Abstract
Hypoxia causes abnormal neonatal pulmonary artery remodeling (PAR) and inhibition of alveolar development (IAD). Transforming growth factor (TGF)-β is an important regulator of lung development and repair from injury. We tested the hypothesis that inhibition of TGF-β signaling attenuates hypoxia-induced PAR and IAD. Mice with an inducible dominant-negative mutation of the TGF-β type II receptor (DNTGFβRII) and nontransgenic wild-type (WT) mice were exposed to hypoxia (12% O2) or air from birth to 14 days of age. Expression of DNTGFβRII was induced by 20 μg/g ZnSO4 given intraperitoneally daily from birth. PAR, IAD, cell proliferation, and expression of extracellular matrix (ECM) proteins were assessed. In WT mice, hypoxia led to thicker, more muscularized resistance pulmonary arteries and impaired alveolarization, accompanied by increases in active TGF-β and phosphorylated Smad2. Hypoxia-induced PAR and IAD were greatly attenuated in DNTGFβRII mice given ZnSO4 compared with WT control mice and DNTGFβRII mice not given ZnSO4. The stimulatory effects of hypoxic exposure on pulmonary arterial cell proliferation and lung ECM proteins were abrogated in DNTGFβRII mice given ZnSO4. These data support the conclusion that TGF-β plays an important role in hypoxia-induced pulmonary vascular adaptation and IAD in the newborn animal model.
Keywords: lung development, infant, persistent pulmonary hypertension of the newborn
chronic hypoxia leads to abnormal pulmonary arterial remodeling (PAR) in neonates and infants and may contribute to the pathogenesis of persistent pulmonary hypertension of the newborn (PPHN) (49), congenital cyanotic heart disease (24, 43), and perhaps bronchopulmonary dysplasia (BPD) (22). Hypoxia soon after birth causes excessive deposition of connective tissue, predominantly collagen and elastin, and the arteries become fixed in an incompletely dilated state (23). The exact mechanisms underlying the increased muscularization are not fully established but may involve recruitment of circulating cells (20) and adventitial cells (44).
Chronic hypoxia also permanently inhibits alveolar development in animals that undergo postnatal alveolar septation (e.g., mice) (8, 29). Alveolar development begins in utero and continues postnatally in humans and is postnatal in mice (45). Human lung development from week 24 of gestation through the first 2 yr of age parallels lung development of mice in the first two postnatal weeks (10).
The expression, tissue localization, and activity of components of the transforming growth factor (TGF)-β signaling machinery are dynamically regulated during late lung development in mouse and human (1, 34). TGF-β signaling, assessed by phosphorylation of Smad2, is seen in vascular and airway smooth muscle, as well as alveolar and airway epithelium throughout late lung development (1). TGF-β is a key regulator of lung repair following injury (50, 52) and may have a role in BPD in preterm infants (28, 46). TGF-β is important in pulmonary vascular remodeling (9, 35). Inhibition of alveolar development (IAD) and increased TGF-β in bronchoalveolar lavage fluid were seen in rats exposed to hypoxia during the first 2 wk of life but not adulthood (47).
We recently used (12) a novel transgenic mouse model that expresses an inducible dominant-negative mutation of the TGF-β type II receptor (DNTGFβRII mouse) (40) to evaluate the effects of inhibiting TGF-β signaling on hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling during hypoxia exposure in adult mice. We demonstrated inhibition of hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling, as well as reduction in extracellular matrix (ECM) accumulation in adult DNTGFβRII mice (12). Hypoxic PAR is more marked in neonates compared with adult animals for the same degree of hypoxia (36), and alveolar development and hypoxic IAD are unique to newborn mice, because alveolarization does not occur in adult mice. Therefore, we wished to determine the effects of inhibition of TGF-β signaling on hypoxia-induced PAR and IAD in a newborn mouse model. We hypothesized that DNTGFβRII mice would have reduction of PAR and right ventricular (RV) hypertrophy and attenuation of IAD.
This work was presented in part at the Pediatric Academic Societies' Meeting, Toronto, Canada, May 4–8, 2007, and the American Thoracic Societies' Meeting, San Francisco, CA, May 18–23, 2007.
METHODS
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and were consistent with the Public Health Service Policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, August 2002) and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 96-01, revised 2002). All experiments, unless otherwise specified, were done with a minimum of six mice from at least two litters for each experimental condition.
Animal model.
DNTGFβRII (on C57BL/6 background) and nontransgenic (C57BL/6) wild-type (WT) mice were exposed to normobaric 12% O2 (hypoxia) or air (normoxia) from soon after birth for 14 days (the period of maximal alveolar development) as described previously (3). Mice were placed in the hypoxia chamber as soon as possible after birth (<12 h of age) along with their dams. Twelve percent O2 was used for hypoxic exposure because we showed earlier (3) that 10% O2, the concentration often used for chronic hypoxic exposures in adult mice, is associated with increased mortality in newborn mice. The DNTGFβRII mouse expresses a cytoplasmically truncated TGFβRII receptor that competes with endogenous receptors for heterodimeric complex (TGFβRI and -RII) formation and is thus a dominant-negative mutant (40). The DNTGFβRII lacks the cytoplasmic kinase domain and has no intrinsic activity. The DNTGFβRII is under the control of a metallothionein-derived promoter that can be induced by Zn2+.
