Abstract
The myeloperoxidase (MPO)-hydrogen peroxide-halide system is an efficient oxygen-dependent antimicrobial component of polymorphonuclear leukocyte (PMN)-mediated host defense. However, MPO deficiency results in few clinical consequences indicating the activation of compensatory mechanisms. Here, we determined possible mechanisms protecting the host using MPO−/− mice challenged with live gram-negative bacterium Escherichia coli. We observed that MPO−/− mice unexpectedly had improved survival compared with wild-type (WT) mice within 5–12 h after intraperitoneal E. coli challenge. Lungs of MPO−/− mice also demonstrated lower bacterial colonization and markedly attenuated increases in microvascular permeability and edema formation after E. coli challenge compared with WT. However, PMN sequestration in lungs of both groups was similar. Basal inducible nitric oxide synthase (iNOS) expression was significantly elevated in lungs and PMNs of MPO−/− mice, and NO production was increased two- to sixfold compared with WT. Nitrotyrosine levels doubled in lungs of WT mice within 1 h after E. coli challenge but did not change in MPO−/− mice. Inhibition of iNOS in MPO−/− mice significantly increased lung edema and reduced their survival after E. coli challenge, but iNOS inhibitor had the opposite effect in WT mice. Thus augmented iNOS expression and NO production in MPO−/− mice compensate for the lack of HOCl-mediated bacterial killing, and the absence of MPO-derived oxidants mitigates E. coli sepsis-induced lung inflammation and injury.
Keywords: inflammation, endotoxin shock, rodent, host defense
generation of reactive oxygen species (ROS) by phagocytic cells is an important host defense mechanism of which NADPH oxidase, myeloperoxidase (MPO), and inducible nitric oxide synthase (iNOS) play critical roles (4, 35). Studies have shown complex interactions among constituents of the NADPH oxidase-MPO-iNOS systems. iNOS protein and iNOS-dependent NO levels are significantly increased in mouse lungs after LPS (23, 36) and can affect MPO function. For example, inhibition of iNOS attenuates ROS production by polymorphonuclear leukocytes (PMNs) after LPS challenge (10, 34). Low levels of NO enhance MPO activity, whereas high levels can inhibit MPO catalysis due to formation of nitrosyl complexes (1, 2). MPO activity in lungs of iNOS-deficient mice is reduced in acute inflammation compared with control mice (9). Conversely, MPO affects NO signaling in multiple ways. For example, MPO is secreted by activated PMNs (32) and undergoes transcytosis across the vessel wall where it oxidizes NO and reduces its bioavailability in tissue (11, 38). MPO consumption of NO increases iNOS activity by reducing NO-mediated feedback inhibition (13). MPO also utilizes the NO breakdown product, NO2−, as a physiological substrate, producing NO-derived ROS that promote tyrosine nitration and lipid peroxidation (30, 43). Mycoplasma infection reduces alveolar fluid clearance by inhibiting epithelial Na+ channels via ROS and nitrogen species in wild-type (WT) mice but not in MPO−/− mice (19). LPS challenge also impairs NO-dependent vascular responses in control mice but not in MPO−/− mice (11).
The response to infection is perturbed in patients with deficiencies of the enzyme-based host defense system. Patients with chronic granulomatous disease, in which NADPH oxidase is defective, are extremely susceptible to bacterial infection (33). In contrast, MPO-deficient patients generally do not have increased frequency of infections (26) despite impaired HOCl production (15). These findings suggest that compensatory mechanisms can confer resistance in the absence of MPO. In the present study, we investigated the relationship between MPO and iNOS in MPO−/− mice using a model of Escherichia coli-induced septicemia. We addressed the possibility that iNOS plays a crucial compensatory role that maintains host defense in the absence of MPO. We observed markedly increased basal iNOS expression and NO production that underlie the ability of MPO−/− mice to resist bacterial infection. Thus, strikingly, lung inflammatory injury was greatly reduced in the absence of the MPO-hydrogen peroxide-halide host defense pathway in MPO−/− mice.
MATERIALS AND METHODS
Animals.
Studies were made using MPO−/− mice (5), and wild-type C57BL/6 mice (WT; The Jackson Laboratory, Bar Harbor, ME) were used as controls. Mice (22–26 g, 9–12 wk old) were housed in specific pathogen-free conditions with free access to food and water in the University of Illinois Animal Care Facility. All experimental procedures complied with institutional and National Institutes of Health guidelines for animal use, and approvals were obtained from Animal Care Committee of the University of Illinois at Chicago.
E. coli infection and survival studies in mice.
Live E. coli were obtained from American Type Culture Collection, Manassas, VA (ATCC 25992). Mice were challenged intraperitoneally with 1 × 109 live E. coli for survival studies and with 0.5 to 2 × 108 live E. coli for other experiments as indicated. Control mice were injected intraperitoneally with an equal volume of PBS.
Pulmonary microvascular permeability.
