Abstract
Nitric oxide (NO) effects are often mediated via S-nitrosothiol (SNO) formation; SNO uptake has recently been shown to be mediated in some cell types via system L-type amino acid transporters (LAT-1, 2). Inhaled NO therapy may exert some biological effects via SNO formation. We therefore sought to determine if pulmonary epithelial SNO uptake depended on LAT or peptide transporter 2 (PEPT2). Both LAT-1 and PEPT2 proteins were detected by immunoblot and immunocytochemistry in L2 cells and rat lung. We tested SNO uptake through the transporters by exposing rat alveolar epithelial cells (L2 and type II) to RSNOs: S-nitrosoglutathione, S-nitrosocysteinylglycine (SNO-Cys-Gly), S-nitrosocysteine (CSNO), and to NO donor diethylamine NONOate (DEA-NONOate). SNO was detected in cell lysates by ozone chemiluminescence. NO uptake was detected by fluorescence in alveolar epithelial cells loaded with 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate cultured in submersion and exposed to RSNOs and DEA NONOate. Addition of l-Cys but not d-Cys to RSNOs or DEA NONOate increased SNO and DAF-FM signal that was inhibited by coincubation with LAT competitors. Incubation of cells with PEPT2 substrate SNO-Cys-Gly showed no increase in SNO or DAF-FM signal unless incubated with l-Cys. This was unaffected by PEPT2 inhibition. We conclude that RSNOs (thionitrites, S-nitrosothiols) and NO enter alveolar epithelial cells predominantly by S-nitrosation of l-Cys, which is then imported through LAT.
Keywords: peptide transporter 2, type II alveolar epithelium, S-nitrosylation, nitric oxide
s-nitrosation of critical cysteine residues forming S-nitrosothiols (SNO) on regulatory proteins is increasingly implicated in a variety of nitric oxide (NO)-dependent cellular processes, consistent with signaling, but with as yet incompletely understood chemistry (6). Inhaled agents capable of generating protein RSNOs (thionitrites, S-nitrosothiols), such as S-nitrosoglutathione (GSNO) (24) and O-nitrosoethanol (ethyl nitrite), have been used experimentally (1, 19) and clinically (18) to treat pulmonary hypertension and other pulmonary conditions, but little is known about the importation of S-nitrosated peptides or amino acids (RSNO) into alveolar epithelium, which could, in turn, affect SNO-dependent pathways.
Recent studies have shown that extracellular NO or RSNO achieve intracellular SNO accumulation in vascular endothelium and smooth muscle cells by transnitrosation of extracellular l-cysteine (l-Cys) to form CSNO, which is then imported via the L-type amino acid transporters (LAT-1, LAT-2) (14, 15, 27, 28). Messenger RNAs for both transporters have been detected in lung, but specific lung cellular localization has not been reported (20, 26).
In addition, pulmonary epithelial SNO uptake could theoretically take place through dipeptide transporters such as peptide transporter 2 (PEPT2), which is expressed and shown to be functionally active in lung (7, 17). Glutathione is the most abundant form of thiol in the alveolar lining fluid, present in high micromolar concentrations, a small proportion of which is S-nitrosated under physiological conditions (5). In the presence of NO gas and ethyl nitrite (19), glutathione can form GSNO. GSNO can be metabolized by γ-glutamyltranspeptidase to SNO-Cys-Gly (4) that could be taken up by PEPT2. The physiological role of PEPT2, if any, in the uptake of SNO-Cys-Gly in alveolar epithelium is unknown.
To determine the potential role of amino acid and/or dipeptide importation in the process of intracellular SNO accumulation in alveolar epithelium, we identified LAT-1 expression in pulmonary tissues and alveolar epithelial cells by Western blotting and immunohistochemistry. To determine transporter function in SNO accumulation, we exposed L2 cells to SNO donors (GSNO, CSNO, SNO-l-Cys-Gly) and NO donor DEA-NONOate in the presence of LAT competitors or PEPT2 competitors. We measured intracellular NO accumulation using a NO-sensitive fluorescent label, and we measured SNO accumulation by triiodide reduction ozone chemiluminescence. Some of the studies were also performed in freshly isolated rat type II alveolar epithelial cells.
