, Lenoble M, Lepape A, Perdrix JP. Production of proinflammatory cytokines and cytokines involved in the TH1/TH2 balance is modulated by pentoxifylline. J Cardiovasc Pharmacol 25, Suppl 2: S80–S84, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR Why does the myocardium fail? Insights from basic science. Lancet 352, Suppl 1: SI8–SI14, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Brown MR, Fisher LA. Corticotropin-releasing factor: effects on the autonomic nervous system and visceral systems. Fed Proc 44: 243–248, 1985. [PubMed] [Google Scholar]
- 5.Catt KJ, Millan MA, Wynn PC, Mendelsohn FA, Aguilera G. Brain receptors for hypothalamic hormones. Adv Biochem Psychopharmacol 43: 51–67, 1987. [PubMed] [Google Scholar]
- 6.Chrousos GP The stress response and immune function: clinical implications. The 1999 Novera H Spector Lecture. Ann NY Acad Sci 917: 38–67, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) Trial. Circulation 107: 3133–3140, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103: 2055–2059, 2001. [DOI] [PubMed] [Google Scholar]
- 9.D'Hellencourt CL, Diaw L, Cornillet P, Guenounou M. Differential regulation of TNF alpha, IL-1β, IL-6, IL-8, TNFβ, and IL-10 by pentoxifylline. Int J Immunopharmacol 18: 739–748, 1996. [DOI] [PubMed] [Google Scholar]
- 10.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Dikalov S, Landmesser U, Harrison DG. Geldanamycin leads to superoxide formation by enzymatic and non-enzymatic redox cycling. Implications for studies of Hsp90 and endothelial cell nitric-oxide synthase. J Biol Chem 277: 25480–25485, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci 17: 7166–7179, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferri CC, Ferguson AV. Prostaglandin E2 mediates cellular effects of interleukin-1β on parvocellular neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol 17: 498–508, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Fisher LA Central actions of corticotropin-releasing factor on autonomic nervous activity and cardiovascular functioning. Ciba Found Symp 172: 243–253, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Fitts DA, Thornton SN, Ruhf AA, Zierath DK, Johnson AK, Thunhorst RL. Effects of central oxytocin receptor blockade on water and saline intake, mean arterial pressure, and c-Fos expression in rats. Am J Physiol Regul Integr Comp Physiol 285: R1331–R1339, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286: H2264–H2271, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Francis J, Wei SG, Weiss RM, Felder RB. Brain angiotensin-converting enzyme activity and autonomic regulation in heart failure. Am J Physiol Heart Circ Physiol 287: H2138–H2146, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol Heart Circ Physiol 281: H2241–H2251, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol 281: R1734–R1745, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am J Physiol Heart Circ Physiol 287: H791–H797, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman WE, Schmid PG. Cardiovascular and antidiuretic effects of central prostaglandin E2. J Physiol 288: 159–169, 1979. [PMC free article] [PubMed] [Google Scholar]
- 22.Huang BS, Ahmad M, Tan J, Leenen FH. Sympathetic hyperactivity and cardiac dysfunction post-MI: Different impact of specific CNS versus general AT(1) receptor blockade. J Mol Cell Cardiol 43: 479–486, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Weiss ML. Characterization of the central cell groups regulating the kidney in the rat. Brain Res 845: 77–91, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Iida S, Chu Y, Weiss RM, Kang YM, Faraci FM, Heistad DD. Vascular effects of a common gene variant of extracellular superoxide dismutase in heart failure. Am J Physiol Heart Circ Physiol 291: H914–H920, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure –Pathophysiological links. Cardiovasc Res 70: 434–445, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: visceral sensory signals and mechanisms of central integration. Front Neuroendocrinol 18: 292–353, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99: 758–766, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res 94: 402–409, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Kaur C, Ling EA. Neuronal and glial response in the rat hypothalamus-neurohypophysis complex with streptozotocin-induced diabetes. Brain Res 925: 42–54, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 109: 1594–1602, 2004. [DOI] [PubMed] [Google Scholar]
- 31.McKinley MJ, Allen AM, Mathai ML, May C, McAllen RM, Oldfield BJ, Weisinger RS. Brain angiotensin and body fluid homeostasis. Jpn J Physiol 51: 281–289, 2001. [DOI] [PubMed] [Google Scholar]
- 32.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl 3: S99–S104, 1996. [PubMed] [Google Scholar]
- 33.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Miller FJ, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Okuno T, Lindheimer MD, Oparil S. Central effects of prostaglandin E2 on blood pressure and plasma renin activity in rats. Role of the sympathoadrenal system and vasopressin. Hypertension 4: 809–816, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13: 139–146, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Palomeque J, Sapia L, Hajjar RJ, Mattiazzi A, Vila Petroff M. Angiotensin II-induced negative inotropy in rat ventricular myocytes: role of reactive oxygen species and p38 MAPK. Am J Physiol Heart Circ Physiol 290: H96–H106, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1986.
- 39.Raasch W, Wittmershaus C, Dendorfer A, Voges I, Pahlke F, Dodt C, Dominiak P, Johren O. Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats. Endocrinology 147: 3539–3546, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 223: 22–38, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept 128: 227–238, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Sliwa K, Woodiwiss A, Kone VN, Candy G, Badenhorst D, Norton G, Zambakides C, Peters F, Essop R. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: results of a randomized study. Circulation 109: 750–755, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Tan J, Wang H, Leenen FH. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am J Physiol Heart Circ Physiol 286: H1665–H1671, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann NY Acad Sci 840: 434–443, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Yndestad A, Kristian Damas J, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure–the whys and wherefores. Heart Fail Rev 11: 83–92, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure. Role of nuclear factor κB. Hypertension 49: 511–518, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang HJ, Xu L, Drake VJ, Xie L, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J 17: 2293–2295, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284: R916–R927, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Zucker IH Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension 48: 1005–1011, 2006. [DOI] [PubMed] [Google Scholar]