Abstract
Nitric oxide (NO) participates in locally mediated vasodilation induced by increased local skin temperature (Tloc) and in sympathetically mediated vasodilation during whole body heat stress. We hypothesized that endothelial NOS (eNOS) participates in the former, but not the latter, response. We tested this hypothesis by examining the effects of the eNOS antagonist NG-amino-l-arginine (l-NAA) on skin blood flow (SkBF) responses to increased Tloc and whole body heat stress. Microdialysis probes were inserted into forearm skin for drug delivery. One microdialysis site was perfused with l-NAA in Ringer solution and a second site with Ringer solution alone. SkBF [laser-Doppler flowmetry (LDF)] and blood pressure [mean arterial pressure (MAP)] were monitored, and cutaneous vascular conductance (CVC) was calculated (CVC = LDF ÷ MAP). In protocol 1, Tloc was controlled with LDF/local heating units. Tloc initially was held at 34°C and then increased to 41.5°C. In protocol 2, after a normothermic period, whole body heat stress was induced (water-perfused suits). At the end of both protocols, 58 mM sodium nitroprusside was perfused at both microdialysis sites to cause maximal vasodilation for data normalization. In protocol 1, CVC at 34°C Tloc did not differ between l-NAA-treated and untreated sites (P > 0.05). Local skin warming to 41.5°C Tloc increased CVC at both sites. This response was attenuated at l-NAA-treated sites (P < 0.05). In protocol 2, during normothermia, CVC did not differ between l-NAA-treated and untreated sites (P > 0.05). During heat stress, CVC rose to similar levels at l-NAA-treated and untreated sites (P > 0.05). We conclude that eNOS is predominantly responsible for NO generation in skin during responses to increased Tloc, but not during reflex responses to whole body heat stress.
Keywords: skin blood flow, microdialysis, nitric oxide, NOS III, laser-Doppler flowmetry
a wide variety of mechanistic roles for nitric oxide (NO) and NO synthase (NOS) in human biology have been elucidated. Indeed, it is now a great scientific challenge to clarify these varied roles and come to a coherent understanding of how NO/NOS systems function in vivo. To accomplish this, studies that address the roles of different NOS isoforms, endothelial NOS (eNOS, NOS III), neuronal NOS (nNOS, NOS I), and inducible NOS (iNOS, NOS II), are required.
For in vivo studies in humans, the skin represents an easily accessible tissue that has several known NO/NOS-dependent control mechanisms. One such mechanism causes changes in skin blood flow (SkBF) in response to local temperature (Tloc) changes. With local warming of skin, as Tloc increases, so does local SkBF. The response is biphasic, with an initial increase mediated by an axon reflex followed by a plateau that requires the generation of NO by NOS (15, 21, 27, 28). If Tloc is held at 42°C for 35–55 min during the plateau phase of the response, SkBF can reach maximal levels (38).
Another NO- and NOS-dependent mechanism of the cutaneous vasculature is the increase in SkBF that occurs during thermoregulatory reflexes elicited by whole body heat stress. This reflex response is mediated by a powerful active vasodilator system that is capable of directing 60% of cardiac output, or ∼8 l/min of blood flow, to the cutaneous circulation during severe heat stress (11, 17, 30). This active vasodilator response is mediated by sympathetic cholinergic nerves and involves neurotransmitters that include acetylcholine (ACh), vasoactive intestinal peptide, and perhaps others, as well as NOS generation of NO (4, 22, 26, 44, 45).
Three main isoforms of NOS exist in mammals; however, only two, eNOS and nNOS, are constitutively expressed. eNOS and nNOS are involved in signal cascades (13), and both are found in human skin (5). Thus, at least one, or perhaps both eNOS and nNOS, must be involved in generation of the NO required for the vasodilation evoked by local skin warming or by whole body heat stress (19). Although iNOS has been detected by immunohistochemical techniques in skin, it is present in minute amounts compared with eNOS and nNOS. The iNOS isoform acts as a regulator and effector of immune responses; hence, iNOS can be excluded as an isoform responsible for generation of NO in normal physiological vasodilation in skin (13, 41).
Although it is clear that NOS production of NO is involved in vasodilation of cutaneous vessels during direct local application of heat to the skin's surface and during sympathetic nervous system-mediated thermoregulatory reflexes in response to whole body heat stress, the roles of the different NOS isoforms in these two processes have only recently been clarified (19, 25, 36). Recent work by our laboratory identified an important mechanistic role for nNOS in the generation of NO during cutaneous active vasodilation as induced by whole body heat stress, but not during skin vasodilation induced by local warming of forearm skin (25). This dichotomous involvement of nNOS in two distinct vasodilatory processes suggested similar dichotomous roles for eNOS in the control of the human forearm cutaneous vasculature.