Expression of DNTGFβRII was induced by administering ZnSO4 (20 μg/g ip) daily from birth to DNTGFβRII pups maintained in air (DT-zinc-air group) or hypoxia (DT-zinc-hypoxia group). DNTGFβRII mice given saline (vehicle control) and maintained in air (DT-saline-air group) or hypoxia (DT-saline-hypoxia group) served as controls. Additional controls were WT mice given saline and WT mice given ZnSO4 (20 μg/g ip) to ensure that this dose of Zn2+ did not affect lung development. To confirm induction of DNTGFβRII in animals given ZnSO4, RNA was isolated from lungs of DNTGFβRII pups given either ZnSO4 or saline vehicle and RT-PCR was done for DNTGFβRII receptor mRNA (primers: 5′-ATC GTC ATC GTC TTT GTA GTC-3′ and 5′-TCC CAC CGC ACG TTC AGA AG-3′). In preliminary studies, we administered ZnSO4 to the dams in order to transmit the Zn2+ to the pups through the milk, but inducing the DNTGFβRII in the dams increased the mortality of the pups because of decreased milk production. Administration of ZnSO4 and induction of the expression of the mutated receptor in the pups alone was well tolerated, and no differences were noted in mortality of WT or DNTGFβRII mice (given either vehicle or ZnSO4) over the 14-day period of the study.
After completion of hypoxia or normoxia exposure, the chest was opened, the lungs were inflation fixed via the trachea with 10% formalin at 20 cmH2O pressure, and the RV was perfused with formalin at the same pressure until the effluent was clear to flush out the blood in the pulmonary vessels. After formalin fixation for 24 h, samples were changed to 70% ethanol to avoid overfixation. Five-micrometer sections were stained with hematoxylin and eosin for pulmonary morphometry. Each section consisted of a coronal section from apex to base of both lungs from a newborn mouse. Additional mice were euthanized after completion of hypoxia or normoxia exposure, and frozen sections were made along the coronal axis.
Immunohistochemical analysis.
Immunohistochemical (IHC) staining for active TGF-β1 was done with a primary rabbit polyclonal antibody [LC(1–30), a gift from Dr. Kathy Flanders NIH, Bethesda, MD] (19) on frozen sections; IHC staining for other proteins of interest was done in paraffin-embedded sections. The primary antibodies for phosphorylated (p)Smad2 [for Smad2 activation, rabbit anti-pSmad2 (Ser465/467); Chemicon International], α-smooth muscle actin (α-SMA, for smooth muscle cell labeling; Sigma-Aldrich), and Ki67 (for cell proliferation; LabVision) were used at 1:100 dilution for 30 min, and the secondary antibody and diaminobenzidine (DAB) staining kit were used as described in the product manual (DAKO Envision+HRP-DAB, DakoCytomation). For paraffin-embedded sections, antigen retrieval (required for pSmad2 and Ki67, not for α-SMA) was performed by heating in pH 6.0 citrate buffer (LabVision) for 20 min before addition of primary antibody. Nonspecific IgG and omission of primary antibody were used as controls for staining specificity. For quantitation, whole lung sections were imaged at ×400. The identity of the sample was masked to observers estimating IHC staining to avoid bias. Six random high-power fields from each of six different animals per group were evaluated for each experiment.
Western blot analysis.
Newborn mouse lungs were homogenized in 1 ml of a tissue protein extraction reagent (T-PER, Pierce Biotechnology) and centrifuged at 7,000 g for 5 min, and the supernatant was frozen at −80°C until analysis. Protein concentrations were measured with the Bio-Rad Bradford Protein Assay. Ten micrograms of protein per lane was fractionated by 10% Tris-glycine SDS-PAGE electrophoresis, followed by transfer to a polyvinylidene difluoride membrane (Millipore). Western blot analysis for pSmad2, pSmad3, Smad2, and β-tubulin was done with specific primary antibodies (developed in rabbit, reactive against mouse) for pSmad2 (Cell Signaling), pSmad3 (Cell Signaling), Smad2 (Cell Signaling), or β-tubulin (Santa Cruz) at 1:1,000–2,000 dilution for 2 h at room temperature (pSmad2, pSmad3, Smad2) or overnight at 4°C (β-tubulin). The secondary antibody was a goat anti-rabbit secondary antibody (Sigma) used at 1:10,000 dilution for 1 h at room temperature. Immunoreactive bands were visualized by treatment with Immun-Star Western blotting detection reagents (Bio-Rad) according to the manufacturer's instructions. Densitometry was done with a Kodak Gel Logic 100 digital imaging system (Carestream Health) and Kodak Molecular Imaging Software (v. 4.5.1, Carestream Health), normalizing for β-tubulin in the same lane [a protein that did not change significantly with hypoxia in this model, unlike total Smad2 and Smad3, which increased slightly with hypoxia (unpublished data)].
ELISA.
All newborn mouse lung homogenates were analyzed as a single batch for TGF-β1 by ELISA as described in the manufacturer's protocol (MB100B, R&D Systems). The range of measurement of this ELISA was 5–2,000 pg/ml. Active and total TGF-β1 were measured separately by ELISA. Total (latent + active) TGF-β1 was measured by the addition of hydrochloric acid to activate latent TGF-β to active TGF-β, followed by neutralization with sodium hydroxide, as described in the product manual. This TGF-β1 ELISA recognizes active TGF-β1 but not the latent form of TGF-β1, TGF-β2, TGF-β3, or any of the bone morphogenic proteins. TGF-β1 concentrations were normalized by protein concentration.
Lung and heart morphometry.
The software package MetaMorph v. 6.2r4 (Universal Imaging) interfaced with a Nikon TE2000U microscope equipped with a QiCam Fast Cooled high-resolution CCD camera (1,392 × 1,040 pixels = 4.15 MB TIF file per image) was used for image analysis for lung vascular or alveolar morphometry, as previously described (3, 5). Pulmonary arteries were defined as vessels that accompanied airways (veins are interlobular). Vessels smaller than 20 μm or larger than 150 μm (not resistance vessels) in external diameter were excluded from analysis (3). At least 20 pulmonary arteries from each section were evaluated. Vessels cut transversely were measured along both axes, and the average wall thickness was obtained. Vessels cut obliquely or longitudinally were measured along the short axis. The wall thickness (%) of each artery was expressed as a percentage of the vessel diameter: wall thickness (%) = 100 × (2 × medial wall thickness)/external diameter.
