Abstract
Transforming growth factor (TGF)-β1 has been reported to cause endothelial cell apoptosis. However, conflicting data have also demonstrated that TGF-β1 promotes endothelial cell survival. In this study, the effect of TGF-β1 on apoptosis of cultured bovine pulmonary artery endothelial cells (PAEC) induced by multiple stimuli was investigated. TGF-β1 protected against apoptosis of bovine PAEC induced by serum deprivation or the VEGF receptor inhibitor SU-5416, but not by UV light exposure or TNFα. Neither caspase-8 nor caspase-12 was activated by serum deprivation or the VEGF receptor blocker. However, blockade of VEGF receptors activated caspase-9, an effect that was abolished by TGF-β1. Furthermore, serum deprivation and inhibition of VEGF receptors significantly decreased the protein level of Bcl-2, an effect that was also abrogated by TGF-β1. In addition, the baseline level of Bcl-2 was enhanced by TGF-β1 and reduced by inhibition of activin receptor-like kinase 5 (ALK5), a TGF-β1 type I receptor. Furthermore, inhibition of ALK5 caused apoptosis of bovine PAEC. These results suggest that TGF-β1 signaling is critical for maintenance of bovine PAEC survival. Finally, the protective effects of TGF-β1 on bovine PAEC apoptosis and Bcl-2 reduction were abolished by ALK5 inhibition, but not by inhibition of non-SMAD signaling pathways. Also, TGF-β1 activated SMAD2 and SMAD1/5, an effect that was abolished by ALK5 inhibition. The results of this study suggest that TGF-β1 protects against bovine PAEC apoptosis, possibly through ALK5-mediated Bcl-2 induction and subsequent inhibition of the mitochondria-mediated intrinsic pathway of apoptosis. Understanding the mechanism by which TGF-β1 promotes endothelial cell survival may provide a better treatment for apoptosis-dependent vascular diseases, such as emphysema.
Keywords: Bcl-2, pulmonary artery hypertension, SMAD
endothelial cells are exposed to multiple stresses because of their location at the interface between the blood and surrounding tissue. One pathological consequence of vascular stresses is the induction of endothelial cell apoptosis. Apoptosis occurs through two fundamental signaling pathways, the extrinsic and the intrinsic pathway. The extrinsic pathway is activated when the apoptotic stimulus acts through a death receptor. Caspase-8 is the major initiator caspase involved in the extrinsic pathway. The intrinsic pathway is triggered by internal apoptotic signals and involves mitochondria. Internal apoptotic signals promote mitochondrial outer membrane permeabilization, resulting in release of apoptosis-inducing proteins from mitochondria to the cytosol, with subsequent activation of caspase-9 (12). In addition, overwhelming endoplasmic reticulum (ER) stress causes caspase-12 activation (9). Activated caspases-8, -9, and -12 subsequently cleave and activate caspase-3 or other effector caspases, culminating in apoptosis (34, 50).
Mitochondria-mediated apoptosis is regulated by the balance of the activities of antiapoptotic B cell lymphoma-2 (Bcl-2) family members and proapoptotic Bcl-2 family members, including Bax and Bad (1, 12). Normally, proapoptotic Bcl-2 proteins are sequestered in the cytoplasm in an inactive state by antiapoptotic Bcl-2 proteins through nonspecific heterodimerization. Stress-induced increase in availability of proapoptotic Bcl-2 family proteins causes translocation of Bax and Bak to the mitochondrial outer membrane, which forms pores, which in turn allow release of apoptosis-inducing proteins to cytosol, and apoptosis occurs (12). Apoptosis mediated through the extrinsic pathway is inhibited by Fas-associated death domain-like inhibitory proteins (FLIPs) (39), a dominant-negative homolog of procaspase-8.
As a multiple functional cytokine, transforming growth factor (TGF)-β1 triggers numerous cellular responses through diverse receptors and intracellular signaling pathways (6, 7, 25, 33). There are three types of TGF-β1 receptors: type I (TβRI), type II (TβRII), and accessory type III (TβRIII). The only known function of TβRII is binding of TGF-β1 and phosphorylation and, thus, activation of TβRI (18). In endothelial cells, there are two isoforms of type I receptors, activin receptor-like kinase (ALK) 1 (ALK1) and ALK5 (18). Activation of ALK1 and ALK5 exhibits distinct effects via different intracellular signaling transducers (18). Activation of ALK1 causes SMAD1/5 activation, whereas activation of ALK5 triggers SMAD2/3 activation (18). The activated SMAD1/5 or SMAD2/3 binds to SMAD4. This SMAD protein complex then translocates to the nucleus and activates target gene expression through interaction with DNA-binding partners and other transcription factors and cofactors (6, 7, 25, 33). In addition, TGF-β1 activates multiple non-SMAD pathways, such as RhoA/Rho-activated kinase (ROCK), phosphatidylinositol-3-kinase (PI3K)/Akt, and mitogen-activated protein kinases, including extracellular signal-regulated protein kinases (ERK1/2), c-Jun NH2-terminal kinase (JNK), and p38 (16, 30). The roles of TGF-β1 in endothelial cell apoptosis are controversial. Several studies suggest that TGF-β1 causes endothelial cell apoptosis (29, 42, 46). Others demonstrate that TGF-β1 promotes endothelial cell survival (27, 35, 43) and protects against hypoxia-induced endothelial cell apoptosis (19). The seemingly controversial findings suggest that the role of TGF-β1 in endothelial cell apoptosis may be cell type specific. The effect of TGF-β1 on apoptosis of endothelial cells derived from pulmonary vascular beds is unknown.
Pulmonary vascular endothelial cells express and secrete abundant amounts of VEGF, an endothelial cell survival factor (44). Defective VEGF signaling, by chronic inhibition of VEGF receptors or genetic deletion of lung VEGF, has been shown to cause alveolar septal cell apoptosis (14, 38). In addition, serum deprivation, UV light exposure, and TNFα can cause endothelial cell apoptosis. In the present study, the effect of TGF-β1 was tested on main pulmonary artery endothelial cell (PAEC) apoptosis induced by distinct insults. TGF-β1 protected against main PAEC apoptosis induced by serum deprivation and blockade of VEGF receptors, but not by UV light exposure or TNFα. In addition, TGF-β1 prevented caspase-9 activation induced by blockade of VEGF receptors. Furthermore, TGF-β1 enhanced the protein level of Bcl-2, but not cFLIP. These findings suggest that TGF-β1 protects against mitochondria-mediated apoptosis, but not extrinsic pathway-mediated apoptosis, in main PAEC.
MATERIALS AND METHODS
Cell lines and reagents.