Given the foregoing observations, we hypothesized that eNOS mediates the plateau phase of vasodilation induced by Tloc increases, but not the cutaneous active vasodilation induced by whole body heat stress. We tested our hypothesis by examining whether increases in SkBF during local skin warming (protocol 1) or whole body heat stress (protocol 2) were attenuated by blockade of eNOS with the mechanism-based eNOS inactivator NG-amino-l-arginine (l-NAA) (1, 6, 8, 37, 43); thus the overall purpose of our work was to elucidate the role(s) of eNOS in cutaneous vasomotor control mechanisms.
METHODS
All subjects were in good health, did not use tobacco products, and took no medications. All subjects gave their written, informed consent to participate in these studies, which conformed to the standards set by the Declaration of Helsinki and were approved by the University of Texas Health Science Center Institutional Review Board. Subjects did not use caffeine-containing beverages on the day of the study.
l-NAA (AXXORA, San Diego, CA) was chosen as a mechanism-based eNOS inactivator (6). In vitro inhibition studies by Wolff and Lubeskie (43) with isolated NOS isoforms found this agent to have 10-fold selectivity for eNOS over nNOS. In subsequent studies, Cooper et al. (8) found that although the isolated nNOS enzyme could be inhibited by l-NAA in vitro, nNOS in pituitary cells was largely refractory to inactivation by l-NAA. Finally, in physiological studies, Stricklett et al. (37) used l-NAA as an eNOS antagonist to successfully define the roles of eNOS and nNOS in NO generation in rat inner medullary collecting ducts. The foregoing suggested that l-NAA could be useful for in vivo studies as long as great care was taken with the concentrations used (1).
For our studies, l-NAA was administered by intradermal microdialysis. This technique allows introduction of pharmacological agents into a small volume of skin without risk of systemic effects. All microdialysis probes were manufactured in our laboratory from polyimide tubing with a 1-cm length of capillary microdialysis membrane (regenerated cellulose, 200 μm diameter, 20 kDa molecular cutoff). Each probe was reinforced with a 51-μm-diameter stainless steel wire inserted throughout the lumen and was gas sterilized before use.
Microdialysis probes were placed 5 cm apart in the ventral forearm skin. This probe separation ensured that drug administration at one site did not influence the other. Placement of each microdialysis probe was accomplished by insertion of a 25-gauge needle through the dermis with sterile technique after ice had been applied to the skin surface for several minutes to achieve short-term, local anesthesia. The microdialysis probe was threaded through the lumen of the needle, which was then removed, leaving the microdialysis probe in place. The microdialysis membrane was placed completely within the skin, so that the polyimide tubing projected from the entry and exit points. The entry and exit points for each probe were ∼2–3 cm apart (20). Using this technique, we have placed probes 0.3–1.0 mm under the epidermal surface in the dermis as verified by ultrasound measurements (21). Microdialysis probes were perfused at a rate of 2 μl/min using a microinfusion pump (Harvard Apparatus, South Natick, MA).
After probe placement, ≥100 min were allowed to elapse before placement of additional instrumentation to ensure that insertion trauma had resolved (2). Subjects were then placed in the supine position and instrumented for LDF from skin at both microdialysis sites (Moorlab Flowmeter, Moor Instruments, Devon, UK). Pulse rate and mean arterial pressure (MAP) were measured from a finger by photoplethysmography (Ohmeda, Madison, WI).
To define an appropriate l-NAA concentration, studies were done to characterize the in vivo effects of l-NAA as administered by intradermal microdialysis. These studies evaluated the attenuation of ACh-induced, eNOS-mediated vasodilation by different concentrations of l-NAA to define an isoform-selective l-NAA concentration to be used in our subsequent physiological experiments (14, 24). l-NAA at 1.25, 2.5, 5, 10, and 20 mM was perfused via intradermal microdialysis probes for 45 min. Then 1.0 mM ACh was added to the perfusate to evoke endothelium-dependent vasodilation. l-NAA at 1.25 and 2.5 mM had no effect on ACh-induced vasodilation. In contrast, ≥5 mM l-NAA significantly attenuated ACh-induced vasodilation. This work demonstrated that 5 mM l-NAA was the minimal perfusate concentration that could reliably attenuate endothelium-dependent vasodilation as induced by exogenous ACh. Because isoform selectivity is more evident at lower antagonist concentrations, we used 5 mM l-NAA, inasmuch as this was the minimal concentration that attenuated eNOS-mediated responses and also minimized the chance of nNOS inhibition (1, 6, 8, 37, 43).
Protocol 1: local skin warming.
