Abstract
Metalloelastase (MMP-12), mainly produced by macrophages, has been shown to play a key role in the pathogenesis of emphysema in animal models. Chronic cigarette smoke increases pulmonary MMP-12, which is closely correlated with an elevation of pulmonary substance P (SP). Because alveolar macrophages (AMs) contain the neurokinin-1 receptor (NK1R), we tested whether SP was able to trigger the upregulation of MMP-12 synthesis in AMs by acting on the NK1R. AMs isolated from bronchoalveolar lavage cells in C3H/HeN mice were cultured with control medium or SP that was coupled without or with NK1R antagonists (CP-99,994 or aprepitant) for 24 h. We found that SP significantly increased the mRNA of MMP-12 and NK1R by 11-fold and 82%, respectively, in AMs (P < 0.05), and these responses were abolished by NK1R antagonists with little change in the cells' viability. Because pulmonary SP is primarily released by bronchopulmonary C-fibers (PCFs), we further asked whether destruction of PCFs would reduce SP and MMP-12. Two groups of mice were pretreated with vehicle and neonatal capsaicin (NCAP) to degenerate PCFs, respectively. Our results show that NCAP treatment significantly decreased mRNA and protein levels of SP associated with a reduction NK1R and MMP-12 in the lungs and AMs. These findings suggest that SP has a modulatory effect on pulmonary MMP-12 by acting on NK1R to trigger MMP-12 syntheses in the AMs.
Keywords: emphysema, neurokinin-1 receptor, bronchopulmonary C-fibers, matrix metalloproteinase-12, chronic obstructive pulmonary disease
metalloelastase (MMP-12) is able to degrade many kinds of extracellular matrix proteins, including elastin (16). Loss of elastic recoil and damage of elastic fibers along with histological changes of emphysema implicate elastin degradation as an important factor in the pathogenesis of chronic obstructive pulmonary disease (COPD) (38). MMP-12 is increased in lung tissue (5) and macrophages (6) of cigarette smoke (CS)-exposed mice and in sputum from COPD patients (7, 40). Furthermore, an increase of the ratio of MMP-12 to the tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) was also observed in COPD animal models (61). TIMP-1 can inhibit all matrix metalloproteinases (MMPs), including MMP-12, resulting in loss of proteolytic activity (27, 54). Most importantly, MMP-12 gene-deficient mice do not develop emphysema after chronic CS exposure (19). Conversely, overexpression of MMP-12 in transgenic mice is associated with spontaneous development of pulmonary emphysema (41). Together, these results strongly suggest that MMP-12 plays a key role in the pathogenesis of COPD, at least in animal models.
Substance P (SP) is a proinflammatory neuropeptide and neurogenic mediator with a high affinity for binding to the neurokinin-1 receptor (NK1R) (48). Previous studies have shown three lines of evidence implying the possible SP modulatory effects on pulmonary MMP-12. First, significantly correlated increases of pulmonary SP and MMP-12 have been observed in CS-exposed mice (61). Second, alveolar macrophages (AMs), a major producer of MMP-12 (27), express NK1R in guinea pigs (2) and humans (1, 22). Third, SP is capable of activating AMs to release inflammatory mediators including interleukin (IL)-1β and tumor necrosis factor (TNF)-α (1), and the latter could stimulate macrophages to release MMP-12 (10). However, to date, the role of SP in regulating synthesis of MMP-12 in AMs and generating pulmonary MMP-12 remains unclear.
The objective of the present study was to test two major hypotheses: 1) SP is able to trigger the upregulation of MMP-12 synthesis in AMs by acting on NK1R; and 2) reduction of SP by degeneration of the bronchopulmonary C-fibers (PCFs) via neonatal capsaicin treatment (NCAP) markedly decreases MMP-12 in lungs and AMs. A large number of previous studies have demonstrated that pulmonary SP is primarily synthesized in the cell bodies of PCFs located in the nodose and jugular ganglia (20) and released from PCF endings into the lungs when stimulated (32). Accordingly, these hypotheses were examined in vitro and in vivo, respectively. Our results showed that SP significantly increased mRNA and protein levels of MMP-12 and NK1R in the AMs isolated from bronchoalveolar lavage (BAL) cells in C3H/HeN mice. Furthermore, PCF degeneration dramatically reduced pulmonary SP (a 75% reduction) that was associated with a significant decrease of MMP-12 in both lung tissues and AMs. Our results provide the experimental evidence of the modulatory effect of SP on pulmonary MMP-12 by acting on NK1R to trigger MMP-12 synthesis in AMs.
MATERIALS AND METHODS
General animal preparations and grouping.
The experimental protocols were approved by the Institutional Animal Care and Use Committee in Lovelace Respiratory Research Institute facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Experiments were performed in 84 C3H/HeN female mice at 26 wk of age for several reasons. First, previous studies have shown that C3H/HeN mice are most vulnerable to developing CS-induced emphysema among other mouse strains. The emphysema susceptibility has been ranked as C3H/HeN > A/J > C57BL/6; female mice are particularly affected (17, 60). Second, a close correlation between pulmonary SP and MMP-12 has been reported in C3H/HeN female mice (61). Third, this species presented a similar blunted ventilatory response to hypercapnia and hypoxia as observed in patients with severe COPD (60). In addition, the majority of the studies described above were performed in 26-wk-old mice (36, 60, 61).
Four series of experiments were carried out in the present study. In the first series of experiments, immunomagnetic isolation of AMs from BAL cells was performed in 40 mice, and the obtained AMs were divided into four groups that are described in detail later. Five trials were performed for each group, and AMs from two mice were utilized in each trial. MMP-12, MMP-9, TIMP-1, and NK1R in AMs and IL-1β and TNF-α in culture supernatant were detected, respectively. Twenty mice were used in the second series for testing the effects of SP and CP-99,994 (n = 10) or aprepitant (n = 10) on the cell viability of AMs. In the third series, 24 mice were divided evenly into two groups: one group was treated with NCAP [capsaicin, 50 mg/kg sc, 97% purity (Sigma-Aldrich, St. Louis, MO)], and the other group was treated with vehicle [CON; 10% ethanol and 10% Tween 80 in 0.9% (wt/vol) NaCl solution] at the second day after birth, as previously described (23). Twenty-six weeks later, BAL fluid (BALF) was collected and the AMs obtained from the BAL were divided into two subgroups, i.e., one for protein (n = 6) and another for mRNA measurement (n = 6) for each group. Subsequently, the right lung in each mouse was harvested. In these cases, the same mRNA and protein measurements, with the additional detection of mRNA preprotachykinin-A (PPT-A; a precursor to SP) and SP, were performed. The preparation and protocols for AMs are detailed below. Just as the MMP-12 proenzyme was detected in the cells (37), the MMP-12 proenzyme (54 kDa) was detected in the present study. In addition to the three series of experiments conducted on AMs, a fourth series was carried out in a macrophage cell line (see Preparation and protocols for macrophage cell lines).