Bovine main PAEC (BPAEC) were isolated and characterized as previously described (4). Human PAEC (HPAEC), endothelial basal medium 2 (EBM2), and endothelial growth medium 2 (EGM2, which contains EBM2, hydrocortisone, human epidermal growth factor, VEGF, human fibroblast growth factor-B, R3-insulin-like growth factor I, FBS, ascorbic acid, and heparin) were purchased from Clonetics (Walkersville, MD). Endothelial cells at passages 3–7 were used in the present study. Recombinant human TGF-β1 was obtained from R & D Systems (Minneapolis, MN). SU-5416, wortmannin, LY-294002, and antibodies directed against actin and vinculin were purchased from Sigma (St. Louis, MO). TNFα, SB-431542, Y-27632, and SB-203580 were purchased from EMD Bioscience (La Jolla, CA), Tocris (Ellisville, MO), Calbiochem (San Diego, CA), and SmithKline Beecham Pharmaceuticals (Philadelphia, PA), respectively. U-0126, PD-98059, and antibodies directed against procaspase-3, cleaved caspase-3, Bax, SMAD5, phosphorylated SMAD1/5 (Ser463/465), and phosphorylated SMAD2 (Ser465/467) were obtained from Cell Signaling Technology (Beverly, MA). Antibodies directed against caspase-9, caspase-12, cFLIP, phosphorylated Bad (Ser136), and Bad were purchased from Stressgen (Ann Arbor, MI). Antibodies directed against Bcl-2, caspase-8, and GAPDH were purchased from BD Transduction Laboratories (San Jose, CA), Stratagene (La Jolla, CA), and Covance Research Products (Richmond, CA), respectively.
Gel electrophoresis and immunoblot analysis.
Lysates were solubilized in Laemmli buffer, and proteins were resolved by SDS-PAGE. The resolved proteins were then transferred to Immunobilon polyvinylidene difluoride membranes and immunoblotted with antibodies, as previously described (22).
Caspase-3 activity assay.
Cells were lysed in caspase lysis buffer [10 mM HEPES (pH 7.5), 40 mM β-glycerophosphate, 50 mM NaCl2, 2 mM MgCl2, and 5 mM EGTA] by freeze-thaw cycles, and caspase-3 activity was assessed and is presented as picomoles per milligram of protein per minute, as previously described (15).
Data analysis.
All experiments were performed at least in triplicate. Values are means ± SE. Differences among groups were determined by ANOVA and unpaired t-test. Differences among means were considered significant when P < 0.05.
RESULTS
TGF-β1 protects against endothelial cell apoptosis induced by serum deprivation or by blockade of VEGF receptors.
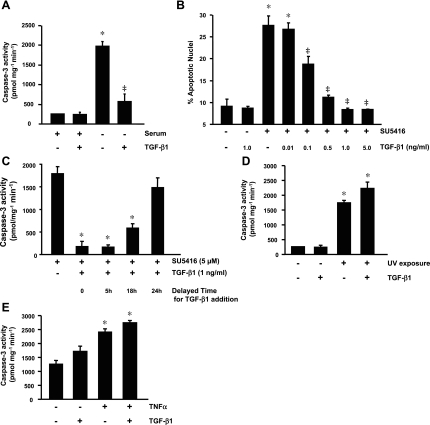
The well-known model of serum deprivation was used to induce endothelial cell apoptosis. As expected, BPAEC incubated with serum-free medium for 48 h displayed a significant increase in caspase-3 activity, an effect that was prevented by coincubation with TGF-β1 (Fig. 1A). These results suggest that TGF-β1 is protective against endothelial cell apoptosis induced by serum deprivation.
Fig. 1.
Effect of transforming growth factor (TGF)-β1 on endothelial cell apoptosis. A: caspase-3 activity in bovine pulmonary artery endothelial cells (BPAEC) incubated with minimal essential medium (MEM) with or without serum (10% FBS) in the absence or presence of TGF-β1 (1 ng/ml) for 48 h. *P < 0.001 vs. MEM + serum; ‡P < 0.001 vs. serum-free MEM without TGF-β1. B: BPAEC were incubated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β1 (0.01, 0.1, 0.5, 1.0, and 5.0 ng/ml) in MEM + 10% FBS for 48 h, and endothelial cell apoptosis was assessed by 4,6-diamidino-2-phenylindole staining of nuclei. *P < 0.001 vs. vehicle; ‡P < 0.001 vs. SU-5416 without TGF-β1. C: BPAEC were incubated with SU-5416 (5 μM) for 0, 5, 18, or 24 h before addition of TGF-β1 (1 ng/ml) and continued in culture in serum-containing MEM up to a total of 48 h. BPAEC incubated with SU-5416 alone without addition of TGF-β1 in the same medium for 48 h were used as a control. Cells were collected, and caspase-3 activity was assessed. *P < 0.001 vs. SU-5416 alone for 48 h. D: BPAEC were not exposed or exposed to UV light for 3 min and then incubated with vehicle or TGF-β (1 ng/ml) in MEM + 10% FBS for 18 h, and caspase-3 activity was assessed. *P < 0.001 vs. no UV exposure. E: caspase-3 activity in BPAEC treated with vehicle or TNFα (20 ng/ml) in the absence or presence of TGF-β1 (1 ng/ml) in HEPES buffer without CO2 for 18 h. *P < 0.001 vs. vehicle alone. Values are means ± SE from ≥3 independent experiments.
VEGF is a survival factor for endothelial cells (44). Inhibition of VEGF signaling by its receptor blocker SU-5416 causes alveolar septal cell apoptosis and emphysema (14). Thus the effect of SU-5416 on apoptosis was studied in cultured BPAEC. In BPAEC exposed to vehicle or SU-5416 in medium containing serum for 24, 48, and 72 h, 48 h of exposure to SU-5416 was necessary and sufficient to significantly increase caspase-3 activity (see supplemental Fig. 1a in the online version of this article). In addition, in BPAEC incubated with various concentrations of SU-5416 for 48 h in medium containing serum, SU-5416 dose dependently enhanced caspase-3 activity, with a maximal effect at 5 μM (see supplemental Fig. 1b). Caspases are activated through cleavage of procaspases. Thus the effect of blockade of VEGF receptors with SU-5416 on caspase-3 activation was also evaluated by assessment of procaspase-3 cleavage (see supplemental Fig. 1c). Similar to the serum deprivation model, the effect of VEGF receptor blockade on caspase-3 activation was completely prevented by addition of TGF-β1 (see supplemental Fig. 1). Cells expressing activated caspase-3 are likely undergoing apoptosis. To further confirm the protective role of TGF-β1 against endothelial cell apoptosis during VEGF receptor blockade, endothelial cell apoptosis was assessed by visualization of nuclear morphology. TGF-β1 dose dependently prevented SU-5416-induced endothelial cell apoptosis, with a maximal effect at 1 ng/ml (Fig. 1B). To further evaluate the protective effect of TGF-β1 on endothelial cell apoptosis induced by blockade of VEGF receptors, BPAEC were incubated with SU-5416 alone for 0, 5, 18, or 24 h before addition of TGF-β1 and continued in culture up to a total of 48 h. BPAEC incubated with SU-5416 alone for 48 h were used as a control. A protective effect against caspase-3 activation was observed when addition of TGF-β1 was delayed for up to 18 h (Fig. 1C), suggesting that TGF-β1 can rescue BPAEC from progression of apoptosis.
