Abstract
The enzymatic activity of CD38, ADP-ribosyl cyclase, synthesizes the calcium mobilizing molecule cyclic ADP-ribose from β-NAD. In human airway smooth muscle (HASM) cells, CD38 expression is augmented by the inflammatory cytokine, TNF-α, causing increased intracellular calcium response to agonists. The transcriptional and posttranscriptional regulation of CD38 expression involves signaling through MAPKs and requires activation of NF-κB and activator protein-1 (AP-1). The cytokine-augmented CD38 expression is decreased by anti-inflammatory glucocorticoids due to inhibition of NF-κB activation and other mechanisms. In this study, we investigated glucocorticoid regulation of CD38 expression in HASM cells through the MKP-1. In HASM cells, dexamethasone and TNF-α induced MKP-1 expression (both mRNA and protein) rapidly. Dexamethasone decreased TNF-α-induced phosphorylation of the major MAPKs, i.e., ERK, p38, and JNK, and decreased the activation of NF-κB and AP-1. Dexamethasone also decreased CD38 expression induced by TNF-α, and part of this effect was attributable to decreased transcript stability. In cells transfected with MKP-1-specific small interfering RNAs (siRNAs), there was significant attenuation of MKP-1 expression and partial, but nonsignificant, reversal of dexamethasone inhibition of CD38 expression. These results indicate that regulation of CD38 expression in HASM cells by glucocorticoids involves decreased signaling through MAPKs and activation of transcription factors. The glucocorticoid effects on decreased CD38 expression and function result from regulation through transcription and transcript stability.
Keywords: mitogen-activated protein kinases, nuclear factor-κB, activator protein-1, adenosine 5′-diphosphate-ribosyl cyclase, tumor necrosis factor-α
glucocorticoids are widely used as immunosuppressive and anti-inflammatory agents. Synthetic glucocorticoids are useful in the treatment of inflammatory diseases such as asthma, rheumatoid arthritis, Crohn's disease, and chronic allergic diseases. The anti-inflammatory effects of the glucocorticoids are mediated by at least two distinct mechanisms: 1) binding to the glucocorticoid response elements (GREs) within the promoter regions of genes as a transcription factor to activate transcription; and 2) as an inhibitor of transcription factors such as NF-κB and activator protein-1 (AP-1) through a protein-protein interaction known as transrepression (2–4, 24, 38–40). Recent investigations have demonstrated that the anti-inflammatory effects of glucocorticoids may be distinct from the effects on gene transcription and may involve the MAPK pathways (1, 17, 27, 36). The actions of proinflammatory cytokines such as TNF-α involve the transcription factors NF-κB and AP-1, and the transcriptional and posttranscriptional changes in gene expression induced by TNF-α are mediated by MAPK pathways (48). MAPKs also regulate the stability of mRNA for several proinflammatory cytokines and agents (6, 11, 20, 21). Recent evidence suggests that glucocorticoids can induce the expression of a dual specificity phosphatase 1 (DUSP1), also known as MKP-1 (16, 17, 22, 23, 27, 50). MKP-1 causes dephosphorylation of the activated MAPKs, resulting in their inactivation. Evidence of regulation of gene expression via induction of MKP-1 by glucocorticoids has been reported in a variety of systems (16, 17, 23, 50). Thus MKP-1 induction is considered as an important anti-inflammatory action of glucocorticoids.
In human airway smooth muscle (HASM) and epithelial cells, TNF-α causes transient activation of the major MAPKs, i.e., p38, JNK, and ERK, resulting in the regulated expression of a variety of genes involved in excitation-contraction coupling (18, 22, 23, 32, 35, 37, 46, 49, 52). We have reported that TNF-α upregulates the expression of CD38, which encodes the 45-kDa transmembrane glycoprotein involved in the synthesis of cyclic ADP-ribose (cADPR), a calcium-mobilizing second messenger molecule (13, 14). This TNF-α-induced CD38 expression is transcriptionally regulated by NF-κB and involves MAPKs (44). Furthermore, dexamethasone, an anti-inflammatory glucocorticoid, inhibits TNF-α-induced CD38 expression by inhibiting NF-κB activation as well as through NF-κB-independent mechanisms (25). In the present study, we investigated the role of MKP-1 in the regulation of CD38 expression in HASM cells.
MATERIALS AND METHODS
Materials.
Tris base, glucose, HEPES, TNF-α, and other chemicals were purchased from Sigma Chemical (St. Louis, MO). HBSS and DMEM were purchased from Gibco BRL (Grand Island, NY). TRIzol, Lipofectamine 2000, SuperScript III RT, and the 100-bp DNA ladder were purchased from Invitrogen (Carlsbad, CA). Antibodies for MKP-1, phospho-c-Jun, c-Jun, and p65 and p50 subunits of NF-κB were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and MAPK family antibody kit (9926) was purchased from Cell Signaling Technology (Danvers, MA). Lane Marker Sample Buffer was obtained from Pierce (Rockford, IL). EMSA kit was obtained from Promega (Madison, WI). The nuclear extraction kit was purchased from Active Motif (Carlsbad, CA). SMARTpool small interfering RNA (siRNA) against human MKP-1 and siCONTROL (nontargeting) siRNA were obtained from Dharmacon (Lafayette, CO).
HASM cell culture.
