Abstract
The molecular events leading to emphysema development include generation of oxidative stress and alveolar cell apoptosis. Oxidative stress upregulates ceramides, proapoptotic signaling sphingolipids that trigger further oxidative stress and alveolar space enlargement, as shown in an experimental model of emphysema due to VEGF blockade. As alveolar cell apoptosis and oxidative stress mutually interact to mediate alveolar destruction, we hypothesized that the oxidative stress generated by ceramide is required for its pathogenic effect on lung alveoli. To model the direct lung effects of ceramide, mice received ceramide intratracheally (Cer12:0 or Cer8:0; 1 mg/kg) or vehicle. Apoptosis was inhibited with a general caspase inhibitor. Ceramide augmentation shown to mimic levels found in human emphysema lungs increased oxidative stress, and decreased, independently of caspase activation, the lung superoxide dismutase activity at 48 h. In contrast to their wild-type littermates, transgenic mice overexpressing human Cu/Zn SOD were significantly protected from ceramide-induced superoxide production, apoptosis, and air space enlargement. Activation of lung acid sphingomyelinase in response to ceramide treatment was abolished in the Cu/Zn SOD transgenic mice. Since cigarette smoke-induced emphysema in mice is similarly ameliorated by the Cu/Zn SOD overexpression, we hypothesized that cigarette smoke may induce ceramides in the mouse lung. Utilizing tandem mass spectrometry, we documented increased lung ceramides in adult mice exposed to cigarette smoke for 4 wk. In conclusion, ceramide-induced superoxide accumulation in the lung may be a critical step in ceramide's proapoptotic effect in the lung. This work implicates excessive lung ceramides as amplifiers of lung injury through redox-dependent mechanisms.
Keywords: sphingolipids, cell death, lung, oxidative stress
emphysema, along with chronic bronchitis, is the foremost pathological component of chronic obstructive pulmonary diseases (COPD). The unique characteristics of emphysematous alveolar destruction are the end result of pathological processes initiated in most cases by cigarette smoking. The synergistic interaction between inflammatory mechanisms, extracellular matrix proteolysis, and alveolar cell apoptosis has been postulated to cause alveolar destruction in emphysema (7, 11, 40, 41). Understanding the molecular basis of their interaction may help to better clarify the unique characteristics of this disease, since similar mechanisms operate in other lung pathological processes, such as acute lung injury or pneumonia, which are not characterized by emphysematous lung destruction.
Oxidative stress, caused by cigarette smoke itself, or by infiltrating inflammatory or injured parenchymal cells, may act as a critical mechanism that links inflammation, excessive matrix proteolysis, and alveolar cell apoptosis. A central role for oxidative stress in emphysema development and pathophysiology has been supported by the documentation of oxidative stress in lungs, bronchoalveolar lavage, and peripheral blood mononuclear cells of COPD patients and in smokers (8, 10, 30, 32, 45). Recent reports confirm the contribution of oxidative stress to emphysema pathophysiology by an observed increase in susceptibility of mice lacking the critical antioxidant transcription factor, nuclear erythroid-related factor-2 (Nrf-2), to cigarette smoke-induced emphysema when compared with wild-type littermates (34).
Although definitive data examining the beneficial effects of antioxidant supplementation in COPD patients have yet to be described, recent reports demonstrate protection against alveolar oxidative stress, alveolar cell apoptosis, and alveolar enlargement by a small molecule superoxide dismutase (SOD) mimetic in the rat model based on VEGF receptor blockade (42), as well as reported protection of Cu/Zn SOD overexpressing mice against alveolar destruction caused by chronic cigarette smoke exposure (12). These studies, together with recent work identifying a key role for ceramides in emphysema-like lung injury (28), led us to investigate whether ceramides and oxidative stress engage each other into stimulatory feedback signaling in the injured lung.
Ceramides are signaling sphingolipids composed of a sphingoid base and a fatty acid side chain that may vary in length and saturation, defining the various ceramide species. The ceramide-initiated signaling activates various stress-response, apoptosis, and cell senescence pathways (15). In turn, the enzymatic regulation of ceramide synthesis occurs via sphingomyelin hydrolysis by neutral or acid sphingomyelinase, via de novo pathway synthesis involving serine palmitoyl-CoA transferase, and to some extent via catabolism. At the cellular level, ceramide accumulation is induced by environmental or oxidative stress, cytokines, or LPS (29). In most models, including cultured pulmonary cells and in the VEGF receptor blockade model of emphysema, ceramides act upstream in the apoptosis process, preceding caspase-3 activation (28, 35, 37, 39). Although several studies have evaluated specific lung effects due to elevated ceramide levels, the distinct mechanisms underlying these effects remain largely undefined. Acutely, within minutes, ceramides increase vascular permeability (13, 36), whereas more delayed effects include apoptosis and alveolar enlargement (28). Findings in cell culture systems where ceramides induced reactive oxygen species (ROS) directly by altering mitochondrial function (2, 31) or indirectly by inactivating antioxidant enzymes such as catalase (16) indicate a potentially complex cross talk between ceramides and oxidative stress in vivo in the lung.
To study these interactions, we modeled lung ceramide increases by directly instilling ceramides intratracheally into the lungs of mice. We have previously demonstrated that direct delivery of ceramide to the lungs increased endogenous ceramides up to twofold, to a range comparable to concentrations seen in human lungs of smoking-induced emphysema (27, 28) and sufficient to trigger both alveolar epithelial and endothelial cell apoptosis and expression of oxidative stress biomarkers such as 8-hydroxyguanosine (28). In this report, using transgenic overexpression of the antioxidant enzyme Cu/Zn SOD and apoptosis inhibitors, we identified that ceramide induces redox-dependent cell death and alveolar enlargement.
MATERIALS AND METHODS
Chemicals and reagents.