In the newborn mouse lung, nearly all the wall thickness is comprised of the tunica media, and hence wall thickness is primarily medial wall thickness. The adventitia was not included because there is very little adventitia in the newborn mouse even with hypoxic exposure. As there is no intimal hypertrophy or hyperplasia in newborn mice exposed to chronic hypoxia, the single layer of endothelium does not contribute significantly to wall thickness.
Muscularization of resistance pulmonary arteries was evaluated in sections stained for α-SMA as described previously (12). Briefly, the percentage of muscularization of each pulmonary artery (20- to 150-μm external diameter) in relation to its circumference was calculated as an index of pulmonary arterial muscularization.
Hearts were sectioned transversely just below the level of the mitral leaflet, and the thickness of the free wall of the RV compared with that of the left ventricle (RV-to-LV free wall thickness ratio) was determined as an index of RV hypertrophy secondary to pulmonary hypertension. The common method in older animals is to compare the ratio of the weights of the dissected RV to the LV + septum, but accurate dissection of the newborn mouse heart into RV and LV is not possible because there is no exact plane of division between the ventricles. RV pressures were not measured, because they are difficult to measure in mice <4 wk of age. A minimum of 12 hearts from at least 3 litters for each group were analyzed.
Alveolar morphometry.
Alveolar development was evaluated by mean linear intercepts (MLI) (30) and radial alveolar counts (RAC) (14) with the MetaMorph image system. Photographs from six random ×100 lung fields were taken from each animal, with one image from the apex, middle, and base of each lung for MLI measurement.
Extracellular matrix protein and cardiac hypertrophy gene expression assessment.
Quantitative real-time PCR was performed with the Bio-Rad iCycler System as described previously (5, 31) on RNA that was extracted from fresh homogenized lungs and microdissected RVs of 7-day-old mice by the TRIzol (Invitrogen) method. The mouse PCR primer sequences for the molecules of interest are listed in Table 1.
Table 1.
Mouse primer sequences (3′-5′) for real-time quantitative RT-PCR
| Primer Name | Sequence |
|---|---|
| 18S forward | GTC TGC CCT ATC AAC TTT CG |
| 18S reverse | ATG TGG TAG CCG TTT CTC A |
| c-fos forward | ATCGGCAGAAGGG GCAAAGTAG |
| c-fos reverse | GCAACGCAGACTT CTCATCTTCAAG |
| Col l a1 forward | CCA AGG GTA ACA GCG GTG AA |
| Col l a1 reverse | CCT CGT TTT CCT TCT TCT CCG |
| Col l a2 forward | TGT TGG CCC ATC TGG TAA AGA |
| Col l a2 reverse | CAG GGA ATC CGA TGT TGC C |
| Col 3 a1 forward | TCA AGT CTG GAG TGG GAG G |
| Col 3 a1 reverse | TCC AGG ATG TCC AGA AGA ACC A |
| Fibronectin forward | ACA GAA ATG ACC ATT GAA GG |
| Fibronectin reverse | TGT CTG GAG AAA GGT TGA TT |
| Lysyl oxidase forward | GTG ACA TTC GCT ACA CAG GAC ATC |
| Lysyl oxidase reverse | CCA AAC ACC AGG TAC GGC TTT ATC |
| α-MHC forward | TGAAAACGGAAAGACGGTGA |
| α-MHC reverse | TCCTTGAGGTTGTACAGCACA |
| β-MHC forward | CTA CAG GCC TGG GCT TAC CT |
| β-MHC reverse | TCT CCT TCT CAG ACT TCC GC |
| Tenascin-C forward | GGA GTT GCT GGT ATC GTC TCT AAG G |
| Tenascin-C reverse | ACA CCT GCC ATC CAA ACA CAT C |
| Tropoelastin forward | TGG TAT TGG TGG CAT CGG |
| Tropoelastin reverse | CCT TGG CTT TGA CTC CTG TG |
Statistical analysis.
Results are expressed as means ± SE. The data were analyzed by two-way ANOVA to test for separate and combined effects of genotype and hypoxia on pulmonary arterial wall thickness, RV-to-LV thickness ratio, alveolar morphometry measurements, and estimates of cell proliferation. Multiple comparison testing (Student-Newman-Keuls) was performed if statistical significance (P < 0.05) was noted by ANOVA.
RESULTS
No change in mortality was noted in the hypoxia-exposed or zinc-supplemented mouse pups, and the mortality rate was ∼10% in all groups.
Hypoxic exposure increased active, but not total, TGF-β1 in neonatal lung.
Hypoxia exposure for 14 days increased active TGF-β1 in lungs of WT mice (Fig. 1), but concentrations of total TGF-β1 were unchanged (WT-air 2,078 ± 205 vs. WT-hypoxia 1,655 ± 270 pg·ml−1·mg protein−1, P = 0.27).
Fig. 1.
Hypoxic exposure increases active transforming growth factor (TGF)-β in newborn lung. A and B: representative photomicrographs of mouse lung sections from wild-type (WT) mice exposed to air (A) or hypoxia (B) for 14 days and stained with an antibody recognizing active TGF-β1 (red) (×400). C: levels of active TGF-β1 measured by ELISA from lung extracts of mice exposed to air or hypoxia from birth to 14 days of age (n = 6 mice/group; means ± SE) *P < 0.05 vs. air.
Induction of DNTGFβRII expression attenuated hypoxia-induced Smad2 phosphorylation in neonatal lung.