Pulmonary capillary filtration coefficient (Kf,c) was measured to determine pulmonary microvascular permeability to liquid as previously described (28). After the standard 20-min equilibration perfusion, the outflow pressure was rapidly elevated by 10 cmH2O for 2 min, and the lung wet weight was monitored. Lung dry weight was determined, and Kf,c (ml·min−1·cmH2O−1·dry g−1) was calculated from the slope of the recorded weight change normalized to the pressure change and lung dry weight. Pulmonary edema formation was measured by continuously monitoring the lung wet weight change for 90 min (36). Because perfusate albumin concentration was constant, and pulmonary arterial pressure did not change, the rate and magnitude of the increase in lung wet weight provided another index of pulmonary microvessel permeability.
The effect of iNOS inhibitor l-N6-(1-iminoethyl)lysine (l-NIL) on lung edema in vivo in WT and MPO−/− mice treated with E. coli was determined by measuring excess lung water. Lungs were excised, weighed, and then homogenized after the addition of 1 ml of double-distilled H2O. The homogenate was centrifuged at 12,000 rpm for 10 min to obtain the supernatant, and the hemoglobin content was determined. Hemoglobin content and hematocrit were determined in a blood sample from the right ventricle. Fractions of homogenate, supernatant, and blood were weighed and then placed in a drying oven at 60°C for 24 h after which the dry weights were determined. The lung wet-to-dry ratio and final excess lung water were calculated as previously described (37).
Lung PMN sequestration.
Lung PMN sequestration was assessed by determining MPO activity and also morphometrically quantifying PMN infiltration as previously described (28). Mouse lungs were inflated with 10% formalin and embedding in paraffin. Hematoxylin and eosin-stained tissue sections were visualized using a high magnification (×100) objective with an oil-immersion numerical aperture. Middle region (∼30 mm2) of the left lung upper lobe was outlined at low magnification (×1.25). At least 5% of the outlined region was measured with a systematic random design of counting frames. The total number of PMNs in the outlined region of lung was determined using the formula n = ∑Q− × section sampling fraction (SSF)/area sampling fraction (ASF), where ∑Q− is the total number of PMN counted by optical evaluation using a random design procedure. The ASF is the counting frame (6,400 μm2), and SSF is the fraction of section sampled in the region of the lung.
PMN isolation.
PMNs from WT or MPO−/− mice were purified from peripheral venous blood after collection into EDTA anticoagulant and 1:1 dilution into Ca2+/Mg2+-free HBSS-BSA, followed by a discontinuous Percoll gradient as previously described (14, 27). This procedure yielded PMN purity of 90–95% and >95% viability assessed by trypan blue exclusion.
Measurement of nitrite concentration.
PMNs from peripheral blood of WT or MPO−/− mice were incubated in arginine (Arg)-free DMEM for 1 h. Fresh DMEM was added containing 1 mM Arg, and cells were then incubated for 3 h at 37°C. Accumulated nitrite + nitrate in the medium of PMNs or in plasma isolated from WT or MPO−/− mice was measured after reduction of nitrate to nitrite using a Cd-Cu Reducer (Nitralyzer II kit, World Precision Instruments) following the manufacturer's instructions. Nitrite was measured in 50-μl aliquots using the Griess reagent (nitrite detection kit; Promega).
Measurement of lung tissue iNOS activity.
Lung slices (1-mm thick) were washed and placed in HBSS with or without 1 mM Arg at 37°C. NO production was measured in real-time using a three-electrode system (6, 36): a porphyrinic microsensor supercoated with Nafion polymer, a platinum counter electrode, and a silver-silver chloride reference electrode. A micromanipulator was used to place the microsensor on the surface of the lung slice, and the baseline was recorded. To determine constitutive NOS (cNOS) activity, lung slices in HBSS containing 1 mM Arg were stimulated with 10 μM calcium ionophore A-23187. To measure the lung iNOS activity, slices were incubated in HBSS without l-arginine (l-Arg) for 60 min, and NO generation was initiated by application of 1 mM l-Arg.
Measurement of lung tissue nitrotyrosine.
Nitrotyrosine content was determined by stable isotope dilution gas chromatography-mass spectrometry (GC/MS) on a Finnigan Voyager quadrupole GC/MS equipped with a chemical ionization probe as previously described (17). Reactions were terminated by forming cell pellets and adding butylated hydroxytoluene. Lipids and salts were removed by extraction with a single-phase solvent mixture of H2O:water-washed diethyl ether:methanol (1:3:7 vol/vol/vol). Internal standards of 3-[13C6]nitrotyrosine were then added, samples were hydrolyzed with HCl, and the nitrotyrosine content in amino acid hydrolysates was determined after reduction to amino tyrosine as an n-propyl per heptafluorobutyryl derivative. Nitrotyrosine content was normalized to the content of tyrosine determined by stable isotope dilution GC/MS using 2H4-labeled tyrosine as the standard. No significant intra-preparative formation of nitrotyrosine occurred as assessed in each sample by determining the 3-[2H3]-nitrotyrosine present.
Bacterial colony counts.
Lungs were removed aseptically and placed in 1 ml of sterile saline and then homogenized in a tissue homogenizer in a vented hood under sterile conditions. Lung homogenates (0.1 ml) were plated in soy base blood agar plates (Difco), the plates were incubated for 18 h at 37°C, and the number of colonies was counted.
Western blotting.