N-octanoyl-d-erythro-sphingosine (Cer8:0 ceramide), N-lauroyl-d-erythro-sphingosine (Cer12:0 ceramide), and N-octanoyl-d-erythro-sphinganine (C8:0 dihydroceramide) were from Avanti Polar Lipids (Alabaster, AL). Primary antibodies utilized were actin (Calbiochem, LaJolla, CA), active caspase-3/7 (Cell Signaling Technology, Beverly, MA and Abcam, Cambridge, MA), ASMase (Santa Cruz Biotechnology, Santa Cruz, CA), Cu/Zn SOD (Upstate, Lake Placid, NY), GAPDH (Abcam), and vinculin (Calbiochem). All other reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Animal studies were approved by the Animal Care and Use Committee of Johns Hopkins University and Indiana University. A transgenic breeding pair of a wild-type C57/Bl6 mouse and a C57/Bl6 mouse overexpressing human Cu/Zn SOD were procured from the Jackson Laboratory (stock #002629). The entire human SOD1 sequence was used for the transgene. Seven copies of the transgene were inserted into chromosome 3 (J:18645). Mice were bred in our American Association for Accreditation of Laboratory Animal Care-accredited animal facility. At 4 wk of age, animals were tagged and tails clipped. DNA from their tail biopsies was isolated for genotyping. Protocols for both mouse tail DNA isolation and genotyping by PCR were provided by Jackson Laboratory. Transgenic animals were identified by the presence of the human Cu/Zn SOD transcript, whereas IL-2 was used as internal wild-type control (Supplementary Fig. 1B. Supplemental data for this article is available online at the AJP-Lung web site.). Experiments such as ceramide administration were performed on male and female mice at 3–4 mo of age.
Fig. 1.
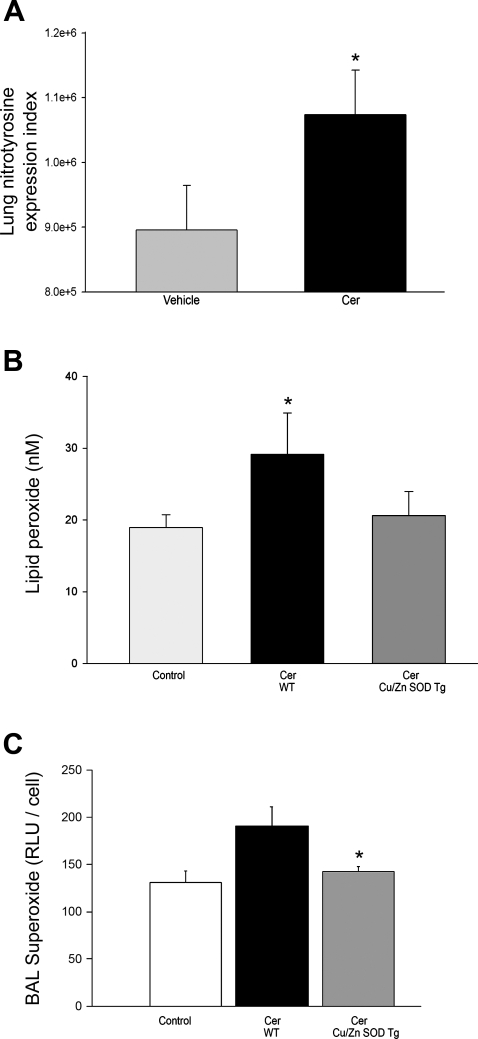
Effect of ceramide on oxidative stress in the mouse lung and modulation by Cu/Zn SOD overexpression. A: nitrotyrosine expression levels detected by immunohistochemistry (IHC) in the lung parenchyma 48 h after intratracheal administration of vehicle (n = 5) or ceramide 12:0 (Cer; 1 mg/kg; n = 5). Paraffin sections from lungs of mice were coded, and images of alveolar tissue were captured in a random blinded fashion. The number of alveolar cells expressing nitrotyrosine and the expression intensity were quantified by image software analysis and expressed as index (means + SD; *P = 0.002, ANOVA). B: lipid peroxide levels measured in the lungs of wild-type (WT; n = 4) or Cu/Zn SOD transgenic (Tg; n = 5) mice 48 h after Cer instillation compared with control (means + SE; *P = 0.050 vs. control). C: superoxide levels in bronchoalveolar (BAL) lavage of WT (n = 4) and Tg (n = 6) mice 48 h after intratracheal Cer instillation (means + SE; *P = 0.01 vs. WT Cer).
Cigarette smoke exposure was performed as previously described (6). DBA2 mice (n = 5 per group) were exposed to cigarette smoke or ambient air for 4 wk. Mice were then euthanized, lungs were dissected, and the lung tissue was snap-frozen and stored. The lipid extraction was performed by a Bligh and Dyer method, and lipid phosphorus was measured as described (28). Ceramides were measured by tandem mass spectroscopy utilizing internal standards C17 (28) and normalized by lipid phosphorus concentration. This method enables one to both measure individual ceramide species and discriminate between ceramides and dihydroceramides, the immediate precursors of ceramide in the de novo pathway of its synthesis.
Ceramide administration was performed as previously described (28). Briefly, bioactive ceramide or dihydroceramide was first solubilized in ethanol and then delivered with perfluorocarbon as vehicle. We performed experimental instillation of ceramide or dihydroceramide (1 mg/kg it) in transgenic mice (3–6/group) and wild-type littermate control mice at 3 mo of age for both females and males. A separate experiment was performed comparing ceramide or vehicle (perfluorocarbon) in transgenic mice (5–8/group) and wild-type littermate control mice at 3 mo of age for both females and males. We performed intratracheal instillation by two techniques, under indirect and direct visualization of the trachea. We have previously documented the efficiency of ceramide delivery with the less invasive, indirect technique, also known as aspiration (20), which generated a similar degree of air space enlargement and apoptosis as the direct approach (28). Given that excessive apoptosis may generate further oxidative stress (42), we dissected the role of caspase-dependent apoptosis on the ceramide-induced oxidative stress. For caspase inhibition in vivo, mice were given a general caspase inhibitor, Z-Asp-CH2-DCB (3 mg/kg; Biomol International, Plymouth Meeting, PA), successfully used in vivo to inhibit lung apoptosis and to attenuate ceramide-induced air space enlargement (18, 28), or DMSO (vehicle) intraperitoneally daily 2× (on day 1, the inhibitor was injected 60 min before ceramide instillation). At the end of experiments, the mice were euthanized, and the tissue was processed as described. In addition, mice underwent bronchoalveolar lavage (BAL) with 0.6 ml of PBS times 3. The whole BAL was then snap-frozen in liquid nitrogen and stored at −80°C for further analysis. An aliquot was used for cell counting.
Lipid extraction and ceramide species measurement by tandem mass spectroscopy.
