Abstract
Interleukin-13 (IL-13) has been strongly implicated in the pathogenesis of allergic asthma through animal models that have shown that IL-13 is both necessary and sufficient to cause airway hyperresponsiveness (AHR). Airway smooth muscle (ASM) is a primary effector of AHR, and IL-13 increases the responsiveness of ASM, by increasing Ca2+ release intracellularly, to bronchoconstrictors such as histamine. The mechanisms and signaling pathways mediating this effect are incompletely understood. We have investigated the pathways through which IL-13 regulates the Ca2+ response to histamine in primary human ASM cell cultures. Functional IL-13 receptors were demonstrated by IL-13-mediated phosphorylation of signal transducer and activator of transcription 6 (STAT6) and mitogen-activated protein kinases (MAPKs). IL-13 increased Ca2+ responses to histamine. The augmentation of Ca2+ signaling was not affected by inhibition of STAT6 or p38 MAPK signaling but was prevented by concurrent inhibition of c-jun N-terminal kinase (JNK) and extracellular signal-related kinase (ERK) MAPKs. This inhibition did not affect the IL-13-induced increase in histamine receptors. We conclude that IL-13 induces potentiation of Ca2+ responses to contractile agonists by affecting mechanisms downstream of receptors. JNK and ERK MAPKs modulate these mechanisms.
Keywords: lung, allergy, inflammation, cytokines, mitogen-activated protein kinase
t helper type 2-biased immune responses are central to the pathogenesis of allergic asthma, and several lines of evidence, mainly from animal models, indicate that interleukin (IL)-13 plays a key role. IL-13-deficient mice are protected from allergen-induced airway hyperresponsiveness (AHR; Ref. 31), whereas administration of IL-13 leads to the development of AHR (11, 19). Similarly, intratracheal administration of IL-13 to mice causes AHR through a leukotriene D4-dependent pathway (30), and the inhibition of IL-13 through the administration of the soluble IL-13 receptor (IL-13Rα2) reverses ovalbumin-induced AHR (22, 32). In human subjects, polymorphisms in the IL-13 gene have been associated with altered plasma levels of IL-13 and with atopy (14, 15). Increased levels of IL-13 are also found in the sputum of asthmatic subjects and correlate with bronchial responsiveness to methacholine (24).
Several studies indicate that IL-13 may cause AHR independently of inflammation, and it is plausible that the effect may result from a direct effect on airway smooth muscle (ASM). ASM cells express functional IL-13 receptors and respond to IL-13 by secreting eotaxin (12) and IL-5 (9). IL-13 is also linked to an augmented ASM contractility in rabbit tracheal strips (9) and to an impaired relaxation of human cultured ASM cells by β-agonists (20). The effects of IL-13 on contractility can, at least partially, be explained by altered intracellular calcium Ca2+ signaling, since it has been shown that IL-13 causes increased Ca2+ mobilization in response to bronchoactive agents in murine and human ASM cells (5) (28). In the latter case, the effect of IL-13 is due to an increased expression of CD38, a bifunctional membrane-bound protein linked to Ca2+ release from intracellular stores (3, 11).
IL-13 triggers several signaling pathways, and published data imply that different effects of IL-13 are mediated through discrete mechanisms. IL-13 is known to act by phosphorylation of Janus-activating kinases (JAK1 and JAK2) and protein tyrosine kinase 2 (TYK2) and subsequent phosphorylation of the transcription factor signal transducer and activator of transcription 6 (STAT6; Refs. 16, 18). IL-13 also triggers signaling involving mitogen-activated protein kinases (MAPK). In human ASM, the stimulation of eotaxin secretion by IL-13 is mediated through STAT6 as well as two MAPKs: p38 and extracellular signal-related kinase (ERK; Ref. 12). The IL-13 effect on β-adrenergic responsiveness in ASM is abrogated by ERK pathway inhibition (20). IL-13 signaling pathway(s) involved in potentiation of ASM calcium responses have not been elucidated. The objective of the current study was to investigate which signaling pathways mediate the effects of IL-13 on human ASM cell calcium signaling in response to histamine.
MATERIALS AND METHODS
Cell cultures.