Eight subjects [4 men and 4 women (2 in the follicular phase and 2 in the luteal phase)] participated in the local skin warming protocol. Their average (mean ± SE) age was 34 ± 5 yr, weight was 71 ± 5 kg, and height was 172 ± 2 cm.
Upon arrival in the laboratory, each subject had two intradermal microdialysis probes placed into the skin on the ventral aspect of one forearm and was instrumented for LDF and MAP monitoring as described above. Each LDF probe was equipped with a special probe holder that contained resistive heating elements and thermocouples to permit simultaneous LDF measurements as well as control and measurement of Tloc (21). After LDF probe placement, perfusion of the microdialysis probes with Ringer solution at a rate of 2 μl/min was begun.
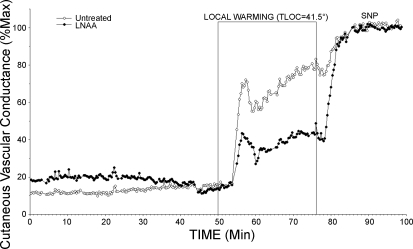
Data collection began with a 5-min control period during which Tloc was maintained at 34°C. The perfusate at one site was then changed to 5 mM l-NAA in Ringer solution while perfusion with Ringer solution was maintained at the second site to serve as an untreated control while Tloc was maintained at 34°C. After ∼45 min, Tloc was raised at a rate of 0.6°C/min to 41.5°C at both sites and maintained at 41.5°C until a clear LDF plateau had been established. A relatively slow rate of warming and a maximal Tloc of 41.5°C were chosen to obviate pain fiber activation, which can cause vasodilation through mechanisms other than NO (21). Finally, the perfusates at both sites were changed to 56 mM sodium nitroprusside (SNP) in Ringer solution for 20–25 min to cause maximal vasodilation for data normalization (23). The protocol is illustrated in Fig. 1.
Fig. 1.
Protocol 1: local skin warming. Two microdialysis sites were used: one was perfused with Ringer solution alone, and the other was perfused with 5 mM NG-amino-l-arginine (l-NAA) in Ringer solution (initiated at 0 min). During the initial phase, local temperature (Tloc) of microdialysis sites was held at 34°C. Local skin heating was then performed to raise Tloc to 41.5°C at both microdialysis sites. Finally, perfusates at both sites were changed to 56 mM sodium nitroprusside (SNP) to cause maximal vasodilation. An initial peak in cutaneous vascular conductance (CVC) was followed by a prolonged plateau at both sites. An initial peak was not observed in all studies.
Values are means ± SE. For data analysis, CVC was calculated as LDF ÷ MAP. Vasomotor responses were analyzed by comparison of the average levels of CVCs during the final 3 min of the 34°C Tloc period with the final 3 min of the 41.5°C Tloc period. CVC responses were analyzed by repeated-measures ANOVA followed by specific means comparisons (21). The level of statistical significance (alpha) was defined as 0.05.
Protocol 2: whole body heat stress.
Nine subjects [4 men and 5 women (3 in the follicular phase and 2 in the luteal phase)] participated in the whole body heat stress protocol. Their average age was 30 ± 4 yr, weight was 69 ± 5 kg, and height was 176 ± 3 cm.
A tube-lined suit was worn to induce thermoregulatory reflexes. A water-impermeable plastic garment worn over the suit insulated the subjects from the room environment and prevented evaporation of sweat. The suit and garment covered the entire body, except the head and the arm from which the measurements were made. The hands and feet were also uncovered. The suit was used to control skin temperature (Tsk) by perfusion with water of different temperatures and, thus, cause periods of normothermia, cold stress, and heat stress (18, 31). The suit was perfused with cold water to decrease Tsk and induce cold stress and warm water to raise Tsk to 38–39°C during heating periods. This technique of indirect whole body heating thus evokes thermoregulatory reflex responses, yet it does not increase Tloc at blood flow measurement sites, which could confound assessment of reflex responses (18, 31).
Internal temperature was monitored with a thermocouple placed in the sublingual sulcus [oral temperature (Tor)]. Subjects were instructed not to speak so as not to confound this measurement. Tsk was recorded as the weighted electrical average from six thermocouples taped on the skin surface (18, 31). Pulse rate and MAP were recorded continuously from a finger (Finapres BP Monitor, Ohmeda).
After arriving in the laboratory on the day of the study, subjects were instrumented with two intradermal microdialysis probes as previously described. SkBF was monitored at both microdialysis sites by LDF.