To determine which ceramide molecular species were present in lung samples from cigarette smoke, we used a modification of the combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) technique by Sullards and Merrill (38). Cellular or lung tissue lipids were extracted, and lipid content was assessed by measurements of total lipid phosphorus (Pi) (28). After lipid extraction, ceramides were eluted as previously described (28). The following individual molecular species of ceramides were monitored: 14:0, 16:0, 18:0, 18:1, 20:0, 24:0, and 24:1-ceramides, utilizing C17 ceramide as internal standard.
Measurements of oxidative stress included detection of nitrotyrosine (42), lipid peroxides, superoxide, and hydrogen peroxide, as well as lung enzymatic activity of superoxide dismutase and catalase. Nitrotyrosine expression was evaluated by immunohistochemistry (IHC) of lung parenchyma (large airways and large blood vessels were omitted). Briefly, 5-μm paraffin slides were incubated with anti-nitrotyrosine antibody (dilution 4:1,000; Zymed, South San Francisco, CA). Slides were then coded, and a blinded individual captured random pictures of the stained slides. Intensity of staining was quantified via image analysis software (Metamorph; Molecular Devices, Downingtown, PA), and an index was calculated from the total pixel count generated. Lipid peroxides were measured in lung lysates using the LPO kit from Cayman Chemical (Ann Arbor, MI). The superoxide levels and SOD activity were measured using kits from Calbiochem and Cayman Chemical, respectively. Superoxide levels were measured in BAL (10 μl) previously snap-frozen via luminol-based chemiluminescence, and results were expressed as relative light units normalized by the number of cells in the BAL. To ensure the specificity of the superoxide detection assay, we used as positive and negative controls the xanthine and xanthine oxidase reaction (25 μM), which generate superoxide anions that oxidize luminol and the neutralization of this reaction with SOD (25 units), respectively. The specificity of the assay for superoxide anion was confirmed by complete neutralization of the luminescence signal generated by the xanthine and xanthine oxidase reaction (447.9 RLU) by the SOD enzyme (200 RLU). The superoxide dismutase activity was measured in total lung lysates and in lysates that were fractionated into cytosolic and mitochondrial components by centrifugation at 10,000 g for 15 min at 4°C (24). The hydrogen peroxide was measured by the Fluoro H2O2 kit from Cell Technology (Mountain View, CA) utilizing aliquots of previously snap-frozen BAL. The catalase activity was measured in lung tissue lysates utilizing the Amplex Red Catalase Assay Kit from Molecular Probes (Eugene, OR) following the manufacturer's instructions.
Morphometric analysis was performed on coded slides as described (1, 42).
Apoptosis was detected in lysates (28) or inflated lung enabling focus on alveoli, rather than large airways and vessels (42) via active caspase-3 IHC (Abcam and Cell Signaling) or in situ labeling of apoptotic DNA on murine lung (28), using rat serum as negative control. The immunostaining for both casapase-3 and TUNEL was followed by DAPI (Molecular Probes) nuclear counterstaining. Executioner caspase (caspase-3 and/or -7) activity was measured with ApoONE Homogeneous Caspase-3/7 assay kit (Promega, Madison, WI) as described (28). Human recombinant caspase-3 (Calbiochem) was utilized as positive control.
Acid sphingomyelinase enzymatic activity determination.
The acid sphingomyelinase activity was measured at pH 5.0 in the absence of added cations as described (28) utilizing the Amplex E Red Sphingomyelinase Assay Kit (Molecular Probes) following the manufacturer's instructions. Results were normalized by the protein content of the lung lysate.
Western blotting.
Lung tissue was homogenized in RIPA buffer with protease inhibitors on ice, and proteins were isolated by centrifugation at 10,000 g for 10 min at 4°C. Proteins were loaded in equal amounts (10 μg, unless otherwise noted) as determined by BCA protein concentration assay (Pierce, Rockford, IL). Total proteins (10–50 μg/lane) were separated by SDS-PAGE using Novex gels (Invitrogen, Carlsbad, CA), followed by immunoblotting for human Cu/Zn SOD as previously described (28). The chemiluminescent signals were quantified by densitometry (ImageQuant; Amersham, Piscataway, NJ) and normalized by housekeeping proteins (actin, GAPDH, or vinculin).
Zymography.
Lung tissues were homogenized in homogenization buffer (50 mM Tris·HCl, pH 7.6, 150 mM NaCl, 5 mM CaCl2, 1% Triton X-100, 0.05% Brij-35) with protease inhibitors (2.3 mM AEBSF, 30 μM pepstatin, 1 μg/ml leupeptin, and 1 μg/ml aprotinin). Homogenates were sonicated using a cell disrupter, and supernatants collected after centrifugation at 10,000 rpm at 4°C. Total protein concentrations were determined by BCA assay. Homogenates (30 μg) were mixed with nondenaturing 5× Laemmli buffer (0.125 M Tris·HCl, pH 6.8, 20% glycerol, 4% SDS, 0.004% bromophenol blue) and resolved on either 10% or 12% gelatin-containing polyacrylamide gels (Bio-Rad). Gels were incubated at room temperature in 50 mM Tris·HCl, pH 7.6, 5 mM CaCl2, 1 μM ZnSO4, 2.5% Triton X-100 for 25 min, and then washed in 50 mM Tris·HCl, pH 7.6, 5 mM CaCl2, and 1 μM ZnSO4. Gels were stained with coomassie blue for 60 min and then destained for detection of MMP activity, which was quantified by densitometry using Image J (NIH) software.
Statistical analysis was performed with SigmaStat software using ANOVA with Student-Newman-Keuls post hoc test. Statistical difference was accepted at P < 0.05.