Induction of the DNTGFβRII receptor mRNA in the lung was confirmed in DNTGFβRII mouse pups treated with ZnSO4 but not saline (Fig. 2). pSmad2 staining was increased in lung sections of DT-saline-hypoxia compared with DT-saline-air pups, confirming that hypoxia exposure increased TGF-β signaling (Fig. 3). The hypoxia-induced pSmad2 staining in lung was attenuated in DT-zinc-hypoxia pups because of the induction of the DNTGFβRII receptor. pSmad2 levels in lungs of DT-zinc-air pups were low and similar to those of DT-saline-air pups (Fig. 3). This reduction in pSmad2 was confirmed by Western blotting (Fig. 3). Similar reductions in pSmad3 were also observed (data not shown).
Fig. 2.
Administration of ZnSO4 (zinc) but not 0.9% saline induces mRNA expression of DNTGFβRII in lungs of mouse pups, as demonstrated by RT-PCR. Each lane represents a sample from a different mouse pup.
Fig. 3.
Phosphorylated (p)Smad2 immunohistochemical staining and quantitation by Western blots in DNTGFβRII mouse pups at 14 days of age. A–D: representative photomicrographs of a resistance pulmonary artery (PA) and a bronchus (Br) from DNTGFβRII mouse pups given either saline (A, B) or zinc (C, D) during 14 days of air (A, C) or hypoxia (B, D) exposure (×400; calibration bars = 50 μm). Nuclear staining of pSmad2, indicating presence of TGF-β signaling, was increased in hypoxia-exposed DNTGFβRII mouse pups given saline (B) and reduced in DNTGFβRII pups that received zinc (C, D). E: representative Western blots of lung homogenates for pSmad2, Smad2, and β-tubulin from DNTGFβRII mouse pups given either saline or zinc during 14 days of air or hypoxia exposure. F and G: pSmad2-to-β-tubulin ratio (F) and pSmad2-to-Smad2 ratio (G) in Western blots quantitated by densitometry (means ± SE; n = 6 mice/group). *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding saline.
Blocking of TGF-β signaling attenuated hypoxia-induced pulmonary arterial remodeling but not RV hypertrophy.
Hypoxia led to increased wall thickness in resistance pulmonary arteries of DT-saline-hypoxia compared with DT-saline-air pups, and this hypoxia-induced increase was attenuated in DT-zinc-hypoxia pups (Fig. 4). ZnSO4 treatment did not block the hypoxia-induced pulmonary vascular hypertrophy in WT-zinc-hypoxia pups (comparable to WT-saline-hypoxia pups; data not shown). Hypoxia led to increased α-SMA staining in pulmonary arteries (external diameter of 20–50 μm as well as 51–150 μm) of DT-saline-hypoxia compared with DT-saline-air pups, and this hypoxia-induced increase was attenuated in DT-zinc-hypoxia pups (Fig. 5). α-SMA staining in DT-zinc-air pups was similar to that in DT-saline-air pups.
Fig. 4.
Pulmonary arterial remodeling and right (RV)-to-left ventricle (LV) ratios in DNTGFβRII mouse pups at 14 days of age. A–D: representative photomicrographs of a resistance pulmonary artery (PA) and a bronchus (Br) from DNTGFβRII mouse pups given either saline (A, B) or zinc (C, D) during 14 days of air (A, C) or hypoxia (B, D) exposure (×400; calibration bars = 50 μm). In mouse pups given saline, pulmonary arterial wall thickness (arrows) is increased by hypoxia (B) compared with air (A). Administration of zinc significantly attenuates the hypoxia-induced increase in wall thickness (D) but does not change wall thickness of air-exposed animals (C). E and F: wall thickness (%) of pulmonary arteries (E) and RV-to-LV thickness ratio (F) at 14 days of age in DNTGFβRII mouse pups given either saline or zinc while being exposed to air or hypoxia (means ± SE; n = 6 mice/group). *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding saline.
Fig. 5.
Pulmonary arterial muscularization as evaluated by α-smooth muscle actin (α-SMA) staining in DNTGFβRII mouse pups at 14 days of age. A–H: representative photomicrographs of lung at low power (×100; A, C, E, G) and a resistance pulmonary artery (arrows) in high-power images (×400; B, D, F, H) from DNTGFβRII mouse pups given either saline (A–D) or zinc (E–H) during 14 days of air (A, B, E, F) or hypoxia (C, D, G, H) exposure (calibration bars = 250 μm in ×100 images and 50 μm in ×400 images). In mouse pups given saline, hypoxia increases α-SMA staining diffusely in the lung (C) and in resistance pulmonary arteries (D) compared with air (A, B). Small pulmonary arterioles (arrowhead) are also muscularized in the hypoxia-saline group (D). Administration of zinc significantly attenuates the hypoxia-induced increase in α-SMA staining in the lung (G) and in pulmonary arteries (H) but does not change staining in air-exposed animals (E, F). I–K: quantitation of muscularization of pulmonary arteries of all pulmonary arteries 20–150 μm (I), smaller (20–50 μm) pulmonary arteries (J), and larger (51–150 μm) pulmonary arteries (K) in DNTGFβRII mouse pups given either saline or zinc while being exposed to air or hypoxia demonstrates that hypoxia increases α-SMA staining of resistance pulmonary arteries, which is attenuated in animals given zinc (means ± SE; n = 80 arteries/group). *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding saline.
Hypoxia led to an increase in RV-to-LV wall thickness ratios in DT-saline-hypoxia compared with DT-saline-air pups, and this index of RV hypertrophy was not significantly attenuated in DT-zinc-hypoxia pups (Fig. 4). Arterial wall thickness and RV-to-LV ratio were comparable in DNTGFβRII pups given saline and WT pups exposed to the same condition (air or hypoxia) (data not shown).
Blocking TGF-β signaling attenuated hypoxia-induced IAD.