The procedures for the isolation and culture of HASM cells are described in earlier publications (12, 13, 25, 44). Briefly, HASM cells isolated from trachealis muscle were plated at a density of 1.0 × 104 cells/cm2 and cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin B. The cells were growth-arrested for 48 h in arresting medium containing no serum but in the presence of transferrin and insulin. To examine MKP-1 expression, the cells were exposed to dexamethasone (concentration range of 1–1,000 nM) for 1 h and processed for determination of mRNA and protein levels. The cells were also treated with different concentrations of dexamethasone for 1 h followed by TNF-α (40 ng/ml) or vehicle (PBS containing 0.1% BSA) for 24 h. At the end of the incubation period, the expression of CD38 by RT-PCR and activity of ADP-ribosyl cyclase by reverse cyclase assay were determined as described below. To measure the activation of MAPKs, the cells were exposed to dexamethasone for 1 h followed by TNF-α, and the cells were collected at 15, 30, and 60 min for the detection of total and phosphorylated MAPKs.
To inhibit MKP-1 expression, HASM cells were transiently transfected with 200 nM MKP-1-specific SMARTpool siRNA or scrambled siRNA (Dharmacon) using Lipofectamine 2000, following the manufacturer's protocols. The cells were plated 24 h before transfection (50,000 cells/well in 24-well format or 500,000 cells/60-mm Petri dish) in antibiotic-free DMEM supplemented with 10% FBS. Briefly, the siRNA duplexes were diluted in low-serum OptiMEM 1 medium, combined with Lipofectamine 2000, and incubated for 20 min at room temperature to allow for complex formation. The complexes were added to the culture medium, and, 6–8 h following transfection, the medium was replaced with serum-free arresting medium. After incubation in arresting medium for 48 h, the cells were exposed to 100 nM dexamethasone for 1 h for the determination of MKP-1 expression and MAPK activation. To determine CD38 expression in the cells following MKP-1 silencing, the cells were exposed to 40 ng/ml TNF-α for 24 h, and CD38 expression was determined by RT-PCR (see below).
RT-PCR.
CD38 expression was determined by RT-PCR as described in earlier publications (12, 13, 15, 25, 44). Total cellular RNA was isolated with TRIzol from control cells or cells treated with TNF-α in the presence or absence of different concentrations of dexamethasone. RNA was quantified, and equal amounts from the different samples were used in the RT reaction using SuperScript III RT. Human CD38, MKP-1, and GAPDH were amplified using the following primer sets: CDUP99F (5′-ACAAACCCTGCTGCCGGCTCTC-3′) and CDUP99R (5′-GCATCGCGCCAGGACGGTCT-3′); MKP-1F (5′-GAAGGACATTTGGGCTGTGT-3′)and MKP-1R (5′-CGTTGAAAGCGAAGAAGGAG-3′); and GAPDHF (5′-GAAGGGAAGGTCGGAGTC-3′) and GAPDHR (5′-GAAGATG GTGATGGGATTTC-3′) for GAPDH as internal controls. The PCR was performed under the following conditions: 94°C for 3 min of denaturing, 30 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 45 s, and a final extension at 72°C for 10 min. The PCR products were separated on 1.2% agarose gel and identified by ethidium bromide staining. The band intensity of scanned gel photograph was determined using LabWorks image acquisition and analysis software (UVP, Upland, CA), and CD38 expression was normalized to GAPDH expression for the corresponding sample.
Reverse cyclase assay.
The ADP-ribosyl cyclase activity of HASM cell lysates was quantified by measuring the reverse cyclase activity of CD38 with modifications (19). HASM cell lysates containing 20 μg of protein were incubated for 2 h at 37°C with or without 10 mM nicotinamide in the presence of 0.45 mM cyclic-ADP ribose in a total volume of 50 μl. The reverse cyclase reaction was stopped by adding 25 μl of 1 M HCl, vacuum-filtered through a protein-binding membrane (0.45 μm, Immobilon; Millipore), and neutralized with 15 μl of 2 M Tris base, and the NAD in the filtrate was quantified by a cycling reaction. The filtrate (40 μl) was incubated with equal volume of reagent containing 2 μM rezasurin, 0.76% vol/vol ethanol, 4 μM flavin mononucleotide, 40 μg/ml alcohol dehydrogenase, and 0.04 U/ml diaphorase in NaH2PO4/Na2HPO4 buffer, pH 8.0, at room temperature. The NAD fluorescence was quantified (excitation at 544 nm and emission at 590 nm) in a fluorometer (FLUOstar Galaxy, BMG LABTECH), and the rate of emission of fluorescence was calculated. The quantity of NAD generated in the reaction was calculated from known NAD standards using a standard curve and expressed as femtomoles of NAD per hour per milligram of total protein.
Western blot detection.
For Western blot detection of the phospho-c-Jun, MKP-1, and phospho-MAPKs, whole cell lysates with equal amount of protein were fractionated on 4–15% gradient gels, and the proteins were transferred electrophoretically onto a polyvinylidene difluoride membrane. For immunostaining, the membranes were blocked in PBS containing 1% milk diluent (KPL, Gaithersburg, MD) and 0.05% Tween-20 for 30 min. Membranes were incubated with primary antibody for phospho-c-Jun, MKP-1, phospho-p44/42, phospho-JNK, or phospho-p38 and then in goat-anti-rabbit IgG conjugated with horseradish peroxidase for 1 h. The membrane was stripped and reprobed with antibodies for c-Jun and p44/42, JNK, p38 MAPKs followed by goat-anti-rabbit IgG conjugated with horseradish peroxidase for 1 h. The signals were visualized on X-ray film after the application of chemiluminescence SuperSignal West Dura Extended Duration Substrate (Pierce).