RESULTS
Augmentation of ceramide levels in the lung triggered oxidative stress. We have previously shown that intratracheal administration of bioactive synthetic ceramide 12:0 (Cer12:0) augments endogenous ceramide levels in a dose-dependent manner (28). A dose of 1 mg/kg resulted in a twofold increase in endogenous lung ceramides and raised the absolute ceramide content to levels comparable to those measured in samples from patients with cigarette smoke-induced emphysema and in emphysematous lungs of mice and rats treated with a VEGF receptor blocker (28). Furthermore, this approach increased 8-hydroxyguanosines detected at 24 h postinstillation, suggesting oxidative damage of nucleic acids in the lung parenchyma (28). We therefore utilized this dose of exogenous ceramide delivered intratracheally to adult mice to further define the selective effect of augmented lung ceramides on the redox state of the lung. Ceramide instillation markedly increased other markers of oxidative damage such as lung nitrotyrosine expression (Fig. 1A) and lung lipid peroxide levels (Fig. 1B). In accordance to alterations seen in COPD patients (33) and rodent models of the disease (34), ceramide treatment was associated with elevation of ROS in the BAL of mice compared with control animals. The average cell number in the BAL in all treatment groups was 17 × 104/ml, with a majority of alveolar macrophages (>90%). There was no significant loss of cell viability in the BAL of ceramide-instilled lungs, as assessed by trypan blue staining. Although difficult to measure due to their instability, ceramide treatment was associated with a trend of increased hydrogen peroxide (Supplementary Fig. 1A) and superoxide (Fig. 1C) levels in the lavage of mice at 48 h.
Ceramide treatment inhibited lung SOD activity.
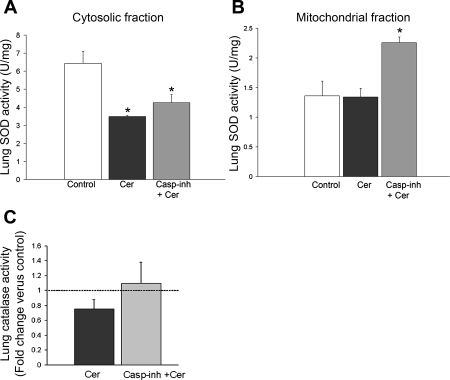
Interestingly, the cytosolic rather than the mitochondrial SOD activity was markedly decreased by ceramide instillation compared with control (Fig. 2, A and B). In addition, a trend towards a decrease in lung catalase activity following ceramide treatment was observed (Fig. 2C). The primary destructive effect of ceramide augmentation in the lung parenchyma appears to be alveolar cell apoptosis (28), which may itself trigger further oxidative stress and disrupt mitochondrial protein, such as mitochondrial SOD (42). To determine whether the effect of ceramide on antioxidant enzymes was apoptosis dependent, we treated mice with a general caspase inhibitor. We have previously demonstrated that caspase inhibition by an identical approach was effective in this model in decreasing ceramide-induced apoptosis (28). However, attenuating caspase activation had no statistically significantly effect on the ceramide-induced modifications of cytosolic SOD (Fig. 2A) or catalase (Fig. 2C) activities. On the other hand, caspase inhibition augmented mitochondrial SOD activity (Fig. 2C), suggesting a cross talk between caspase and mitochondrial SOD antioxidant activities or mitochondrial integrity. The finding that ceramides decreased cytosolic SOD activity and increased superoxide production in the lungs of mice prompted us to investigate the effects of Cu/Zn SOD activity augmentation on the ceramide-induced redox state of the lung.
Fig. 2.
Antioxidant enzymatic activities in response to ceramide augmentation in the lung and modulation by caspase inhibitors. Lung SOD activity levels measured 24 h after intratracheal dihydroceramide (control; white bar) or ceramide (Cer) instillation (1 mg/kg) without (black bar) or with Z-Asp-CH2-DCB (3 mg/kg ip daily ×2; gray bar). SOD activity was measured in lung lysates after fractionation into cytosolic (A) and mitochondrial components (B), respectively, followed by normalization by protein concentration (means + SE; *P < 0.05 vs. control). C: catalase activity levels in the mouse lung measured 24 h after intratracheal dihydroceramide (control; n = 3) or ceramide (Cer; n = 4) instillation (1 mg/kg) without (black bar) or with Z-Asp-CH2-DCB (3 mg/kg ip daily ×2; gray bar). Results were expressed as mean fold change compared with control (dotted line) +SE.
Cu/Zn SOD overexpression prevented ceramide-induced lung oxidative stress.
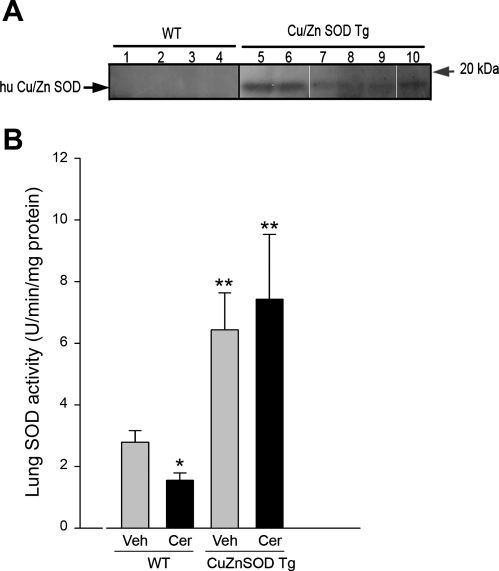
As expected (43), transgenic mice overexpressing human Cu/Zn SOD, demonstrated by genotyping and protein analysis (Supplementary Fig. 1B and Fig. 3A, respectively), exhibited marked increases in lung SOD activity compared with wild-type littermates (Fig. 3B). In this setting of augmented SOD, lung ceramide augmentation failed to inhibit SOD activity (Fig. 3B). The Cu/Zn SOD transgenic overexpression was associated with significant protection against ceramide-induced oxidative stress in the lung (Figs. 1, B and C, and Supplementary Fig. 1A).
Fig. 3.
SOD activity modulation by ceramides and Cu/Zn SOD transgenic overexpression. A: expression levels of human Cu/Zn SOD in the lung of wild-type (WT) mice (lanes 1–4) and transgenic (Tg) mice (lanes 5–10) detected by Western blot with species-specific Cu/Zn SOD antibody. B: SOD activity levels in the lungs of WT and Cu/Zn SOD transgenic mice measured 48 h after intratracheal vehicle (Veh) or ceramide (Cer) instillation. SOD activity was measured in lung lysates and normalized by protein concentration (means + SD; *P = 0.03 vs. Veh-Wt; **P < 0.05 vs. WT). Number of animals studied: WT + Veh, n = 5; WT + Cer, n = 4; Tg + Veh, n = 5; Tg + Cer, n = 8.
Cu/Zn SOD overexpression attenuated ceramide-induced caspase-3 activation in the lung.