Hypoxia inhibited alveolar development, as evidenced by an increase in MLI and a reduction in RAC in DT-saline-hypoxia compared with DT-saline-air pups (Fig. 6). DT-zinc-hypoxia pups had significantly lower MLI and increased RAC compared with DT-saline-hypoxia pups, but the MLI was still not equivalent to that in DT-saline-air pups, indicating that inhibition of TGF-β signaling partially attenuated the hypoxia-induced IAD. DNTGFβRII pups given saline were comparable in alveolar development to WT pups exposed to the same condition (air or hypoxia) (data not shown).
Fig. 6.
Alveolar development in DNTGFβRII mouse pups at 14 days of age. A–D: representative photomicrographs of hematoxylin and eosin-stained sections of lungs from DNTGFβRII mouse pups given either saline (A, B) or zinc (C, D) during 14 days of air (A, C) or hypoxia (B, D) exposure (×100; calibration bars = 250 μm). In mouse pups given saline, alveolar size is larger in hypoxia-exposed (B) compared with air-exposed (A) mice, indicating delay in septation. Administration of zinc significantly attenuates the hypoxia-induced increase in alveolar size (D) but does not change alveoli of air-exposed animals (C). E and F: mean linear intercept (E) and radial alveolar count (F) at 14 days of age in DNTGFβRII mouse pups given either saline or zinc while being exposed to air or hypoxia (means ± SE; n = 6 mice/group). *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding saline.
Blocking of TGF-β signaling attenuated hypoxia-induced increases in cell proliferation in pulmonary arteries.
Hypoxia increased vascular cell proliferation in DT-saline-hypoxia compared with DT-saline-air pups (Fig. 7), and this increased cell proliferation was attenuated in DT-zinc-hypoxia pups. Hypoxia did not significantly change the number of Ki67+ cells in the alveolar interstitium, but both DT-zinc-air and DT-zinc-hypoxia pups had increased numbers of Ki67+ cells in the alveolar interstitium (Fig. 7), indicating increased alveolar cell proliferation with blocking of TGF-β signaling.
Fig. 7.
Cell proliferation in DNTGFβRII mouse pups at 14 days of age as determined by Ki67 staining. A–D: representative photomicrographs of Ki67-stained sections of lungs from DNTGFβRII mouse pups given either saline (A, B) or zinc (C, D) during 14 days of air (A, C) or hypoxia (B, D) exposure (×400; calibration bars = 50 μm). In mouse pups given saline, increased numbers of stained nuclei are seen in walls of pulmonary arteries (PA) in hypoxia-exposed (B) compared with air-exposed (A) mice, indicating increased vascular cell proliferation. Administration of zinc significantly attenuates the hypoxia-induced increase in vascular cell proliferation (D). E and F: quantitation of Ki67 staining in pulmonary arterial wall, expressed as number of Ki67+ nuclei per 1,000 μm2 of vessel wall (E) and quantitation of Ki67 staining in alveoli, expressed as number of Ki67+ nuclei per 50,000 μm2 of lung cross-sectional area (F) at 14 days of age in DNTGFβRII mouse pups given either saline or zinc while being exposed to air or hypoxia (means ± SE; n = 6 mice/group). *P < 0.05 vs. corresponding air; #P < 0.05 vs. corresponding saline.
Blocking of TGF-β signaling attenuated hypoxia-induced changes in collagen, tropoelastin, and tenascin-C but not fibronectin gene expression in neonatal lung.
Hypoxia increased lung collagen I and III mRNA levels in DT-saline-hypoxia pups compared with DT-saline-air pups (Fig. 8). Inhibition of TGF-β signaling did not alter collagen I or III mRNA levels in lung of DT-zinc-air pups but attenuated the hypoxia-induced increases in collagen I and III mRNA levels in lung of DT-zinc-hypoxia pups (Fig. 8). Hypoxia also increased lung tropoelastin mRNA levels in DT-saline-hypoxia pups compared with DT-saline-air pups, and blocking of TGF-β signaling attenuated the hypoxia-induced increases in tropoelastin expression in DT-zinc-hypoxia pups (Fig. 8). Lysyl oxidase, which is important in elastin deposition, did not change with hypoxia or inhibition of TGF-β signaling. Tenascin-C expression was reduced in DT-saline-hypoxia lungs compared with DT-saline-air lungs, and inhibition of TGF-β signaling in DT-zinc-hypoxia pups blocked the hypoxia-induced decreases in tenascin-C expression. Hypoxia reduced fibronectin mRNA in DT-saline-hypoxia lungs compared with DT-saline-air lungs, and inhibition of TGF-β signaling increased fibronectin expression slightly in DT-zinc-air lungs but not in DT-zinc-hypoxia lungs (Fig. 8). Briefly, blocking TGF-β signaling attenuated the hypoxia-induced increases in collagen and tropoelastin expression and the hypoxia-induced decrease in tenascin-C expression but not the hypoxia-induced decrease in fibronectin expression.
Fig. 8.
Collagen, tropoelastin, lysyl oxidase, tenascin-C, and fibronectin mRNA levels measured by real-time quantitative RT-PCR in homogenized lungs from mice exposed to air or hypoxia from birth to 7 days (n = 6 mice/group; means ± SE). *P < 0.05 vs. corresponding air at same time point; #P < 0.05 vs. corresponding saline.
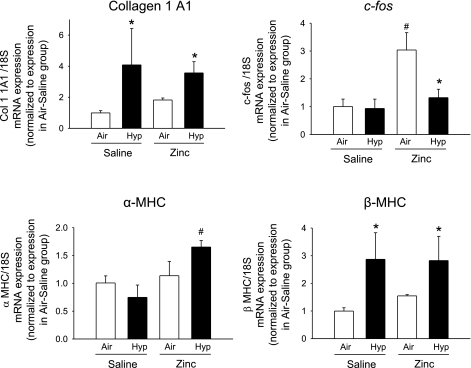
Blocking of TGF-β signaling does not attenuate hypoxia-induced increases in β-myosin heavy chain or collagen I gene expression in neonatal right ventricle.