To determine whether the protective effect of TGF-β1 against endothelial cell apoptosis is universal, the effects of TGF-β1 on apoptosis caused by UV radiation and TNFα were tested. As expected, in BPAEC exposed to UV light for 3 min and then incubated with medium containing serum for 18 h, caspase-3 activity was significantly increased (Fig. 1D). In contrast to the effect on the models of serum deprivation and VEGF receptor blockade, addition of TGF-β1 did not prevent caspase-3 activation induced by UV exposure (Fig. 1D). Because TNFα concomitantly activates the apoptosis and the antiapoptosis pathway, such as NF-κB and activator protein 1, TNFα does not cause cell apoptosis under normal culture conditions (37, 45). Kramer et al. (15) showed that TNFα caused BPAEC apoptosis in HEPES buffer at 37°C without CO2 (15); therefore, this established condition was used for the present study. Similar to the effect during UV light exposure, TGF-β1 failed to abrogate TNFα-induced caspase-3 activation (Fig. 1E). These findings indicate that the protective effect of TGF-β1 against endothelial cell apoptosis is dependent on the stimulus.
Whether TGF-β1 plays a similar role in HPAEC was also investigated. Apoptosis was not significantly increased in HPAEC exposed to 0.1–10 μM SU-5416 in EGM2 for up to 72 h compared with vehicle-treated cells (data not shown). In addition, serum deprivation alone for up to 72 h did not cause apoptosis in HPAEC cultured in EGM2 without serum (data not shown). However, apoptosis occurred at 24 h when HPAEC were cultured in EBM2, an effect that was not rescued by addition of various concentrations of TGF-β1 (see supplemental Fig. 2).
Fig. 2.
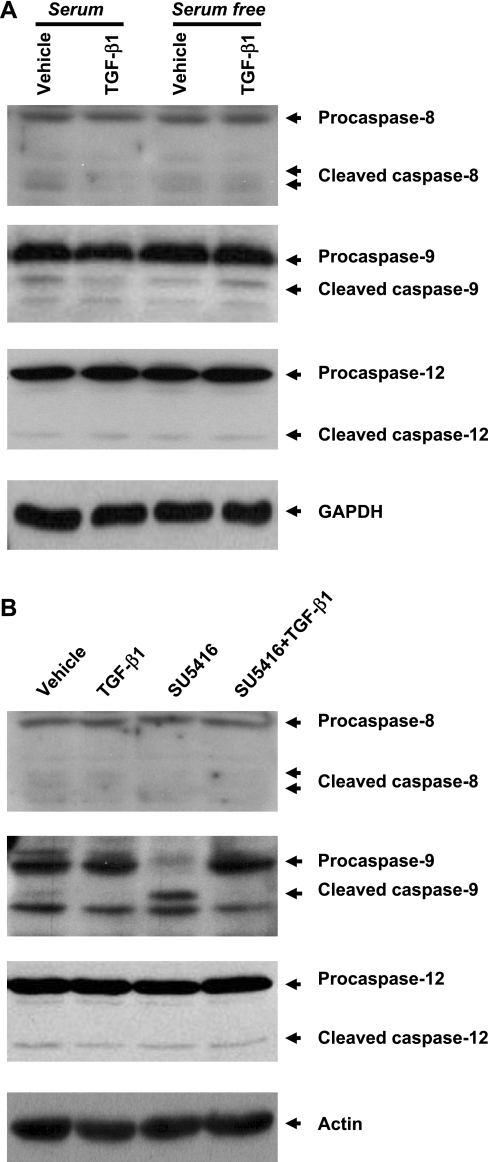
Effects of TGF-β1 on cleavage of initial caspases in BPAEC subjected to serum deprivation and blockade of VEGF receptors. A: BPAEC were incubated with MEM with or without serum (10% FBS) in the absence or presence of TGF-β1 (1 ng/ml) for 48 h. B: BPAEC were incubated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β (1 ng/ml) in MEM + 10% FBS for 48 h. Cleavage of procaspases-8, -9, and -12 was assessed in equivalent amounts of lysate by immunoblot analysis using antibodies directed against pro- and cleaved caspases-8, -9, and -12, respectively. Expression levels of GAPDH and actin were used as controls for protein loading. Blots represent results from 3 independent experiments.
TGF-β1 prevents caspase-9 activation induced by blockade of VEGF receptors.
Apoptosis occurs through several pathways, including extrinsic, intrinsic, and ER stress pathways mediated through activation of caspases-8, -9, and -12, respectively. To elucidate the mechanism underlying TGF-β1 protection against endothelial cell apoptosis, the cleavage of these procaspases was examined. Cleavage of these procaspases was not increased by serum deprivation or coincubation with TGF-β1 (Fig. 2A). However, a significant increase in cleavage of procaspase-9, but not procaspase-8 or -12, was observed on blockade of VEGF receptors (Fig. 2B). The effect of VEGF receptor blockade on caspase-9 activation was abolished by coincubation with TGF-β1 (Fig. 2B). These results suggest that TGF-β1 plays a protective role in the mitochondria-mediated intrinsic pathway of apoptosis.
TGF-β1 modulation of Bcl-2.
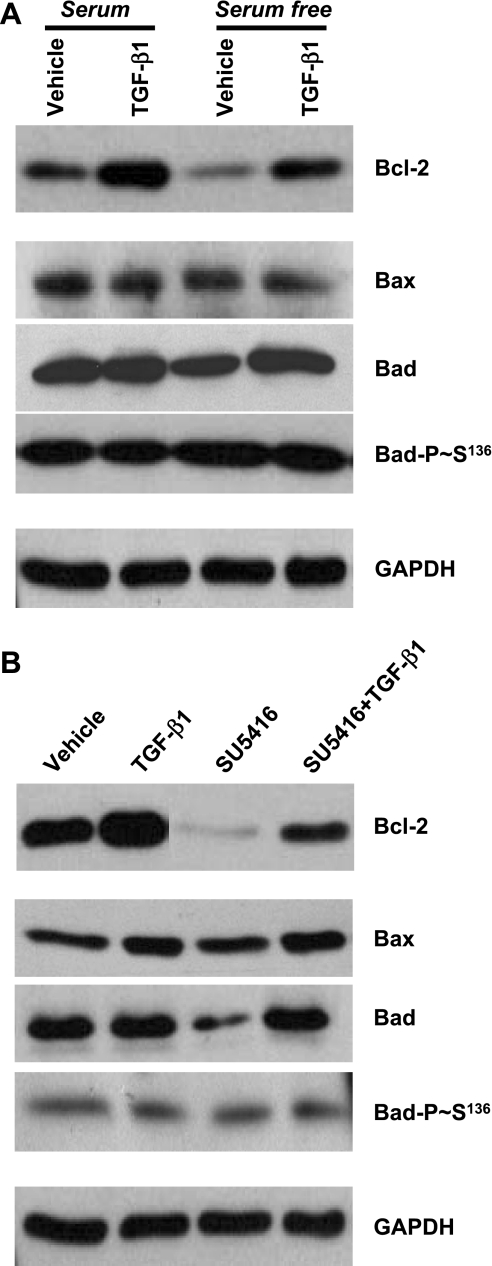
Mitochondria-mediated apoptosis is regulated by the balance of pro- and antiapoptotic Bcl-2 family members. Next, the effect of TGF-β1 on protein expression and posttranslational modification of these mitochondria-associated Bcl-2 family members was examined in cultured BPAEC subjected to serum deprivation or blockade of VEGF receptors. TGF-β1 dramatically increased the protein level of antiapoptotic Bcl-2 in endothelial cells (Fig. 3). Serum deprivation and blockade of VEGF receptors significantly diminished the baseline levels of Bcl-2, effects that were prevented by coincubation with TGF-β1 (Fig. 3). These results suggest that the protective effect of TGF-β1 on endothelial cell apoptosis may act via induction of Bcl-2.