We found that SNO or NO donor treatment of L2 cells achieved maximum intracellular NO or SNO levels only in the presence of l-Cys and that this effect was inhibitable by competition with l-leucine (l-Leu), a LAT substrate, and 2-amino-2-norborane carboxylic acid (BCH), another LAT competitor. Treatment with SNO-Cys-Gly achieved significant intracellular NO or SNO levels only when coincubated with l-Cys. Parallel results were found in freshly isolated alveolar epithelial cells.
METHODS
Materials.
Reagents were obtained from Sigma (St. Louis, MO) except where noted. GSNO was synthesized as described previously (15) or purchased from Alexis Biochemicals (Lausen, Switzerland). S-nitrosocysteine and S-nitrosocysteinylglycine were similarly prepared just before use. Hanks' balanced salt solution (HBSS) was purchased from GIBCO (Carlsbad, CA). Polyclonal rabbit anti-human L-type amino acid LAT-1 was purchased from Trans Genic (Minatojimaminami-machi, Chuo-ku, Kobe, Japan). Polyclonal rabbit anti-PEPT2 antiserum was a generous gift of David Smith, Univ. of Michigan. Secondary antibodies were obtained from Vector (Burlingame, CA). PVDF membranes, chemiluminescence substrate (WestPico), and autoradiography film were obtained from Pierce-Endogen (Rockford, IL). Diethylamine NONOate (DEA-NONOate) was from Cayman Chemical (Ann Arbor, MI).
Rat lung tissue preparation.
Rat lungs were removed after euthanizing Sprague-Dawley rats (Charles River, Raleigh, NC) with pentobarbital sodium in accord with protocols approved by the Institutional Animal Care and Use Committee. For tissue extraction, lungs were perfused via the pulmonary artery with PBS plus 1 mM EDTA, pH 7.4, snap frozen, and subjected to protein extraction as described previously (1). For immunohistochemistry, lungs were inflation fixed with 10% phosphate buffered formalin for 30 min, followed by immersion in fixative overnight at 4°C, then paraffin embedded and sectioned.
LAT1, PEPT2 expression.
To detect expression in cells, equal protein masses of lysate supernatants were loaded onto precast 4–15% gradient SDS-PAGE gels (Bio-Rad, Hercules, CA) after denaturing in SDS and reducing in dithiothreitol as previously described (2). Gels were electroblotted onto PVDF membranes overnight in glycine-methanol transfer buffer (2), and membranes were then detected with anti-LAT-1 (1:5,000) or anti-PEPT-2 (1:400 of preadsorbed antiserum) followed by goat-anti-rabbit peroxidase (1:10,000) and detection with chemiluminescence substrate (SuperSignal, WestPico, Pierce-Endogen) exposed to film (CL-Xposure, Pierce-Endogen). PEPT2 antiserum was preadsorbed in equal volume of rat serum overnight at 4°C and then precipitated for 10 min at 10,000 g. Supernatants were used at dilutions specified.
Expression was detected in cells and tissue by immunohistochemistry. Sections were dewaxed and subjected to antigen retrieval as previously described (1). For anti-LAT-1, tissues were incubated in primary antibody (1:200), for PEPT2, anti-serum 1:200, and for negative control, nonimmune serum, followed by biotinylated goat anti-rabbit, and detected using avidin-biotin amplification as previously described (ABC Elite, Vector), with diaminobenzidine or Nova Red chromagen (Vector) according to the manufacturer's directions. Photomicrographs were obtained with a digital camera at ×1,000 final magnification under oil immersion. Type II alveolar epithelial cells were centrifuged onto glass slides (Cytospin, Thermo Fischer, Waltham, MA) and detected using anti-pro-SP-C as previously described in detail (3). Imaging was performed using a Nikon E400 microscope connected to a digital camera (DP11; Olympus, Melville, NY).
L2 cell culture.
L2 cells were purchased from American Type Culture Collection (Manassas, VA). Cells were grown in Gibco's F-12 Kaighn's Nutrient Mixture supplemented with 10% fetal bovine serum, 10,000 units of penicillin, and 10 mg of streptomycin/ml in 10-cm plastic dishes (Costar, Corning, NY) after being coated in poly-d-lysine. For the NO accumulation studies, cells were plated onto similarly coated 96-well transparent plates with black sidewalls for fluorescence microplate analysis. L2 cells were used at passages 43–50. In preliminary studies, trypan blue was added to cells to measure viability under our experimental conditions after adding NO donors and inhibitors. Measurement of pH of the cell media after adding amino acids and inhibitors was performed.