Primary cultures of human ASM cells were prepared from lung transplant specimens as described previously (8). Briefly, tissue digestion was performed by gently shaking the tissues in HBSS (in mM: 5 KCl, 0.3 KH2PO4, 138 NaCl, 4 NaHCO3, and 5.6 Na2HPO4), containing collagenase type IV (0.4 mg/ml), elastase (0.38 mg/ml), and soybean trypsin inhibitor (1 mg/ml) at 37°C for 90 min. The dissociated cells were collected by filtering through 125-μm Nytex mesh followed by centrifugation. The pellet was reconstituted in growth medium consisting of DMEM-Ham's F-12 medium supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin, and cells were plated in a 25-cm2 flask. Confluent cells were detached with a 0.025% trypsin solution containing 0.02% EDTA. Cells were grown on 25-mm glass coverslips for single cell imaging of Ca2+ transients and in sixwell culture dishes for protein and RNA extraction. The cells were growth arrested 24 h before experiments by being incubated in medium without FBS, supplemented with 1% BSA, 5.7 mg/l insulin, and 5 mg/l transferrin.
Western blotting.
STAT6 and histamine 1 (H1) receptor expression and STAT6 and MAPK phosphorylation was quantified by Western analysis. Cells were incubated with IL-13 for different times as indicated in results, washed with ice cold PBS with sodium orthovanadate, and lysed with lysis buffer (62.5 mM Tris·HCl, pH 6.8, 2% SDS, 10% glycerol, and 50 mM DTT) on ice. Electrophoresis was carried out by using 8 or 12% polyacrylamide gels, and proteins were transferred to nitrocellulose or polyvinylidene difluoride membranes. Membranes were blocked for 1 h at room temperature with 5% powdered milk in Tris·HCl buffer containing Tween 20 (TTBS) or 1% BSA with EDTA and NaCl in TTBS. Primary antibodies were diluted in TTBS and added as follows: anti-STAT6 and anti-phospho-STAT6 (rabbit polyclonal IgG, dilution 1:1,000; Upstate, Lake Placid, NY); anti-β-actin (mouse monoclonal, dilution 1:10,000; Sigma-Aldrich, St, Louis, MO); anti-JNK and anti-phospho-JNK, anti-ERK 1/2, anti-phospho-ERK1/2, and anti-phospho-p38 (rabbit polyclonal IgG, dilution 1:1,000; Cell Signaling Technology, Danvers, MA); and anti-H1 receptor (rabbit polyclonal and goat polyclonal, dilution 1:2,000 to 1:500; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were incubated with primary antibodies overnight at 4°C followed by incubation with secondary antibodies [goat anti-rabbit IgG horseradish perioxidase (HRP) conjugated (dilution 1:1,500; Upstate,); donkey anti-mouse IgG HRP conjugated (dilution 1:10,000; Jackson ImmunoResearch Laboratories, West Grove, PA); or donkey anti-goat IgG HRP conjugated (dilution 1:2,000 to 1:10,000, as appropriate; Santa Cruz Biotechnology)] at room temperature for 1 h. Blots were developed by chemiluminescence (Amersham Bioscience, Buckinghamshire, England) and visualized using a Fluoro 800 Advanced Fluorescence Imager (Alpha Innotech, Montreal, QC, Canada), and the densitometry was performed with commercial software (Fluorochem). Resting levels of phosphorylation were assessed in vehicle-treated cells. Negative controls omitting primary antibody were performed for all primary antibodies.
Measurement of intracellular Ca2+.
Cytosolic calcium measurements were performed as described previously (29). ASM cells were incubated for 30 min at 37°C with HBSS (in mM: 137 NaCl, 4.2 NaHCO3, 10 glucose, 3 Na2HPO4, 5.4 KCl, 0.4 KH2PO4, 1.3 CaCl2, 0.5 MgCl2, 0.8 MgSO4, and 5 HEPES) in the presence of 5 μM fura-2-acetoxymethylester. The loaded cells were washed, and the coverslips were placed in a Leiden chamber (Medical Systems, Greenville, NY) containing 450 μl of HBSS on the stage of an inverted microscope equipped for cell imaging with a ×40 oil immersion objective (Nikon, Tokyo, Japan). The images of the cells before and after stimulation with histamine were obtained using an intensified camera (Videoscope IC 200) and PTI (Photon Technology International, London, ON, Canada) software at a single emission wavelength (510 nm) with double excitatory wavelengths (345 and 380 nm). The fluorescence ratio (345/380) was measured in individual cells (n = 8 per slide), and the free Ca2+ was calculated using Grynkiewicz's formula (10).