Hypoxia increased β-myosin heavy chain (MHC) and collagen I mRNA levels in RVs of DT-saline-hypoxia pups compared with DT-saline-air pups, but blocking of TGF-β signaling in DT-zinc-hypoxia pups did not change the hypoxia-induced increases of these genes (Fig. 9). α-MHC showed a trend toward reduction with hypoxia in DT-saline-hypoxia compared with DT-saline-air pups (P = 0.2), and inhibition of TGF-β signaling significantly increased α-MHC in DT-zinc-hypoxia pups compared with DT-saline-hypoxia pups (Fig. 9). Hypoxia did not change c-fos mRNA levels in DT-saline-hypoxia pups compared with DT-saline-air pups. However, inhibition of TGF-β signaling significantly increased c-fos mRNA in DT-zinc-air pups compared with the other three groups (Fig. 9).
Fig. 9.
Collagen 1, c-fos, α-myosin heavy chain (MHC), and β-MHC mRNA levels measured by real-time quantitative RT-PCR in homogenized RV of mice exposed to air or hypoxia from birth to 7 days (n = 12 mice/group; means ± SE). *P < 0.05 vs. corresponding air at same time point; #P < 0.05 vs. corresponding saline.
DISCUSSION
The present study is the first to test the effects of chronic postnatal hypoxia on PAR and alveolar development in an animal with an inducible defect of the TGF-β signaling pathway. Previous in vivo studies of the functional role of TGF-β in normal lung development and in the response to hypoxia have been limited, because null mutations of TGF-β isoforms and TGF-β receptor II are either lethal (26, 38, 39, 42) or result in multifocal inflammatory disease and early death (TGF-β1-null mice) (42). The inducible DNTGFβRII mouse has the advantage of not disrupting critical TGF-β signaling pathways during embryonic lung development, while permitting the disruption of TGF-β signaling by both Smad-dependent and -independent pathways during a chosen postnatal time period. The most important finding in our study is that blocking TGF-β signaling attenuated both hypoxia-induced inhibition of normal postnatal PAR and hypoxia-induced IAD, indicating that TGF-β plays a role in mediating both of these processes in the developing lung.
This study is clinically relevant, because chronic hypoxia-induced PAR is considered a model of PAR associated with PPHN and some forms of congenital heart disease. Our investigation suggests that TGF-β may mediate pulmonary vascular changes in PPHN and congenital heart disease. Newborn mice that conditionally overexpress bioactive TGF-β1 have poor alveolar development, with thick hypercellular septae, increased collagen and α-SMA, as well as abnormal vascular development (48). Newborn rat pups with transient gene transfer of active TGF-β1 by an adenoviral vector also demonstrated large distorted alveoli and interstitial fibrosis (21). Vicencio et al. (47) demonstrated IAD and increased TGF-β in bronchoalveolar lavage fluid in rats exposed to hypoxia during the first 2 wk of life but not during adulthood. Our study confirms that chronic hypoxia leads to increased TGF-β activity in newborn lung, and extends this observation by demonstrating that inhibition of increased TGF-β signaling attenuates hypoxia-induced abnormal vascular remodeling and IAD.
Our study demonstrates that inhibition of TGF-β signaling attenuates vascular remodeling but not RV hypertrophy, suggesting that these processes are not necessarily interrelated. A similar observation was recently noted in rosiglitazone-treated hypoxic adult rats, which have decreased vascular remodeling without a reduction in pulmonary hypertension (15). One possible explanation is that hypoxia-induced vasoconstriction leading to pulmonary hypertension and RV hypertrophy may persist despite strategies that inhibit abnormal vascular remodeling. We showed earlier that endothelin (ET)-A receptor blockade, which attenuates hypoxia-induced vasoconstriction (6) as well as vascular remodeling (3), is able to prevent RV hypertrophy (3). ET-1 can regulate TGF-β synthesis in the newborn lung (25) and may thereby regulate both hypoxia-induced vasoconstriction (directly) as well as vascular remodeling (indirectly via TGF-β). We have noted a disconnect between pulmonary vascular and RV remodeling in other newborn mouse models: newborn Mmp2−/− mice in air have thicker-walled pulmonary arteries but no RV hypertrophy (5). Hypoxic PAR is more marked in neonates compared with adult animals for the same degree of hypoxia (36) because of multiple developmental differences between the neonatal and adult pulmonary circulations (16, 27). Our study adds to these differences, because we observed that inhibition of TGF-β signaling attenuates RV hypertrophy in adult (12) but not neonatal mice. Another possible explanation is that RV hypertrophy in the hypoxia-zinc mice reflects the complex interaction of the effects of pulmonary vascular resistance and the direct effects of hypoxia on the heart with the effect of inhibiting TGF-β signaling in the heart. The different mRNA expression patterns of c-fos, α-MHC, β-MHC, and collagen 1A1, genes generally considered to be involved in RV hypertrophy (17, 41), demonstrate the need for further investigation of cardiac hypertrophy genes in the neonatal RV during hypoxic stress and during inhibition of TGF-β signaling.
We observed an increase in cell proliferation in the tunica media of hypoxia-exposed newborn mice, which was prevented by inhibition of TGF-β signaling. A hypoxia-induced increase in neonatal pulmonary arterial cell proliferation has been described by other investigators (7). Our results extend these findings by suggesting that this increase is mediated through TGF-β signaling, a novel observation that merits further investigation.