Fig. 3.
Effects of TGF-β1 on protein expression and phosphorylation of Bcl-2 family members in BPAEC subjected to serum deprivation and blockade of VEGF receptors. A: BPAEC were incubated with MEM with or without serum (10% FBS) in the absence or presence of TGF-β1 (1 ng/ml) for 48 h. B: BPAEC were incubated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β (1 ng/ml) in MEM + 10% FBS for 48 h. Protein levels of Bcl-2, Bax, Bad, and phosphorylated Bad (Bad-P-Ser136) were assessed by immunoblot analysis using respective antibodies. Expression levels of GAPDH were used as controls for protein loading. Blots represent results from 3 independent experiments.
Next, the effect of TGF-β1 on proapoptotic Bcl-2 family members was investigated. The protein level of Bax was not altered by serum deprivation, blockade of VEGF receptors, or TGF-β1 (Fig. 3). However, Bad protein level was reduced by blockade of VEGF receptors, an effect that was prevented by coincubation with TGF-β1 (Fig. 3). Furthermore, Bad phosphorylation at Ser136 was not changed by serum deprivation, blockade of VEGF receptors, or TGF-β1 (Fig. 3).
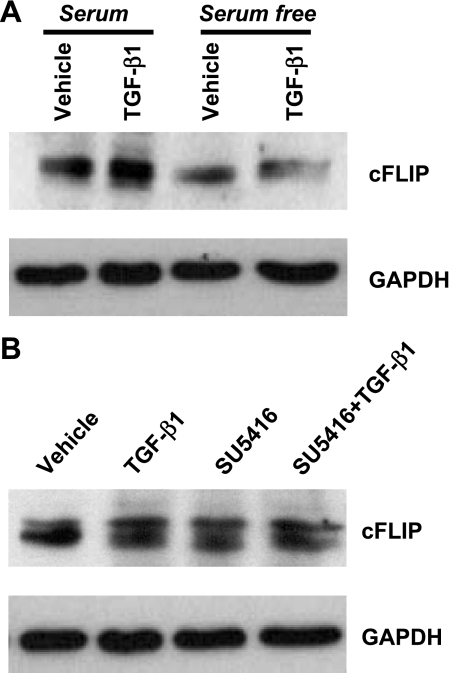
TGF-β1 does not alter protein level of cFLIP.
In human umbilical vein endothelial cells, serum deprivation decreased protein content of the caspase-8 inhibitor cFLIP, an effect prevented by VEGF (36). To determine whether cFLIP participated in TGF-β1 protection against endothelial cell apoptosis, cFLIP expression level was assessed. Consistent with previous findings (36), serum deprivation induced a significant decrease in cFLIP protein content in BPAEC (Fig. 4A). However, inhibition of VEGF signaling with SU-5416 did not alter cFLIP expression (Fig. 4B). In addition, TGF-β1 did not alter cFLIP expression in the presence or absence of serum (Fig. 4A), which is consistent with the finding that TGF-β1 does not alter caspase-8 activity (Fig. 2). These results suggest that TGF-β1 does not protect against caspase-8-mediated endothelial cell apoptosis.
Fig. 4.
Effects of TGF-β1 on protein expression of Fas-associated death domain-like inhibitory proteins (cFLIP) in BPAEC subjected to serum deprivation and blockade of VEGF receptors. A: BPAEC were incubated with MEM with or without serum (10% FBS) in the absence or presence of TGF-β1 (1 ng/ml) for 48 h. B: BPAEC were incubated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β (1 ng/ml) in MEM + 10% FBS for 48 h. Protein levels of cFLIP were assessed by immunoblot analysis, with GAPDH used as a control for protein loading. Blots represent results from 3 independent experiments.
ALK5 activity is required for maintenance of endothelial cell survival and for TGF-β1 protection against endothelial cell apoptosis.
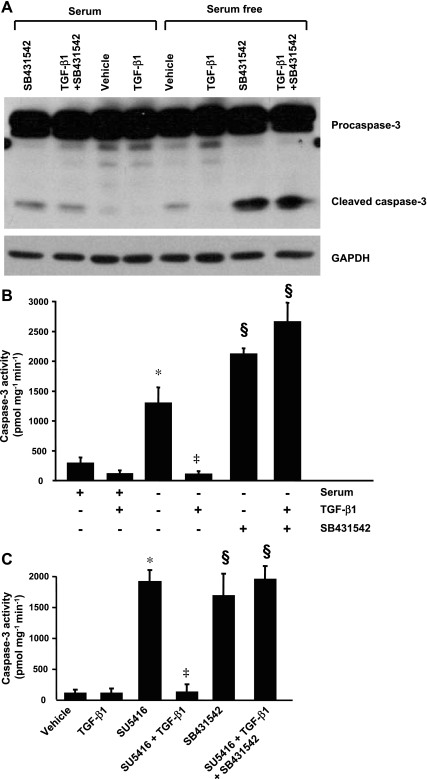
To determine which TGF-β1 receptors mediate TGF-β1 protection against endothelial cell apoptosis, ALK5 activity was chemically inhibited with SB-431542. In BPAEC incubated ith SB-431542 alone or in combination with TGF-β1 in serum-free medium, inhibition of ALK5 abrogated the protective effect of TGF-β1 on cleavage and activity of caspase-3 induced by serum deprivation (Fig. 5, A and B). Also, SB-431542 alone exacerbated the procaspase-3 cleavage and activation induced by serum deprivation (Fig. 5, A and B). Similarly, inhibition of ALK5 not only abolished the protective effect of TGF-β1 against VEGF inhibition-induced caspase-3 activation (Fig. 5C), but it also activated caspase-3 in endothelial cells incubated with serum-containing medium (Fig. 5, A and C). Taken together, these findings suggest that ALK5 is critical for maintenance of endothelial cell survival and TGF-β1 protection against endothelial cell apoptosis.
Fig. 5.