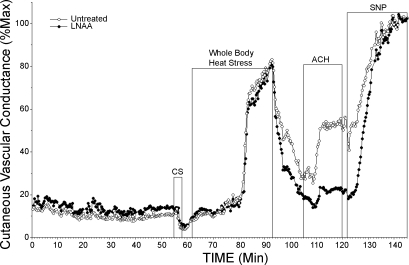
Data collection began with a control 5- to 10-min period during which Tsk was maintained at a normothermic level of 34°C. During this period, both microdialysis sites were perfused with Ringer solution at 2 μl/min; then the perfusate at one microdialysis site was changed to 5 mM l-NAA in Ringer solution at 2 μl/min. After ∼45–50 min of normothermia with l-NAA perfusion, Tsk was decreased to induce cold stress for 3 min to verify that active vasoconstrictor function was intact at the microdialysis sites and, thereby, exclude unanticipated effects of l-NAA. After cold stress, Tsk was raised to 38–39°C and maintained at that level to induce heat stress and activate the vasodilator system. Whole body heating was maintained for ∼25–40 min until Tor had increased by ∼1°C and SkBF had stabilized at an elevated level. After heat stress, subjects were returned to normothermia. In four studies, exogenous ACh was perfused through the microdialysis probes to verify l-NAA inactivation of eNOS. At the conclusion of all studies, both microdialysis sites were perfused with 56 mM SNP to cause maximal vasodilation. The protocol is illustrated in Fig. 2.
Fig. 2.
Protocol 2: whole body heat stress. Two microdialysis sites were used. After an initial normothermic control period when both microdialysis sites were perfused with Ringer solution, perfusate at one site was changed to 5 mM l-NAA in Ringer solution (initiated at 5 min). Perfusion with Ringer solution alone was maintained at the other site. After ∼45 min of perfusion with l-NAA, skin temperature (Tsk) was decreased to induce whole body cold stress. Tsk was then increased to induce whole body heat stress, after which the subjects were cooled to normothermia. In this example, exogenous ACh was administered to both untreated and l-NAA-treated sites following heat stress to verify l-NAA effects. Finally, perfusates at both sites were changed to 56 mM SNP to cause maximal vasodilation.
Values are means ± SE. For data analysis, CVC values at l-NAA-treated and untreated sites were normalized to their respective maxima as elicited by SNP to facilitate comparisons between l-NAA-treated and untreated control sites within and among subjects. The vasomotor and NO responses to heat stress were analyzed by comparison of the internal temperature thresholds for the initial increases in CVC at the two microdialysis sites. The internal temperature threshold for the onset of vasodilation for each site was defined as the level of Tor at which a sustained increase in CVC began, after Tsk had been increased to 38°C. Tor thresholds were chosen from individual graphs of CVC vs. time by an investigator blinded to the conditions, subjects, and antagonist treatment. The thresholds for cutaneous vasodilation were compared by ANOVA for repeated measures. Comparisons of CVC values, normalized to maxima, from the normothermic baseline, during the final minute of cold stress, and from the final 3 min of heat stress were made by ANOVA followed by Student-Newman-Keuls test. The level of statistical significance (alpha) was defined as 0.05.
RESULTS
The physical characteristics (age, height, weight) of the subject groups in protocols 1 and 2 were not statistically different. Responses of the two sexes within each protocol were not different; therefore, data for men and women were combined for data analysis.
Protocol 1: local skin warming.
When Tloc was held at 34°C, CVC averaged 19 ± 4%max at sites perfused with Ringer solution only and 16 ± 2%max at sites perfused with 5 mM l-NAA. There were no significant differences between these values at 34°C Tloc (P > 0.05 between sites).
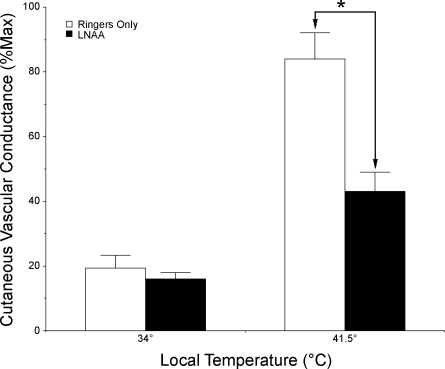
In response to increasing Tloc, an initial peak in SkBF was observed in only three of the eight subjects, although when it did happen, similar responses were observed simultaneously at the untreated and l-NAA-treated sites. As Tloc was increased to 41.5°C, CVC increased significantly and achieved stable plateaus at both sites (P < 0.05, 34°C vs. 41.5°C for both sites). During the plateau phase, with Tloc maintained at 41.5°C, CVC averaged 84 ± 8%max at sites perfused with Ringer solution only, while CVC at sites treated with 5 mM l-NAA only rose to 43 ± 6%max. At 41.5°C Tloc, CVC was significantly less at l-NAA-treated than at untreated control sites (P < 0.05 between sites). These results are summarized in Fig. 3.
Fig. 3.