Inhibition of IL-13 signaling.
Several specific inhibitors were used to investigate the role of different signaling pathways in IL-13-augmented histamine signaling. All inhibitors were given for 1 h before treatment with IL-13 or vehicle. All experiments were repeated at least three times, and total of 100–200 cells was analyzed for each treatment. To inhibit JNK activation, we used SP600125 (20 μM; Tocris Cookson, Ellsville, MO) and cell-permeable biologically active JNK blocking peptide (25 μM; Calbiochem, San Diego, CA) as well as a related peptide (1–100 μM; Biomer Technology Hayward, CA) in which the HIV-TAT protein transduction domain was replaced by PTD4 protein transduction domain (13). Vehicle or control peptides, respectively, were used as controls. The sequences were as follows: inhibitory peptide: YARAAAQARA-GG-RPK TLNLEP QVPRSQ DT; and control peptide: YARAAAQARA-GG-RPK GGNGEP QVPRSQ DT. To assess the role of other MAP kinases cells were treated with p38 inhibitor SB202190 (3 and 30 μM; Calbiochem, San Diego, CA) or a corresponding control molecule SB202474 and the MEK inhibitor U0126 (1 μM; Cell Signaling Technology). The JAK-STAT pathway was inhibited by the JAK2 inhibitor AG490 (50 μM; Calbiochem) or the STAT6 inhibitor leflunomide (100 μM; Sigma-Aldrich). To target the STAT6 pathway more specifically, a previously published antisense methodology (12) was used. Briefly, growth-arrested, subconfluent cells were transfected using FuGENE (Roche, Indianapolis, IN) with antisense, scrambled, or sense oligodeoxynucletides (Invitrogen, Burlington, ON, Canada). The oligodeoxynucletides sequences were as follows: antisense: 5′-gtG AGG TCC TGT TCA Gtg gg-3′; sense: 5′-ccC ACT GAA CAG GAG Ctc ac-3′; and scrambled: 5′-caC TCC AGG ACA AGT Cac cc-3′ (lowercase denotes phosphorothioate-modified bases).
Real-time quantitative PCR for H1 receptor.
To investigate the mRNA expression of H1 receptor, we performed real-time quantitative PCR using standard techniques. Briefly, total RNA was extracted from cultured ASM cells, and real-time PCR was performed using a LightCycler (Roche Diagnostic, Mannhein, Germany). Reaction mixtures contained 2 μl of LightCycler FastStart DNA master mixture for SYBR green I (Roche Diagnostic), 0.5 μM each primer, 4 mM MgCl2, and 1 μl of cDNA template. After activation of polymerase (95°C for 5 min), 45 cycles were run with 5-s denaturation at 95°C, 8-s annealing (at 69°C for H1 receptor, 55°C for S9), and extension at 72°C (for 8 s for H1 receptor and 13 s for S9). The double-stranded PCR product was quantified using SYBR green (Qiagen, Mississauga, ON, Canada). The expression of H1 receptor mRNA was standardized by the housekeeping gene S9, and the method of normalization was based on generating standard curves for each gene using PCR products of known copy number.
The primer design for human H1 receptor (Invitrogen, Burlington, ON, Canada) was based on published sequences (7). The sequences of each pair of primers were as follows: H1 receptor, forward: 5′-AAGTCACCATCCCAAACCCCCAAG-3′; and reverse: 5′-TCAGGCCCTGCTCATCTGTCTTGA-3′. The size of the PCR product was 196 bp. Ribosomal protein S9, forward: 5′-TGCTGACGCTTGATGAGAAG-3′; and reverse: 5′-CGCAGAGAGAAGTCGATGTG-3′. The size of the PCR product was 307 bp.
Statistical analysis.
ANOVA and the post hoc Newman-Keuls test were applied to compare multiple means, unless data were not normally distributed in which case nonparametric equivalent tests were used. Values of P < 0.05 were considered significant.