Collection of BALF.
All mice were anesthetized with Nembutal (50 mg/kg ip), and the trachea was cannulated as described previously (61). The lungs were perfused through the right ventricle of the heart with buffered salt solution (125 mM NaCl, 5 mM KCl, 2.5 mM Na2HPO4, 17 mM HEPES, 10 μg/ml gentamicin, and 1 mg/ml dextrose, pH 7.4) to remove blood cells from the pulmonary vasculature. The intact lungs were then lavaged 10 times with an infusion of cold 0.7-ml aliquots of buffered PBS (pH 7.2) containing 5% BSA and 2 mM EDTA, similar to the procedures previously reported (35). The lavage was centrifuged at 1,000 rpm for 10 min at 4°C. The cell pellet was resuspended in 0.8 ml RBC lysis buffer (Sigma-Aldrich), incubated at room temperature for 10 min, and neutralized by the addition of 8 ml of PBS, and the suspension was centrifuged again. Donor cells were resuspended in a total of 1 ml of PBS. Total and differential cell counts were performed as described previously (61).
Immunomagnetic isolation of macrophages in BAL cells.
CD11b MicroBeads (Miltenyi Biotec, Auburn, CA) were able to specifically bind with macrophages in mouse, by which method the macrophages in spleen were isolated using magnetic-activated cell sorting (MACS) techniques (43). The same approach was applied in the present study to isolate AMs from BAL cells. Briefly, a MACS separation column system (Miltenyi Biotec, Bergisch Gladbach, Germany) was assembled by placing a MiniMACS separation MS column into the magnetic component and washing with 1 ml of depletion buffer [PBS, pH 7.2, supplemented with 0.5% heat-inactivated FBS and 2 mM EDTA by diluting MACS BSA stock solution in autoMACS rinsing solution (Miltenyi Biotec)]. Pooled BAL cells were resuspended in 10 μl of CD11b MicroBeads in 90 μl of depletion buffer and incubated for 15 min at 4°C. Cells were washed with depletion buffer, centrifuged at 1,000 rpm for 10 min, and diluted in 500 μl of depletion buffer. Labeled cells were placed in the MS column in the magnetic field, which was rinsed three times with 500 μl of depletion buffer to wash out cells without binding with CD11b. The column was then removed from the magnetic field. One milliliter of depletion buffer was pipetted onto the column to collect the CD11b+ cell fraction. Finally, the amount of live cells and the percentage of purified AMs were determined by utilizing trypan blue exclusion and cytocentrifuge preparations stained with Wright-Giemsa, respectively (61). The recovery portion of AMs was calculated as the ratio of isolated AMs to the total macrophages in BALF. We found that the percentages of live cell rate, purification, and recovery of AMs were 95, 98, and 70%, respectively, indicating a high efficiency of the magnetic isolation used in the present study.
Culture of AMs with and without SP and NK1R antagonists.
AMs depleted from BAL cells were stored at −80°C for later use or pooled and resuspended in 0.5 ml o culture medium at 2 × 105 cells/well into 24-well plates and maintained at 37°C in a 5% CO2 incubator overnight. The culture medium consisted of RPMI 1640 medium (Sigma-Aldrich) supplemented with 1% glutamine, 0.1 mM nonessential amino acids (Life Technology, Grand Island, NY), 50 mM 2-mercaptoethanol (Sigma-Aldrich), 1% penicillin-streptomycin (Sigma-Aldrich), and 10% heat-inactivated FBS (ATCC, Manassas, VA). After being washed twice, cells were resuspended in the wells with 0.5 ml of serum starved medium. As mentioned above, AMs obtained from four groups of mice were incubated with control medium; SP (10−8 M; Sigma-Aldrich); CP-99,994 (10 ng/ml; Pfizer), a NK1R antagonist (39); and SP plus CP-99,994; respectively. The gene and protein levels of MMP-12, MMP-9, TIMP-1, and NK1R in AMs were detected in the first and second groups, respectively. The same protocols were repeated in the third and fourth groups, with the exception that CP-99,994 was replaced by aprepitant (10−8 M; Merck), another NK1R antagonist (18, 33) used in clinic (21). The doses of SP, CP-99,994, and aprepitant were chosen because several investigators have shown that they could sufficiently activate or block NK1R in the cells (28, 33, 56). For example, SP at 10−8 M for 24 h is a threshold dose that significantly evokes upregulation of TNF-α in AMs in humans (1). After being cultured for 24 h at 37°C in a 5% CO2 incubator, the supernatants were removed and the cells were collected and pelleted by centrifugation at 4,000 rpm for 10 min.
Preparation and protocols for macrophage cell lines.
This set of experiments was designed to test the dose and time dependence of SP upregulation of MMP-12 by using the RAW 264.7 macrophages. Five groups of macrophages obtained from the American Type Culture Collection were cultured with 1 ml of serum-starved medium at 4 × 105 cells/well into 12-well plates. They were treated without (control) or with four different SP concentrations from 10−10 to 10−7 M for 24 h. The macrophages in six other groups were harvested at 0, 1.5, 3, 6, 12, and 24 h after incubation with SP at 10−8 M. The same experiments in the studies described above were repeated six times, and the mRNA levels of MMP-12 were measured.
Measurement of cell viability.
Viability of AMs was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich) test (42). Immediately after culture without and with SP and/or the NK1R antagonists mentioned above for 24 h, the AMs in each well were washed twice with PBS. Subsequently, culture medium (0.5 ml) and MTT solution (0.25 mg) were sequentially added in each well, and the cells were incubated for 4 h at 37°C in 5% CO2. After being washed twice with PBS, the cells were solubilized in DMSO (100 μl/well). The formation of the purple formazan within the cells was assessed using a microplate reader (Molecular Devices, Sunnyvale, CA) at a wavelength of 570 nm. The viability of AMs was expressed as a percentage versus control.
RNA extraction, RT-PCR, and real-time PCR analysis.