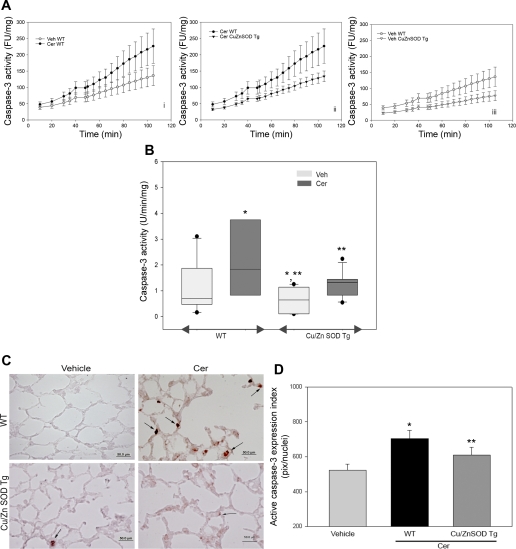
We have previously demonstrated that intratracheal ceramide instillation induced lung alveolar cell apoptosis, characterized by caspase-3 activation, elevated TUNEL-positive cells, and bax activation (28). Therefore, in this study, we chose the caspase-3 activity as the endpoint marker for apoptosis of lung parenchyma. This endpoint allows for both quantitative assessment and localization studies of cellular apoptosis to alveolar spaces rather than large airways or vessels. In line with our previous results, ceramide treatment significantly enhanced caspase-3 activation in lungs of wild-type littermates of transgenic mice compared with vehicle control (Fig. 4, A and B). The extent of ceramide-induced alveolar cell apoptosis was markedly attenuated in the Cu/Zn SOD transgenic mice (Fig. 4, A and B). Furthermore, the Cu/Zn SOD overexpressing mice manifested a reduction in the baseline caspase-3 activity measured in vehicle-treated animals (Fig. 4, A and B). To ascertain whether the increase in caspase-3 activity occurred in alveolar septal cells, alveolar cell apoptosis was determined by immunohistochemical detection of active caspase-3. Indeed, alveolar cells of wild-type mice treated with ceramide exhibited elevated active caspase-3 compared with vehicle-treated wild-type mice, whereas Cu/Zn SOD mice exhibited reduced levels of alveolar cell apoptosis in response to ceramide instillation (Fig. 4, C and D).
Fig. 4.
Effect of Cu/Zn SOD transgenic overexpression on ceramide-triggered caspase-3 activation in the mouse lung. A: kinetics of caspase-3 activity in mouse lung lysates incubated with a fluorescently labeled specific caspase substrate. Results were expressed in units of activity of recombinant human caspase-3, normalized by protein concentration; means ± SD. B: boxplot of caspase-3 activities measured in lung lysates from wild-type (WT) and Cu/Zn SOD transgenic (Tg) mice after vehicle (light boxes) or ceramide (Cer; dark boxes; n = 7) instillation. Horizontal line represents median activity normalized by protein concentration, with dots showing outliers and whiskers showing the 5th and 95th percentile values, respectively. (ANOVA followed by Bonferroni t-test; *P < 0.05 vs. Veh-Wt; **P < 0.05 vs. Cer-Wt). C: expression levels of active caspase-3 (brown, arrows) detected by IHC with specific active caspase-3 antibody in the lung parenchyma of wild-type and Cu/Zn SOD transgenic (Tg) mice 48 h after intratracheal instillation of vehicle or ceramide (1 mg/kg). D: quantification of caspase-3 immunostaining. Random sections were imaged from coded slides, followed by computation of the intensity and areas of caspase-3 activation by image analysis software, normalized by total nuclei (DAPI-stained; means + SE, *P < 0.05 compared with Cer-Wt. Ctl represented Veh-treated lungs). Number of animals studied: Wt + Veh, n = 5; Wt + Cer, n = 4; Tg + Veh, n = 5; Tg + Cer, n = 8.
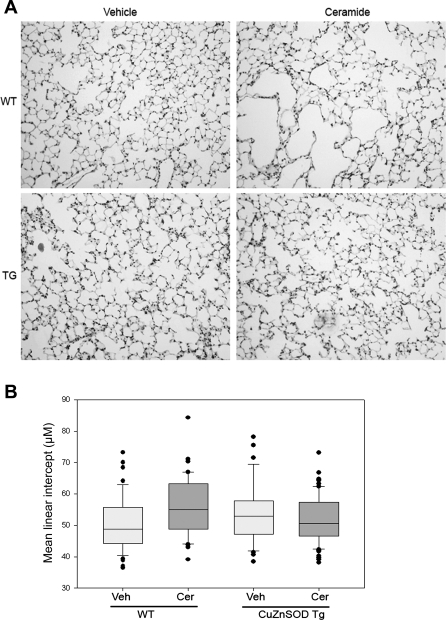
Ceramide-induced air space enlargement was inhibited in Cu/Zn SOD transgenic mice.
Mice overexpressing Cu/Zn SOD were protected against ceramide-induced air space enlargement at 48 h (Fig. 5, A and B). We have shown previously (28) that ceramide instillation in the lung increases alveolar mean linear intercept associated with an activation of matrix proteases such as MMP-12 at 24 h. In addition, MMP-9 activity was significantly increased in the lungs of mice 48 h following ceramide instillation (Supplementary Fig. 2). Ceramide treatment increased the mean linear intercept from 50.4 ± 1.1 μm (mean ± SE) in controls to 54.5 ± 1 in wild-type mice, but only to 52.4 ± 0.9 in Cu/Zn SOD transgenic mice (P = 0.012 by 2-way ANOVA with post hoc Bonferroni t-test) showing a statistically significant increase in alveolar diameters after ceramide administration in wild-type (P = 0.015), but not in transgenic mice (P = 0.255). The significant protection conferred by the overexpression of Cu/Zn SOD against ceramide-induced lung apoptosis and air space enlargement suggested that ROS generated by ceramides in the lung directly contributed to alveolar enlargement.
Fig. 5.
Effect of Cu/Zn SOD overexpression on air space enlargement in ceramide-treated mice. A: microphotographs of hematoxylin-eosin-stained paraffin-embedded sections of mouse lung fixed at a constant inflation pressure. Compared with vehicle, ceramide instillation increased the air space size in wild-type mice (WT) but not Cu/Zn SOD transgenic (TG) mice at 48 h. B: standardized morphometric measurement of mean linear intercepts of lung parenchyma prepared as described in A and presented as boxplot with the horizontal line depicting median values, whiskers showing the 5th and 95th percentiles and dots depicting outliers. (Wt + Veh, n = 5; Wt + Cer, n = 4; Tg + Veh, n = 5; Tg + Cer, n = 8; P < 0.05, ANOVA).