Nuclear protein extraction.
For the preparation of nuclear extracts, HASM cells were grown to confluence, washed with ice-cold PBS containing phosphatase inhibitors, and scraped in the same buffer. The cell pellets obtained by centrifugation were resuspended in 500 μl of 1× hypotonic buffer by pipetting several times, transferred to a prechilled microcentrifuge tube, and incubated for 15 min on ice. Twenty-five microliters of detergent was added, vortexed for 10 s, and centrifuged at 14,000 g for 30 s at 4°C. The nuclear pellet was resuspended in 50 μl of complete lysis buffer, vortexed, and incubated on ice for 30 min, vortexed briefly, and centrifuged at 14,000 g for 10 min at 4°C. Protein concentration in the nuclear extract was quantitated using the Bradford protein assay kit (Bio-Rad, Hercules, CA).
EMSA.
EMSA was performed as described in an earlier publication (25). The double-stranded oligonucleotides containing the consensus binding sites for NF-κB (5′-AGTTGA GGGGACTTTCCCAGGC-3′) and one of the putative binding sites for AP-1 that exhibits very strong binding on exposure to TNF-α (AP-1 site 4 in the cd38 promoter, referred to as AP-1–4; 5′-ATCATCTTTGACTCATCTCTTTC-3′) were labeled with [γ-32P]ATP (3,000 Ci/mmol at 10 mCi/ml) by T4 Polynucleotide Kinase (Promega). Five micrograms of the nuclear extract was incubated for 30 min at room temperature with 0.4 pmol of double-stranded 32P-labeled oligonucleotide containing the consensus binding sites in a total volume of 10 μl in the following buffer: 20% glycerol, 5 mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris·HCl (pH 7.5), and 0.25 mg/ml poly(dI-dC). The samples were separated on nonreducing 4% polyacrylamide gels using 0.5 M TBE buffer (107.8 g of Tris base, 55 g of boric acid, and 7.44 g of disodium EDTA·2H2O in 1 l of water). The gels were dried and autoradiographed with intensifying screens at −70°C. A 100-fold excess of unlabeled NF-κB or AP-1 probe or the SP1 probe as a nonspecific competitor was used to confirm specificity of binding of nuclear proteins.
mRNA stability determination.
HASM cells were treated with TNF-α for 12 h and incubated for an additional 12 h in the absence of TNF-α but in the presence of 5 μg/ml actinomycin D to arrest further transcription. Dexamethasone was added 30 min before the addition of TNF-α. CD38 expression was determined as described above at various time points (0 min, 15 min, 30 min, 1 h, 2 h, and 6 h) following the arrest of transcription.
Statistical analysis.
HASM cells from at least three different donors were used in the studies. The EMSA experiments were repeated three times. The quantitative PCR results and ADP-ribosyl cyclase activities in the various samples were compared by one-way ANOVA with Bonferroni test for multiple comparisons. Statistical analyses were done using the GraphPad Prism software. Two means were considered significantly different when P was ≤0.05.
RESULTS
Effect of dexamethasone on MKP-1 expression and activation of MAPKs.
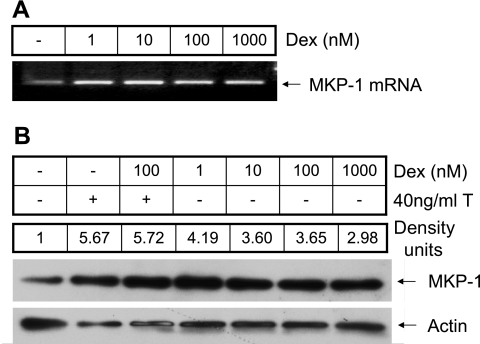
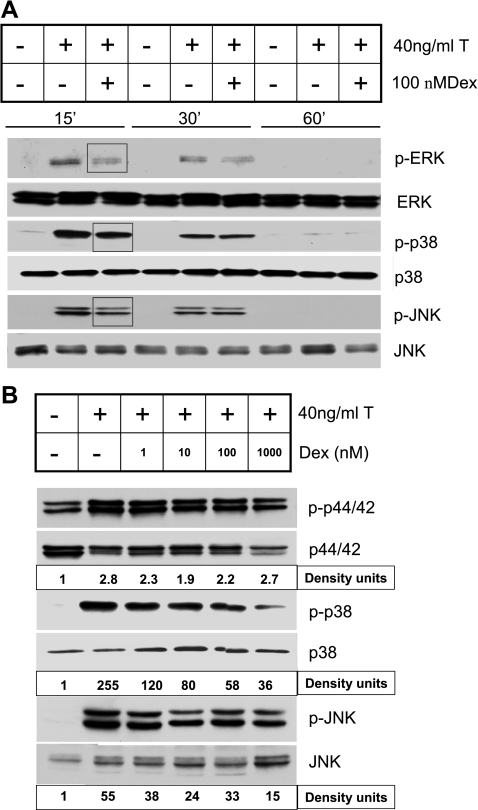
In many cells, deactivation of MAPKs is mediated by MAPK phosphatases such as MKP-1 (17, 22, 27, 50). To investigate whether dexamethasone regulates the expression of MKP-1 in HASM cells, the expression of MKP-1 mRNA and protein were determined following exposure to different concentrations (1–1,000 nM) of dexamethasone. MKP-1 protein expression in the presence of TNF-α and/or dexamethasone was normalized to the level expressed constitutively in the cells. MKP-1 mRNA levels increased following treatment with dexamethasone in as early as 1 h (Fig. 1). This increase was seen even at the lowest concentration (1 nM) of dexamethasone used in the study. There was significant increase in the MKP-1 protein expression in cells treated with TNF-α and dexamethasone compared with levels in untreated control cells (Fig. 1). To determine whether the induction of MKP-1 by dexamethasone resulted in decreased activation of the MAPKs, we measured the phosphorylation of ERK (p42/44), JNK, and p38 MAPKs in HASM cells following exposure to TNF-α. TNF-α-induced activation of the MAPKs was decreased in a concentration-dependent manner by dexamethasone (Fig. 2, A and B). Inhibition of p38 and JNK MAPK phosphorylation occurred at a dexamethasone concentration of 1 nM, whereas a significant and consistent decrease in the phosphorylation of ERK occurred at a dexamethasone concentration of ≥10 nM, suggesting a differential sensitivity of the MAPKs to the effects of glucocorticoids in HASM cells.