Total RNA from the caudal lobe of the right lung, AMs, and cultured AMs was isolated as previously described (11, 61) using TRI reagents (Molecular Research Center, Cincinnati, OH) according to the manufacturer's protocol. RNA (2 μg) was converted to cDNA by reverse transcription using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Foster, CA) with an oligo(dT)15 primer (Promega, Madison, WI). MMP-9 oligonucleotide primers were 5′-TTC TCT GGA CGT CAA ATG TGG-3′ (sense) and 5′-CAA AGA AGG AGC CCT AGT TCA AGG-3′ (antisense). Primers for MMP-12, TIMP-1, PPT-A (a precursor of SP), NK1R, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and for PCR amplification were the same as describe previously (61). The amplified DNA fragments were analyzed by electrophoresis on a 1.5% agarose gel and stained with ethidium bromide. MMP-12, MMP-9, TIMP-1, PPT-A, NK1R, and GAPDH mRNA analyses were performed with a TaqMan real-time PCR assay on an ABI PRISM 7700 sequence detector (Applied Biosystems, Foster, CA) as described previously (61). All primers and probes for real-time PCR were supplied by Applied Biosystems. One microliter of cDNA sample from each group was used for real-time PCR assay. The results for MMP-12, MMP-9, TIMP-1, PPT-A, and NK1R are expressed as the ratio to GAPDH.
Protein preparation and EIA for SP.
After preparation of homogenate buffer [PBS; 20 mM Tris·HCl, pH 7.6, 0.5% Ipegal (Sigma-Aldrich); 250 mM NaCl; 3 mM EDTA and EGTA; 100 mM DTT and PMSF; 1 M β-glycerol phosphate; 0.2 M sodium vanadate; and 1 mg/ml leupeptin], 30 mg of cranial and accessory lobes of the right lung and AMs pellets were suspended in the buffer and homogenized as described previously (57, 61). Protease inhibitor cocktail (PI cocktail IV, 1:100 dilution; Calbiochem) was added to the supernatant and stored at −80°C. Protein concentration of the supernatants was determined using the Bradford method with a Coomassie protein assay kit (Pierce, Rockford, IL). SP in the lung extractions and AMs was quantitatively determined using a commercial EIA kit (Cayman Chemical, Ann Arbor, MI) as described (61). Data are expressed as picograms per milligram of total protein.
Western blotting for MMP-12, MMP-9, TIMP-1, NK1R, and β-actin.
Western blotting analyses were performed as described (61). Whole lung cells, AM proteins (15 μg), and cultured AMs (7.5 μg) were separated by electrophoresis in a 12% SDS-PAGE gel and transferred to nitrocellulose membranes (Amersham, Piscataway, NJ). After being blocked for 1 h at room temperature with 5% milk, the membranes were incubated overnight at 4°C in 5% BSA with an MMP-12 goat polyclonal IgG (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with horseradish peroxidase-conjugated mouse anti-goat IgG (1:1,000; Santa Cruz Biotechnology), antibody-bound proteins were developed using an electrochemiluminescent method (ECL; Amersham Biosciences). After detection of MMP-12, membranes were stripped and reprobed with MMP-9 rabbit anti-rat polyclonal antibody (1:300; Chemicon), TIMP-1 rabbit polyclonal IgG (1:200; Santa Cruz Biotechnology), NK1R rabbit polyclonal antibody (1:300; Sigma-Aldrich), and β-actin rabbit polyclonal IgG (1:1,000; Sigma), respectively. The antibody-bound proteins were visualized after incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000; Sigma).
Detecting IL-1β and TNF-α using ELISA.
When AMs were cultured without and with SP, CP-99,994, or a combination of both as described above, the protein levels of IL-1β and TNF-α in culture supernatants were quantified using the ELISA kit (R&D Systems). The measurements were performed according to the manufacturer's instructions. Results are expressed in picograms per milliliter.
Data analysis.
Data are expressed as absolute values with the exception that cell viability is expressed as a percentage of control. All data are means ± SE. The difference between the data obtained from SP dose and time dependence and from CON and NCAP-treated mice was analyzed using one-way ANOVA. The data obtained from AMs or the macrophages from the RAW 264.7 cell line incubated in the culture medium with and without the presence of SP or NK1R antagonists (CP-99,994 or aprepitant) were compared using two-way ANOVA. If ANOVA indicated a significant difference, the data were analyzed using Tukey's method as a post hoc test for the difference between groups. P values <0.05 were considered significant.
RESULTS
SP increases MMP-12 mRNA expression and protein levels in AMs in vitro.
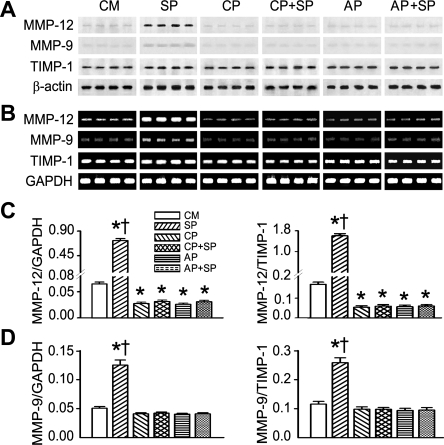
As shown in Fig. 1, we found that, compared with the control culture medium, adding SP obviously upregulated MMP-12 mRNA expression and protein levels in AMs. SP elevated MMP-12 mRNA expression of AMs 11-fold and the ratio of MMP-12 to TIMP-1 9-fold compared with control culture medium, respectively. To demonstrate whether SP uniquely upregulates MMP-12 synthesis in AMs, we also detected the MMP-9 response to SP. Our data showed that SP enhanced gene expression of MMP-9 and MMP-9/TIMP-1 1.2–1.5-fold. Two selective NK1R antagonists (CP-99,994 and aprepitant) were applied to further specify the role of NK1R in SP upregulation of MMP-12 in AMs, and these experiments led to three important results. First, SP-induced mRNA expression and protein levels of MMP-12/MMP-9 were abolished by both NK1R blockades. Second, mRNA expression and protein levels of MMP-12/MMP-9 were not different between AMs treated with CP-99,994 and aprepitant or between those treated with NK1R antagonist alone or coupled with SP. Third, most importantly, compared with AMs incubated with the control culture medium, adding NK1R blockades in the control culture medium caused a significant decrease in MMP-12 rather than MMP-9 mRNA and protein production.
Fig. 1.