Effect of inhibition of activin receptor-like kinase (ALK) 5 (ALK5) on TGF-β1 protection against endothelial cell apoptosis. A and B: BPAEC were incubated with or without TGF-β1 (1 ng/ml) in the absence or presence of the ALK5 inhibitor SB-431542 (10 μM) in MEM with or without serum (10% FBS) for 48 h. A: procaspase-3 cleavage. Blot represents results from 3 independent experiments. B: caspase-3 activity. Values are means ± SE from 3 independent experiments. *P < 0.001 vs. MEM + 10% FBS; ‡P < 0.001 vs. serum-free medium in the absence of TGF-β1; §P < 0.001 vs. serum-free medium in the absence and presence of TGF-β1. C: caspase-3 activity in BPAEC treated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β (1 ng/ml) or incubated with SB-431542 (10 μM) or a combination of SU-5416, TGF-β1, and SB-431542 in MEM + 10% FBS for 48 h. Values are means ± SE from 3 independent experiments. *P < 0.001 vs. vehicle; ‡P < 0.001 vs. SU-5416; §P < 0.001 vs. vehicle or SU-5416 + TGF-β1.
ALK5 activity is necessary for baseline and TGF-β1-induced expression of Bcl-2 and Bad.
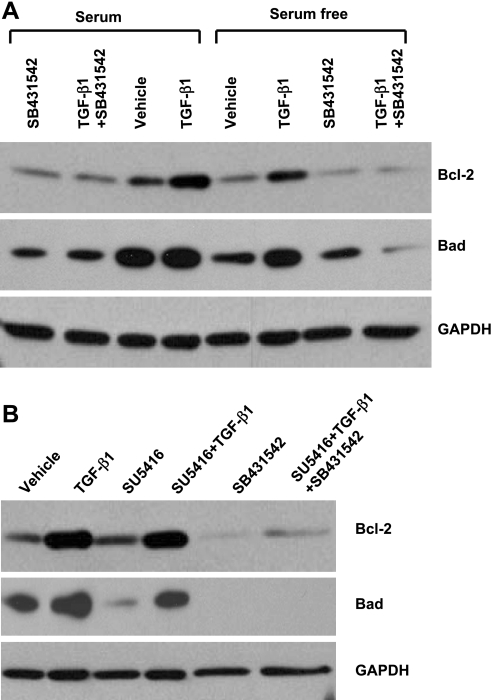
To further determine whether ALK5 activation is crucial for TGF-β1 induction of Bcl-2 and Bad, BPAEC subjected to serum deprivation or inhibition of VEGF receptors were incubated with SB-431542 alone or in combination with TGF-β1. Inhibition of ALK5 abolished the effect of TGF-β1 on induction of Bcl-2 and Bad (Fig. 6). Also, inhibition of ALK5 alone decreased baseline protein levels of Bcl-2 and Bad in cells incubated in serum-containing medium (Fig. 6). ALK5 inhibition had no effect on cFLIP protein expression and Bad phosphorylation at Ser136 in BPAEC subjected to serum deprivation or blockade of VEGF receptors (data not shown). These findings suggest that ALK5 activity is necessary for baseline and TGF-β1-induced protein expression of Bcl-2 and Bad.
Fig. 6.
Effects of ALK5 inhibition on protein expression of Bcl-2 and Bad in BPAEC subjected to serum deprivation and blockade of VEGF receptors. A: BPAEC were incubated with or without TGF-β1 (1 ng/ml) in the absence or presence of SB-431542 (10 μM) in MEM with or without 10% FBS for 48 h. B: BPAEC were incubated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β (1 ng/ml), SB-431542 (10 μM), or a combination of SU-5416, TGF-β1, and SB431542 in MEM + 10% FBS for 48 h, and protein levels of Bcl-2 and Bad were assessed by immunoblot analysis, with GAPDH used as a control for protein loading. Blots represent results from 3 independent experiments.
Non-SMAD-signaling pathways are not involved in TGF-β1 protection against endothelial cell apoptosis.
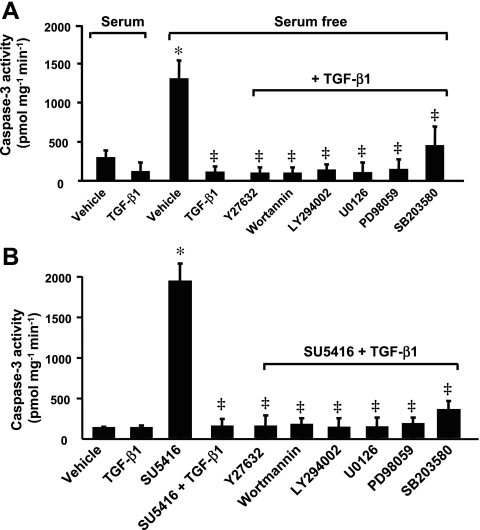
TGF-β1 activates multiple non-SMAD-signaling pathways. To explore whether TGF-β1 is mediating its effects through a non-SMAD pathway, chemical inhibitors were used to assess the requirements of RhoA/ROCK, PI3K/Akt, ERK1/2, and p38 for TGF-β1 protection against endothelial cell apoptosis. ROCK was inhibited by Y-27632, PI3K activity by the irreversible chemical inhibitor wortmannin and a structurally unrelated inhibitor LY-294002, ERK1/2 by U-0126 and PD-98059, and p38 by SB-203580. In BPAEC incubated with TGF-β1 in the absence or presence of these inhibitors in serum-free medium, none of these inhibitors altered the protective effect of TGF-β1 on caspase-3 activation induced by serum deprivation (Fig. 7A). In BPAEC incubated with inhibitor in serum-free medium, caspase-3 activity was similar to that in cells subjected to serum deprivation alone (data not shown). Although inhibition of RhoA/ROCK, PI3K/Akt, ERK1/2, or p38 slightly increased caspase-3 activity compared with vehicle (serum-containing medium; data not shown), none of these inhibitors effectively blunted the protective effect of TGF-β1 on caspase-3 activation induced by blockade of VEGF receptors (Fig. 7B). These results suggest that these non-SMAD pathways are not involved in TGF-β1 protection against endothelial cell apoptosis.
Fig. 7.
Effects of non-SMAD pathways on TGF-β1 protection against endothelial cell apoptosis. A: caspase-3 activity in BPAEC incubated with or without TGF-β1 (1 ng/ml) in the absence or presence of Y-27632 (10 μM), wortmannin (500 nM), LY-294002 (10 μM), U-0126 (10 μM), PD-98059 (10 μM), or SB-203580 (5 μM) in serum-free medium for 48 h. Cells incubated with MEM + 10% FBS in the absence or presence of TGF-β1 (1 ng/ml) for 48 h were used as controls. *P < 0.001 vs. MEM containing serum; ‡P < 0.001 vs. serum-free MEM without TGF-β1. B: caspase-3 activity in BPAEC treated with vehicle or SU-5416 (5 μM) in the absence or presence of TGF-β1 (1 ng/ml) alone or TGF-β1 in combination with either inhibitor for 48 h. *P < 0.001 vs. vehicle; ‡P < 0.001 vs. SU-5416 alone. Values are means ± SE from 3 independent experiments.