The project was approved by the Institutional Review Board of McGill University Hospital Center and the University of Montreal Hospital Center. Signed informed consent was obtained from cell donors.
RESULTS
IL-13 responsiveness of human ASM cells.
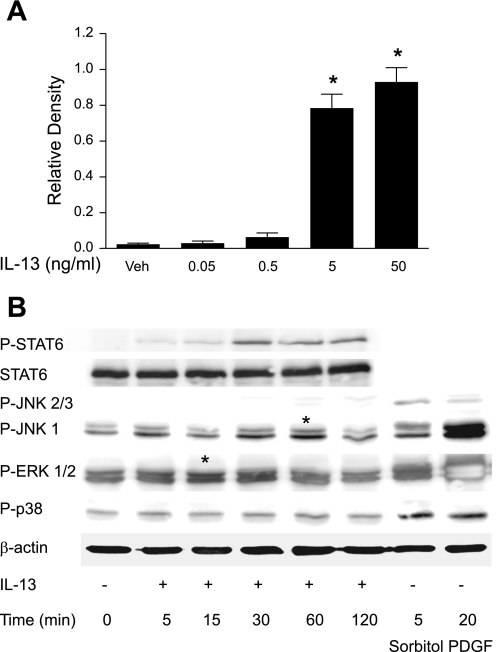
To confirm that ASM cells were capable of responding to IL-13 stimulation, we performed Western analysis for phosphorylated STAT6. We observed robust phosphorylation of STAT6 by 5 ng/ml of IL-13 (Fig. 1A) and chose this concentration for the subsequent studies. STAT6 phosphorylation reached a peak at 30 min and was still detectable at 2 h poststimulation (Fig. 1B). IL-13 activation of both JNK and ERK MAPK pathways was also evident, but no significant activation of p38 MAPK could be demonstrated at the time points tested (Fig. 1B).
Fig. 1.
IL-13-induced activation signal transducer and activator of transcription 6 (STAT6) and MAPK. A: STAT6 phosphorylation in human airway smooth muscle (HASM) cells treated for 30 min with different concentrations of IL-13. Densitometry measurements were obtained from 3 blots representing independent experiments. *P < 0.05 vs. vehicle (Veh)-treated group. B: representative time course of STAT6 and MAPKs ( ERK1/2, p38, and JNK) phosphorylation in HASM cells incubated with 5 ng/ml IL-13. Sorbitol and PDGF were used as positive controls. Similar results were obtained in 2 other experiments. *Bands representing increased phosphorylation of ERK and JNK MAPK.
Effect of IL-13 on histamine-induced Ca2+ signals in human ASM.
Acute application of IL-13 alone did not lead to the mobilization of intracellular Ca2+ in human ASM cells. A 24-h exposure to IL-13 did not change baseline levels of Ca2+ but affected histamine-induced Ca2+ transients. Histamine induced a typical, rapid increase in Ca2+ followed by a sustained elevated plateau (Fig. 2A). The peak Ca2+ signals evoked by submaximal concentrations of histamine (10−7 M to 10−5 M) were significantly greater in IL-13-pretreated cells (Fig. 2B).
Fig. 2.
Effect of IL-13 on histamine-induced Ca2+ responses of HASM cells. A: representative curve showing Ca2+ response of a HASM cell to 10−6 M histamine. B: mean peak Ca2+ responses to different concentrations of histamine in cells treated for 24 h with vehicle (PBS/BSA) or 5 ng/ml IL-13. *P < 0.05 vs. vehicle-treated group.
Effects of inhibition of IL-13 signaling pathways on augmented histamine signaling.
To identify which signaling pathways regulate IL-13-dependent increases in Ca2+ responses, the cells were treated with inhibitors of the JAK-STAT and MAPK pathways. A STAT6 inhibitor leflunomide (100 μM) and JAK2 inhibitor AG490 (50 μM) failed to inhibit the IL-13- induced potentiation of Ca2+ signals (Fig. 3, A and B), although both inhibitors reduced STAT6 phosphorylation induced by IL-13 (Fig. 3C). Similarly, the antisense oligonucleotide to STAT6, despite reducing STAT6 expression (Fig. 3D) did not affect IL-13-induced potentiation of the Ca2+ signal in response to histamine (Fig. 3E), although it reduced Ca2+ responses in both IL-13 and control groups.