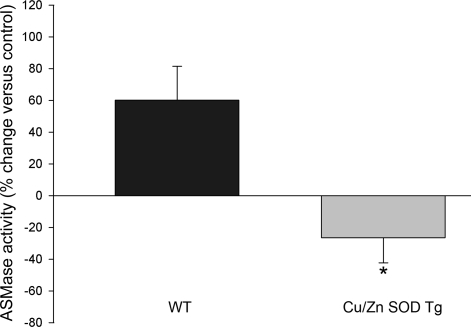
Exogenous ceramides fail to activate acid sphingomyelinase in the Cu/Zn SOD transgenic lungs.
We have shown that ceramides administered intratracheally to mice (with a fatty acid chain of 12 carbons) caused an increase in endogenous ceramides (with fatty acid chains of 14 carbons and higher) concomitant with ASMase activation (28). We postulated that the ASMase activation generates more ceramides, thus leading to a positive feedback mechanism causing the amplification of lung injury (illustrated in schematic in Supplementary Fig. 3). To investigate whether the protective action of Cu/Zn SOD overexpression was associated with an inability of exogenous ceramides to amplify further ceramide synthesis, we measured the ASMase activity in the lungs of wild-type and Cu/Zn SOD transgenic animals 48 h following intratracheal exogenous ceramide 12:0 or vehicle administration. Unlike wild-type mice, which manifested a 50% increase in ASMase activity, the Cu/Zn SOD overexpressing mice failed to increase the lung ASMase activity in response to exogenous ceramide (Fig. 6).
Fig. 6.
Effect of Cu/Zn SOD overexpression on acid sphingomyelinase (ASM) activation in ceramide-treated mice. ASM activity was measured in wild-type mice (WT) or Cu/Zn SOD transgenic (Tg) mice 48 h after intratracheal ceramide instillation utilizing a fluorometric activity assay. The enzymatic activity was normalized by protein concentration, and results were expressed as average % change compared with the vehicle control for each genotype (% +SE; *P = 0.01, Student's t-test). Number of animals studied: Wt + Veh, n = 5; Wt + Cer, n = 4; Tg + Veh, n = 5; Tg + Cer, n = 8.
Cigarette smoke exposure increases ceramides in the lung.
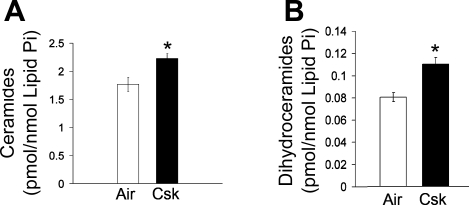
Cigarette smoke is the most common etiological insult in emphysema, and Cu/Zn SOD overexpression has been shown to attenuate both the oxidative stress and alveolar enlargement from chronic cigarette smoke exposure in mice (12). Since our data implicated ceramides in the upregulation of oxidative stress in the lung, which was prevented by Cu/Zn SOD overexpression, we asked whether ceramides are upregulated in vivo by cigarette smoke. Cigarette smoke has been shown to upregulate ceramide levels in lung endothelial cells (28). Furthermore, ceramides were elevated in a model of apoptosis-dependent emphysema, induced by VEGF receptor blockade in mice and rats (28). However, the contribution of cigarette smoke to the upregulation of endogenous ceramides in vivo remains undefined. We measured ceramides by tandem mass spectroscopy in the lungs of mice exposed to cigarette smoke extract for 4 wk. Cigarette smoke exposure significantly upregulated lung ceramides compared with control lungs, increasing the ceramide concentration per unit of phospholipids by 26% (P = 0.01) (Fig. 7A). Interestingly, dihydroceramides were also increased by cigarette smoke (by 37%) (Fig. 7B), suggesting activation of the de novo pathway of ceramide synthesis as a potential mechanism of initial ceramide upregulation.
Fig. 7.
Lung ceramide levels in response to cigarette smoke exposure in mice. A: levels of endogenous ceramides measured by tandem mass spectrometry and normalized by lipid phosphorus in lungs of mice exposed to air (n = 5) or cigarette smoke (Csk; n = 5) for 4 wk (means ± SE; *P = 0.01). B: levels of endogenous dihydroceramides, immediate precursors of ceramides in the de novo synthetic pathway, measured by tandem mass spectrometry and normalized by lipid phosphorus in the lungs of mice exposed to air or Csk for 4 wk (means ± SE; *P = 0.003).
DISCUSSION
The present study shows that selective increases in lung ceramides by airway instillation of bioactive synthetic ceramides inhibited SOD activity, subsequently causing increased oxidative stress in the lung. In turn, the oxidative stress directly contributed to caspase activation and alveolar space enlargement as documented by the protective effects of overexpressing Cu/Zn SOD in mice. In follow-up of our initial observation that ceramide induces the activation of ASMase (28), an enzyme responsible for further increases in endogenous ceramides, we documented in the current study that the lung ASMase activation is redox dependent. Overall, these results for the first time directly implicate oxidative stress as a downstream pathological mediator of alveolar enlargement caused by increased lung ceramides, a finding with potential relevance to cigarette smoke-induced lung injury.
Ceramides are upregulated in the lungs of patients with severe emphysema, as well as in lungs from mice and rats treated with VEGF receptor 2 inhibitors, a model of apoptosis-dependent emphysema (27, 28). We report here the novel finding that cigarette smoke increases ceramides in the lungs of mice after 4 wk of intermittent exposure (5 h a day, 5 days a wk). This timepoint precedes the development of morphological changes of emphysema that typically occur after 4–6 mo of exposure in DBA2 or C57/Bl6 mice, respectively (3). It remains to be determined the role of ceramide to the contribution of concomitant inflammation, oxidative stress, apoptosis, and extracellular matrix remodeling in the setting of cigarette smoking. To address the direct effects of increased lung ceramide levels, we augmented ceramide levels via instillation of bioactive ceramide and noted that it triggered oxidative stress, apoptosis, macrophage influx in the lung parenchyma, and activation of extracellular matrix proteases MMP-12 (28), and, as shown in the present studies, MMP-9. While many of these findings recapitulate phenotypically the chronic manifestations of experimental emphysema, other changes, including the extent and reversibility of the extracellular matrix degradation, remain to be determined. Notwithstanding these potential limitations, our experimental approach based on intratracheal delivery of ceramide in the lung allowed us to link ceramide-specific signaling mechanisms and redox balance in the lung. In line with our findings, overexpression of Cu/Zn SOD resulted in protection against cigarette smoke-induced air space enlargement in mice (12). It is therefore conceivable that ceramide might participate in the generation of oxidative stress via generation of superoxide, thus contributing to cigarette smoke-induced lung injury.