Effects of substance P (SP) and CP-99,994 (CP) or aprepitant (AP) on metalloelastase (MMP-12), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of matrix metalloproteinase (TIMP)-1 mRNA expression and protein levels in alveolar macrophages (AMs) in vitro. The representative mRNA bands of MMP-12, MMP-9, and TIMP-1 (172, 390, and 333 bp, respectively) and their protein bands (54, 98, and 27 kDa, respectively) are displayed in A and B, respectively. The mRNA group data for MMP-12 and MMP-9 (the ratio of MMP-12/MMP-9 to TIMP-1) are shown in C and D, respectively. Values are means ± SE; n = 5 trials. *P < 0.01 compared with control culture medium (CM). †P < 0.01, SP vs. CP, CP+SP, AP, or AP+SP.
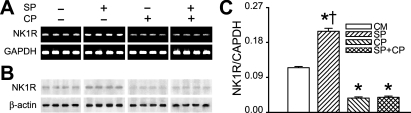
SP increases NK1R expression in AMs in vitro.
NK1R mRNA and protein levels were upregulated in AMs incubated with SP for 24 h (Fig. 2, A and B). With the use of real-time PCR, the results further showed that SP increased NK1R mRNA expression by 82% (Fig. 2C). Similar to the effects on MMP-12, NK1R blockade abolished the effect of SP on NK1R mRNA expression and significantly reduced NK1R mRNA expression compared with the control culture medium.
Fig. 2.
Effects of SP and CP on neurokinin-1 receptor (NK1R) mRNA expression and protein level in AMs in vitro. The representative NK1R mRNA (365 bp) and protein (53 kDa) from AMs are displayed in A and B, respectively. The group data of NK1R mRNA are shown in C. Values are means ± SE; n = 5 trials. *P < 0.01 compared with CM. †P < 0.01, SP vs. CP or CP+SP.
SP enhances IL-1β and TNF-α secreted from AMs in vitro.
As shown in Fig. 3, the protein levels of IL-1β and TNF-α secreted from AMs were doubled by SP compared with control. IL-1β, but not TNF-α, was significantly lower in the AMs treated with CP-99,994 than in those without treatment, indicating that endogenously released SP is able to increase IL-1β rather than TNF-α. Similar responses were also observed after CP-99,994 was applied before SP. In other words, SP-induced IL-1β and TNF-α secretion from AMs was abolished by pretreatment with CP-99,994.
Fig. 3.
Effects of SP and CP on protein levels of interleukin (IL)-1β (A) and tumor necrosis factor (TNF)-α (B) secreted from AMs in vitro. Values are means ± SE; n = 5 trials. *P < 0.01 compared with CM. †P < 0.01, SP vs. CP or CP+SP.
SP and NK1R antagonist have no cytotoxic effect on AMs in vitro.
To clarify whether the SP-induced changes in MMP-12 and NK1R was due to possible SP cytotoxic effects, we examined the viability of AMs incubated with the SP and/or NK1R antagonists by using the MTT assay as a marker. Previous studies have shown that this is a sensitive index to reflect cell viability and growth (42). We found no significant differences in MTT among the AMs treated with or without SP and/or NK1R antagonists (Fig. 4).
Fig. 4.
Effects of SP, CP, and AP on the viability of AMs in vitro. Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and expressed as the percentage of control (dashed line). Values are means ± SE; n = 5 trials for CP and AP, respectively.
SP upregulation of synthesis of MMP-12 in AMs is dose and time dependent in macrophage cell lines.
SP upregulation of MMP-12 mRNA expression appeared dose dependent. In the concentration ranging from 10−10 to 10−8 M, the higher the dose applied, the greater the responses of MMP-12 mRNA expression observed. However, for a dose >10−8 M, the response reached a plateau, i.e., the response was not significantly different between the cells exposed to SP at 10−8 and 10−7 M (Fig. 5A). With respect to the time course, compared with control, MMP-12 mRNA was significantly increased after 3 h and reached peak response at 6 h, and the increase persisted up to 24 h after administration of SP (Fig. 5B). This SP dose and time dependence of upregulation of MMP-12 was further supported by mRNA expression using RT-PCR (Fig. 5, C and D).
Fig. 5.
Dose and time dependence of SP-induced MMP-12 mRNA expression in the RAW264.7 cell line. The group data for dose and time dependence of SP-induced modulation of MMP-12 mRNA measured using real-time PCR are exhibited in A and B, respectively, whereas the representative bands of MMP-12 mRNA (172 bp) detected using RT-PCR are shown in C and D. Values are means ± SE; n = 6 trials. *P < 0.01 compared with control. †P < 0.01 compared with 10−10 M SP in A and 1.5 h in B. ‡P < 0.01 compared with 10−9 M SP in A and 3.0 h in B. Note that the data for MMP-12 mRNA obtained after 3 h of SP culture are significantly higher than those for the control (P < 0.05) in B.
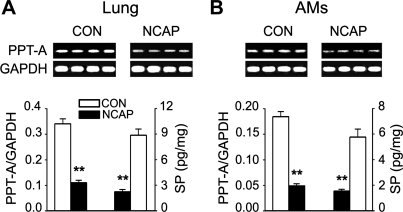
NCAP treatment reduces SP in the lungs and AMs.
We compared the levels of SP and PPT-A in lung tissues of the CON and NCAP-treated mice to ensure the effect of NCAP treatment on pulmonary SP depletion. We found that NCAP treatment resulted in downregulation of PPT-A mRNA expression in the lung tissue (Fig. 6A, top). Interestingly, a similar reduction of PPT-A was also observed in AMs (Fig. 6B, top). As shown in Fig. 6, A and B, bottom, the group data showed that NCAP treatment decreased PPT-A mRNA expression by 68% in the lung tissue and by 72% in AMs. The level of SP protein was reduced by 75% in the lung tissue and by 78% in AMs, respectively, after NCAP treatment. These results indicated that NCAP treatment was able to deplete pulmonary SP.
Fig. 6.
Effect of neonatal capsaicin (NCAP) treatment on preprotachykinin-A (PPT-A) mRNA expression and SP protein levels in lung tissues and AMs. A and B, top: 4 representative mRNA expressions of PPT-A (364 bp) in lungs (A) and AMs (B) of control (CON) and NCAP-treated mice. Bottom: relevant group data for PPT-A mRNA and SP protein. Values are means ± SE; n = 12 and 6 per group for lungs and AMs, respectively. **P < 0.01, CON vs. NCAP-treated mice.