Fig. 3.
Effect of JAK/STAT signaling pathway inhibitors on IL-13-augmented Ca2+ responses to histamine. The inhibitors were added 1 h before administration of IL-13 (5 ng/ml). Phosphorylation was assessed by Western blotting after 30 min and Ca2+ responses to 10−6 M histamine after 24 h of IL-13 treatment. A: Ca2+ responses in the absence and presence of the STAT6 inhibitor leflunomide (100 μM). *P < 0.05 vs. vehicle (PBS/BSA)-treated cells. B: Ca2+ responses in the absence and presence of the JAK 2 inhibitor AG490 (50 μM). *P < 0.05 vs. vehicle (PBS/BSA)-treated cells. C: effect of leflunomide and AG490 on IL-13-induced STAT6 phosphorylation. Similar results were obtained in 3 experiments. D: STAT6 expression in cells treated with sense, antisense, or scrambled STAT6 oligodeoxynucletides (ODN). Expression of β-actin was used as a control. E: Ca2+ responses of cells treated with STAT6 ODNs. *P < 0.05 vs. vehicle-treated groups.
We also assessed the possible involvement of MAPKs in regulating this response. The JNK inhibitor SP600125 effectively abrogated IL-13-induced potentiation of the Ca2+ signal evoked by histamine (Fig. 4A). To target JNK more specifically, we used a cell-permeable JNK blocking peptide at the concentration known to inhibit c-jun activation (2). Surprisingly, it did not affect IL-13-induced augmentation of Ca2+ responses to histamine (Fig. 4B). Similarly, a peptide with a different transduction domain also had no effect on the augmented Ca2+ responses to histamine at concentrations up to 100 μM (data not shown). Western blotting showed that SP600125 at the concentration regarded as specific for JNK and used in our Ca2+ measurements, as well as at a lower concentration, inhibited not only JNK (Fig. 4C) but also ERK activation (Fig. 4, D and E), suggesting that the inhibitory effect may be due to its effect on the ERK pathway. To test the involvement of ERK MAPK, we used U0126, an inhibitor of the upstream kinase MEK. U0126, at the concentration previously shown to inhibit ERK in human ASM cells (21), did not abolish the IL-13-dependent augmentation of peak Ca2+ responses to histamine, although it caused a decrease in responses in both groups (Fig. 5). This suggests that the activation of both ERK and JNK are necessary for IL-13 to augment Ca2+ responses to histamine. To verify this, the ERK pathway inhibitor U0126 and JNK inhibitory peptide were both added to cell cultures 1 h before IL-13 treatment. Simultaneous inhibition of ERK and JNK pathways resulted in almost complete inhibition of IL-13-induced augmentation of Ca2+ responses to histamine (Fig. 6). Consistent with the lack of effect of IL-13 on p38 MAPK phosphorylation, the p38 inhibitor SB202190 did not affect IL-13-induced augmentation of Ca2+ responses (Fig. 7 ).
Fig. 4.
Effect of JNK inhibition on Ca2+ responses to histamine in control and IL-13 treated cells. A: effect of pharmacological JNK inhibitor, SP600125 (20 μM) on Ca2+ responses in control and IL-13-treated cells. *P < 0.05 vs. vehicle-treated group. B: The effect of control and JNK inhibitory peptides on Ca2+ responses in control and IL-13-treated cells. *P < 0.05 vs. vehicle-treated groups. C: representative example showing effect of SP600125 on JNK phosphorylation. D: representative example showing effect of SP600125 on ERK phosphorylation. E: densitometry measurements of IL-13-induced ERK phosphorylation in cells treated with JNK inhibitor, SP600125 in 3 independent experiments. *P < 0.05 vs. vehicle-treated group; #P < 0.05 vs. IL-13-treated group.
Fig. 5.
Effect of ERK inhibition on Ca2+ responses to histamine in control and IL-13-treated cells. U0126 significantly affected the histamine response in both control and IL-13-treated cells, but there was no statistically significant effect on the IL-13 response per se. *P < 0.05 vs. vehicle-treated group.