The inability of the transgenic Cu/Zn SOD mouse to augment the ASMase activity from its baseline in response to exogenous ceramide may imply that Cu/Zn SOD exerted its protective effects by limiting the amplification of ceramide synthesis in the ceramide-challenged lung. Furthermore, these findings indicate a redox-dependent regulation of lung ASMase activity. Such an effect, first reported by Lavrentiadou et al. (22) who noted that in A549 cells glutathione modulated hydrogen peroxide-induced ASMase activity, was recently confirmed in endothelial cells (47). There is growing evidence that ASMase mediates cellular injury in the liver (21) and inflammatory effects in the lung induced by platelet-activating factor or Pseudomonas infection (14). Similar to our previous report (28), ceramide instillation increased lung ASMase activity in wild-type mice. Future studies will determine whether cigarette smoke initiates a similar mechanism of ROS-dependent ASMase upregulation and amplification of (paracellular) ceramide synthesis in the lung, similar to our finding of extra- or paracellular pools of ceramide contributing to alveolar cell apoptosis in the VEGF receptor blockade model (28).
The prooxidative impact of ceramides in the lung may stem from a direct effect on mitochondrial electron transport chain and/or an inhibitory effect on lung antioxidant enzymes (16, 17, 22, 31). Enhanced ceramide concentrations in the lung were sufficient to increase superoxide (O2−) release as a critical step in ceramide-induced lung apoptosis and alveolar enlargement. Since the protection conferred by Cu/Zn SOD overexpression was complete in the majority of animals studied, and the inhibitory effect of ceramide on catalase was not robust, we speculate that the excess in hydrogen peroxide (H2O2) was rapidly neutralized by either catalases or glutathione peroxidases in the transgenic model. Whereas ceramide's effect on mitochondria and antioxidant catalases may be related to apoptotic activities as previously shown (16) and as suggested in our data, its inhibitory effect on the cytosolic antioxidant SOD was caspase independent. The human genome expresses three SOD genes: the cytosolic Cu/Zn SOD (SOD1), the mitochondrial Mn SOD (SOD2), and the extracellular SOD3 (19). Transgenic mice overexpressing either Mn SOD or Cu/Zn SOD, when exposed to oxidative stress such as hyperoxia, show increased survival and decreased lung injury (43, 44), suggesting efficient superoxide scavenging in the lungs of these animals. We focused on Cu/Zn SOD for several reasons. First, cytosolic, rather than mitochondrial, levels of SOD activity were primarily affected by ceramide augmentation in the lung. Second, Foronjy et al. (12) reported remarkable protective effect of Cu/Zn SOD overexpression on cigarette smoke-induced inflammatory markers and air space enlargement in mice. Utilizing a similar transgenic model of human Cu/Zn SOD to increase the mouse lung SOD activity to more than twice normal levels was sufficient to attenuate the oxidative stress induced by ceramides and its downstream deleterious effects on caspase-3 activation and air space enlargement. For unclear reasons, which may reflect events at the lung developmental stage, we and others (12) noted a statistically insignificant higher mean linear intercept in the control Cu/Zn SOD transgenic mice compared with untreated wild-type littermates. Overall, the significant protective effects of Cu/Zn SOD do not rule out the potential effectiveness of increasing the activity of other SODs to prevent ceramide-induced lung injury.
The mechanism by which ceramide (Cer12:0 or Cer8:0) decreased Cu/Zn SOD activity in the lung is not clear. This effect may be organ or ceramide-species specific, as in cultured neurons ceramide Cer2:0 was found to increase Cu/Zn SOD activity (4). Similar to our study in which lungs of Cu/Zn SOD transgenic animals were protected from ceramide-induced apoptosis, isolated neurons (4) or carotid arteries (9) from these transgenic mice were protected from ceramide-induced cell death or endothelial dysfunction, respectively. Interestingly, Mn SOD was found to be transcriptionally activated by ceramide in several cell lines, and it also had a protective role against ceramide-induced apoptosis (26). While we could detect no significant effect of ceramide on the mitochondrial SOD, cotreatment with a general caspase inhibitor did increase its activity. While establishing the mechanism of this increase was beyond the scope of this report, we speculate the Mn SOD could have been activated by a potential increase in ROS triggered by caspase inhibition. Indeed, caspase inhibition with ZVAD-fmk has been shown to stimulate ROS accumulation via autophagic-induced catalase degradation (46). It is therefore possible that ZVAD treatment increased ROS causing a stimulation of Mn SOD activity, whereas the Cu/Zn SOD and catalase activities were not increased due to a direct suppression from ceramide and ZVAD, respectively.
Numerous reports have implicated excessive levels of oxidative stress in the pathogenesis of emphysema, usually attributed to cigarette smoke or phagocyte activation (5, 25). Erythrocytes and alveolar macrophages from smokers show altered activities of the antioxidant enzymes, which are hypothesized to be initially activated, but eventually overwhelmed, in their ability to scavenge the excess free radicals (25, 45). ROS are well known to play various roles in the cellular stress responses, from physiological intracellular signaling, to injury and cell death. The type of cell death induced by oxidative stress depends on the cell type and amounts of ROS released. Classically, high levels of ROS are associated with necrosis, whereas moderate to low levels of ROS cause apoptosis (23). More recently, apoptosis itself has been incriminated in further augmentation of the oxidant stress (42), presumed through the activation of mitochondrial sources of ROS, or through secondary necrosis, although the precise mechanisms are yet to be elucidated. The findings of our study support the notion that excessive oxidative stress and apoptosis mutually interact and contribute to alveolar space enlargement.