The hypoxia-induced IAD was attenuated by inhibition of TGF-β signaling, indicating that excessive TGF-β signaling may be responsible for the diminished alveolarization associated with hypoxic exposure. TGF-β has also been shown to contribute to hyperoxia-induced IAD (2, 32), indicating that excessive TGF-β signaling may result from either extreme of oxygen exposure. Similar to our findings in the hypoxia model, inhibition of TGF-β signaling in the hyperoxia model with anti-TGF-β antibodies attenuated pSmad2 expression and improved alveolar development and ECM assembly (32). Impairment of distal alveolar septation associated with dysregulated TGF-β signaling, which is reversible with anti-TGF-β antibodies, has also been noted in fibrillin-1-deficient mice (a mouse model of Marfan syndrome) (33). Our interesting observation that alveolar cell proliferation increased on inhibition of TGF-β signaling suggests that endogenous TGF-β activity might inhibit alveolar epithelial or endothelial cell proliferation even in normoxia.
We previously observed (12) that hypoxia increases mRNA for collagens I and III in adult mouse lung, and that this was prevented by inhibition of TGF-β signaling. In the present study, we observed similar changes in collagen gene expression in neonatal mice, suggesting that similar mechanisms may regulate these collagen subtypes in both the neonate and the adult. Tropoelastin expression, which we showed previously (4) to be increased by hypoxia and decreased by ET-A receptor blockade, was also reduced by inhibition of TGF-β signaling, indicating a convergence of ET-A receptor signaling and TGF-β signaling on elastin expression under hypoxic conditions.
Tenascin-C is important in lung development, being produced mainly by lung fibroblasts and endothelial cells, and is located along alveolar septal walls and concentrated at secondary septal tips (51). The reduction of tenascin-C gene expression by hypoxia and the attenuation of this effect by inhibition of TGF-β signaling suggest that hypoxia-induced TGF-β signaling suppresses tenascin-C, which in turn may inhibit alveolar development.
Fibronectin expression was also attenuated by hypoxia. Fibronectin is known to have important roles in alveolar cell differentiation and lung growth and maturation (37), and hypoxia-induced inhibition of fibronectin expression may contribute to the impairment in lung development. Inhibition of TGF-β signaling did not attenuate the hypoxia-induced decrease in fibronectin expression, suggesting that this effect is not mediated through TGF-β. This may, at least in part, account for the observation that inhibition of TGF-β signaling did not completely restore the hypoxia-induced defect in alveolarization. The observation of a hypoxia-induced decrease in lung fibronectin expression contrasts with our previous demonstration (11) that lung fibronectin increases with hypoxia in adult mice, supporting the concept that hypoxia-induced effects on fibronectin differ in neonatal versus mature lungs.
A limitation of our study is that the inhibition of TGF-β activation could not be precisely regulated or targeted in the lung. These results generate hypotheses that can be tested with specific neutralizing antibodies or transgenic models with targeting to lung. Other limitations are that the newborn mouse model may not closely resemble the human because of interspecies differences and the fact that chronic hypoxia, while a good reproducible model in animals, is not the main etiologic factor underlying PPHN or BPD in human infants. BPD is characterized by an early inhibition of alveolarization (13), but pulmonary vascular muscularization and pulmonary hypertension is usually a late finding (22).
Our investigations indicate that TGF-β signaling is a critical mediator of the effects of hypoxia on the newborn lung and suggest that inhibition of TGF-β signaling may be a possible therapeutic strategy in attenuating the abnormal lung development and vascular remodeling observed in hypoxic critically ill neonates and infants. Further investigations are required to delineate the differences in downstream TGF-β signaling mediated by pSmad2 and pSmad3, which may signal through different nuclear mechanisms (18). The DNTGFβRII transgenic mouse may also be useful in evaluating the role of TGF-β in newborn mouse models of other neonatal disorders that are characterized by excessive TGF-β activation (e.g., hyperoxic lung injury, necrotizing enterocolitis, and hydrocephalus), because of the ability to reduce TGF-β signaling at selected time periods.
GRANTS
This study is supported in part by National Institutes of Health Grants R01-HL-092906, K08-HD-046513, HL-44195, HL-50147, HL-45990, HL-07457, HL-56046, HL-86706, and C06-RR-15490, a Children's Center for Research and Innovation grant, American Thoracic Society/Pulmonary Hypertension Association Grant ATS PH-06-006, Research Facilities Improvement Program Grant C06-RR-15490, and an American Heart Association-0455197B Grant.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alejandre-Alcázar MA, Michiels-Corsten M, Vicencio AG, Reiss I, Ryu J, de Krijger RR, Haddad GG, Tibboel D, Seeger W, Eickelberg O, Morty RE. TGF-beta signaling is dynamically regulated during the alveolarization of rodent and human lungs. Dev Dyn 237: 259–269, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res 57: 631–636, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Li P, Bulger A, Murphy-Ullrich J, Anderson PG, Oparil S, Chen YF. Endothelin-1 mediates hypoxia-induced increases in vascular collagen in the newborn mouse lung. Pediatr Res 61: 559–564, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res 63: 26–32, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambalavanan N, Philips JB III, Bulger A, Oparil S, Chen YF. Endothelin-A receptor blockade in neonatal porcine pulmonary hypertension. Pediatr Res 52: 913–921, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Belknap JK, Orton EC, Ensley B, Tucker A, Stenmark KR. Hypoxia increases bromodeoxyuridine labeling indices in bovine neonatal pulmonary arteries. Am J Respir Cell Mol Biol 16: 366–371, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Blanco LN, Massaro D, Massaro GD. Alveolar size, number, and surface area: developmentally dependent response to 13% O2. Am J Physiol Lung Cell Mol Physiol 261: L370–L377, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension: potential role for transforming growth factor-beta. Am J Pathol 144: 286–295, 1994. [PMC free article] [PubMed] [Google Scholar]
- 10.Burri P Structural aspects of prenatal and postnatal development and growth for the lung. In: Lung Growth and Development, edited by McDonald JA. New York: Dekker, 1997, p. 1–35.