Primary type II cell isolation and culture.
Type II rat alveolar epithelial cells were isolated using the method of Chen et al. (3). Briefly, the lungs of adult male Sprague Dawley rats, 300–400 g, were perfused with RPMI medium (GIBCO) through the pulmonary artery after clipping the left atrial appendage, then lavaged with buffer followed by lavages with elastase in RPMI. Lungs were then minced, filtered, pelleted, and resuspended in medium. Macrophages were removed by plating the cell suspension on rat IgG-coated plastic Petri dishes. Cell suspensions were treated with mouse anti-rat CD45 leukocyte common antigen OX-30 (Accurate Chemical, Westbury, NY), and leukocytes were magnetically removed using Dynal CELLection anti-mouse IgG-coated beads (Invitrogen). Type I cells were separated from the resulting cell suspension by treatment with rabbit anti-rat T1α (Sigma, KS-17) and magnetic removal using goat anti-rabbit IgG-coated beads (BioMax; Qiagen, Valencia, CA), leaving type II cells, which were identified using primary antibodies to pro-SP-C (Santa Cruz Biotechnology, Santa Cruz, CA). SP-C-positive cells were in excess of 90%. Experiments exposing freshly isolated type II cells seeded onto 96-well microplates were performed within 48 h of isolation.
Determination of S-nitrosothiol intracellular accumulation.
L2 cells were grown to confluence in 100× 20-mm polystyrene culture dishes at which time they were washed three times with HBSS buffered with 25 mM HEPES (HBSH, pH 7.4). The cells were then incubated in the presence of GSNO, CSNO, SNO-Cys-Gly, or DEA-NONOate ± transporter inhibitors/competitors (LAT: l-Leu, BCH; PEPT2: cefadroxil, Gly-Sar) at the indicated concentrations at 37°C for 15 min. After incubation, the cells were washed four times with ice-cold HBSH. Cells were then scraped into PBS containing 20 mM N-ethylmaleimide. Samples were then sonicated for 10 s each and centrifuged at 10,000 g for 10 min at 4°C in a microcentrifuge. Sulfanilamide (50 mg/ml) was added to sample supernatants to remove nitrite, and 200 μl of each sample was injected into a mixture of KI and I2 in glacial acetate in a reflux apparatus as previously used by us (15). Released NO gas reacted with ozone, detected by chemiluminescence using an NO analyzer (Sievers, Boulder, CO).
Standard curves were generated with known concentrations of GSNO freshly prepared before analysis. SNO content was determined as the HgCl2-labile NO signal, and duplicate measurements were obtained in each experiment for samples and standards.
Intracellular NO accumulation.
Incubations and buffers were all at 37°C, 5% CO2. Cells were plated in 96-well flat bottom plates (Costar) precoated with poly-d-lysine, cultured 48 h, loaded with 5 μM 4-amino-5-methylamino-2′7′-difluoro-fluorescein (DAF-FM) diacetate for 45 min, and rinsed by incubating in HBSS for 15 min. DAF-FM diacetate undergoes deesterification by cellular esterases to form DAF-FM, impermeant to cell membranes, which reacts with NO to yield a fluorescent product. Cells were then incubated in the presence of GSNO, CSNO, SNO-Cys-Gly, or DEA-NONOate ± transporter inhibitors/competitors as described above at the indicated concentrations for up to 30 min. Plates were read at 490 nm using a fluorescent microplate reader (Safire; Tecan, Salzburg, Austria). Mean fluorescence values from 4–8 wells/condition were normalized to the mean maximum fluorescence after subtracting background fluorescence in buffer-only control wells.
Statistical analysis.
Intergroup differences were compared by ANOVA, and significant differences were determined using Tukey's Highly Significant Difference as a post hoc test using KaleidaGraph V.4 (Synergy Software, Reading, PA).
RESULTS
LAT-1 and PEPT-2 expression in rat lung epithelial cells.
