Abstract
We previously reported that neutrophil elastase (NE) downregulates transforming growth factor-β (TGF-β)-maintained tropoelastin mRNA levels in lung fibroblasts through transactivation of the epidermal growth factor (EGF) receptor (EGFR)/Mek/Erk pathway, which is dependent on the NE-initiated release of soluble EGFR ligands. In the present study, we investigated the mechanism by which EGF downregulates tropoelastin expression. We found that EGF downregulates tropoelastin expression through inhibition of TGF-β signaling. We show that EGF does not prevent the TGF-β-induced nuclear accumulation of Smad2/3; rather, EGF stabilizes the short-lived Smad transcriptional corepressor TG-interacting factor (TGIF) via EGFR/Mek/Erk-mediated phosphorylation of TGIF. Elevation of TGIF levels, either by TGIF overexpression or prevention of TGIF degradation, is sufficient to inhibit TGF-β-induced tropoelastin expression. Moreover, TGIF is essential for EGF-mediated downregulation of tropoelastin expression, inasmuch as small interfering RNA knockdown of TGIF blocked EGF-induced downregulation of tropoelastin. Finally, we demonstrated that NE treatment, which releases EGF-like growth factors, causes stabilization of TGIF through the EGFR/Mek/Erk pathway. These results suggest that EGFR/Mek/Erk signaling specifically antagonizes the proelastogenic action of TGF-β in lung fibroblasts by stabilizing the Smad transcriptional corepressor TGIF.
Keywords: elastin, neutrophil elastase
elastin, a principal component of elastic fibers, maintains the resilience and structural integrity of airways and blood vessels in the mature lung (5, 26). Elastin is a remarkably durable polymer that is assembled from cross-linked monomers of tropoelastin, its soluble precursor. Once synthesized during the fetal and early postnatal phases of lung development, elastin turns over very slowly in healthy lung during the life span of the organism. Emphysema, however, is characterized by irreversible destruction of elastic fibers, which is the consequence of uncontrolled elastolysis and insufficient repair of interstitial elastin (1, 33). The mechanisms underlying the insufficient repair of elastin are still under investigation.
Transforming growth factor-β (TGF-β) is a potent proelastogenic factor (15, 24, 25). TGF-β signaling is initiated by binding of the ligand to TGF-β type I and type II receptors (TβRI and TβRII) on the cell surface and transduced to the nucleus through the nuclear translocation of its intracellular effectors, the Smad family (11, 34). Binding of TGF-β to TβRII induces the oligomerization of TβRII with TβRI, leading to phosphorylation and activation of TβRI. Smad2 and Smad3 are directly phosphorylated and activated by TβRI. The phosphorylated Smad2/3, in complex with Smad4, translocates to the nucleus. On translocation to the nucleus, activated Smad complexes interact with coactivators or corepressors and, hence, transactivate or suppress target gene transcription. TGF-β signaling could be modulated by an array of growth factors. Of particular interest, epidermal growth factor (EGF) has been shown to inhibit TGF-β signaling through blockade of the TGF-β-induced translocation of Smad2/3 via EGF receptor (EGFR)/Ras/Mek/Erk-mediated phosphorylation of Smad2/3 at the linker region (16, 17). EGF signaling can also inhibit TGF-β signaling via EGFR/Ras/Mek/Erk-mediated phosphorylation/stabilization of a short-lived nuclear protein, TG-interacting factor (TGIF) (22), a Smad transcriptional corepressor (35).
It has been well documented that TGF-β upregulates tropoelastin expression through increasing tropoelastin mRNA stability (15, 18, 24). TGF-β has been shown to stabilize tropoelastin mRNA in a Smad-dependent manner (19). Lungs of Smad3-null mice exhibit downregulated levels of tropoelastin mRNA from the end of alveolarization through adulthood and decrease in interstitial elastin deposition and develop destructive emphysematous changes (7). We recently demonstrated that tropoelastin expression in RFL-6 fibroblasts is primarily maintained by autocrine TGF-β (10), so it is plausible that deficient endogenous TGF-β signaling could contribute to the insufficient resynthesis/repair of elastin in the emphysematous lung.
We previously demonstrated that neutrophil elastase (NE) downregulates tropoelastin mRNA levels in lung fibroblasts through the EGFR/Mek/Erk pathway (10). EGF-like growth factors, released by elastase, are required for the downregulation of tropoelastin expression (9, 10, 21). EGF has been shown to downregulate tropoelastin expression through the EGFR/Mek/Erk pathway (9, 21). We recently demonstrated that NE and EGF downregulate tropoelastin expression by decreasing tropoelastin mRNA stability (10). However, the mechanism by which EGF suppresses tropoelastin expression remains largely unknown. Since endogenous TGF-β has been shown to be responsible for tropoelastin expression in RFL-6 fibroblasts and EGF has been shown to inhibit TGF-β signaling, we hypothesize that EGF suppresses tropoelastin expression through inhibition of endogenous TGF-β signaling.