Lungs were homogenized in 0.05 M Tris, 0.138 M NaCl, 0.0027 M KCl (pH 8) containing a protease inhibitor mixture (Sigma-Aldrich). Neutrophils were purified from mouse bone marrow using a discontinuous Percoll gradient. Protein concentration was measured, and equal amounts of total protein were loaded per lane. Proteins were separated by SDS-PAGE gradient gels (5–20%), transferred to nitrocellulose, blocked with 5% nonfat milk, and probed using antibody specific for endothelial NOS (eNOS) or iNOS (BD Transduction Laboratories, Lexington, KY).
Statistical analysis.
Data are expressed as the means ± SE or SD as indicated in the figure legends. Statistical analysis was performed using the two-way ANOVA and Newman-Keuls test for multiple comparisons. The value of P < 0.05 was considered significant. Kaplan-Meier survival curves (Figs. 1A and 5) were compared using a log-rank test to determine significance.
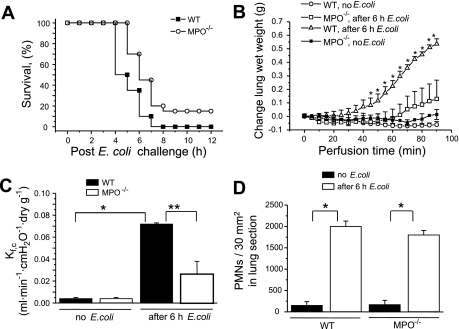
Fig. 1.
A: Kaplan-Meier survival plots for wild-type (WT) and myeloperoxidase MPO−/− mice. Mice were challenged with 109 Escherichia coli per mouse via intraperitoneal injection. Survival curves were generated from 2 independent experiments with a total of 20 mice per group. The difference in survival between the WT and MPO−/− mice was significant (P = 0.02 by the log-rank test). B: changes in lung wet weight (grams). WT and MPO−/− mice were untreated or challenged with 108 E. coli intraperitoneally for 6 h. Lungs were removed and perfused, and lung wet weights were continuously monitored. Points indicate means ± SE (n = 5). *P < 0.05 compared with untreated WT mice. C: lung microvessel permeability (measured as capillary filtration coefficient, Kf,c) in WT and MPO−/− mice 6 h after intraperitoneal E. coli challenge (108 cells). Bars indicate means ± SE (n = 5). *P < 0.05 compared with no E. coli challenge; **P < 0.05 compared with MPO−/− mice after 6 h E. coli. D: morphometric analysis of polymorphonuclear leukocytes (PMNs) in lung interstitium from WT and MPO−/− in controls and 6 h after E. coli challenge. Bars indicate means ± SE (n = 5). *P < 0.05 compared with mice before E. coli challenge.
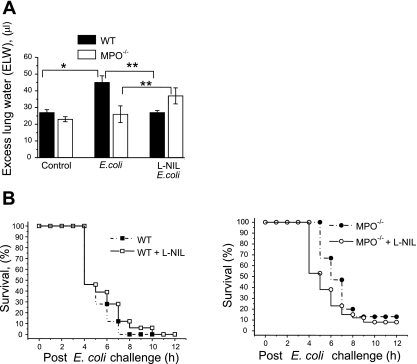
Fig. 5.
Effect of iNOS inhibitor on lung edema and survival after E. coli challenge. A: WT or MPO−/− mice without or with pretreatment with 5 mg/kg l-NIL for 20 min were treated with 5 × 107 CFU E. coli via intraperitoneal injection. After 15 h, lungs were removed, and excess lung water was determined as described in material and methods. Bars indicate means ± SE (n = 4–5). *P < 0.05 vs. WT control; **P < 0.05 vs. corresponding point with l-NIL pretreatment. B: WT or MPO−/− mice without or with pretreatment with 5 mg/kg l-NIL for 20 min were challenged with 109 E. coli via intraperitoneal injection. Kaplan-Meier curves were generated from experiments with a total of 20 mice per group. The increase in survival of WT mice and decrease in survival of MPO−/− mice with l-NIL pretreatment was significant (P < 0.05 by the log-rank test).
RESULTS
Mortality studies.
WT and MPO−/− mice were challenged with intraperitoneal injection of live E. coli (1 × 109 cells). Approximately 50% of WT mice died within 5 h after E. coli challenge, whereas all MPO−/− mice were alive at this time (Fig. 1A). All WT mice died within 8 h after challenge; in contrast, 20% of the MPO−/− mice were still alive at 12 h.
Lung microvascular permeability and edema formation.
Water content was continuously monitored in perfused lungs from control animals or mice challenged for 6 h with E. coli (1 × 108 cells). All mice were alive at 6 h after this dosage. The weight of lungs from control WT or MPO−/− mice remained stable over the 90-min perfusion, whereas lungs from WT mice treated with E. coli had significantly increased water accumulation after 60 min of monitoring, which continued to rise over 90 min (Fig. 1B). In contrast, lungs from MPO−/− mice showed almost no wet weight gain (Fig. 1B). This difference was particularly striking at 90 min. We determined pulmonary capillary filtration coefficient (Kf,c), a measure of vascular permeability. WT and MPO−/− mice demonstrated similar lung microvascular permeability values under basal condition (Fig. 1C). However, at 6 h after E. coli challenge, Kf,c increased significantly in both groups, but the response was threefold greater in lungs from WT than MPO−/− mice (Fig. 1C).