Summary of CVC responses to local warming of the skin. With a Tloc of 34°C, CVC did not differ between sites perfused with Ringer solution alone and sites perfused with 5 mM l-NAA in Ringer solution (P > 0.05 between sites). With local heating to 41.5°C, CVC increased significantly at both sites (P < 0.05, 34°C vs. 41.5C); however, responses were significantly different (*P < 0.05 between sites); thus endothelial nitric oxide synthase (eNOS) antagonism attenuated vasodilation induced by local warming of the skin.
Protocol 2: whole body heat stress.
In normothermia, CVC averaged 13 ± 2%max at untreated sites and 15 ± 2%max at l-NAA-treated sites (P > 0.05 between sites).
During whole body cooling, CVC fell significantly at both microdialysis sites (P < 0.05, normothermia vs. cold stress). During the final minute of whole body cooling, CVC fell to an average of 8 ± 2%max at untreated sites and to 9 ± 2%max at l-NAA-treated sites. These responses were not significantly different (P > 0.05 between sites).
Under normothermic conditions, Tor averaged 36.89 ± 0.12°C. In response to whole body heating, CVC began to rise at the untreated site when Tor reached a threshold of 37.07 ± 0.11°C, whereas CVC began to increase at the l-NAA-treated site when Tor reached 37.06 ± 0.12°C. These Tor threshold values did not differ between sites (P > 0.80). At the peak of heat stress, Tor reached a maximal level of 37.87 ± 0.10°C (P < 0.01 vs. normothermia). Under normothermic conditions, MAP averaged 74 ± 3 mmHg and did not change significantly during heat stress (74 ± 4 mmHg, P > 0.80, normothermia vs. peak heat stress). Pulse rate averaged 62 ± 3 beats/min in normothermia and increased significantly to 96 ± 3 beats/min by the end of whole body heating (P < 0.01, normothermia vs. peak heat stress).
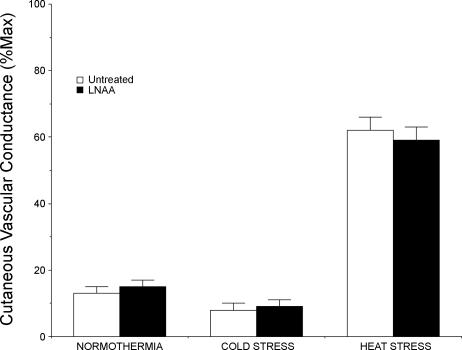
In response to whole body heating, CVC rose significantly at untreated and l-NAA-treated sites (P < 0.05, normothermia vs. peak heat stress). At the peak of heat stress, CVC had risen to 62 ± 4%max CVC at the untreated sites and to 59 ± 4%max CVC at the l-NAA-treated sites. These responses were not significantly different between sites (P > 0.05, untreated vs. l-NAA). The overall CVC results for protocol 2 are illustrated in Fig. 4.
Fig. 4.
Summary of CVC responses to whole body heat stress. Under normothermic conditions, CVC did not differ between sites perfused with Ringer solution only and sites perfused with 5 mM l-NAA in Ringer solution (P > 0.05 between sites). During whole body cold stress, CVC fell at both sites (P < 0.05). Responses at the sites were not statistically different (P > 0.05 between sites). In response to whole body heating, CVC rose at both sites (P < 0.05). Responses at sites perfused with Ringer solution only and sites perfused with 5 mM l-NAA in Ringer solution were not statistically different (P > 0.05 between sites); thus eNOS antagonism did not attenuate cutaneous active vasodilator response to whole body heat stress.
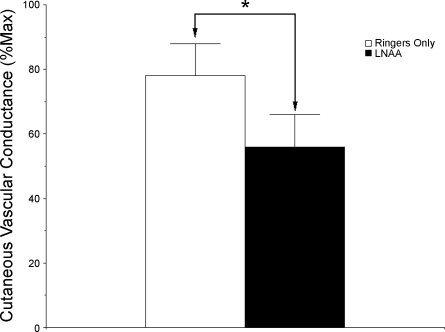
After heat stress, exogenous ACh increased CVC to 78 ± 10%max CVC at untreated sites and to 56 ± 10%max CVC at l-NNA-treated sites. These responses were significantly different between sites (P < 0.05, untreated vs. l-NAA). These CVC results are illustrated in Fig. 5.
Fig. 5.
Summary of CVC responses to exogenous ACh after whole body heat stress. Vasodilatory response to 1.0 mM ACh was significantly attenuated by 5 mM l-NAA, thus verifying eNOS antagonism. *P ≤ 0.05 between sites.