Fig. 6.
Combined effect of the JNK inhibitory peptide and ERK inhibitior U0126 on Ca2+ responses to histamine. *P < 0.05 vs. IL-13-treated group in the absence of inhibitors.
Fig. 7.
Effect of the p38 inhibitor SB202190 (1 μg/ml) on Ca2+ responses to histamine in control and IL-13-treated cells. SB202474 was used as a negative control. *P < 0.05 vs. vehicle-treated group.
To test whether the effect of combined JNK and ERK inhibition on IL-13-induced augmentation of Ca2+ signaling is due to modulation of histamine receptor expression, we examined H1 receptor expression in control and IL-13-treated cells in the absence or presence of SP600125. Although IL-13 increased the expression of H1 receptor mRNA (Fig. 8A), inhibition of JNK/ERK did not affect it (Fig. 8B). Due to the poor quality of commercially available antibodies, we were not able to show convincing H1 receptor expression at the protein level. Thus we do not know if IL-13 induces an increase of H1 receptor protein nor if JNK/ERK inhibition affects it.
Fig. 8.
Effect of IL-13 on H1 receptor expression in the presence and absence of SP600125. A: cells were treated with 5 ng/ml of IL-13 for different time points, and H1 mRNA expression was measured by RTPCR and expressed as a ratio to housekeeping gene. B: cells were treated with SP600125 or vehicle 1 h before application of IL-13, and H1 expression was assessed 12 h later. *P ≤ 0.05 vs. vehicle control. Data are means of 5 experiments.
DISCUSSION
IL-13 is one of the putative mediators of AHR in asthma. In this study, we explored the mechanisms that mediate the IL-13-induced increase in human ASM cells Ca2+ responses to histamine. We demonstrate that 24-h exposure to IL-13 at relatively low concentrations, and therefore likely of physiological relevance, is sufficient to affect Ca2+ mobilization in ASM cells. This effect of IL-13 is regulated by coordinated activity of the MAPK (JNK and ERK) enzymes.
IL-13 has been strongly linked to AHR based on studies of animal models. IL-13-deficient mice are protected from allergen-induced AHR (31), and the effects of IL-13 are notably independent of eosinophilia (5). The latter effect can be explained by IL-13 affecting ASM directly. Indeed, we have previously shown that in murine ASM IL-13 itself causes contraction and Ca2+ mobilization, as well as augmentation of Ca2+ responses to 5-HT and leukotriene D4 (5). In the present study, we have observed that in human ASM cells, IL-13 does not have a direct Ca2+-mobilizing effect but, consistent with previously published data, induces augmentation of Ca2+ response to histamine (28). These data indicate that there are significant differences between murine ASM and HUMAN ASM responsiveness to IL-13. Furthermore, the potentiating effect of IL-13 on responses to contractile agonists in human ASM does not seem to be related to the mechanisms of Ca2+ mobilization in murine cells.
The signaling pathways activated by IL-13 and important for modulation of Ca2+ responses to contractile agonists are largely unexplored. It has been proposed that JAK/STAT6 pathway inhibition may be a relatively specific target for novel therapeutic strategies in asthma (19), and the importance of this signaling pathway in mediating certain direct effects of IL-13 on ASM is supported by the observation that STAT6 activation is involved in eotaxin secretion in cultured ASM cells (25). Consistent with other studies, we have confirmed that IL-13 induces STAT6 activation in human ASM cells. However, partial inhibition of STAT6 phosphorylation (activation) by leflunomide or of STAT6 expression by antisense oligodeoxynucleotides did not affect IL-13-induced augmentation of Ca2+ signaling even though the same antisense methodology effectively inhibited IL-13-induced eotaxin secretion (25). Furthermore, inhibition of JAK2, which resulted in a significant decrease of STAT6 activation by IL-13, did not affect modulation of Ca2+ responses either. These observations suggest that the JAK/STAT pathway does not play a major role in IL-13-induced potentiation of Ca2+ signaling. Of interest in this regard is the finding that not all animal models could confirm the necessity of STAT6 activation for AHR. Although STAT6-deficient mice have been shown to be protected from allergen-induced AHR (19), STAT6-dependent mechanisms of AHR appear to be more important for acute models (33) and may not be essential for chronic models (6). A chronic model based on low level exposure to ovalbumin demonstrated IL-4/IL-13-dependent but STAT6-independent AHR and airway remodeling (6). Similarly, repeated exposures to Aspergillus fumigatus induced IL-13-dependent AHR and airway fibrosis in STAT6 deficient mice (1). However, the contribution of this pathway in the regulation of ASM Ca2+ responses cannot be completely excluded given that there was only partial inhibition of STAT6 by the strategies used in the current study.