TGF-β1 activated transcription factors, SMAD2 and SMAD1/5.
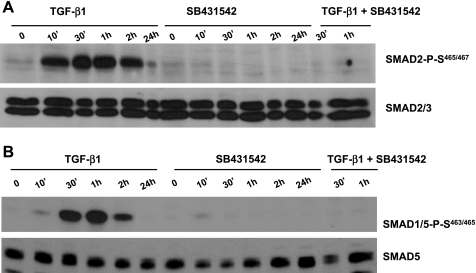
To investigate the potential roles of SMAD pathways in TGF-β1 protection against endothelial cell apoptosis, BPAEC were exposed to vehicle, TGF-β1, SB-431542, or TGF-β1 + SB-431542 for various times in serum-containing medium, and activation of SMAD2 and SMAD1/5 was measured by examination of phosphorylation of SMAD2 at Ser465/467 and phosphorylation of SMAD1/5 at Ser463/465, respectively. A rapid activation of SMAD2 and SMAD1/5 was noted in cells exposed to TGF-β1 (Fig. 8). SMAD2/3 are the downstream transcription factors from ALK5. As expected, ALK5 inhibition completely abolished TGF-β1-induced SMAD2 activation (Fig. 8A). ALK5 inhibition also prevented TGF-β1-induced SMAD1/5 activation (Fig. 8B). This result is consistent with a previous report that ALK5 activity is important for TGF-β1-induced ALK1 activation (8).
Fig. 8.
Effects of TGF-β1 on activation of SMAD2 and SMAD1/5. BPAEC were exposed to TGF-β1 (1 ng/ml), SB-431542 (10 μM), or TGF-β1 (1 ng/ml) + SB-431542 (10 μM) in serum-containing MEM for 0–24 h, and activation of SMAD2 and SMAD1/5 was assessed by measurement of phosphorylation of SMAD2 at Ser465/467 and SMAD1/5 at Ser463/465, respectively. Expression of SMAD2/3 and SMAD5 was also examined in the same blot. Blots represent results from 2 independent experiments.
DISCUSSION
TGF-β1 has been reported to cause endothelial cell apoptosis or promote endothelial cell survival, depending on cells studied. In the present study, cultured BPAEC were used to demonstrate that TGF-β1 protected against endothelial cell apoptosis induced by serum deprivation and blockade of VEGF receptors, but not by UV light exposure or TNFα. Furthermore, ALK5, but not non-SMAD pathways, is critical for TGF-β1 protection against endothelial cell apoptosis. In addition, TGF-β1 enhanced Bcl-2 expression and prevented caspase-9 activation induced by blockade of VEGF receptors through ALK5. TGF-β1 signaling did not alter cFLIP protein level. Finally, TGF-β1 was involved in activation of SMAD2 and SMAD1/5, which was dependent on ALK5. These results suggest that ALK5-dependent Bcl-2 induction, possibly through SMAD2 and SMAD1/5, may be a mechanism underlying TGF-β1 protection against endothelial cell apoptosis.
Apoptosis occurs through the caspase-8-dependent extrinsic pathway, the caspase-9-dependent intrinsic pathway, and the caspase-12-dependent ER stress pathway. Neither caspase-8 nor caspase-12 was activated by serum deprivation or blockade of VEGF receptors, suggesting that serum deprivation and blockade of VEGF receptors do not activate the extrinsic and ER stress pathways. Blockade of VEGF receptors significantly increased caspase-9 activation, an effect that was prevented by TGF-β1, suggesting that TGF-β1 protects against apoptosis mediated through mitochondrial dysfunction. This notion was further supported by the observations that TGF-β1 prevented reduction of Bcl-2 induced by serum deprivation and blockade of VEGF receptors. UV light exposure has been shown to cause apoptosis through activation of the intrinsic pathway (28), as well as activation of the CD-95/caspase-8-mediated extrinsic pathway (31). TNFα-induced apoptosis acts through TNFα receptor 1/caspase-8 activation (10). In addition, Lu et al. (24) showed that chemical inhibition of isoprenylcysteine-o-carboxyl methyltransferase by AGGC caused endothelial cell apoptosis through ER stress-induced dysfunction of unfolded protein response. AGGC-induced endothelial cell apoptosis was not rescued by TGF-β1 (data not shown). In the present study, TGF-β1 protected against endothelial cell apoptosis induced by serum deprivation and VEGF receptor blockade, but not by UV light, TNFα, or AGGC, which demonstrates that TGF-β1 protects against endothelial cell apoptosis mediated through the mitochondria-dependent intrinsic pathway, but not the extrinsic or the ER stress-mediated pathway. The conclusion that TGF-β1 does not protect against extrinsic pathway-mediated apoptosis is also supported by the observation that TGF-β1 did not alter cFLIP protein level.
Serum deprivation has been reported to decrease cFLIP expression in endothelial cells (36). Consistently, cFLIP expression was diminished in BPAEC exposed to serum deprivation. However, serum deprivation did not increase caspase-8 activation, suggesting that cFLIP may not inhibit caspase-8 activity in PAEC. In fact, the role of cFLIP in regulation of caspase-8 activation and apoptosis is controversial, with some investigators reporting that cFLIPs act as death activators and others reporting that they inhibit apoptosis (36). These seemingly contradictory results may be due to multiple FLIP isoforms from alternative splicing of a single gene (36). It is also possible that other endothelial cell mechanisms modulate caspase-8 activity. It has been shown that inhibitors of apoptotic proteins inhibit caspase activation by direct binding (10). Perhaps inhibitors of apoptotic proteins prevent activation of the extrinsic pathway, even though cFLIP was reduced by serum deprivation.
The balance between pro- and antiapoptotic Bcl-2 family proteins determines the fate of cells. In the present study, TGF-β1 not only prevented reduction of Bcl-2 induced by serum deprivation and blockade of VEGF receptors, but it also enhanced the baseline level of Bcl-2. In addition, inhibition of ALK5 significantly suppressed Bcl-2 protein level and caused endothelial cell apoptosis. These findings suggest that induction of Bcl-2 mediated through TGF-β1/ALK5 signaling is critical for maintenance of endothelial cell survival and prevention of endothelial cell apoptosis. Decease in proapoptotic Bcl-2 proteins may also contribute to cell survival. In the present study, the protein content of Bax, a proapoptotic Bcl-2 protein, was not altered by serum deprivation, blockade of VEGF receptors, or TGF-β1, suggesting that Bax does not play a role in TGF-β1 prevention against endothelial cell apoptosis. It has been shown that Bad phosphorylation at Ser136 causes inactivation of the protein, contributing to cell survival. TGF-β1 did not alter Bad phosphorylation at Ser136, suggesting that TGF-β1 protection against apoptosis does not occur via Bad phosphorylation. The effect of Bad on apoptosis is dependent on cell type (3). In the present study, blockade of VEGF receptors reduced Bad protein level concomitantly with occurrence of apoptosis. These results suggest that enhanced Bad protein level is not associated with occurrence of apoptosis in PAEC.