DISCUSSION
In protocol 1, l-NAA attenuated CVC increases induced by local skin warming. In direct contrast, in protocol 2, l-NAA altered neither the Tor threshold for the onset of cutaneous active vasodilation nor the CVC increases induced by whole body heat stress. Given that 1) in prior studies, l-NAA has proven to be an effective mechanism-based inactivator of eNOS (1, 6, 8, 37, 43), 2) in the present study, l-NAA inhibited vasodilation induced by exogenous ACh, and 3) in the present study, l-NAA had completely dichotomous effects on two physiological vasodilator processes known to be NO dependent, our findings strongly support the notion that eNOS effects a significant portion of the cutaneous vasodilation induced by local warming on the skin but does not effect a measurable portion of the cutaneous active vasodilator response to whole body heat stress in humans.
In the present study, our finding that eNOS inactivation attenuated the NO-dependent SkBF plateau caused by local skin warming is consistent with findings from a prior study from our laboratory with the specific nNOS inhibitor 7-nitroindazole (7-NI). We previously found that nNOS inhibition had no attenuating effect on SkBF plateau responses to local skin warming, which suggests that eNOS mediates the response (25). Taken together, the results of these studies provide complementary evidence that eNOS, rather than nNOS, effects an increase in NO generation during prolonged local warming of forearm skin.
Prior work by Shastry and Joyner (32) implicates eNOS as a mediator of NO production during the prolonged SkBF plateau phase of local skin warming. They examined the effect of the heat shock protein 90 (HSP90) inhibitor geldanamycin on SkBF increases induced by increased Tloc. HSP90 has been shown to bind eNOS and enhance its activation, leading to increased NO generation (12). Shastry and Joyner reasoned that if eNOS production of NO was involved in increasing SkBF in response to increased Tloc, inhibition of the interaction of HSP90 with eNOS would attenuate SkBF responses. They found that geldanamycin attenuated the SkBF plateau by ∼20% (32). In the present study, we found that inhibition of eNOS attenuated the SkBF plateau by ∼50% and suggest that eNOS could be activated by means beyond interaction with HSP90 alone (10). One possibility is that eNOS could be activated by a direct effect of temperature; however, studies with isolated NOS isoforms suggest that this is not likely. Venturini et al. (40) found that temperature directly influenced nNOS and iNOS activity independent of substrate concentration; however, eNOS activity was essentially unaltered by temperature. Another observation related to the complexity of the local response to skin warming was made by Minson and colleagues (28). They observed that the timing of administration of the competitive NOS antagonist nitro-l-arginine methyl ester (l-NAME) altered the degree of inhibition observed during skin warming. Administration of l-NAME before and throughout local skin warming reduced the plateau phase to ∼40%max CVC. Administration of l-NAME after the plateau phase was clearly established reduced the plateau to ∼20%max CVC. These observations suggest that mechanisms activated early in the local warming response can alter NO dependence and suggest involvement of multiple interdependent mechanisms. Overall, it is thus likely that the effect of local skin warming on SkBF is mediated by multiple complex control mechanisms, including substrate availability, HSP90, and other intracellular protein interactions, but surprisingly not by direct thermal effects on eNOS activity (10, 40).
As elegantly delineated by Minson et al. (27), the cutaneous vascular response to local skin warming is biphasic and consists of an initial rapid increase in SkBF followed by a temporary decline and then another increase to the prolonged SkBF plateau. In contrast to the plateau portion of the response, the initial transient phase of the cutaneous vascular response to local skin warming appears to be mediated mainly by antidromic release of neurotransmitters from afferent sensory nerves through activation of temperature-sensitive vanilloid receptors (27, 35). This phase does involve a modest NO-mediated component through NOS activation by the released neurotransmitters (27). Our slow local warming protocol did not elicit this phase except in a few of our subjects and, thus, precluded defining which NOS isoform was involved in this phase; however, when the initial phase did occur, it appeared at untreated and l-NAA-treated sites. This is consistent with the finding by Shastry and Joyner (32) that this phase was unaltered by geldanamycin inhibition of HSP90 and suggested that eNOS does not effect this relatively modest NO-dependent component.
Although l-NAA attenuated SkBF responses to local skin warming, the same concentration of l-NAA did not alter cutaneous vascular responses to whole body heat stress, a reflex response known to be dependent in part on NO generation by NOS (20, 24, 33, 34, 42). Neither the Tor threshold for the onset of cutaneous active vasodilation nor SkBF increases during whole body heat stress were altered by l-NAA. In contrast, increases in SkBF during whole body heat stress are attenuated by non-isoform-specific NOS inhibitors, such as l-NAME (20, 33, 34), and the nNOS isoform-specific antagonist 7-NI (25). Neither isoform-specific or nonspecific NOS inhibitors appear to alter the Tor threshold for the onset of cutaneous active vasodilation (20, 33, 34). When considered together, the results of the present study suggest that the eNOS isoform is not the major NOS isoform responsible for NO generation during whole body heat stress and provide complementary support for nNOS as the major generator of NO during cutaneous active vasodilation (25).