PMN sequestration in lungs.
After E. coli challenge, lung tissue MPO activity (a measure of PMN sequestration) in WT mice increased sixfold at 6 h; predictably, MPO activity was not detected in lungs from MPO−/− mice (data not shown). Thus we determined PMN sequestration in lungs of MPO−/− mice by morphometric analysis (Fig. 1D). PMN uptake in lungs of MPO−/− mice increased ninefold at 6 h after E. coli challenge, which was similar to the response in lungs of WT mice (Fig. 1D).
NO production.
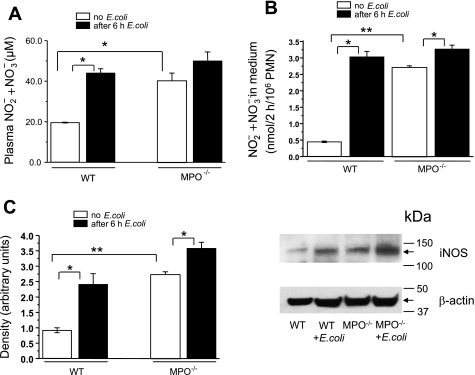
Blood plasma nitrite + nitrate concentration, the stable NO breakdown products, in MPO−/− mice before E. coli challenge was twofold greater than WT mice (Fig. 2A). At 6 h after E. coli challenge, plasma nitrite + nitrate concentration in WT mice increased twofold. E. coli challenge of MPO−/− mice slightly increased the nitrite + nitrate concentration, and it rose to the same final level as in WT mice (Fig. 2A). Because plasma nitrite + nitrate concentration only provides a general index of overall NO production that can be derived from several sources, we directly evaluated NO production and iNOS protein expression in two important targets, peripheral blood PMNs and lungs of WT and MPO−/− mice before and after E. coli challenge. To measure iNOS, which, once expressed, has calcium-independent constitutive activity that requires extracellular l-Arg (8), PMNs were incubated for 1 h in l-Arg-free medium to stop basal iNOS activity. l-Arg (1 mM) was then added to initiate iNOS-mediated NO production followed by incubation for an additional 3 h. Basal NO2− + NO3− produced by control PMNs from MPO−/− mice was sixfold greater than WT PMNs. E. coli challenge resulted in significant increase in NO production in both cases, but the increase was much greater in WT mice (Fig. 2B). Western blot analysis of iNOS protein showed PMNs from MPO−/− mice had much higher iNOS expression than WT mice and that E. coli challenge significantly increased in iNOS expression in both WT and MPO−/− PMNs with a larger increase seen in WT mice (Fig. 2C).
Fig. 2.
A: plasma nitrite + nitrate accumulation in WT and MPO−/− mice after E. coli challenge. Plasma nitrite + nitrate levels were measured before or 6 h after E. coli challenge (108 E. coli). Bars indicate means ± SE (n = 5). *P < 0.05 compared with WT before E. coli challenge. B: NO production from isolated peripheral blood PMN before and after E. coli challenge. PMNs, equilibrated for 60 min in l-arginine (l-Arg)-free medium, were incubated for 3 h in medium with 1 mM l-Arg, and nitrite + nitrate accumulation was measured. Bars indicate means ± SE (n = 5). *P < 0.05 compared with mice before E. coli challenge; **P < 0.05 compared with WT mice before E. coli challenge. C: E. coli-induced inducible nitric oxide synthase (iNOS) expression in mouse PMNs was measured by Western blot analysis. Right shows blot representative of 3 experiments. Left shows quantitation by densitometry, normalized for protein loading (β-actin staining). Bars indicate means ± SE (n = 3 each). *P < 0.05 compared with mice before E. coli challenge; **P < 0.05 compared with WT mice before E. coli challenge.
NO production in lungs of WT and MPO−/− mice.
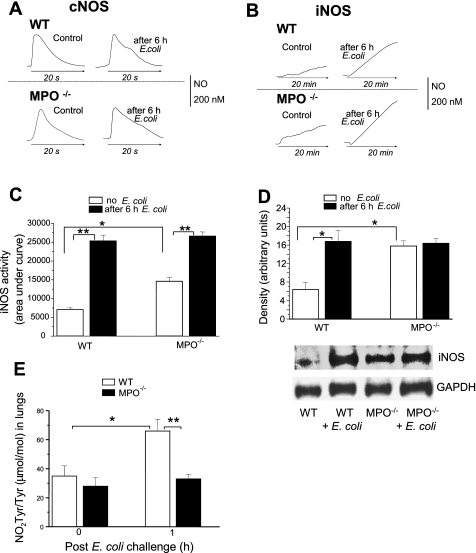
NO released from lungs could be an alternative source of increased NO2− in the blood of MPO−/− mice. To measure Ca2+-regulated constitutive isoforms e/nNOS (cNOS), lung slices were incubated in medium containing 1 mM l-Arg and then treated with calcium ionophore A-23187. This resulted in rapid increase of NO production that returned to baseline in 20 s (Fig. 3A). To measure the Ca2+-independent iNOS activity, lung slices were preincubated in Arg-free medium to stop iNOS activity, and then 1 mM l-Arg was added to initiate NO production. This resulted in a marked and prolonged increase in NO concentration still increasing at 20 min (Fig. 3B) followed by a plateau at 45–60 min and then slowly declining to baseline by 90 min (data not shown). Although the peak concentration of NO achieved by cNOS activity was similar to that derived from iNOS-dependent activity at 20 min, the total output of iNOS-derived NO was much greater because of the prolonged time course compared with the short, 20-s burst provided by cNOS (Fig. 3). cNOS activity in lungs of WT or MPO−/− mice was similar and did not change after E. coli challenge (Fig. 3A). In contrast, basal iNOS activity was twofold greater in lungs from MPO−/− mice compared with WT, and E. coli challenge in both cases resulted in significant increases in iNOS-dependent NO production (Fig. 3, B and C). Western blot analysis (Fig. 3D) showed that the basal iNOS protein level in lung tissue from MPO−/− mice was greater than WT and that E. coli challenge induced similar final levels of iNOS protein in lungs of WT and MPO−/− mice. In contrast, eNOS protein levels were similar in WT and MPO−/− lungs and did not increase after E. coli (data not shown), similar to our previous findings (36).