Fig. 1.
Dexamethasone (Dex) increases MKP-1 expression. Human airway smooth muscle (HASM) cells were treated with 1–1,000 nM dexamethasone for 1 h, and MKP-1 mRNA and protein levels were measured by RT-PCR and Western blot analysis, respectively. A: note that dexamethasone increases the expression of MKP-1 mRNA over the entire concentration range used in the study. B: a representative Western blot showing MKP-1 protein expression in the presence of different concentrations of dexamethasone and in response to TNF-α with or without 100 nM dexamethasone. The band intensities (density units) of the control and dexamethasone-treated samples were normalized to actin expression. Density units represent expression in the treated samples relative to expression in the control sample. Representative of 3 separate experiments.
Fig. 2.
Inhibition of TNF-α-induced activation of MAPKs by dexamethasone in HASM cells. HASM cells were treated with TNF-α in the presence or absence of dexamethasone (1–1,000 nM). Representative Western blots showing expressions of phosphorylated (p-) and total MAPKs [p-p44/42 (p-ERK) and p44/42 (ERK), p-p38 and p38, and p-JNK and JNK]. A: cells were pretreated with 100 nM dexamethasone and collected at 15, 30, and 60 min after TNF-α treatment. Note rapid activation of the MAPKs and a decrease in their activation in the presence of dexamethasone (highlighted by square boxes). B: cells were pretreated with 1–1,000 nM dexamethasone and collected 15 min after TNF-α treatment. The band intensities of the phosphorylated MAPKs were normalized to the total MAPKs and shown as density units. Note dexamethasone concentration-dependent decrease in the activation of MAPKs. Representative of 3 separate experiments.
Effect of dexamethasone on activation of NF-κB and AP-1 and CD38 expression and activity.
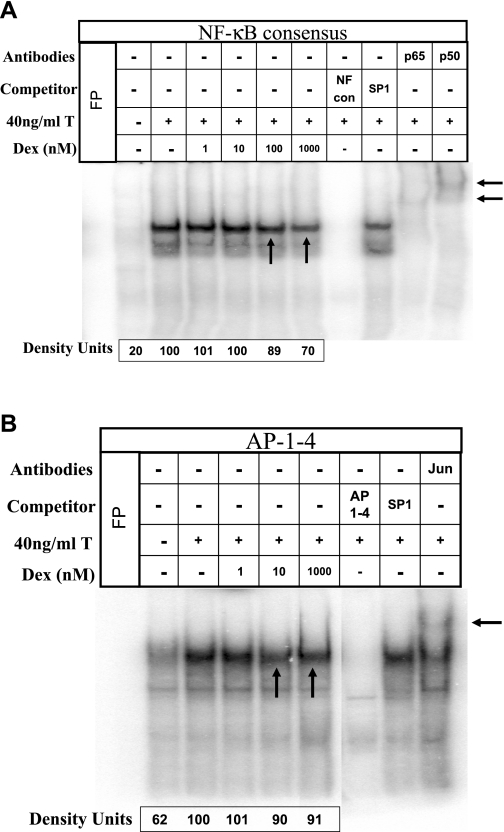
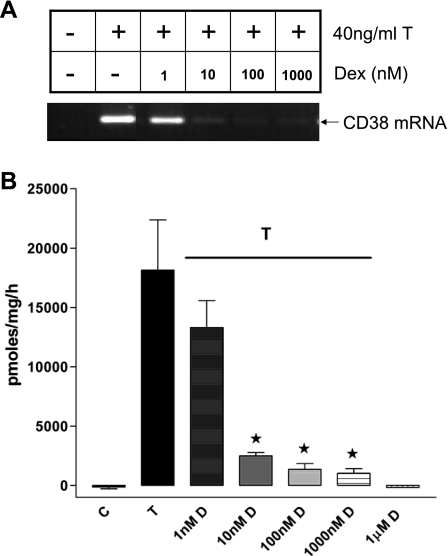
MAPK-mediated changes in the profile of gene expression involve transcription factors such as NF-κB and AP-1 (18). In HASM cells, MAPK-mediated regulation of CD38 expression involves NF-κB and AP-1 (44). Sequence analysis of the promoter region of the cd38 gene reveals one NF-κB and several AP-1 binding motifs. In preliminary studies, we noticed that the binding of nuclear proteins obtained from TNF-α-treated HASM cells to one of the cd38 putative AP-1 sites (referred to as AP-1–4) was very strong compared with binding to the other AP-1 sites. To investigate whether the induction of MKP-1 expression by dexamethasone has an effect on the activation of NF-κB and AP-1, we measured the binding of nuclear proteins to oligonucleotides encoding the consensus NF-κB and the putative cd38 AP-1–4 binding sequences (Fig. 3, A and B). Nuclear proteins were obtained from cells exposed to TNF-α in the presence of various concentrations of dexamethasone (1–1,000 nM). TNF-α increased the binding of nuclear proteins to the consensus NF-κB and the AP-1–4 oligonucleotides, which was inhibited by dexamethasone (Fig. 3). The decreased activation of NF-κB and AP-1 was evident at a dexamethasone concentration of ≥10 nM. Dexamethasone decreased TNF-α-induced CD38 expression and ADP-ribosyl cyclase activity in a concentration-dependent fashion (Fig. 4, A and B). The decrease in the CD38 mRNA level was evident even at a dexamethasone concentration of 1 nM.