Our conclusions in regard to the roles of eNOS in the control of human cutaneous blood vessels are based on the inactivation of eNOS, rather than nNOS, by l-NAA. Isolated enzyme studies demonstrated that l-NAA has 10-fold greater inhibitor constant for eNOS than nNOS and, thus, a relative selectivity for eNOS over nNOS (1, 43). In addition, inhibition studies in whole cells found that nNOS generation of NO in pituitary cells was largely unaltered by l-NAA (8, 43). Finally, l-NAA has been used successfully to delineate the isoform responsible for NO production by endothelin B receptors in kidney collecting ducts (37). Thus prior published work is consistent with selective eNOS inactivation by l-NAA at appropriately defined concentrations.
Because the isoform specificity of NOS antagonists declines with increasing concentration, we performed studies that defined the lowest l-NAA concentration that attenuated the vasodilator response to exogenous ACh that is in part NO dependent and mediated by eNOS (14, 24). These studies demonstrated that 5 mM was the lowest l-NAA concentration that would reliably attenuate endothelium-dependent vasodilation caused by exogenous ACh and yet also be the least likely to inactivate nNOS.
In the present study, we found that the same concentration of l-NAA that significantly attenuated skin vasodilation induced by both local skin warming and exogenous ACh failed to attenuate cutaneous active vasodilation during whole body heat stress. Both of these responses are known to be dependent, at least in part, on NO generation by NOS (15, 20, 21, 24, 25, 27, 28, 33, 34). The divergent findings of 1) an attenuating effect of 5 mM l-NAA on vasodilation induced by local skin warming and 2) no attenuating effect of 5 mM l-NAA on cutaneous active vasodilation in whole body heat stress show that selective eNOS inactivation had been achieved. If only nonselective NOS isoform blockade had been achieved, the vasodilatory responses induced by both of these physiological processes would have been attenuated; that was not the case.
What is the cellular location of the eNOS that is activated by direct temperature increases? The eNOS isoform is expressed in low levels in skin fibroblasts, melanocytes, and dermal papilla cells; however, the predominant site of eNOS localization in skin is within endothelial cells (7). Previous observations that the vasodilatory plateau induced by increased Tloc is refractory to botulinum toxin or cutaneous anesthesia (22, 29) indicate that neural processes are not involved. Thus it is quite likely that increased Tloc acts directly on endothelial cells to cause eNOS activation, increased NO production, and, consequently, local vasodilation. How this thermal signal is transduced is unknown.
Whether the foregoing mechanisms are present in body regions other than the forearm is unclear. The present study, as well as our investigation with 7-NI, was done in forearm skin with single periods of local skin warming (25), in contrast to those of Stewart et al. (36), which were done in skin of the leg with repeated periods of local skin warming. In their protocol, Stewart et al. compared the vasodilation induced by local skin warming during an initial untreated control period with that induced by a second period of local skin warming at the same site during treatment with Nω-nitro-l-arginine-2,4-l-diaminobutyric amide (Nω), an nNOS antagonist, based on in vitro studies (16). Nω was introduced into the skin of the calf by intradermal microdialysis. Compared with the SkBF response during the initial warming period, Nω attenuated the plateau phase of a second period of local skin warming in the calf. The extent of the attenuation was the same as that achieved with a non-isoform-specific NOS antagonist. This work suggests the possibility that nNOS mediates local skin warming responses in the calf, but not in the forearm (25). Alternatively, vasodilation induced by single periods of local skin warming may be mediated by eNOS, whereas different mechanisms involving nNOS activity are evoked by repeated periods of local warming.
Finally, what are the clinical implications of these findings? Simple and reliable techniques to evaluate endothelial function have been sought by clinical practitioners for years, yet they remain unestablished (3). The use of LDF to monitor cutaneous vascular responses to local skin warming has been suggested, and it certainly represents a simple approach, albeit one that is limited by some technical challenges such as the variability inherent in LDF (9). The present study provides additional rationale for using the plateau phase of the response to local skin warming to specifically evaluate eNOS function in humans in vivo; however, because LDF provides only a relative index of SkBF, and not absolute quantification, it is likely that a technique other than LDF will be needed to reproducibly monitor these vasomotor responses in useful ways in clinical settings.