Fig. 3.
A: NO release in lungs of WT and MPO−/− mice. Representative tracings of constitutive NOS (cNOS)-dependent NO release stimulated by 10 μM A-23187. NO production was measured in real-time using a porphyrinic microsensor as described in materials and methods. B: representative tracings of iNOS-dependent NO release stimulated by application of 1 mM l-Arg. NO production was measured in real-time using a porphyrinic microsensor as described in materials and methods. C: quantification of iNOS-dependent NO release from lungs measured as in B. Bars indicate means ± SE of area under the NO curve for 20 min (n = 4–6). **P < 0.05 vs. mice before E. coli challenge; *P < 0.05 vs. WT control mice. D: Western blot analysis of E. coli-induced iNOS expression in lungs from WT and MPO−/− mice. Results are representative of 3 separate experiments. Densitometric quantitation of bands was normalized for protein loading by staining for GAPDH. Values are means ± SE (n = 3 each). *P < 0.05 vs. WT control. E: effects of E. coli challenge on lung nitrotyrosine in WT and MPO−/− mice. NO2-Tyr/Tyr ratio was measured by stable isotope dilution gas chromatography-mass spectrometry as described in materials and methods. Bars indicate means ± SE (n = 5). *P < 0.05 vs. WT control; **P < 0.05 vs. MPO−/− 1 h after E. coli challenge.
Nitrotyrosine generation in lungs.
Nitrotyrosine is generated by peroxynitrite (ONOO−) and ·NO2, an oxidant produced by MPO conversion of NO2− (12, 17). We evaluated nitrotyrosine production in lungs from WT and MPO−/− mice. The ratio of NO2-Tyr/Tyr increased from 35 ± 7 to 66 ± 8 1 h after E. coli challenge in WT lungs (Fig. 3E). In contrast, basal NO2-Tyr/Tyr ratio in MPO−/− mice did not differ from WT and did not increase 1 h after E. coli (Fig. 3E) indicating that MPO plays an important role in nitrotyrosine formation.
Effects of iNOS inhibition.
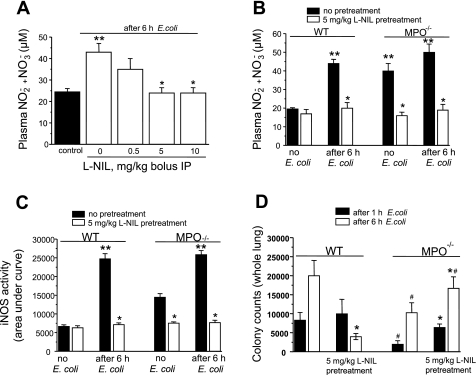
Treatment of WT mice with different doses of the iNOS-specific inhibitor l-NIL 20 min before E. coli challenge induced concentration-dependent decrease in sepsis-induced accumulation of plasma nitrite + nitrate (Fig. 4A) with a maximum effect seen at 5 mg/kg. Pretreatment with 5 mg/kg l-NIL did not change basal nitrite + nitrate levels in WT mice but reduced the elevated plasma nitrite + nitrate concentration in MPO−/− mice to that of WT mice (Fig. 4B), indicating that high basal nitrite + nitrate concentration in MPO−/− mice is derived from iNOS activity. l-NIL pretreatment also blocked nitrite + nitrate accumulation in response to E. coli in both WT and MPO−/− mice (Fig. 4B). Treatment with l-NIL also blocked iNOS activity in lungs of control MPO−/− mice and in WT and MPO−/− mice after E. coli challenge (Fig. 4C).
Fig. 4.