Fig. 3.
Dexamethasone decreases NF-κB and activator protein-1 (AP-1) activation following exposure to TNF-α. EMSAs demonstrating DNA binding of NF-κB to consensus oligonucleotide probe and AP-1 to cd38 putative oligonucleotide (AP-1–4) probe. Nuclear proteins were extracted from HASM cells treated with TNF-α in the presence of 1–1,000 nM dexamethasone. The specific complexes formed can be competed by 100-fold molar excess of unlabeled specific oligonucleotide probes [NF-κB consensus (NF-κB con) or AP-1–4]. A nonspecific oligonucleotide probe (SP1) was used as an internal control. A: a representative EMSA for NF-κB binding to consensus oligonucleotide sequences. Note increased NF-κB activation by TNF-α, which is decreased in the presence of dexamethasone (at ≥100 nM) (vertical arrows). Note gel supershift in the presence of anti-p65 and -p50 antibodies (labeled p65 and p50), demonstrating specificity of the assay (horizontal arrows). B: a representative EMSA for AP-1 binding to cd38 putative oligonucleotide sequences. Note increased AP-1 activation by TNF-α, which is decreased by dexamethasone (at ≥10 nM) (vertical arrows). Note gel supershift in the presence of anti-c-Jun antibody (labeled Jun) (horizontal arrow). These results are representative of 4 experiments. T, TNF-α. FP, Free Probe.
Fig. 4.
Effect of dexamethasone on TNF-α-induced CD38 expression in growth-arrested HASM cells. A: a representative gel image showing CD38 expression induced by TNF-α with or without dexamethasone (D) (1–1,000 nM). B: ADP-ribosyl cyclase activity shown as pmol·mg−1·h−1 in lysates of HASM cells treated with TNF-α (T) and dexamethasone. Note significant decreases in ADP-ribosyl cyclase activity in cells exposed to dexamethasone in a concentration-dependent manner. Results are expressed as means ± SE of 4–6 different experiments. C, control. * Significant decrease in ADP-ribosyl cyclase activity.
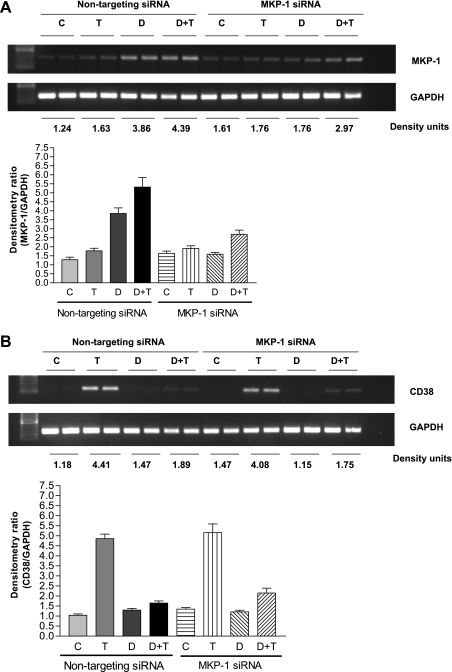
In cells transiently transfected with the MKP-1 SMARTpool siRNA, there was a significant reduction (P ' 0.05; n = 6) in MKP-1 expression following exposure to dexamethasone and/or TNF-α compared with expression in cells transfected with the nontargeting siRNA (Fig. 5A). In the MKP-1 siRNA-transfected HASM cells, there was a partial nonsignificant reversal of the inhibitory effect of dexamethasone on TNF-α-induced CD38 expression (Fig. 5B).
Fig. 5.