TGF-β1 initiates numerous cellular responses through SMAD and non-SMAD pathways (7). In the present study, non-SMAD pathways, including RhoA/ROCK, PI3K/Akt, ERK1/2, and p38, are not involved in TGF-β1 protection against endothelial cell apoptosis. Lu et al. (23) showed that TGF-β1 causes SMAD2 activation in PAEC. It is known that SMAD2/3 are activated via ALK5 (18). The results of the present study confirmed the ALK5-dependent activation of SMAD2 by TGF-β1. In addition, TGF-β1-induced SMAD1/5 activation was also dependent on ALK5. A recent study showed that SMAD3/4 are recruited to the Bcl-2 promoter (21), suggesting that SMAD3/4 may control Bcl-2 transcription. The results of the present study demonstrate that ALK5 is critical for maintenance of endothelial cell survival and TGF-β1 protection of endothelial cells from apoptosis. Thus, perhaps ALK5-dependent activation of SMAD2 and SMAD1/5 mediates the protective effect of TGF-β1 against PAEC apoptosis through induction of Bcl-2. Others have reported that ALK5/SMAD2/3 activation is implicated in TGF-β1-induced apoptosis of bone marrow-derived peritubular capillary endothelial cells (20). Heterogeneity of endothelial cells from different vascular beds may explain the difference between the results of the present study and those of Li et al. (20).
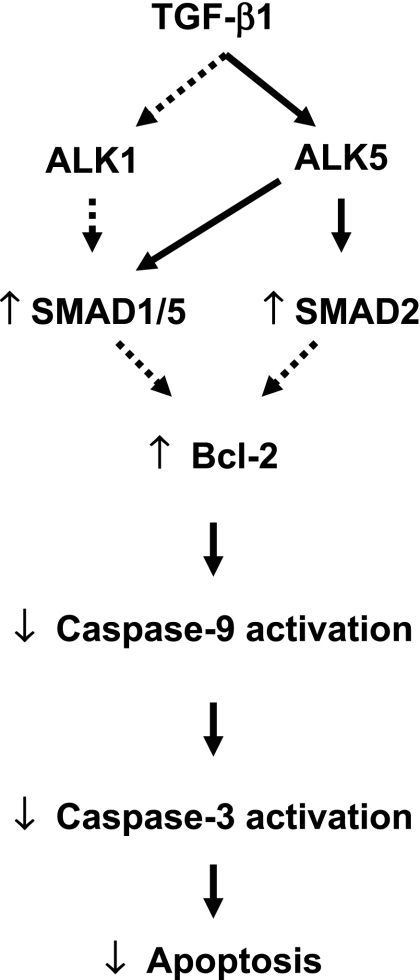
Taken together, the results of the present study demonstrate that TGF-β1 protects against BPAEC apoptosis induced by serum deprivation and blockade of VEGF receptors through inhibition of the mitochondria-dependent intrinsic pathway via ALK5 (Fig. 9). Since endothelial cell apoptosis is important in the pathogenesis of emphysema, these results suggest that strategies to increase TGF-β1 signaling via ALK5 may be protective against the development of vascular injuries characterized by apoptosis, such as emphysema.
Fig. 9.
Schematic representation of intracellular signaling events involved in TGF-β1 protection against endothelial cell apoptosis. Solid lines, defined pathways; dashed lines, speculative pathways.
In the present study, it was not possible to duplicate the models of apoptosis using cultured HPAEC. Neither serum deprivation nor the VEGF receptor blocker SU-5416 caused apoptosis when HPAEC were cultured in EGM2, as recommended by the manufacturer. Apoptosis occurred at 24 h when HPAEC were cultured in EBM2, an effect that was not rescued by addition of various concentrations of TGF-β1. The results of the present study suggest that TGF-β1 has the capability to protect apoptosis mediated through the intrinsic pathway, but not the extrinsic pathway and the ER stress pathway. Incubation of HPAEC with EBM2 may activate multiple apoptosis pathways as a result of deprivation of serum and various growth factors. Thus it is not surprising that TGF-β1 did not protect HPAEC against EBM2-induced apoptosis. Therefore, a better system is needed to demonstrate whether the data in the bovine system could apply to the human cells.
Abnormal TGF-β signaling has been linked to the pathogenesis of pulmonary artery hypertension (PAH). For example, germline mutations of the bone morphogenetic protein receptor II have been identified in patients with familial and sporadic PAH (5, 17, 40). Mutations in genes encoding ALK1 and endoglin have also been found in PAH patients with hereditary hemorrhagic telangiectasia (11, 41). Recent studies have revealed somatic mutations of TβRII in endothelial cells within plexiform lesions of patients with PAH (47). Furthermore, ALK5/SMAD2/3 signaling is impaired in monocrotaline (MCT)-induced PAH in rats (49). In addition, endothelial cells within plexiform lesions of human patients with PAH lack expression and activation of the key components (TβRI, TβRII, SMAD1/5, SMAD2/3, and SMAD4) of TGF-β1 signaling (32, 47). These findings suggest that impairment of TGF-β1 signaling may contribute to plexiform lesion formation and PAH. In contrast, elevated levels of TGF-β1 have been reported to be associated with PAH (2, 13, 26). More recently, Zaiman and colleagues (48) reported that ALK5/SMAD2 signaling is activated in pulmonary vascular cells, which correlated with enhanced pulmonary vascular cell apoptosis, in MCT-induced PAH in rats. They also demonstrated a partial reversal/small improvement of several hemodynamic parameters and remodeling in these PAH rats on administration of ALK5 inhibitor (48). These results suggest that activation of ALK5/SMAD2 signaling may cause vascular endothelial cell apoptosis, leading to PAH. TGF-β1 has been demonstrated to cause lung microvascular endothelial cell apoptosis (unpublished observations), an effect that was prevented by ALK5 inhibition. However, in the present study, TGF-β1 protected against apoptosis of main PAEC via ALK5. The heterogeneous responses to TGF-β1 signaling in endothelial cells from the conduit pulmonary arteries and the lung microcirculation may explain why inhibition of ALK5 was not as effective in reversing MCT-induced PAH. ALK5 inhibition-induced apoptosis of PAEC may contribute to the progression of the disease in these MCT-treated rats. The results of the present study add to understanding of the complexity of TGF-β1 actions in pulmonary hypertension.
GRANTS
This work was supported by a Rhode Island Foundation Medical Research Grant, American Lung Association Research Grant RG-1140-N, a Parker B. Francis Fellowship (Q. Lu), National Heart, Lung, and Blood Institute Grant HL-64936 and Veterans Affairs Merit Review (S. Rounds), and National Heart, Lung, and Blood Institute Grant HL-67795 and Veterans Affairs Merit Review (E. O. Harrington).
Supplementary Material
Acknowledgments
The author thanks Drs. Sharon Rounds and Elizabeth O. Harrington for advice and support and Angela Yang and Julie Newton for technical assistance.