NCAP treatment decreases MMP-12 mRNA and protein expression in the lung tissues and AMs.
In the present study, NCAP treatment induced an obvious downregulation of MMP-12 mRNA and protein expression with little change in TIMP-1 in the lung tissues and AMs (Fig. 7, A and B). Group data showed that, compared with CON, NCAP-treated mice displayed a significantly lower MMP-12 mRNA expression, detected using real-time PCR, in the lung tissues and AMs (48% and 55%, respectively; Fig. 7C). Similar results were also observed in the ratio of MMP-12 to TIMP-1 (43% in the lung tissues and 62% in AMs; Fig. 7D).
Fig. 7.
Effect of NCAP treatment on MMP-12 and TIMP-1 mRNA expression and protein levels in lung tissues and AMs. Four representative mRNA expressions of MMP-12 and TIMP-1 (172 and 333 bp, respectively) and their protein bands (54 and 27 kDa, respectively) are displayed in A and B, respectively. The group data for MMP-12 and the ratio of MMP-12 to TIMP-1 are exhibited in C and D, respectively. Values are means ± SE; n = 12 and 6 per group for lungs and AMs, respectively. *P < 0.05; **P < 0.01, CON vs. NCAP-treated mice.
NK1R mRNA and protein expression in lungs and AMs are reduced by NCAP treatment.
We also examined whether NCAP treatment would change mRNA expression and protein levels of NK1R. NCAP treatment induced the downregulation of NK1R protein and mRNA levels in the lung tissues and in AMs (Fig. 8, A and B). The corresponding group data, obtained using real-time PCR, showed that NCAP treatment decreased NK1R mRNA expression by 63% and 65% in the lung tissue and AMs, respectively (Fig. 8C).
Fig. 8.
Comparison of NK1R mRNA and protein in lung tissues and AMs between CON and NCAP-treated mice. Four representative NK1R protein (53 kDa) and gene (365 bp) expressions in lungs and AMs are displayed in A and B, respectively. The group data for the NK1R mRNA expression in the lung (left) and AMs (right) are shown in C. Values are means ± SE; n = 12 and 6 per group for lungs and AMs, respectively. **P < 0.01, CON vs. NCAP-treated mice.
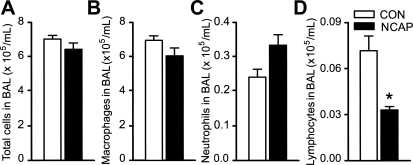
NCAP treatment alters inflammatory cells in BALF.
Compared with CON, NCAP treatment slightly decreased BAL total cells (Fig. 9A; P > 0.05). Analyzing the cells by category, we found that NCAP treatment significantly reduced lymphocytes (from 0.86 ± 0.07 to 0.52 ± 0.09%, P < 0.05; Fig. 9D) with little effect on AMs (Fig. 9B) and neutrophils (Fig. 9C).
Fig. 9.
Comparison of bronchoalveolar lavage (BAL) total (A) and differential cells [AMs (B), neutrophils (C), and lymphocytes (D)] between CON and NCAP-treated mice. Values are means ± SE; n = 6 per group. *P < 0.05, CON vs. NCAP-treated mice.
DISCUSSION
The most important finding in the present study is that SP is capable of triggering upregulation of MMP-12 expression in AMs by acting on NK1R. Numerous studies have demonstrated the key role MMP-12 plays in developing the CS-induced emphysema in animals (5, 19). Because AMs are established as the major producer of MMP-12 (27), attention has been intensively paid to the modulation of MMP-12 in AMs. Investigators have shown that MMP-12 gene and protein expression in macrophages in vitro are promoted by some cytokines, such as TNF-α and IL-1β (10). Although AMs contain NK1Rs in animals and humans (1, 2), SP is rich in the lung (48), and the chronic CS-induced increase of pulmonary SP is closely correlated to MMP-12 (61), the modulatory effect of SP on the synthesis of MMP-12 in AMs has not been explored. In the present study, we found that the levels of MMP-12 mRNA in AMs were increased 11-fold after incubation with SP, and a similar upregulation was also observed in MMP-12 protein levels. Furthermore, SP upregulation of MMP-12 in AMs was achieved by its acting on NK1Rs in AMs, because these responses did not occur when SP was added after CP-99,994 or aprepitant, the selective NK1R antagonist. A specific interesting finding in this study is that, compared with control, both MMP-12 protein and mRNA levels in AMs were significantly lower after blockade of NK1Rs, clearly indicating a role of endogenously released SP in this modulation. This small, but significant, effect of endogenously released SP on upregulation of MMP-12 in AMs is in agreement with our data on dose dependence of exogenous SP. There are two lines of evidence supporting the unique role of SP in upregulation of MMP-12 in AMs. First, exogenous SP used in this study produced an 11-fold increase in MMP-12 but only a 1.5-fold increase in MMP-9. Second, as mentioned above, endogenously released SP, revealed using NK1R antagonist (CP-99,994 or aprepitant), is able to specifically upregulate MMP-12, rather than MMP-9, because compared with control, MMP-9 gene and protein levels were not affected by adding NK1R antagonist. Nevertheless, the present study provides the experimental evidence that SP is capable of triggering upregulation of MMP-12 expression in AMs by acting on NK1R in vitro. Together with the important pathological role MMP-12 plays in animal models of COPD, our finding implies that SP might be involved in the development of emphysema and pulmonary remodeling by modulating MMP-12. To clarify the possible cytotoxic effects of SP and CP-99,994/aprepitant on AMs that may have contributed to upregulation of MMP-12, we tested the viability of AMs using the MTT assay. Our data showed that cell viability was not impaired by SP and the NK1R antagonists, suggesting that there was no cytotoxic effect of these agents on cultured AMs.