A: nitrite + nitrate production in blood plasma of WT mice after E. coli challenge. Different doses of l-N6-(1-iminoethyl)lysine (l-NIL) were administrated to WT mice 20 min before E. coli challenge, and blood plasma levels were measured at 6 h after E. coli. Bars indicate means ± SE (n = 3). **P < 0.05 vs. control; *P < 0.05 vs. 0 mg/kg l-NIL. B: effects of iNOS inhibition on nitrite + nitrate levels in plasma of WT and MPO−/− mice after E. coli challenge. WT and MPO−/− mice were pretreated without or with 5 mg/kg l-NIL for 20 min, and nitrite + nitrate levels were measured before or 6 h after E. coli challenge. l-NIL blocked the increase in plasma nitrite + nitrate in WT mice after E. coli and reduced basal and post-E. coli nitrite + nitrate levels in MPO−/− mice. Bars indicate means ± SE (n = 4–6). *P < 0.05 vs. without l-NIL pretreatment; **P < 0.05 vs. WT, no E. coli. C: iNOS activity in lungs from mice treated as in B was measured in real-time using a porphyrinic microsensor as described in material and methods. Data are presented as relative area under NO curve for 20 min. Bars indicate means ± SE (n = 4–6). *P < 0.05 vs. without l-NIL pretreatment; **P < 0.05 vs. no E. coli. D: lung bacterial clearance at 1 and 6 h following intraperitoneal administration of E. coli [2 × 108 colony-forming units (CFU)] in WT and MPO−/− mice were measured as described in materials and methods. Bars indicate means ± SE (n = 5). *P < 0.05 vs. corresponding point without l-NIL treatment; #P < 0.05 vs. corresponding point in WT mice.
Next, we compared the bacterial clearance capacity of WT and MPO−/− mice. The bacterial burden in lungs at 1 or 6 h after E. coli challenge was significantly less in MPO−/− mice compared with WT (Fig. 4D). Pretreatment with iNOS-specific inhibitor l-NIL significantly reduced bacterial colony counts 6 h after E. coli in WT mice, whereas in MPO−/− mice, the same pretreatment increased bacterial colony counts at 1 and 6 h compared with mice without iNOS inhibitor (Fig. 4D). These data suggest a role for iNOS-derived NO in bacterial clearance in MPO−/− mice.
To determine the role of iNOS-derived NO on lung injury, we measured the effect of E. coli challenge on excess lung water in animals treated with the iNOS inhibitor l-NIL. E. coli treatment resulted in increased lung edema in WT mice but did not cause a significant increase in MPO−/− mice (Fig. 5A). Interestingly, inhibition of iNOS led to a decrease in lung edema in WT mice after E. coli treatment, whereas in MPO-null mice, l-NIL resulted in increased edema (Fig. 5A), consistent with the effects of iNOS inhibition on bacterial clearance (Fig. 4D).
Because MPO deletion conferred a significant survival advantage to lethal E. coli challenge (Fig. 1A), we investigated the effect of iNOS inhibition on the response. Pretreatment of WT mice with l-NIL resulted in an increase in survival, whereas pretreatment of MPO−/− mice with l-NIL resulted in decreased survival (Fig. 5B). Taken together, these data suggest a role for iNOS-derived NO in MPO−/− mice that results in increased bacterial clearance, reduced lung injury, and increased survival in this model of E. coli-induced sepsis.
DISCUSSION
NADPH oxidase, MPO, and iNOS are important enzymes regulating antimicrobial host defense (see Refs. 22, 31, 41). MPO produces HOCl, a potent bactericidal agent that is also a proinflammatory oxidant capable of inducing tissue injury and inflammation (15, 22, 42). MPO also catalyzes nitrotyrosine formation and lipid peroxidation by converting NO2− to the potent oxidant ·NO2 (22, 30, 43). Despite the presumed importance of MPO in host defense, patients with MPO deficiency exhibit almost normal resistance to infection (15, 26). Although this led to claims that MPO does not play a crucial role in host defense, a more likely explanation is that compensatory mechanisms substitute for its loss (16, 22). However, the nature and relative importance of these compensatory effects remain controversial (16, 22).
In the present study, we used MPO-null mice to investigate mechanisms responsible for protection of the host in the absence of a functional peroxide-halide pathway. We observed, surprisingly, that MPO−/− mice had enhanced bacterial clearance, reduced lung injury and edema formation, as well as greater survival following E. coli challenge compared with WT mice. The results show that increased iNOS expression and NO production in MPO−/− mice were crucial in mitigating the effects of MPO deficiency. Several lines of evidence support this contention. First, basal expression of iNOS and NO production were significantly enhanced in PMNs and lungs of MPO−/− mice as reflected in the higher iNOS protein levels, iNOS activity in lung tissue and isolated PMNs, and plasma NO2− levels. Second, bacterial colony counts in lungs of MPO−/− mice were significantly lower than in WT mice at both 1 and 6 h after E. coli administration. Third, inhibition of iNOS significantly increased bacterial colony counts in lungs of MPO−/− mice after E. coli administration. Fourth, inhibition of iNOS significantly reduced the survival of MPO−/− mice in response to E. coli challenge.