Effect of MKP-1 downregulation in HASM cells by transient transfection with SMARTpool small interfering RNA (siRNA) on CD38 expression. A, top trace: representative gel image showing MKP-1 expression in HASM cells following transient transfection with nontargeting siRNA or MKP-1 siRNA. The band intensities of MKP-1 are normalized to GAPDH and shown as density units. A, bottom trace: MKP-1 mRNA expression normalized to GAPDH expression in HASM cells. C, control (unstimulated cells); T, cells exposed to 40 ng/ml TNF-α for 24 h; D, cells exposed to 100 nM dexamethasone for 1 h; D+T, cells exposed to dexamethasone for 1 h followed by TNF-α for 24 h. Note significant decrease in MKP-1 expression in cells transfected with MKP-1 siRNA. Results are expressed as means ± SE of 3 different experiments. B, top trace: representative gel image of CD38 expression in HASM cells following transient transfection with nontargeting siRNA or MKP-1 siRNA. The band intensities of CD38 are normalized to GAPDH and shown as density units. B, bottom trace: CD38 expression in HASM cells following transfection with nontargeting siRNA or MKP-1 siRNA. Results are expressed as means ± SE of 3 different experiments.
Effect of dexamethasone on mRNA stability.
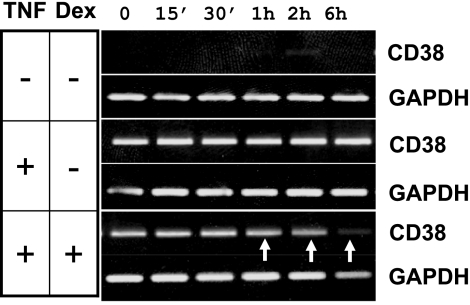
In addition to its role in the transcriptional regulation of gene expression, MAPKs exert posttranscriptional control through transcript stability (6, 11, 20, 21, 44). We assessed the effect of dexamethasone on CD38 transcript stability following its induction with TNF-α. Cells were exposed initially to TNF-α in the absence or presence of dexamethasone, and CD38 expression was determined at different times following the arrest of transcription with actinomycin D. In TNF-α-treated cells, the CD38 mRNA level was maintained at or above basal levels over the 6-h period following the removal of TNF-α (Fig. 6). The transcript level decreased in as early as 1 h in cells treated with dexamethasone (Fig. 6). These results suggest that regulation of CD38 expression in HASM cells by glucocorticoids occurs through both transcriptional and posttranscriptional mechanisms.
Fig. 6.
Effect of dexamethasone on CD38 transcript stability. HASM cells were treated with TNF-α in the presence of 100 nM dexamethasone for 12 h. Cells were washed, replaced with fresh media containing actinomycin D to inhibit further transcription, and collected at 0 min, 15 min, 30 min, 1 h, 2 h, and 6 h for the determination of CD38 mRNA expression. Gel images show CD38 mRNA expression. GAPDH expression is used as an internal control. Note a rapid decline in the mRNA levels in the presence of dexamethasone (indicated by arrows). Representative of 3 experiments.
DISCUSSION
In the present study, we investigated the role of the MKP-1 in the regulation of CD38 expression. In HASM cells, treatment with dexamethasone resulted in a significant and rapid increase in the expression of MKP-1. TNF-α also increased MKP-1 expression, and, in the combined presence of TNF-α and dexamethasone, MKP-1 expression increased further but comparable to that seen in the presence of TNF-α alone. The increased MKP-1 expression induced by dexamethasone was associated with decreased activation of all three MAPKs with a significant reduction in the expression of CD38 and ADP-ribosyl cyclase activity. Dexamethasone decreased the TNF-α-induced activation of NF-κB and AP-1. In the presence of dexamethasone, the CD38 transcript levels also declined compared with temporal controls. These results provide evidence that glucocorticoids inhibit CD38 expression in airway smooth muscle cells by decreasing MAPK activation resulting from the induction of MKP-1. Reduction of MKP-1 expression through siRNA only partially reversed the inhibitory effect of dexamethasone on CD38 expression induced by TNF-α. The inhibitory effects of glucocorticoids on CD38 expression through the induction of MKP-1 result from decreased NF-κB- and AP-1-dependent transcription and through CD38 transcript stability.
Glucocorticoids are used in the treatment of many airway inflammatory conditions including asthma (3, 4, 9). The effects of glucocorticoids in regulating the expression of genes involve the glucocorticoid receptor (GR) (5). Binding of glucocorticoid to its receptor results in the translocation of the GR complex to the nucleus where it acts as a transcription factor or as an inhibitor of other transcription factors, such as NF-κB or AP-1 (7, 24, 28, 31, 41, 51). With respect to CD38, sequence analysis of a cloned putative 3-kb promoter fragment of the human cd38 reveals several transcription factor binding sites (GenBank acc. no. DQ091293). We have identified at least six putative AP-1 binding sites scattered throughout the proximal promoter region and one NF-κB site. In addition, there are four GREs in the promoter region. Other reports from the human and rabbit cd38 confirm the presence of some of these transcription factor binding sites in the promoter (42). The presence of these response elements provides a basis for the direct transcriptional regulation of CD38 expression in HASM cells. Previous studies have shown that TNF-α-induced CD38 expression is decreased in a concentration-dependent manner by the anti-inflammatory glucocorticoid dexamethasone (25). This inhibition involves decreased NF-κB activation following exposure to TNF-α. The decreased NF-κB activation results from a multitude of mechanisms, including increased expression of the inhibitory molecule IκB. Other studies have reported induction of GR-β, a dominant negative GR, by a combination of cytokines, resulting in glucocorticoid resistance to CD38 expression in HASM cells (45). Although these studies provide evidence for direct transcriptional regulation of CD38 expression in HASM cells by glucocorticoids, the results of the present study demonstrate another mechanism of regulation that involves the induction of MKP-1 (see below). The consequence of this induction is evident at both transcriptional (activation of transcription factors) and posttranscriptional (transcript stability) levels. It is worth noting that dexamethasone inhibits TNF-α-induced CD38 mRNA expression at very low concentrations, whereas inhibition of NF-κB and AP-1 activation occurs at relatively high dexamethasone concentrations.