This work was carried out at the Providence Veterans Affairs Medical Center.
Some of these results were presented at the American Thoracic Society International Conference, 18–23 May 2007, San Francisco, CA, and have been published in abstract form (Am J Respir Crit Care Med 175: A530, 2007).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams J, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant-negative mutation of the TGF-β receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 100: 564–571, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Dawicki DD, Chatterjee D, Wyche J, Rounds S. Extracellular ATP and adenosine cause apoptosis of pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 273: L485–L494, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67: 737–744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-β responses. Cell 95: 737–740, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFβ/ALK5 signaling. Mol Cell 12: 817–828, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Groenendyk J, Michalak M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim Pol 52: 381–395, 2005. [PubMed] [Google Scholar]
- 10.Harrington EO, Lu Q, Rounds S. Endothelial cell apoptosis. In: Endothelial Biomedicine. New York: Cambridge University Press, 2007, p. 1081–1097.
- 11.Harrison RE, Berger R, Haworth SG, Tulloh R, Mache CJ, Morrell NW, Aldred MA, Trembath RC. Transforming growth factor-β receptor mutations and pulmonary arterial hypertension in childhood. Circulation 111: 435–441, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hung R, Chow A. Dissecting the “end game”: clinical relevance, molecular mechanisms and laboratory assessment of apoptosis. Clin Invest Med 27: 324–344, 2004. [PubMed] [Google Scholar]
- 13.Jiang Y, Dai A, Li Q, Hu R. Hypoxia induces transforming growth factor-β1 gene expression in the pulmonary artery of rats via hypoxia-inducible factor-1α. Acta Biochim Biophys Sin 39: 73–80, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer K, Harrington EO, Lu Q, Bellas R, Newton J, Sheahan KL, Rounds S. Isoprenylcysteine carboxyl methyltransferase activity modulates endothelial cell apoptosis. Mol Biol Cell 14: 848–857, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamouille S, Derynck R. Cell size and invasion in TGF-β-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 178: 437–451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips A Jr, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 26: 81–84, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Lebrin F, Deckers M, Bertolino P, ten Dijke P. TGF-β receptor function in the endothelium. Cardiovasc Res 65: 599–608, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 116: 2677–2685, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Deane JA, Campanale NV, Bertram JF, Ricardo SD. Blockade of p38 mitogen-activated protein kinase and TGF-β1/Smad signaling pathways rescues bone marrow-derived peritubular capillary endothelial cells in adriamycin-induced nephrosis. J Am Soc Nephrol 17: 2799–2811, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Dashwood WM, Zhong X, Nakagama H, Dashwood RH. Bcl-2 overexpression in PhIP-induced colon tumors: cloning of the rat Bcl-2 promoter and characterization of a pathway involving β-catenin, c-Myc and E2F1. Oncogene 26: 6194–6202, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Q, Harrington EO, Hai CM, Newton J, Garber M, Hirase T, Rounds S. Isoprenylcysteine carboxyl methyltransferase (ICMT) modulates endothelial monolayer permeability: involvement of RhoA carboxyl methylation. Circ Res 94: 306–315, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-β1-induced endothelial barrier dysfunction involves SMAD2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol 101: 375–384, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lu Q, Harrington EO, Newton J, Jankowich M, Rounds S. Inhibition of ICMT induces endothelial cell apoptosis through GRP94. Am J Respir Cell Mol Biol 37: 20–30, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massague J TGFβ signal transduction. Annu Rev Biochem 67: 753–791, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Mata-Greenwood E, Meyrick B, Steinhorn RH, Fineman JR, Black SM. Alterations in TGF-β1 expression in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 285: L209–L221, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Mauro M, Kim J, Costello C, Laurence J. Role of transforming growth factor-β1 in microvascular endothelial cell apoptosis associated with thrombotic thrombocytopenic purpura and hemolytic-uremic syndrome. Am J Hematol 66: 12–22, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev 17: 1475–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollman MJ, Naumovski L, Gibbons GH. Vascular cell apoptosis: cell type-specific modulation by transforming growth factor-β1 in endothelial cells versus smooth muscle cells. Circulation 99: 2019–2026, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Rahimi RA, Leof EB. TGF-β signaling: a tale of two responses. J Cell Biochem 102: 593–608, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1). J Biol Chem 272: 25783–25786, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor-β signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 170: 1340–1348, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Slee E, Harte M, Kluck R, Wolf B, Casiano C, Newmeyer D. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspase-2, -3, -6, -7, and -10 in a caspase-9-dependent manner. J Cell Biol 144: 281–292, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solovyan VT, Keski-Oja J. Apoptosis of human endothelial cells is accompanied by proteolytic processing of latent TGF-β binding proteins and activation of TGF-β. Cell Death Differ 12: 815–826, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Suhara T, Mano T, Oliveira BE, Walsh K. Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP). Circ Res 89: 13–19, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Tamatani M, Che Y, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFκB activation in primary hippocampal neurons. J Biol Chem 274: 8531–8538, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 97: 1559–1566, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J, Schroter M, Scaffidi C, Krammer P, Peter M, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386: 517–521, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, Newman J, Wheeler L, Higenbottam T, Gibbs JS, Egan J, Crozier A, Peacock A, Allcock R, Corris P, Loyd JE, Trembath RC, Nichols WC. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J Med Genet 37: 741–745, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, Nichols WC, Morrell NW, Berg J, Manes A, McGaughran J, Pauciulo MW, Wheeler L. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 345: 325–334, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Tsukada T, Eguchi K, Migita K, Kawabe Y, Kawakami A, Matsuoka N, Takashima H, Mizokami A, Nagataki S. Transforming growth factor-β1 induces apoptotic cell death in cultured human umbilical vein endothelial cells with down-regulated expression of bcl-2. Biochem Biophys Res Commun 210: 1076–1082, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Vinals F, Pouyssegur J. Transforming growth factor-β1 (TGF-β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-α signaling. Mol Cell Biol 21: 7218–7230, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 290: L209–L221, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Mayo M, Korneluk R, Goeddel D, Baldwin AJ. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680–1683, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Yan Q, Sage EH. Transforming growth factor-β1 induces apoptotic cell death in cultured retinal endothelial cells but not pericytes: association with decreased expression of p21waf1/cip1. J Cell Biochem 70: 70–83, 1998. [PubMed] [Google Scholar]
- 47.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 88: E2–E11, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, Chakravarty S, Protter A, Sehgal PB, Champion HC, Tuder RM. Role of TGF-β/ALK5 kinase in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med 177: 896–905, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakrzewicz A, Kouri FM, Nejman B, Kwapiszewska G, Hecker M, Sandu R, Dony E, Seeger W, Schermuly RT, Eickelberg O, Morty RE. The transforming growth factor-β/ Smad2,3 signalling axis is impaired in experimental pulmonary hypertension. Eur Respir J 29: 1094–1104, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Zou H, Li Y, Liu X, Wang X. An APAF1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274: 11549–11556, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.