IL-13 also signals through activation of several MAP kinases, namely ERK, p38, and JNK. U0126, the inhibitor of MEK, the kinase immediately upstream of ERK has recently been shown to reduce AHR in a murine model of asthma, although this effect was attributed to the observed decrease in airway inflammation (4). ERK and p38 kinases are involved in IL-13-induced eotaxin release (12), and ERK activation mediates a IL-13-dependent decrease in ASM cell relaxation (20). Our results indicate that IL-13-induced augmentation of Ca2+ signaling in response to contractile agents requires activation of both ERK and JNK and that inhibition of either of these kinases alone is not sufficient to abrogate the effects of IL-13. Interestingly, it has been recently reported that JNK is involved in the regulation of TNF-α-induced increased expression of CD38, a molecule implicated in increased Ca2+ mobilization, at a transcriptional level, and ERK at a posttranscriptional level (27). The importance of this mechanism for AHR is supported by the observation that the pharmacological inhibitor of JNK, SP600125, inhibits AHR in murine models (23). However, our study demonstrates that this inhibitor blocks activation of both JNK and ERK MAPKs and abrogates IL-13-induced increases in Ca2+ responses to contractile agonist.
Since gene chip experiments have suggested that the H1 receptor is an IL-13-regulated gene (17, 26), one could postulate that IL-13-mediated potentiation of Ca2+ responses is controlled by regulation of receptor expression. Our data confirm that IL-13 increases the expression of histamine H1 mRNA by about twofold but inhibition of JNK and ERK by SP600125, in concentrations sufficient to abrogate augmentation of Ca2+ responses, does not affect H1 receptor mRNA expression. This observation indicates that different signaling pathways are responsible for Ca2+ responses and receptor expression and suggest that augmentation of the Ca2+ signal does not result from the increased number of receptors. However, we cannot exclude that H1 receptor expression may be regulated by ERK at posttranscriptional level similar to expression of CD38 (27).
In conclusion, we have shown that low concentrations of IL-13 augment histamine-induced calcium transients in HUMAN ASM through coordinate actions of JNK and ERK signaling pathways, modulating the response downstream of the receptor. Our findings support the potential use of JNK and ERK1/2 inhibition in preventing IL-13-induced alterations in ASM contractile responses.
GRANTS
This work was supported by Canadian Institutes of Health Research Grant MOP-77749 and the JT Costello Memorial Fund.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blease K, Schuh JM, Jakubzick C, Lukacs NW, Kunkel SL, Joshi BH, Puri RK, Kaplan MH, Hogaboam CM. Stat6-deficient mice develop airway hyperresponsiveness and peribronchial fibrosis during chronic fungal asthma. Am J Pathol 160: 481–490, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9: 1180–1186, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 31: 36–42, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Anti-inflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol 172: 7053–7059, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Eum SY, Maghni K, Tolloczko B, Eidelman DH, Martin JG. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288: L576–L584, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Foster PS, Webb DC, Yang M, Herbert C, Kumar RK. Dissociation of T helper type 2 cytokine-dependent airway lesions from signal transducer and activator of transcription 6 signalling in experimental chronic asthma. Clin Exp Allergy 33: 688–695, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Gantner F, Sakai K, Tusche MW, Cruikshank WW, Center DM, Bacon KB. Histamine h(4) and h(2) receptors control histamine-induced interleukin-16 release from human CD8(+) T cells. J Pharmacol Exp Ther 303: 300–307, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Govindaraju V, Michoud MC, Al Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol 291: C957–C965, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Grunstein MM, Hakonarson H, Leiter J, Chen M, Whelan R, Grunstein JS, Chuang S. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 282: L520–L528, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 11.Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 291: L1286–L1293, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hirst SJ, Hallsworth MP, Peng Q, Lee TH. Selective induction of eotaxin release by interleukin-13 or interleukin-4 in human airway smooth muscle cells is synergistic with interleukin-1beta and is mediated by the interleukin-4 receptor alpha-chain. Am J Respir Crit Care Med 165: 1161–1171, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res 61: 474–477, 2001. [PubMed] [Google Scholar]