High basal expression of iNOS in MPO-null mice could help to explain differences in survival seen in MPO−/− and WT mice after E. coli challenge. Lungs and PMNs of MPO−/− mice constitutively produced higher levels of NO than WT mice. The preexisting higher level of NO, which has antimicrobial activity (29, 40), may act to kill or slow the replication of the E. coli from the time of administration compared with WT mice in which iNOS upregulation was not maximal until 6 h. NO itself is generally not toxic to host tissue but may become damaging in combination with ROS (30), which would become prominent at later times during sepsis. Thus the high NO existing before E. coli challenge in MPO−/− mice in this case would be protective at the early stage of sepsis, whereas in WT mice, high NO generated later in the response could combine with ROS that are also present to cause host tissue damage (30). This possibility is consistent with our present finding that inhibition of iNOS results in less lung injury and increased survival in WT mice as well as our previous study (36) showing that NOS inhibition reduced macrophage inflammatory protein-2 (MIP-2) expression and lung edema associated with E. coli-induced sepsis in WT mice. Reactive oxygen and nitrogen species were also shown to impair the ability of the lung to clear alveolar fluid by inhibiting epithelial Na+ channels in mycoplasma-infected mice (19). Interestingly, MPO−/− mice maintained their ability to clear alveolar fluid after mycoplasma infection, likely due to lack of generation of reactive oxidants (19), consistent with our findings of reduced lung injury and edema in response to E. coli infection in MPO-deficient mice.
MPO-derived ·NO2 causes tyrosine nitration as well as lipid peroxidation, resulting in alteration of protein function, cell membrane injury, and generation of proinflammatory mediators (30, 43). Because NO terminates lipid peroxidation (20), and MPO scavenges and inactivates NO (1), MPO−/− mice are expected to have reduced lipid peroxidation. In our studies, we observed that E. coli challenge resulted in a marked increase in nitrotyrosine production in lungs of WT mice, but no increase was seen in MPO−/− mice. In addition to MPO-derived ·NO2, nitrotyrosine is generated from reaction of tyrosine residues with ONOO− derived from NO and superoxide (30). It was shown that MPO−/− mice injected intraperitoneally with thioglycolate or zymosan produced significantly less nitrotyrosine compared with WT mice (43). Thus, in some inflammatory models, MPO appears to play a dominant role in nitrotyrosine formation. In the E. coli sepsis model in the present study, however, ONOO− did not appear to contribute to nitration of tyrosine in MPO−/− lungs at 1 h after E. coli challenge despite high basal expression of iNOS and NO production. This finding helps to explain the reduction in lung inflammatory injury and mortality in MPO−/− mice compared with WT mice. In addition, the lack of HOCl production during sepsis in MPO−/− mice could reduce tissue injury compared with WT (22). These data are consistent with a previous study showing no difference in lung nitrotyrosine formation between WT and iNOS−/− mice after mycoplasma infection and a reduction in nitrotyrosine to uninfected levels in mice depleted of PMNs by cyclophosphamide, indicating the importance of PMN-derived MPO in this process (18). MPO also oxidizes glutathione (39), an important cellular antioxidant; thus augmentation of this antioxidant defense in MPO−/− mice could be another factor contributing to their protection.
We considered the possibility that alterations in eNOS could have contributed to the higher NO production and protection seen in MPO−/− mice. Studies have reported varying effects of inflammation on eNOS expression (7). For example, eNOS promoter activity and mRNA expression were reduced by TNF-α in cultured bovine endothelial cells (3) and human endothelial cells (25), whereas IFN/LPS treatment of bovine endothelial cells resulted in increased expression of eNOS mRNA (21). However, in our previous study (36) and the present study, we observed that eNOS immunoreactivity in mouse lungs remained constant for up to 6 h after in vivo E. coli challenge, indicating that eNOS expression is not altered in response to gram-negative septicemia. In addition, the iNOS-specific inhibitor, l-NIL, blocked the excess NO production in MPO−/− mice. Thus the present results cannot be explained by alterations in the activity of eNOS.
A key unresolved question is the mechanism of upregulation of iNOS expression in the MPO−/− mice. Studies showed that MPO inhibited iNOS expression in IFN-γ/LPS-treated monocyte macrophages, and it was proposed that MPO scavenges the low levels of NO that are necessary for the induction of iNOS (24). It is thus possible that iNOS is upregulated in MPO−/− mice secondary to absence of such an inhibitory effect of MPO on iNOS expression. However, this question has not been addressed in the MPO-null mouse model.
In summary, our data show that augmentation of iNOS-dependent NO production in the absence of MPO is a crucial compensatory mechanism regulating host defense and thus prevents lung inflammation and injury induced by E. coli septicemia in MPO-null mice.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-45638 and P01-HL-77806 to A. B. Malik, HL-60678 to R. A. Skidgel, and P01-HL-076491, P01-HL-77107, and HL-70621 to S. L. Hazen.