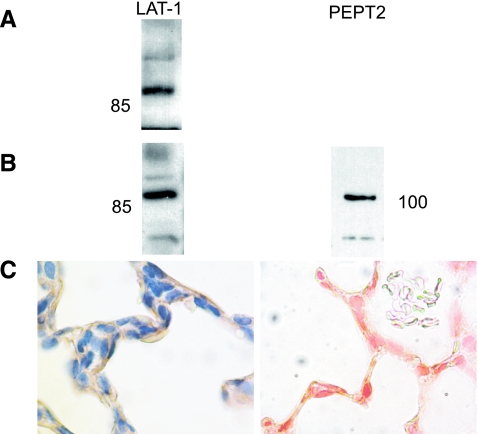
LAT-1 was detected in whole lung homogenate and L2 cell lysates by Western blot. PEPT2 was detected in L2 lysates by Western blot (previously shown to be expressed in whole lung) (7) and in L2 and type II alveolar epithelial cells by immunohistochemistry. LAT-1 expression in lung sections was apparent at the apical surfaces of cells lining alveoli, consistent with expression in both type I and type II epithelium (Fig. 1). No staining was seen in sections incubated with nonimmune serum.
Fig. 1.
L-type amino acid transporter 1 (LAT-1) and peptide transporter 2 (PEPT2) expression. A: LAT-1 expression, whole rat lung immunoblot, 50 μg protein/lane. B, left: LAT-1 expression in L2 cell lysates, 50 μg protein/lane. B, right: PEPT2 expression in L2 cell lysates, 40 μg protein/lane. C: LAT-1 (left) and PEPT2 (right) expression in rat lung.
SNO and NO uptake in alveolar epithelial cells treated with RSNO ± LAT competitors.
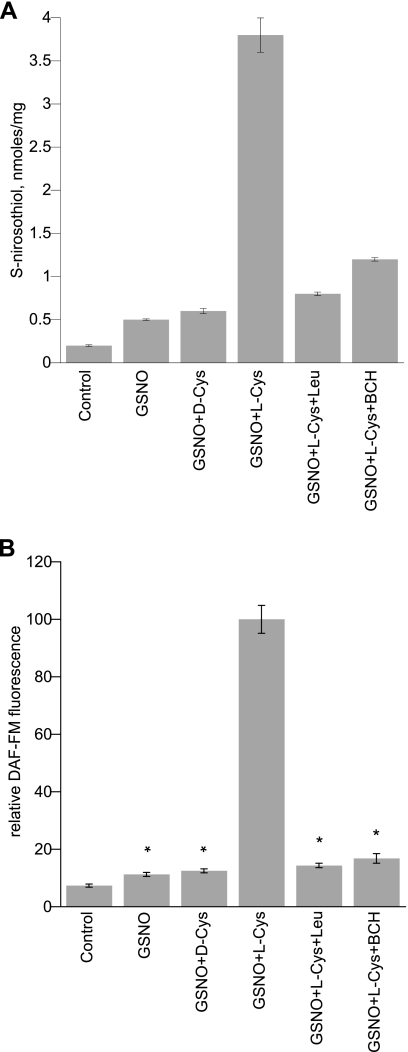
There were no significant effects on cell viability over the 30-min incubations. L2 cells treated with GSNO and l-Cys showed a significant increase in SNO signal analyzed by triiodide/chemiluminescence over cells treated with GSNO alone (Fig. 2). When coincubated with l-Leu or BCH, competitive inhibitors of LAT, intracellular SNO accumulation was significantly decreased (Fig. 2). Substitution of l-Cys with d-Cys eliminated the augmentation of intracellular SNO accumulation. Similarly, measurement of NO accumulation showed a parallel trend with DAF-FM fluorescence greatest in the GSNO + l-Cys-treated cells (Fig. 2). This effect was likewise inhibited by coincubation with LAT competitors l-Leu or BCH. Parallel results for NO accumulation were obtained in freshly isolated rat alveolar type II cells (Fig. 3). The pH of the media after addition of amino acids and inhibitors ranged between 6.9 and 7.2.
Fig. 2.
S-nitrosothiol (SNO)/nitric oxide (NO) uptake by L2 cells treated with RSNO ± LAT inhibition. A: SNO detected by triiodide reduction of NO in cell lysates via ozone chemiluminescence after incubation with 200 μM GSNO ± 50 μMd-Cys, 200 μM GSNO + 50 μMl-Cys, 200 μM GSNO + 50 μMl-Cys + 10 mMl-Leu, 200 μM GSNO + 50 μMl-Cys + 10 mM BCH (competitive inhibitors of LAT-1). Data are means ± SE representative of 3 experiments with samples analyzed in duplicate. B: DAF-FM fluorescence signal in L2 cells treated with RSNO ± LAT inhibition. Effect of 100 μMl-Cys on 200 μM GSNO induced intracellular DAF-FM signal ± 10 mM l-Leu or 10 mM BCH (competitive inhibitors of LAT-1). Values were normalized to the maximum signal. Data are means ± SE, n = 8/condition, *P < 0.001 vs. maximum.