A concept of “nontranscriptional” anti-inflammatory action of glucocorticoids has been proposed in which the effects of glucocorticoids on repression of gene transcription are achieved without the direct action of GR on the target gene (30). Dexamethasone induces the expression of MKP-1, a dual specificity phosphatase that inactivates MAPKs in different cell types (16, 22, 27, 29, 50). MAPKs are inactivated by dephosphorylation of the phosphothreonine and/or phosphotyrosine residues in their catalytic domain. Phosphatases that inactivate MAPKs seem to exert their effects on the magnitude and duration of MAPK activation, their subcellular distribution, and the phosphorylation of specific substrates (33, 34, 47). There are at least 10 mammalian MKPs, and among them MKP-1 has been studied extensively (43). Dexamethasone causes the induction of MKP-1 by binding to the promoter sites of MKP-1 (27). The target of MKP-1 action on MAPKs appears to depend on the cell type. In this context, Furst et al. (17) demonstrated that, in the presence of dexamethasone, TNF-α-induced E-selectin expression in the human endothelial cells is inhibited by MKP-1-mediated inactivation of p38 MAPK. On the other hand, in U937 cells, a breast cancer cell line, MKP-1 inactivates both p38 and JNK MAPKs (16). In a recent study, Issa et al. (22) demonstrated the selective inhibition of JNK activation by glucocorticoids, resulting in inhibition of growth-related oncogene protein-α expression induced by IL-1β and TNF-α in HASM cells. In our study, we found that dexamethasone decreases the activation of all three MAPKs induced by TNF-α at relatively low concentrations.
There are three distinct members of MAPKs, ERK (also called ERK MAPK), p38 MAPK, and JNK (8, 10, 18, 26). Activation of the MAPKs by TNF-α can cause the nuclear translocation and activation of AP-1 and NF-κB (48). Once activated, MAPKs regulate gene expression both at transcriptional and posttranscriptional levels. Our recent study demonstrated that TNF-α increases CD38 expression through the activation of MAPKs (44). This results in transcriptional regulation of CD38 expression involving p38 and JNK MAPKs and NF-κB and AP-1 and transcript stability involving the p38 and ERK MAPKs. In the present study, we have provided evidence that dexamethasone decreases CD38 expression by inducing the MKP-1 and thereby decreasing the activation of the MAPKs. This inhibition is evident even at the lowest dexamethasone concentration used in the study (1 nM), a concentration that caused significant dephosphorylation of p38 and JNK MAPKs. The consequence of this regulation involves control of gene expression through transcriptional and posttranscriptional (transcript stability) mechanisms. It is likely that the regulation of CD38 expression by glucocorticoids at low concentrations results from transcript stability and is mediated largely through the p38 MAPK. This conclusion is based on the fact that inhibition of transcription factor activation requires high dexamethasone concentrations. A surprising finding of the present studies is that MKP-1 knockdown by siRNA did not significantly reverse the inhibitory effects of dexamethasone on CD38 expression. It is likely that the level of downregulation achieved in these studies by transient transfection is not sufficient. This is also reflected by the fact that TNF-α-induced activation of the p38 and ERK MAPKs was diminished but still present in these cells following transfection with the MKP-1 siRNA (data not shown). The results of the present study provide evidence for regulation of CD38 expression in HASM cells by transcriptional and posttranscriptional (stability) mechanisms involving activation of the MAPKs. However, our studies have not provided a direct link between induction of MKP-1 by glucocorticoids and inhibition of CD38 expression since downregulation of MKP-1 expression did not result in the expected reversal of the effects of dexamethasone. These results also do not rule out the contribution of other transcription factor(s) in the regulation of CD38 expression in HASM cells.