The mechanisms underlying SP-induced upregulation of MMP-12 synthesis in AMs remain unknown. SP significantly increases MMP-12 synthesis in AMs as we observed in vitro, suggesting that SP is involved in upregulation of MMP-12 synthesis in AMs. To define the threshold time required to initiate the response of MMP-12 in AMs, we measured MMP-12 in AMs in vitro at different time points after SP incubation. We found that 3 h were necessary to detect an upregulation of MMP-12. Because of this time window, other factors may also be involved in the process due to the possible cascade of effects triggered by SP. It has been well documented that SP exerts its biological activities on binding to a G protein-coupled NK1R on AMs to trigger multiple responses. For example, activating the NK1R in human macrophages by SP promotes release of TNF-α and IL-1β (1). Importantly, these cytokines are known to upregulate MMP-12 in macrophages (10). Thus SP-triggered upregulation of MMP-12 in AMs could be achieved through at least two routes. First, SP directly promotes MMP-12 synthesis in AMs by acting on NK1R and the following intracellular signaling pathways. In addition, our data have revealed that NK1Rs in AMs are elevated by increasing SP but are reduced by a NK1R antagonist. Therefore, the elevation of NK1R by SP seems to partially participate in the pathways mentioned above. Second, SP stimulates AMs to release cytokines that promote AMs to upregulate MMP-12. This assumption is supported by two lines of evidence noted in this study. Compared with control, the AMs treated with CP-99,994 presented significantly lower levels of MMP-12 and IL-1β, but not TNF-α. Thus this IL-1β elevation may partially participate in the endogenously released SP-induced upregulation of MMP-12 via activating NK1R. On the other hand, exogenous SP increased MMP-12 associated with elevation of IL-1β and TNF-α, and these responses were abolished by CP-99,994, suggesting a possible contribution of both mediators to exogenous SP-induced MMP-12 upregulation via activating NK1Rs. These findings imply the possible and different involvement of these mediators in endogenous and exogenous SP-triggered upregulation of MMP-12 synthesis in AMs. Ultimately, further studies are required to delineate the SP direct and indirect effects on the MMP-12 synthesis in AMs.
Another major finding in the present study is that NCAP treatment significantly reduces pulmonary SP and MMP-12 in the lung tissue and AMs in vivo. Pulmonary SP is primarily synthesized in the cell bodies of the PCFs located in the nodose and jugular ganglia (20). When stimulated, PCFs release SP from PCF endings into the lungs (32). Many studies have demonstrated that CS vigorously stimulates PCFs (9, 29–31), leading to a significant increase of pulmonary SP in guinea pigs (26), mice (61), and COPD patients (53). NCAP treatment has been reported to reduce pulmonary SP protein by 75–90% and efficiently cause PCF degeneration in rats (12, 23, 55). Consistent with these results, we found that SP and PPT-A mRNA in the lungs were diminished by 75 and 68%, respectively. Importantly, this pulmonary SP reduction was associated with a striking reduction of MMP-12 in the lungs (48%) and AMs (55%). The NCAP treatment effects on MMP-12 are strengthened by its downregulation of the ratio of MMP-12 to TIMP-1. It has been well documented that the imbalance of MMPs to TIMPs is likely involved in the pathogenesis of emphysema (27). Our data showed that manipulation of SP levels greatly altered the ratio of MMP-12 to TIMP-1 in AMs, clearly demonstrating a powerful role for SP in regulating MMP-12 and the ratio of MMP-12 to TIMP-1. Since SP triggers upregulation of MMP-12 expression in AMs by acting on NK1R in vitro, we reason that the downregulation of MMP-12 in the lungs and AMs by NCAP treatment, at least partially, results from the downregulation of SP. Because the increases of pulmonary SP and MMP-12 are closely correlated in the CS-induced emphysematous mice (61), our data showing that MMP-12 in AMs and lungs is modulated by SP in vivo and in vitro again suggest that SP may play a role in the pathogenesis of emphysema and airway remodeling through modulation of MMP-12. In addition to SP, PCFs also contain neurokinin A and B, which mainly act on the neurokinin-2 and -3 receptors (NK2R and NK3R), respectively (59), and these receptors exist in AMs (2, 3). One may question whether neurokinin A and B are also involved in modulation of synthesis of MMP-12 in AMs. SP could bind with neurokinin receptors with the rank order NK1R > NK2R > NK3R (13, 47). In the present study, MMP-12 mRNA expression was increased 11-fold in the AMs treated with SP, and more importantly, these responses were completely abolished by CP-99,994, a selective NK1R antagonist. These data allow us to believe that the modulatory effects of neurokinin A and B on synthesis of MMP-12 in AMs, if it exists, are limited.
Are the NCAP treatment-induced SP changes fully contributed to PCF degeneration? A number of studies have demonstrated that in addition to release of SP from PCFs, AMs and lymphocytes (44) also can release SP, especially in inflammatory airway diseases (22). In agreement, our data also revealed a spontaneous release of SP from AMs. We compared MMP-12 mRNA and protein levels in the AMs incubated with control culture medium and the medium containing an NK1R antagonist and found a significant downregulation of MMP-12 in the latter, indirectly suggesting a spontaneous release of SP from AMs. Because a slight reduction of AMs and significant decrease in lymphocyte recruitment were induced by NCAP treatment in our experiments, these alterations may be partially related to the NCAP treatment-induced SP reduction. It was reported that degradation of SP involves two enzymes, i.e., neural endopeptidase and angiotensin-converting enzyme (8, 52). Without defining the activity of these enzymes, we cannot rule out the possible elevation of their activity after NCAP treatment. Collectively, we believe that PCF degeneration is the trigger and that other non-PCF factors are the participants in the downregulation of the pulmonary SP we observed.
Pulmonary SP results in a plasticity of NK1R in the lung tissues and AMs. In the present study, we found that the changes of SP were always associated with a parallel alteration in NK1R. For example, adding exogenous SP into the culture medium significantly increased the NK1R gene and protein expression of AMs in vitro. Conversely, blocking NK1R with its antagonist diminished NK1R expression in the AMs. These findings were further strengthened by a study in vivo. It was found in the present study that reduction of pulmonary SP by NCAP treatment led to a downregulation of the expression of NK1R in the AMs. In agreement with our findings, CS produces an upregulation of pulmonary SP that is associated with an increase of NK1R in the lungs (61). Moreover, NK1R expression was upregulated in a human monocytic cell line after 16 h of incubation with SP (14), but it was decreased in the spinal cord of rats after NCAP treatment (45). These results support our finding that extracellular SP is able to modulate the expression of NK1R in AMs. Again, because SP could promote the release of cytokines from AMs, as mentioned above, whether the upregulation of NK1R in the AMs is caused by the direct or indirect effects of SP awaits further clarification.