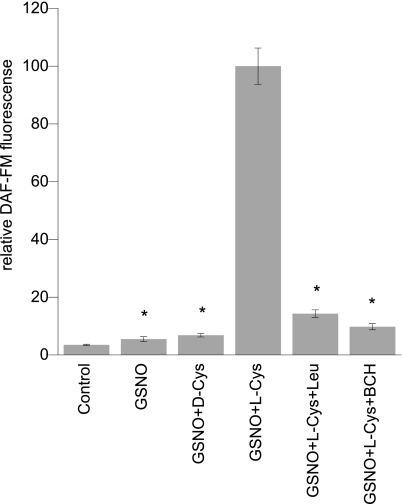
Fig. 3.
NO uptake in isolated rat type II cells treated with RSNO ± LAT inhibition. Effect of 100 μM l- and d-Cys on 200 μM GSNO induced intracellular DAF-FM signal. Conditions include 200 μM GSNO + 100 μM l-Cys + 10 mM l-Leu or 10 mM BCH (competitive inhibitors of LAT-1). Values normalized to maximum signal. Data are means ± SE, n = 8/condition, *P < 0.001 vs. maximum.
SNO and NO uptake in L2 cells treated with DEA NONOate.
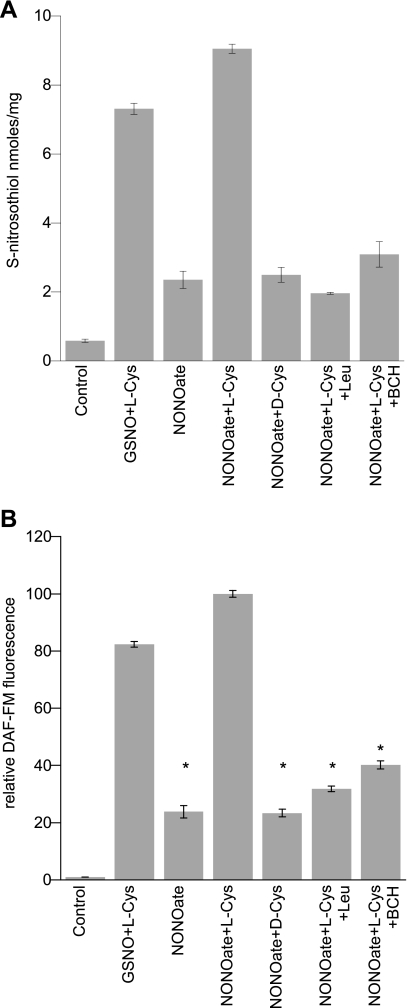
We incubated L2 cells with DEA NONOate alone or with l-Cys. We chose concentrations of DEA NONOate (50–100 μM) similar to GSNO. When l-Cys was added, the intracellular SNO signal increased indicating that DEA NONOate was able to donate NO groups to cysteine and utilize LAT for SNO entry into cells comparable to GSNO (Fig. 4A). This effect was blocked by coincubation with l-Leu indicating that SNO accumulation after exposure to 50 μM DEA NONOate required importation via LAT. We found parallel results for NO uptake (DAF-FM fluorescence), with augmentation by l-Cys but not d-Cys (Fig. 4B).
Fig. 4.
SNO/NO accumulation in L2 cells treated with DEA NONOate. A: SNO detected by ozone chemiluminescence after incubating with 100 μM GSNO + 100 μM l-Cys, 100 μM DEA NONOate ± 100 μM d- or l-Cys, and 100 μM DEA NONOate + 100 μM l-Cys ± LAT-1 competitors l-Leu and BCH (10 mM). Data are means ± SE, n = 6. B: DAF-FM signal in L2 cells treated with NONOate. L2 cells incubated with 100 μM GSNO + 100 μM l-Cys, 100 μM DEA NONOate ± 100 μM d-Cys, 100 μM DEA NONOate + 100 μM l-Cys ± LAT-1 competitors l-Leu and BCH (10 mM). Data are normalized to maximum signal. Data are means ± SE, n = 8/condition, *P < 0.001 vs. maximum.