GRANTS
This study was supported by National Institutes of Health Grants HL-057498 (to M. S. Kannan and T. F. Walseth), DA-11806 (to T. F. Walseth), and HL-081824 (to R. A. Panettieri, Jr.) and a Grant-in-Aid from the University of Minnesota Graduate School (to M. S. Kannan).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med 203: 1883–1889, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ Corticosteroid effects on cell signalling. Eur Respir J 27: 413–426, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ Corticosteroids: the drugs to beat. Eur J Pharmacol 533: 2–14, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol 148: 245–254, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beato M Gene regulation by steroid hormones. Cell 56: 335–344, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Brook M, Sully G, Clark AR, Saklatvala J. Regulation of tumour necrosis factor alpha mRNA stability by the mitogen-activated protein kinase p38 signalling cascade. FEBS Lett 483: 57–61, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Brostjan C, Anrather J, Csizmadia V, Stroka D, Soares M, Bach FH, Winkler H. Glucocorticoid-mediated repression of NFkappaB activity in endothelial cells does not involve induction of IkappaBalpha synthesis. J Biol Chem 271: 19612–19616, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chikanza IC, Kozaci D, Chernajovsky Y. The molecular and cellular basis of corticosteroid resistance. J Endocrinol 179: 301–310, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Davis RJ Transcriptional regulation by MAP kinases. Mol Reprod Dev 42: 459–467, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Dean JL, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem 274: 264–269, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol 31: 36–42, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 17: 452–454, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288: L773–L788, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Dogan S, Deshpande DA, Kannan MS, Walseth TF. Changes in CD38 expression and ADP-ribosyl cyclase activity in rat myometrium during pregnancy: influence of sex steroid hormones. Biol Reprod 71: 97–103, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem 272: 16917–16923, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Furst R, Schroeder T, Eilken HM, Bubik MF, Kiemer AK, Zahler S, Vollmar AM. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB J 21: 74–80, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gerthoffer WT, Singer CA. MAPK regulation of gene expression in airway smooth muscle. Respir Physiol Neurobiol 137: 237–250, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Graeff R, Lee HC. A novel cycling assay for nicotinic acid-adenine dinucleotide phosphate with nanomolar sensitivity. Biochem J 367: 163–168, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henness S, van Thoor E, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A acts via p38 MAPK to increase stability of TNF-α-induced IL-8 mRNA in human ASM. Am J Physiol Lung Cell Mol Physiol 290: L1283–L1290, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal J, Kumar K, Sun L, Zaidi M. Selective upregulation of the ADP-ribosyl cyclases CD38 and CD157 by TNF but not by RANK-L reveals differences in downstream signaling. Am J Physiol Renal Physiol 291: F557–F566, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Issa R, Xie S, Khorasani N, Sukkar M, Adcock IM, Lee KY, Chung KF. Corticosteroid inhibition of growth-related oncogene protein-alpha via mitogen-activated kinase phosphatase-1 in airway smooth muscle cells. J Immunol 178: 7366–7375, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Issa R, Xie S, Lee KY, Stanbridge RD, Bhavsar P, Sukkar MB, Chung KF. GRO-α regulation in airway smooth muscle by IL-1β and TNF-α: role of NF-κB and MAP kinases. Am J Physiol Lung Cell Mol Physiol 291: L66–L74, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Jonat C, Rahmsdorf HJ, Park KK, Cato AC, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell 62: 1189–1204, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Kang BN, Tirumurugaan KG, Deshpande DA, Amrani Y, Panettieri RA, Walseth TF, Kannan MS. Transcriptional regulation of CD38 expression by tumor necrosis factor-alpha in human airway smooth muscle cells: role of NF-kappaB and sensitivity to glucocorticoids. FASEB J 20: 1000–1002, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Karin M The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270: 16483–16486, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kassel O, Sancono A, Kratzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J 20: 7108–7116, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerppola TK, Luk D, Curran T. Fos is a preferential target of glucocorticoid receptor inhibition of AP-1 activity in vitro. Mol Cell Biol 13: 3782–3791, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol 22: 7802–7811, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limbourg FP, Liao JK. Nontranscriptional actions of the glucocorticoid receptor. J Mol Med 81: 168–174, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol 12: 45–56, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Moore PE, Church TL, Chism DD, Panettieri RA Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol 282: L847–L853, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19: 91–118, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31: 268–275, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol Lung Cell Mol Physiol 277: L479–L488, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26: 3203–3213, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Ramakrishnan M, Musa NL, Li J, Liu PT, Pestell RG, Hershenson MB. Catalytic activation of extracellular signal-regulated kinases induces cyclin D1 expression in primary tracheal myocytes. Am J Respir Cell Mol Biol 18: 736–740, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA 91: 752–756, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270: 283–286, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS Jr. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol 15: 943–953, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schule R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Yang N, Verma IM, Evans RM. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell 62: 1217–1226, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Sun L, Iqbal J, Zaidi S, Zhu LL, Zhang X, Peng Y, Moonga BS, Zaidi M. Structure and functional regulation of the CD38 promoter. Biochem Biophys Res Commun 341: 804–809, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol 3: REVIEWS3009, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-α induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-κB and AP-1. Am J Physiol Lung Cell Mol Physiol 292: L1385–L1395, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Tliba O, Cidlowski JA, Amrani Y. CD38 expression is insensitive to steroid action in cells treated with tumor necrosis factor-alpha and interferon-gamma by a mechanism involving the up-regulation of the glucocorticoid receptor beta isoform. Mol Pharmacol 69: 588–596, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Tliba O, Panettieri RA Jr, Tliba S, Walseth TF, Amrani Y. Tumor necrosis factor-alpha differentially regulates the expression of proinflammatory genes in human airway smooth muscle cells by activation of interferon-beta-dependent CD38 pathway. Mol Pharmacol 66: 322–329, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Volmat V, Pouyssegur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol Cell 93: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Wang CC, Lin WN, Lee CW, Lin CC, Luo SF, Wang JS, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK, and NF-κB in IL-1β-induced VCAM-1 expression in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 288: L227–L237, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem 280: 4117–4124, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Yang-Yen HF, Chambard JC, Sun YL, Smeal T, Schmidt TJ, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62: 1205–1215, 1990. [DOI] [PubMed] [Google Scholar]
- 52.Zhou L, Tan A, Iasvovskaia S, Li J, Lin A, Hershenson MB. Ras and mitogen-activated protein kinase kinase kinase-1 coregulate activator protein-1- and nuclear factor-kappaB-mediated gene expression in airway epithelial cells. Am J Respir Cell Mol Biol 28: 762–769, 2003. [DOI] [PubMed] [Google Scholar]