In conclusion, ceramide upregulation in the lung increased oxidative stress and apoptosis, whereas preservation of antioxidant defenses, particularly of Cu/Zn SOD, inhibited caspase activation and interrupted the activation of acid sphingomyelinase and thus potentially the endogenous upregulation of ceramide. These observations may be pertinent to human emphysema, where pathogenic positive feedback loops such as those between oxidative stress and apoptosis may explain the relentless progression of alveolar destruction, even after patients abstain from smoking.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-077328 (I. Petrache) and R01-HL-66554 (R. M. Tuder) and American Lung Association/Maryland Lung Association Grant C-03-006.
Supplementary Material
Acknowledgments
We gratefully acknowledge Carol A. Hargreaves for technical assistance with lipid peroxide determination.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aherne WA, Dunnill MS. Morphometry. London: Edward Arnold, 1982, p. 205.
- 2.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med 31: 717–728, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bartalesi B, Cavarra E, Fineschi S, Lucattelli M, Lunghi B, Martorana PA, Lungarella G. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur Respir J 25: 15–22, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Borg J, London J. Copper/zinc superoxide dismutase overexpression promotes survival of cortical neurons exposed to neurotoxins in vitro. J Neurosci Res 70: 180–189, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cantin A, Crystal RG. Oxidants, antioxidants and the pathogenesis of emphysema. Eur J Respir Dis Suppl 139: 7–17, 1985. [PubMed] [Google Scholar]
- 6.Cavarra E, Bartalesi B, Lucattelli M, Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA, Lungarella G. Effects of cigarette smoke in mice with different levels of alpha(1)-proteinase inhibitor and sensitivity to oxidants. Am J Respir Crit Care Med 164: 886–890, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Churg A, Wright JL. Proteases and emphysema. Curr Opin Pulm Med 11: 153–159, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Dekhuijzen PN, Aben KK, Dekker I, Aarts LP, Wielders PL, van Herwaarden CL, Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 154: 813–816, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Didion SP, Faraci FM. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler Thromb Vasc Biol 25: 90–95, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, MacNee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 60: 293–300, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elias JA, Kang MJ, Crouthers K, Homer R, Lee CG. State of the art. Mechanistic heterogeneity in chronic obstructive pulmonary disease: insights from transgenic mice. Proc Am Thorac Soc 3: 494–498, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D'Armiento JM. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med 173: 623–631, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 10: 155–160, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med 9: 322–330, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277: 25847–25850, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Iwai K, Kondo T, Watanabe M, Yabu T, Kitano T, Taguchi Y, Umehara H, Takahashi A, Uchiyama T, Okazaki T. Ceramide increases oxidative damage due to inhibition of catalase by caspase-3-dependent proteolysis in HL-60 cell apoptosis. J Biol Chem 278: 9813–9822, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Kannan R, Jin M, Gamulescu MA, Hinton DR. Ceramide-induced apoptosis: role of catalase and hepatocyte growth factor. Free Radic Biol Med 37: 166–175, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res 32: 181–199, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med 13: 164–170, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Lavrentiadou SN, Chan C, Kawcak T, Ravid T, Tsaba A, van der Vliet A, Rasooly R, Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am J Respir Cell Mol Biol 25: 676–684, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantell LL, Horowitz S, Davis JM, Kazzaz JA. Hyperoxia-induced cell death in the lung–the correlation of apoptosis, necrosis, and inflammation. Ann NY Acad Sci 887: 171–180, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem 277: 29626–29633, 2002. [DOI] [PubMed] [Google Scholar]
- 25.McCusker K, Hoidal J. Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamsters. Am Rev Respir Dis 141: 678–682, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Pahan K, Dobashi K, Ghosh B, Singh I. Induction of the manganese superoxide dismutase gene by sphingomyelinase and ceramide. J Neurochem 73: 513–520, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Petrache I, Natarajan V, Zhen L, Medler TR, Richter A, Berdyshev EV, Tuder RM. Ceramide causes pulmonary cell apoptosis and emphysema: a role for sphingolipid homeostasis in the maintenance of alveolar cells. Proc Am Thorac Soc 3: 510, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11: 491–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585: 114–125, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Pinamonti S, Leis M, Barbieri A, Leoni D, Muzzoli M, Sostero S, Chicca MC, Carrieri A, Ravenna F, Fabbri LM, Ciaccia A. Detection of xanthine oxidase activity products by EPR and HPLC in bronchoalveolar lavage fluid from patients with chronic obstructive pulmonary disease. Free Radic Biol Med 25: 771–779, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem 272: 21388–21395, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 154: 1055–1060, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 490–495, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravid T, Tsaba A, Gee P, Rasooly R, Medina EA, Goldkorn T. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol 284: L1082–L1092, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan AJ, McCoy DM, McGowan SE, Salome RG, Mallampalli RK. Alveolar sphingolipids generated in response to TNF-α modifies surfactant biophysical activity. J Appl Physiol 94: 253–258, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Sawada M, Nakashima S, Kiyono T, Yamada J, Hara S, Nakagawa M, Shinoda J, Sakai N. Acid sphingomyelinase activation requires caspase-8 but not p53 nor reactive oxygen species during Fas-induced apoptosis in human glioma cells. Exp Cell Res 273: 157–168, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Sullards MC, Merrill AH Jr. Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci STKE 2001: PL1, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Tepper AD, Cock JG, de Vries E, Borst J, van Blitterswijk WJ. CD95/Fas-induced ceramide formation proceeds with slow kinetics and is not blocked by caspase-3/CPP32 inhibition. J Biol Chem 272: 24308–24312, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and emphysema: the missing link. Am J Respir Cell Mol Biol 28: 551–554, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc 3: 503–510, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 29: 88–97, 2003. [DOI] [PubMed] [Google Scholar]
- 43.White CW, Avraham KB, Shanley PF, Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest 87: 2162–2168, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wispe JR, Warner BB, Clark JC, Dey CR, Neuman J, Glasser SW, Crapo JD, Chang LY, Whitsett JA. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem 267: 23937–23941, 1992. [PubMed] [Google Scholar]
- 45.Yildiz L, Kayaoglu N, Aksoy H. The changes of superoxide dismutase, catalase and glutathione peroxidase activities in erythrocytes of active and passive smokers. Clin Chem Lab Med 40: 612–615, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA 103: 4952–4957, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang AY, Yi F, Jin S, Xia M, Chen QZ, Gulbins E, Li PL. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal 9: 817–828, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.