SNO-dipeptide uptake via PEPT2 in alveolar epithelial cells.
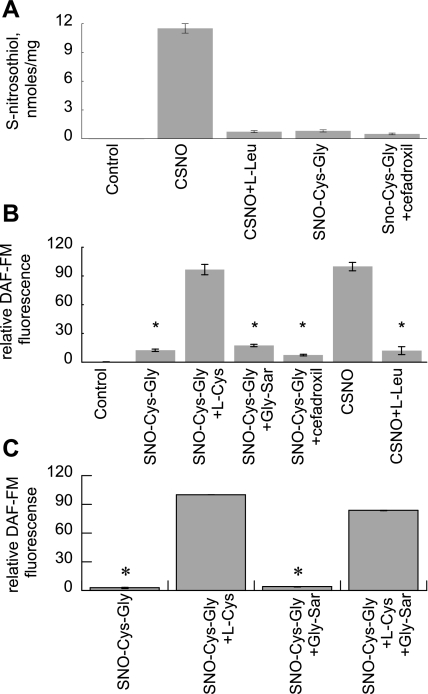
Since glutathione would be the most abundant thiol target for inhaled NO or NO donors to form SNOs in the airway lining fluid, and because glutathione is metabolized to Cys-Gly by dipeptidases in the lung (7), we sought to determine if uptake of SNO-Cys-Gly via PEPT2 could represent a significant route for SNO accumulation in pulmonary epithelium. Using SNO-Cys-Gly, we found minimal SNO accumulation or DAF-FM fluorescence, in contrast with cells incubated with CSNO (Fig. 5, A and B). Coincubation with LAT competitive inhibitor l-Leu significantly decreased the CSNO peak. Inhibition of PEPT2 with cefadroxil or competition with Gly-Sar had no significant effect on SNO or DAF-FM signal. We found parallel results for DAF-FM in primary type II cells (Fig. 5C).
Fig. 5.
PEPT2 mediated uptake of SNO/NO in alveolar epithelium. A: SNO in lysates from L2 cells treated with 200 μM SNO-Cys-Gly ± 100 μM l-Cys or 10 mM Gly-Sar. B: DAF-FM fluorescence in L2 cells treated with SNO-Cys-Gly ± Gly-Sar, with CSNO ± l-Leu for comparison. C: DAF-FM fluorescence in type II cells treated with SNO-Cys-Gly + Gly-Sar or l-Cys for comparison. Data are means ± SE, n = 3/condition, representative of 3 replicate experiments, *P < 0.001 vs. maximum.
DISCUSSION
SNO modification of protein function is increasingly recognized as central to many pulmonary biological responses to NO (6), but little is known about SNO trafficking in alveolar epithelium. This is important since it represents the site through which inhaled NO gains systemic entry. Inhaled NO has been approved by the U. S. Food and Drug Administration for treatment of persistent pulmonary hypertension in the newborn (12), but its benefits in other conditions have yet to be established. It has been assumed without rigorous testing that inhaled NO gas readily diffuses into pulmonary epithelium. Our studies aimed to establish whether or not amino acid/dipeptide transporters are important to the uptake of NO into alveolar epithelium to form SNO, since alveolar epithelium is the route of entry for inhaled NO and other NO-containing agents like GSNO (24) and ethyl nitrite (19). We found that both LAT-1 and PEPT2 are expressed in rat alveolar epithelium. Although PEPT2 expression in rat lung has been previously described (7), ours is the first report, to our knowledge, localizing LAT-1 to alveolar epithelium.
In our studies of transporter function, we found that maximum intracellular SNO levels were achieved in rat alveolar L2 epithelial cells by exposing them to extracellular NO sources in the presence of l-Cys but not d-Cys, with parallel results in the studies of NO accumulation (DAF-FM fluorescence), confirming stereoselectivity. We also found parallel results in freshly isolated type II cells. l-Cys augmented SNO and NO uptake were blocked by coincubation with l-Leu and BCH, indicating that uptake was taking place through LAT. This is consistent with earlier studies in other cell types (10, 14, 15, 28). We cannot distinguish whether alveolar epithelial uptake was predominantly through LAT-1 or LAT-2, but in other cell types LAT-2 demonstrates high specificity but low capacity for amino acid substrate transport (21, 22), so we suspect that most of the uptake occurred through LAT-1.