Acknowledgments
We thank Xiaoming Fu for help in carrying out the nitrotyrosine analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem 275: 37524–37532, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem 275: 5425–5430, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem 279: 963–969, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan C Of microbes, macrophages and nitric oxide. Behring Inst Mitt: 58–72, 1997. [PubMed]
- 5.Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest 107: 419–430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brovkovych V, Stolarczyk E, Oman J, Tomboulian P, Malinski T. Direct electrochemical measurement of nitric oxide in vascular endothelium. J Pharm Biomed Anal 19: 135–143, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Cirino G, Fiorucci S, Sessa WC. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol Sci 24: 91–95, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Closs EI, Scheld JS, Sharafi M, Forstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol 57: 68–74, 2000. [PubMed] [Google Scholar]
- 9.Cuzzocrea S, Mazzon E, Dugo L, Barbera A, Centorrino T, Ciccolo A, Fonti MT, Caputi AP. Inducible nitric oxide synthase knockout mice exhibit resistance to the multiple organ failure induced by zymosan. Shock 16: 51–58, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Dikshit M, Sharma P. Nitric oxide mediated modulation of free radical generation response in the rat polymorphonuclear leukocytes: a flowcytometric study. Methods Cell Sci 24: 69–76, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 296: 2391–2394, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391: 393–397, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Galijasevic S, Saed GM, Diamond MP, Abu-Soud HM. Myeloperoxidase up-regulates the catalytic activity of inducible nitric oxide synthase by preventing nitric oxide feedback inhibition. Proc Natl Acad Sci USA 100: 14766–14771, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao XP, Zhu X, Fu J, Liu Q, Frey RS, Malik AB. Blockade of class IA phosphoinositide 3-kinase in neutrophils prevents NADPH oxidase activation- and adhesion-dependent inflammation. J Biol Chem 282: 6116–6125, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92: 3007–3017, 1998. [PubMed] [Google Scholar]
- 16.Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun 64: 3512–3517, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazen SL, Zhang R, Shen Z, Wu W, Podrez EA, MacPherson JC, Schmitt D, Mitra SN, Mukhopadhyay C, Chen Y, Cohen PA, Hoff HF, Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res 85: 950–958, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Hickman-Davis JM, Lindsey JR, Matalon S. Cyclophosphamide decreases nitrotyrosine formation and inhibits nitric oxide production by alveolar macrophages in mycoplasmosis. Infect Immun 69: 6401–6410, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 173: 334–344, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogg N, Kalyanaraman B. Nitric oxide and lipid peroxidation. Biochim Biophys Acta 1411: 378–384, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kaku Y, Nanri H, Sakimura T, Ejima K, Kuroiwa A, Ikeda M. Differential induction of constitutive and inducible nitric oxide synthases by distinct inflammatory stimuli in bovine aortic endothelial cells. Biochim Biophys Acta 1356: 43–52, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Klebanoff SJ Myeloperoxidase: friend and foe. J Leukoc Biol 77: 598–625, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med 158: 1883–1889, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Kumar AP, Ryan C, Cordy V, Reynolds WF. Inducible nitric oxide synthase expression is inhibited by myeloperoxidase. Nitric Oxide 13: 42–53, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Lai PF, Mohamed F, Monge JC, Stewart DJ. Downregulation of eNOS mRNA expression by TNFalpha: identification and functional characterization of RNA-protein interactions in the 3′UTR. Cardiovasc Res 59: 160–168, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lanza F Clinical manifestation of myeloperoxidase deficiency. J Mol Med 76: 676–681, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Lowell C, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol 133: 895–910, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong ES, Gao XP, Xu N, Predescu D, Rahman A, Broman MT, Jho DH, Malik AB. E. coli pneumonia induces CD18-independent airway neutrophil migration in the absence of increased lung vascular permeability. Am J Physiol Lung Cell Mol Physiol 285: L879–L888, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Pacelli R, Wink DA, Cook JA, Krishna MC, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell JB. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J Exp Med 182: 1469–1479, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 76: 760–781, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Schmekel B, Venge P. The distribution of myeloperoxidase, eosinophil cationic protein, albumin and urea in sequential bronchoalveolar lavage. Eur Respir J 4: 517–523, 1991. [PubMed] [Google Scholar]
- 33.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine 79: 170–200, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Sethi S, Sharma P, Dikshit M. Nitric oxide- and oxygen-derived free radical generation from control and lipopolysaccharide-treated rat polymorphonuclear leukocyte. Nitric Oxide 5: 482–493, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Shepherd VL The role of the respiratory burst of phagocytes in host defense. Semin Respir Infect 1: 99–106, 1986. [PubMed] [Google Scholar]
- 36.Skidgel RA, Gao XP, Brovkovych V, Rahman A, Jho D, Predescu S, Standiford TJ, Malik AB. Nitric oxide stimulates macrophage inflammatory protein-2 expression in sepsis. J Immunol 169: 2093–2101, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol 37: 186–192, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci USA 101: 7699–7704, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsan MF, Turkall RM. Degradation and oxidation of methionine enkephalin by human neutrophils. J Reticuloendothel Soc 31: 353–360, 1982. [PubMed] [Google Scholar]
- 40.Virta M, Karp M, Vuorinen P. Nitric oxide donor-mediated killing of bioluminescent Escherichia coli. Antimicrob Agents Chemother 38: 2775–2779, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahl SM, McCartney-Francis N, Chan J, Dionne R, Ta L, Orenstein JM. Nitric oxide in experimental joint inflammation. Benefit or detriment? Cells Tissues Organs 174: 26–33, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Whiteman M, Chu SH, Siau JL, Rose P, Sabapathy K, Schantz JT, Cheung NS, Spencer JP, Armstrong JS. The pro-inflammatory oxidant hypochlorous acid induces Bax-dependent mitochondrial permeabilisation and cell death through AIF-/EndoG-dependent pathways. Cell Signal 19: 705–714, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 277: 46116–46122, 2002. [DOI] [PubMed] [Google Scholar]