- 11.Chen YF, Feng JA, Li P, Xing D, Ambalavanan N, Oparil S. Atrial natriuretic peptide-dependent modulation of hypoxia-induced pulmonary vascular remodeling. Life Sci 79: 1357–1365, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant negative mutation of the TGF-β receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol 100: 564–571, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Coalson JJ Pathology of new bronchopulmonary dysplasia. Semin Neonatol 8: 73–81, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1—postnatal lung growth. Thorax 37:572–579, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossno JT, Garat CV, Reusch JEB, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Das M, Stenmark KR, Dempsey EC. Enhanced growth of fetal and neonatal pulmonary artery adventitial fibroblasts is dependent on protein kinase C. Am J Physiol Lung Cell Mol Physiol 269: L660–L667, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med 4: 1269–1275, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Flanders KC Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85: 47–64, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flanders KC, Thompson NL, Cissel DS, Van Obberghen-Schilling E, Baker CC, Kass ME, Ellingsworth LR, Roberts AB, Sporn MB. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol 108: 653–660, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163: 2575–2584, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman G, Perkin RM, Anas NG, Sperling DR, Hicks DA, Rowen M. Pulmonary hypertension in infants with bronchopulmonary dysplasia. J Pediatr 112: 67–71, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Haworth SG, Hislop AA. Lung development—the effects of chronic hypoxia. Semin Neonatol 8: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmeister HM, Apitz J, Hoffmeister HE, Fischbach H. The correlation between blood pressure and morphometric findings in children with congenital heart disease and pulmonary hypertension. Basic Res Cardiol 76: 647–656, 1981. [DOI] [PubMed] [Google Scholar]
- 25.Jain R, Shaul PW, Borok Z, Willis BC. Endothelin-1 induces alveolar epithelial-mesenchymal transition through endothelin type A receptor-mediated production of TGF-beta1. Am J Respir Cell Mol Biol 37: 38–47, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11: 415–421, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kelly DA, Hislop AA, Hall SM, Haworth SG. Correlation of pulmonary arterial smooth muscle structure and reactivity during adaptation to extrauterine life. J Vasc Res 39: 30–40, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kwong KY, Niang S, Literat A, Zhu NL, Ramanathan R, Jones CA, Minoo P. Expression of transforming growth factor beta (TGF-β1) by human preterm lung inflammatory cells. Life Sci 79: 2349–2356, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Massaro D, Massaro GD. Pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol 282: L345–L358, 2002. [DOI] [PubMed] [Google Scholar]
- 30.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 23: 162–167, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Miller AP, Feng W, Xing D, Feng W, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 110: 1664–1669, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD Jr. TGF-β-neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Pelton RW, Johnson MD, Perkett EA, Gold LI, Moses HL. Expression of transforming growth factor-beta1, -beta2, and -beta3 mRNA and protein in the murine lung. Am J Respir Cell Mol Biol 5: 522–530, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Perkett EA, Pelton RW, Meyrick B, Gold LI, Miller DA. Expression of transforming growth factor-beta mRNAs and proteins in pulmonary vascular remodeling in the sheep air embolization model of pulmonary hypertension. Am J Respir Cell Mol Biol 11: 16–24, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol 240: H62–H72, 1981. [DOI] [PubMed] [Google Scholar]
- 37.Roman J Fibronectin and fibronectin receptors in lung development. Exp Lung Res 23: 147–159, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Rotzer D, Roth M, Lutz M, Lindemann D, Sebald W, Knaus P. Type III TGF-beta receptor-independent signalling of TGF-beta2 via TbetaRII-B, an alternatively spliced TGF-beta type II receptor. EMBO J 20: 480–490, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124: 2659–2670, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol 139: 541–552, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma S, Taegtmeyer H, Adrogue J, Razeghi P, Sen S, Ngumbela K, Essop MF. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 286: H1185–H1192, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinhorn RH, Fineman JR. The pathophysiology of pulmonary hypertension in congenital heart disease. Artif Organs 23: 970–974, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Stenmark KR, Bouchey D, Nemenoff R, Dempsey EC, Das M. Hypoxia-induced pulmonary vascular remodeling: contribution of the adventitial fibroblasts. Physiol Res 49: 503–517, 2000. [PubMed] [Google Scholar]
- 45.Thurlbeck WM Postnatal growth and development of the lung. Am Rev Respir Dis 111: 803–844, 1975. [DOI] [PubMed] [Google Scholar]
- 46.Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol 24: 22–28, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Vicencio AG, Eickelberg O, Stankewich MC, Kashgarian M, Haddad GG. Regulation of TGF-β ligand and receptor expression in neonatal rat lungs exposed to chronic hypoxia. J Appl Physiol 93: 1123–1130, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Walsh-Sukys MC Persistent pulmonary hypertension of the newborn. The black box revisited. Clin Perinatol 20: 127–143, 1993. [PubMed] [Google Scholar]
- 50.Zhao Y, Young SL. Expression of transforming growth factor-β type II receptor in rat lung is regulated during development. Am J Physiol Lung Cell Mol Physiol 269: L419–L426, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Young SL. Tenascin in rat lung development: in situ localization and cellular sources. Am J Physiol Lung Cell Mol Physiol 269: L482–L491, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Young SL, McIntosh JC, Steele MP, Silbajoris R. Ontogeny and localization of TGF-β type I receptor expression during lung development. Am J Physiol Lung Cell Mol Physiol 278: L1231–L1239, 2000. [DOI] [PubMed] [Google Scholar]