- 14.Howard TD, Whittaker PA, Zaiman AL, Koppelman GH, Xu J, Hanley MT, Meyers DA, Postma DS, Bleecker ER. Identification and association of polymorphisms in the interleukin-13 gene with asthma and atopy in a Dutch population. Am J Respir Cell Mol Biol 25: 377–384, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Hunninghake GM, Soto-Quiros ME, Avila L, Su J, Murphy A, Demeo DL, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Lange C, Raby BA, Silverman EK, Celedon JC. Polymorphisms in IL13, total IgE, eosinophilia, and asthma exacerbations in childhood. J Allergy Clin Immunol 120: 84–90, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ip WK, Wong CK, Lam CW. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin Exp Immunol 145: 162–172, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarai G, Sukkar M, Garrett S, Duroudier N, Westwick J, Adcock I, Chung KF. Effects of interleukin-1beta, interleukin-13 and transforming growth factor-beta on gene expression in human airway smooth muscle using gene microarrays. Eur J Pharmacol 497: 255–265, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol 105: 1063–1070, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med 187: 939–948, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA Jr, Kinet JP, Shore SA. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med 164: 141–148, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Liang KC, Lee CW, Lin WN, Lin CC, Wu CB, Luo SF, Yang CM. Interleukin-1beta induces MMP-9 expression via p42/p44 MAPK, p38 MAPK, JNK, and nuclear factor-kappaB signaling pathways in human tracheal smooth muscle cells. J Cell Physiol 211: 759–770, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Morse B, Sypek JP, Donaldson DD, Haley KJ, Lilly CM. Effects of IL-13 on airway responses in the guinea pig. Am J Physiol Lung Cell Mol Physiol 282: L44–L49, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Nath P, Eynott P, Leung SY, Adcock IM, Bennett BL, Chung KF. Potential role of c-Jun NH2-terminal kinase in allergic airway inflammation and remodelling: effects of SP600125. Eur J Pharmacol 506: 273–283, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Park SW, Jangm HK, An MH, Min JW, Jang AS, Lee JH, Park CS. Interleukin-13 and interleukin-5 in induced sputum of eosinophilic bronchitis: comparison with asthma. Chest 128: 1921–1927, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Peng Q, Matsuda T, Hirst SJ. Signaling pathways regulating interleukin-13-stimulated chemokine release from airway smooth muscle. Am J Respir Crit Care Med 169: 596–603, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Syed F, Panettieri RA Jr, Tliba O, Huang C, Li K, Bracht M, Amegadzie B, Griswold D, Li L, Amrani Y. The effect of IL-13 and IL-13R130Q, a naturally occurring IL-13 polymorphism, on the gene expression of human airway smooth muscle cells. Respir Res 6: 9, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-alpha induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-κB and AP-1. Am J Physiol Lung Cell Mol Physiol 292: L1385–L1395, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Tliba O, Deshpande D, Chen H, Van Besien C, Kannan M, Panettieri RA Jr, Amrani Y. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br J Pharmacol 140: 1159–1162, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolloczko B, Turkewitsch P, Choudry S, Bisotto S, Fixman ED, Martin JG. Src modulates serotonin-induced calcium signaling by regulating phosphatidylinositol 4,5-bisphosphate. Am J Physiol Lung Cell Mol Physiol 282: L1305–L1313, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Vargaftig BB, Singer M. Leukotrienes, IL-13, and chemokines cooperate to induce BHR and mucus in allergic mouse lungs. Am J Physiol Lung Cell Mol Physiol 284: L260–L269, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 167: 4668–4675, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 282: 2258–2261, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol 25: 522–530, 2001. [DOI] [PubMed] [Google Scholar]