The RSNO/NO agents we used generated the highest intracellular SNO signal only when coincubated with l-Cys, which was inhibited by competition with LAT substrates l-Leu and BCH. This may have implications for NO-based inhalation treatments, since the presence of l-Cys would be required to achieve maximum epithelial intracellular SNO. Conditions in which l-Cys levels in airway lining fluid were substantially reduced would therefore be expected to alter uptake of SNOs generated in alveoli. We do not know the lower limit of l-Cys concentration in airway lining fluid that would be necessary to achieve CSNO levels sufficient to be biologically relevant. Although a few reports have described individual amino acid levels in bronchoalveolar lavage (9) or sputum (25), to our knowledge there are no reports describing l-Cys levels in the alveolar lining fluid in health and disease.
l-Cys in the alveolar lining fluid could be derived from glutathione. Glutathione or GSNO could be metabolized through γ-glutamyltranspeptidase to form Cys-Gly or SNO-Cys-Gly, respectively, and then by dipeptidases to yield l-Cys or CSNO. Both γ-glutamyltranspeptidase and membrane-bound dipeptidases capable of metabolizing Cys-Gly are known to be expressed in lung (8), although precise cellular localization has not been reported. However, we did not observe time-dependent increases in intracellular NO signal accumulation in L2 cells with the addition of 200 μM GSNO for up to 60 min (data not shown). Conceivably other cells, type I alveolar epithelium, bronchiolar epithelium, could metabolize GSNO and take up CSNO or SNO-Cys-Gly.
The mechanisms governing available l-Cys in airway lining fluid are complex and only partially understood. l-Cys levels in airway lining fluid are likely to be actively regulated by alveolar epithelial cells. Both system L (13) and system XAG amino acid transporters (11) have been shown to regulate l-Cys uptake in isolated type II alveolar epithelial cells. Sodium and chloride-dependent amino acid transport system ATB0+, which has broad amino acid specificity, including cysteine (11), has been described in type I epithelium, but neither its general function, nor its role in SNO uptake, if any, has been reported (23). Previous reports showed that CSNO uptake was sodium independent in endothelial and smooth muscle cells (14), but we did not directly test this in our studies of pulmonary epithelium.
In other cell types, cysteine uptake by the xCT amino acid transporter contributes to l-Cys export and SNO uptake through LAT (29). Type II cells have been shown to possess functional xCT transporters (13), but the magnitude of the contribution of xCT to l-Cys export (and thus SNO import) is unknown.
Decreased function of LAT could theoretically pose a barrier to NO uptake in alveolar epithelium, which could affect downstream signaling. To our knowledge, there are no reports of naturally occurring functional deficits in system l-amino acid transporters, and we speculate that absolute deficiency is likely to be lethal. Functional mutations in the y+LAT-1 system have been linked to lysinuric protein intolerance (26). Normal developmental regulation of LAT function has not been described to our knowledge.
Since glutathione is an abundant potential reservoir for SNO equivalents such as GSNO in alveolar lining fluid, we thought it was important to determine whether or not the GSNO metabolite SNO-Cys-Gly produced by γ-glutamyl transpeptidase could achieve significant intracellular SNO accumulation through an alternative path, namely uptake through PEPT2. GSNO metabolism by γ-glutamyl transpeptidase has been implicated in physiological roles of GSNO such as control of respiration (16). PEPT2 has been shown to be expressed in rat lung (7), and our studies have shown that it is present in isolated rat alveolar epithelial (L2 and type II) cells as well. Incubation with SNO-l-Cys-l-Gly did not produce significant accumulation of NO signal or SNO signal in L2 cells, unless coincubated with l-Cys. This was unaffected by coincubation with PEPT2 competitor Gly-Sar or with PEPT2 antagonist cefadroxil, confirming the importance of LAT to SNO uptake in type II alveolar epithelium. We did not test the role of PEPT2 in type I alveolar epithelium, which has by far the greater exposure to alveolar lining fluid and inhaled gases.
In summary, our findings lead us to conclude that NO uptake by type II alveolar epithelium takes place predominantly via LAT. NO gains entry via S-nitrosation of extracellular l-Cys, which is then transported through LAT-1 or LAT-2, rather than through metabolism of GSNO to SNO-Cys-Gly and uptake through PEPT2. This could have important implications for the use of NO or NO-based inhalation therapy in conditions where extracellular l-Cys is relatively deficient or where LAT function is decreased.