In summary, we found that the mechanism-based eNOS inactivator l-NAA attenuated the SkBF plateau phase of local skin warming. An identical concentration of l-NAA did not alter the cutaneous active vasodilator response to whole body heat stress. This result demonstrates a specific role for eNOS activation in generating the increase in NO requisite for skin vasodilation due to increased Tloc. In contrast, eNOS activation appears not to play a substantial mechanistic role in cutaneous active vasodilation as induced by whole body heat stress.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-065599.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser-Doppler perfusion imaging. J Invest Dermatol 102: 808–811, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Barac A, Campia U, Panza JA. Methods for evaluating endothelial function in humans. Hypertension 49: 748–760, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL Jr. Evidence for a role for vasoactive intestinal peptide in active vasodilation in the cutaneous vasculature in humans. J Physiol 552: 223–232, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruch-Gerharz D, Ruzicka T, Kolb-Bachofen V. Nitric oxide in human skin: current status and future prospects. J Invest Dermatol 110: 1–7, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Bryk R, Wolff DJ. Pharmacological modulation of nitric oxide synthesis by mechanism-based inactivators and related inhibitors. Pharmacol Ther 84: 157–178, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Cals-Grierson MM, Ormerod AD. Nitric oxide function in the skin. Nitric Oxide 10: 179–193, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cooper GR, Barr A, Wolff DJ. Neuronal nitric oxide synthase is refractory to mechanism-based inactivation in GH3 pituitary cells. Arch Biochem Biophys 357: 195–206, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 27: 503–508, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res 75: 247–260, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilation. J Physiol 142: 219–232, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 812–824, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Haynes V, Elfering S, Traaseth N, Giulivi C. Mitochondrial nitric-oxide synthase: enzyme expression, characterization, and regulation. J Bioenerg Biomembr 36: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilation in young and aged human skin. J Physiol 563: 965–973, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol 572: 811–820, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Martesek P, Roman LJ, Silverman RB. Synthesis and evaluation of peptidomimetics as selective inhibitors and active site probes of nitric oxide synthases. J Med Chem 43: 2938–2945, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JM How does skin blood flow get so high? J Physiol 577: 768, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol 50: 814–818, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Kellogg DL Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol 100: 1709–1718, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol 85: 824–829, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg DL Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol 86: 1185–1190, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Kellogg DL Jr, Pérgola PE, Kosiba WA, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve co-transmission. Circ Res 77: 1222–1228, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Kellogg DL Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kellogg DL Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol 94: 1971–1977, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DL Jr, Zhao JL, Wu Y. Neuronal nitric oxide synthase mechanisms in the cutaneous vasculature of humans in vivo. J Physiol 586: 847–857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol 91: 1619–1626, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Pérgola PE, Kellogg DL Jr, Johnson JM, Kosiba WA. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol 265: H785–H792, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Rowell LB Reflex control of the cutaneous vasculature. J Invest Dermatol 69: 154–166, 1977. [DOI] [PubMed] [Google Scholar]
- 31.Rowell LB, Murray JA, Brengelmann GL, Kraning KK 2nd. Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Circ Res 24: 711–724, 1969. [DOI] [PubMed] [Google Scholar]
- 32.Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol 282: H232–H236, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Shastry S, Minson CT, Wilson SA, Dietz NK, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol 88: 467–472, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Shastry S, Reed AS, Halliwill JR, Dietz NM, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol 85: 830–834, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol 281: R894–R901, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Stewart JM, Medow MS, Minson CT, Taneja I. Cutaneous neuronal nitric oxide is specifically decreased in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 293: H2161–H2167, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol 290: F1315–F1319, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Taylor WF, Johnson JM, O'Leary D, Park MK. Effect of high local temperature on reflex cutaneous vasodilation. J Appl Physiol 57: 191–196, 1984. [DOI] [PubMed] [Google Scholar]
- 39.Ventura S, Bavetta S, Milner P, Ralevic V, Burnstock G. Nitric oxide synthase is co-localized with vasoactive intestinal polypeptide in postganglionic parasympathetic nerves innervating the rat vas deferens. Neuroscience 83: 607–616, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Venturini G, Colasanti M, Fioravanti E, Bianchini A, Pascenzi P. Direct effect of temperature on the catalytic activity of nitric oxide synthases types I, II and III. Nitric Oxide Biol Chem 3: 375–382, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Ghahary A, Shen YJ, Scott PG, Tredget EE. Human dermal fibroblasts produce both nitric oxide and express both constitutive and inducible nitric oxide synthase isoforms. J Invest Dermatol 106: 419–427, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Wilkins BW, Holowatz LA, Wong BJ, Minson CT. Nitric oxide is not permissive for cutaneous active vasodilation in humans. J Physiol 548: 963–969, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff DJ, Lubeskie A. Inactivation of nitric oxide synthase isoforms by diaminoguanidine and NG-amino-l-arginine. Arch Biochem Biophys 325: 227–234, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilation in humans. J Physiol 577: 1043–1051, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]