Our time- and dose-dependent experiments were conducted in RAW 264.7 macrophages mainly because using AMs would be very costly. On the other hand, although the behavior of RAW 264.7 macrophages is not fully the same as that of AMs (4, 24), the majority of reports support the behavioral similarity between the two types of cells. For example, both AMs and RAW 264.7 macrophages presented similar responses (nitric oxide, TNF-α, IL-1β, IL-12, IL-18, and macrophage inflammatory protein-2 and -1α) to lipopolysaccharide (24, 25, 34), hypoxia-reoxygenation (51), or diesel exhaust particles (49). Moreover, the RAW 264.7 macrophages have been utilized to investigate AMs in depth, including time and dose dependence of a given response (4, 15, 50, 51) and the relevant molecular mechanisms (34, 46, 58, 62). In fact, the similarities of both types of cells have been observed in the signal pathways responsible for interferon-γ-induced suppression of c-Jun NH2-terminal kinase activation (46), and time and dose dependence of lipopolysaccharide-produced nitric oxide (34). Therefore, it is likely that the time- and dose-dependent responses we observed in RAW 264.7 macrophages would be qualitatively similar to those in AMs.
In summary, our study provides experimental evidence that SP is able to trigger MMP-12 synthesis by acting on NK1R as well as promoting NK1R expression in AMs in vitro. Our data in vivo also show that the NCAP treatment-induced reduction of pulmonary SP is associated with downregulation of MMP-12 and NK1R in the lung tissues and AMs. We conclude that an increase in pulmonary SP triggers upregulation of MMP-12, at least partially, by acting on NK1R in the AMs.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-74183 and the Master Tobacco Settlement.
Acknowledgments
We thank Pfizer Inc. and Merck & Co., Inc. for providing the NK1R antagonist as a gift and S. Shinnick and J. C. Pena-Philippides for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bardelli C, Gunella G, Varsaldi F, Balbo P, Del Boca E, Bernardone IS, Amoruso A, Brunelleschi S. Expression of functional NK1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-kappaB pathway. Br J Pharmacol 145: 385–396, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunelleschi S, Vanni L, Ledda F, Giotti A, Maggi CA, Fantozzi R. Tachykinins activate guinea-pig alveolar macrophages: involvement of NK2 and NK1 receptors. Br J Pharmacol 100: 417–420, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brylla E, Aust G, Geyer M, Uckermann O, Loffler S, Spanel-Borowski K. Coexpression of preprotachykinin A and B transcripts in the bovine corpus luteum and evidence for functional neurokinin receptor activity in luteal endothelial cells and ovarian macrophages. Regul Pept 125: 125–133, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Chu L, Peng JW, Jiang HY, Zeng QF. Expression of early growth response gene-1 in macrophages stimulated by silicon dioxide [in Chinese]. Zhonghua Bing Li Xue Za Zhi 32: 558–562, 2003. [PubMed] [Google Scholar]
- 5.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 170: 492–498, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Da Hora K, Valenca SS, Porto LC. Immunohistochemical study of tumor necrosis factor-alpha, matrix metalloproteinase-12, and tissue inhibitor of metalloproteinase-2 on alveolar macrophages of BALB/c mice exposed to short-term cigarette smoke. Exp Lung Res 31: 759–770, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Demedts IK, Morel-Montero A, Lebecque S, Pacheco Y, Cataldo D, Joos GF, Pauwels RA, Brusselle GG. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax 61: 196–201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusser DJ, Djokic TD, Borson DB, Nadel JA. Cigarette smoke induces bronchoconstrictor hyperresponsiveness to substance P and inactivates airway neutral endopeptidase in the guinea pig. Possible role of free radicals. J Clin Invest 84: 900–906, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang LB, Morton RF, Wang AL, Lee LY. Bronchoconstriction and delayed rapid shallow breathing induced by cigarette smoke inhalation in anesthetized rats. Lung 169: 153–164, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg MW, Jain MK, Werner F, Sibinga NE, Wiesel P, Wang H, Topper JN, Perrella MA, Lee ME. Transforming growth factor-beta 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J Biol Chem 275: 25766–25773, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Foster JE, Gott K, Schuyler MR, Kozak W, Tesfaigzi Y. LPS-induced neutrophilic inflammation and Bcl-2 expression in metaplastic mucous cells. Am J Physiol Lung Cell Mol Physiol 285: L405–L414, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Franco-Penteado CF, De Souza IA, Camargo EA, Teixeira SA, Muscara MN, De Nucci G, Antunes E. Mechanisms involved in the enhancement of allergic airways neutrophil influx by permanent C-fiber degeneration in rats. J Pharmacol Exp Ther 313: 440–448, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Gerard NP, Garraway LA, Eddy RL Jr, Shows TB, Iijima H, Paquet JL, Gerard C. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry 30: 10640–10646, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Germonpre PR, Bullock GR, Lambrecht BN, Van De Velde V, Luyten WH, Joos GF, Pauwels RA. Presence of substance P and neurokinin 1 receptors in human sputum macrophages and U-937 cells. Eur Respir J 14: 776–782, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Govoni G, Gauthier S, Billia F, Iscove NN, Gros P. Cell-specific and inducible Nramp1 gene expression in mouse macrophages in vitro and in vivo. J Leukoc Biol 62: 277–286, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Gronski TJ Jr, Martin RL, Kobayashi DK, Walsh BC, Holman MC, Huber M, Van Wart HE, Shapiro SD. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem 272: 12189–12194, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, Cosio MG. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hale JJ, Mills SG, MacCoss M, Finke PE, Cascieri MA, Sadowski S, Ber E, Chicchi GG, Kurtz M, Metzger J, Eiermann G, Tsou NN, Tattersall FD, Rupniak NM, Williams AR, Rycroft W, Hargreaves R, MacIntyre DE. Structural optimization affording 2-(R)-(1-(R)-3, 5-bis(trifluoromethyl)phenylethoxy)-3-(S)-(4-fluoro)phenyl-4-(3-oxo-1,2,4-triazol-5-yl)methylmorpholine, a potent, orally active, long-acting morpholine acetal human NK-1 receptor antagonist. J Med Chem 41: 4607–4614, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277: 2002–2004, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Helke CJ, O'Donohue TL, Jacobowitz DM. Substance P as a baro- and chemoreceptor afferent neurotransmitter: immunocytochemical and neurochemical evidence in the rat. Peptides 1: 1–9, 1980. [DOI] [PubMed] [Google Scholar]
- 21.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21: 4112–4119, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol 159: 5654–5660, 1997. [PubMed] [Google Scholar]