GRANTS
This work was supported in part by the Children's Miracle Network, the Jean and George Brumley Neonatal-Perinatal Research Institute (R. L. Auten), National Heart, Lung, and Blood Institute Grants HL-61377 and HL-42444 (A. R. Whorton), and the American Heart Association Beginning Grants-in-Aid 0665388U (S. Li) and 0565381U (T. J. McMahon).
Acknowledgments
We express gratitude to Dr. David E. Smith for the gift of anti-PEPT2 antiserum.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 176: 291–299, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L32–L40, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest 84: 727–735, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Dringen R, Hamprecht B, Broer S. The peptide transporter PepT2 mediates the uptake of the glutathione precursor CysGly in astroglia-rich primary cultures. J Neurochem 71: 388–393, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ, et al. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci USA 90: 10957–10961, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 173: 1186–1193, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groneberg DA, Nickolaus M, Springer J, Doring F, Daniel H, Fischer A. Localization of the peptide transporter PEPT2 in the lung: implications for pulmonary oligopeptide uptake. Am J Pathol 158: 707–714, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habib GM, Barrios R, Shi ZZ, Lieberman MW. Four distinct membrane-bound dipeptidase RNAs are differentially expressed and show discordant regulation with gamma-glutamyl transpeptidase. J Biol Chem 271: 16273–16280, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Hallman M, Merritt TA, Akino T, Bry K. Surfactant protein A, phosphatidylcholine, and surfactant inhibitors in epithelial lining fluid. Correlation with surface activity, severity of respiratory distress syndrome, and outcome in small premature infants. Am Rev Respir Dis 144: 1376–1384, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Hogg N, Broniowska KA, Novalija J, Kettenhofen NJ, Novalija E. Role of S-nitrosothiol transport in the cardioprotective effects of S-nitrosocysteine in rat hearts. Free Radic Biol Med 43: 1086–1094, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Jiang X, Ingbar DH, O'Grady SM. Selectivity properties of a Na-dependent amino acid cotransport system in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 279: L911–L915, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Kinsella JP Clinical trials of inhaled nitric oxide therapy in the newborn. Pediatr Rev 20: e110–e113, 1999. [PubMed] [Google Scholar]
- 13.Knickelbein RG, Seres T, Lam G, Johnston RB Jr, Warshaw JB. Characterization of multiple cysteine and cystine transporters in rat alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 273: L1147–L1155, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Whorton AR. Functional characterization of two S-nitroso-l-cysteine transporters, which mediate movement of NO equivalents into vascular cells. Am J Physiol Cell Physiol 292: C1263–C1271, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Whorton AR. Identification of stereoselective transporters for S-nitroso-l-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J Biol Chem 280: 20102–20110, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 413: 171–174, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Klaassen C. Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mRNA in rodents. Peptides 27: 850–857, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet 360: 141–143, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Moya MP, Gow AJ, McMahon TJ, Toone EJ, Cheifetz IM, Goldberg RN, Stamler JS. S-nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc Natl Acad Sci USA 98: 5792–5797, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem 274: 19738–19744, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn LC. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem 274: 34948–34954, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 274: 19745–19751, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Sloan JL, Grubb BR, Mager S. Expression of the amino acid transporter ATB 0+ in lung: possible role in luminal protein removal. Am J Physiol Lung Cell Mol Physiol 284: L39–L49, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med 165: 922–926, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SR, Ray A, Hodson ME, Pitt TL. Increased sputum amino acid concentrations and auxotrophy of Pseudomonas aeruginosa in severe cystic fibrosis lung disease. Thorax 55: 795–797, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrents D, Estevez R, Pineda M, Fernandez E, Lloberas J, Shi YB, Zorzano A, Palacin M. Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem 273: 32437–32445, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA 101: 7891–7896, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Hogg N. S-nitrosothiols: cellular formation and transport. Free Radic Biol Med 38: 831–838, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Li S, Marshall ZM, Whorton AR. A cystine-cysteine shuttle mediated by xCT facilitates cellular responses to S-nitrosoalbumin. Am J Physiol Cell Physiol 294: C1012–C1020, 2008. [DOI] [PubMed] [Google Scholar]