- 23.Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270: 741–743, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Jones BW, Heldwein KA, Means TK, Saukkonen JJ, Fenton MJ. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann Rheum Dis 60, Suppl 3: iii6–iii12, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JL, Lee K, Castranova V. Nitric oxide up-regulates DNA-binding activity of nuclear factor-kappaB in macrophages stimulated with silica and inflammatory stimulants. Mol Cell Biochem 215: 1–9, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Kwong K, Wu ZX, Kashon ML, Krajnak KM, Wise PM, Lee LY. Chronic smoking enhances tachykinin synthesis and airway responsiveness in guinea pigs. Am J Respir Cell Mol Biol 25: 299–305, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lagente V, Manoury B, Nenan S, Le Quement C, Martin-Chouly C, Boichot E. Role of matrix metalloproteinases in the development of airway inflammation and remodeling. Braz J Med Biol Res 38: 1521–1530, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Lee HR, Ho WZ, Douglas SD. Substance P augments tumor necrosis factor release in human monocyte-derived macrophages. Clin Diagn Lab Immunol 1: 419–423, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee LY, Beck ER, Morton RF, Kou YR, Frazier DT. Role of bronchopulmonary C-fiber afferents in the apneic response to cigarette smoke. J Appl Physiol 63: 1366–1373, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, Coleridge JC. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol 66: 2032–2038, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Lee LY, Morton RF, Kou YR. Acute effects of cigarette smoke on breathing in rats: vagal and nonvagal mechanisms. J Appl Physiol 68: 955–961, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Lee LY, Shuei Lin Y, Gu Q, Chung E, Ho CY. Functional morphology and physiological properties of bronchopulmonary C-fiber afferents. Anat Rec A Discov Mol Cell Evol Biol 270: 17–24, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Lindstrom E, von Mentzer B, Pahlman I, Ahlstedt I, Uvebrant A, Kristensson E, Martinsson R, Noven A, de Verdier J, Vauquelin G. Neurokinin 1 receptor antagonists: correlation between in vitro receptor interaction and in vivo efficacy. J Pharmacol Exp Ther 322: 1286–1293, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Liu CF, Chen YL, Chang WT, Shieh CC, Yu CK, Reid KB, Wang JY. Mite allergen induces nitric oxide production in alveolar macrophage cell lines via CD14/Toll-like receptor 4, and is inhibited by surfactant protein D. Clin Exp Allergy 35: 1615–1624, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, Kim KC. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 176: 3890–3894, 2006. [DOI] [PubMed] [Google Scholar]
- 36.March TH, Bowen LE, Finch GL, Nikula KJ, Wayne BJ, Hobbs CH. Effects of strain and treatment with inhaled aII-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. COPD 2: 289–302, 2005. [PubMed] [Google Scholar]
- 37.Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol 153: 109–119, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCusker K Mechanisms of respiratory tissue injury from cigarette smoking. Am J Med 93: 18S–21S, 1992. [DOI] [PubMed] [Google Scholar]
- 39.McLean S, Ganong A, Seymour PA, Snider RM, Desai MC, Rosen T, Bryce DK, Longo KP, Reynolds LS, Robinson G. Pharmacology of CP-99,994; a nonpeptide antagonist of the tachykinin neurokinin-1 receptor. J Pharmacol Exp Ther 267: 472–479, 1993. [PubMed] [Google Scholar]
- 40.Molet S, Belleguic C, Lena H, Germain N, Bertrand CP, Shapiro SD, Planquois JM, Delaval P, Lagente V. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res 54: 31–36, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 422: 169–173, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Mosmann T Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983. [DOI] [PubMed] [Google Scholar]
- 43.Nagatani K, Dohi M, To Y, Tanaka R, Okunishi K, Nakagome K, Sagawa K, Tanno Y, Komagata Y, Yamamoto K. Splenic dendritic cells induced by oral antigen administration are important for the transfer of oral tolerance in an experimental model of asthma. J Immunol 176: 1481–1489, 2006. [DOI] [PubMed] [Google Scholar]
- 44.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol 201: 167–180, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Ohtori S, Chiba T, Takahashi K, Ino H, Yamagata M, Sameda H, Murata Y, Moriya H. Neonatal capsaicin treatment decreased substance P receptor immunoreactivity in lamina III neurons of the dorsal horn. Neurosci Res 38: 147–154, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc Natl Acad Sci USA 97: 14382–14387, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci 74: 1445–1463, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Piedimonte G Tachykinin peptides, receptors, and peptidases in airway disease. Exp Lung Res 21: 809–834, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Saito Y, Azuma A, Kudo S, Takizawa H, Sugawara I. Effects of diesel exhaust on murine alveolar macrophages and a macrophage cell line. Exp Lung Res 28: 201–217, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol 171: 417–425, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 293: L105–L113, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Skidgel RA, Engelbrecht S, Johnson AR, Erdos EG. Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides 5: 769–776, 1984. [DOI] [PubMed] [Google Scholar]
- 53.Springer J, Groneberg DA, Pregla R, Fischer A. Inflammatory cells as source of tachykinin-induced mucus secretion in chronic bronchitis. Regul Pept 124: 195–201, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem 264: 17374–17378, 1989. [PubMed] [Google Scholar]
- 55.Sun NN, Wong SS, Keith I, Witten ML. Tachykinin substance P depletion by capsaicin exacerbates inflammatory response to sidestream cigarette smoke in rats. Toxicology 201: 39–50, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki R, Furuno T, McKay DM, Wolvers D, Teshima R, Nakanishi M, Bienenstock J. Direct neurite-mast cell communication in vitro occurs via the neuropeptide substance P. J Immunol 163: 2410–2415, 1999. [PubMed] [Google Scholar]
- 57.Tesfaigzi J, Smith-Harrison W, Carlson DM. A simple method for reusing western blots on PVDF membranes. Biotechniques 17: 268–269, 1994. [PubMed] [Google Scholar]
- 58.Wang XM, Kim HP, Song R, Choi AM. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol 34: 434–442, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Widdicombe JG Neural control of airway vasculature and edema. Am Rev Respir Dis 143: S18–S21, 1991. [DOI] [PubMed] [Google Scholar]
- 60.Xu F, Zhuang J, Wang R, Seagrave JC, March TH. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke-induced emphysema. Respir Physiol Neurobiol 158: 5–13, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Xu F, Wang R, Seagrave J, Lin Y, March TH. Cigarette smoke-induced hypercapnic emphysema in C3H mice is associated with increases of macrophage metalloelastase and substance P in the lungs. Exp Lung Res 33: 197–215, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Zsengeller Z, Otake K, Hossain SA, Berclaz PY, Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol 74: 9655–9667, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]