In the present study, we probed the molecular mechanism by which NE downregulates tropoelastin expression. Since NE downregulates tropoelastin expression in lung fibroblasts in an EGFR ligand-dependent manner, we use EGF to investigate the pathway. We demonstrate that EGF, by activating the EGFR/Mek/Erk pathway, suppresses tropoelastin expression through inhibition of TGF-β signaling. We found that EGF does not prevent TGF-β-induced nuclear accumulation of Smad2/3 in RFL-6 fibroblasts. Instead, we show that EGF signaling stabilizes the Smad corepressor TGIF through EGFR/Mek/Erk-mediated phosphorylation of TGIF. Elevated TGIF levels, either by TGIF overexpression or by prevention of TGIF degradation with a proteasome inhibitor, are sufficient to prevent TGF-β-induced tropoelastin expression. Moreover, elevated TGIF levels are essential for EGF-mediated downregulation of tropoelastin expression, since RNA interference (RNAi) knockdown of TGIF makes cells resistant to EGF-mediated downregulation of tropoelastin. Finally, we demonstrated that NE treatment of RFL-6 fibroblasts stabilizes TGIF through the EGFR/Mek/Erk pathway. These data strongly indicate that TGIF is a key downstream effector of the EGFR/Mek/Erk signaling cascade that antagonizes TGF-β-induced tropoelastin expression in lung fibroblasts.
MATERIALS AND METHODS
Materials.
Human NE was used as described previously (9). Murine EGF (catalog no. 53003-018) was obtained from Invitrogen; rabbit polyclonal anti-ERK (COOH-terminal domain; catalog no. 06-182), rabbit polyclonal anti-mouse EGFR (COOH-terminal domain; catalog no. 06-847), and rabbit polyclonal anti-Smad2/3 (catalog no. 07-408) antibodies from Upstate Biotechnology; rabbit polyclonal anti-phosphorylated ERK antibodies (catalog no. 9101) and U0126 from Cell Signaling Technology; recombinant human TGF-β1 (catalog no. 240-B) and mouse monoclonal neutralizing anti-TGF-β1-3 antibody (MAb 1835) from R & D Systems; goat polyclonal anti-rat lung α-elastin antiserum (catalog no. RA75) from Elastin Products; DMSO, AG1478, and clastro lactacystin β-lactone (catalog no. 426102) from Calbiochem; diisopropylfluorophosphate (DFP), phenylmethylsulfonyl fluoride (PMSF), sodium orthovanadate, Triton X-100, Tween 20, Nonidet P-40, mouse monoclonal anti-β-actin antibody (catalog no. A5441), and peroxidase-conjugated goat anti-rabbit antibodies (catalog no. A6154) from Sigma; and rabbit polyclonal anti-TGIF antibody (COOH terminus, amino acids 100-272; catalog no. sc-9084), peroxidase-conjugated goat anti-mouse antibodies (catalog no. sc-2055), mouse monoclonal anti-hemagglutinin protein antibody (HA probe, catalog no. sc-7392), and peroxidase-conjugated donkey anti-goat antibodies (catalog no. sc-2020) from Santa Cruz Biotechnology. Tissue culture reagents were obtained from Invitrogen and Sigma unless otherwise specified.
Cell culture.
RFL-6 (catalog no. 192-CCL, American Type Culture Collection, Rockville, MD) is a fibroblast cell line derived from the lung tissue of normal-gestation day 18 Sprague-Dawley rat fetuses. RFL-6 cells express high levels of tropoelastin mRNA and protein and readily deposit insoluble elastin when they are in a postconfluent state. This elastogenic state is marked by the coinduction of other “proelastogenic” genes, such as lysyl oxidase, fibrillin 1, or fibulin 5, and by a decline in expression of proliferation markers, such as histone 3.2b and proliferating cell nuclear antigen-3, and phosphorylated ERK1/2 in RFL-6 cells (data not shown). These culture conditions allow investigation of tropoelastin regulation under conditions where it is constitutively expressed. Cells were seeded at 1 × 106 cells per T75 flask and maintained in Dulbecco's modified Eagle's medium (JRBioscience) containing 100 U/ml penicillin and 100 μg/ml streptomycin and supplemented with 10% FBS (Atlas Biologicals), 1 mM sodium pyruvate, and 100 μM nonessential amino acids at 37°C in a humidified 5% CO2 atmosphere. For experiments, cells were seeded into 12- or 6-well cluster plates at 5 × 104 or 1 × 105 cells per well or dish, respectively, and cultured for 5–6 days. Once they were in a postconfluent state, cells were placed in medium supplemented with 0.5% serum. After 24 h, cells were placed in serum-free, antibiotic-free medium 1 h before treatment.
Treatment of cell cultures with growth factors.
Unless otherwise specified, serum-free cell cultures were challenged with 10 ng/ml EGF and/or 2 ng/ml TGF-β1 and inhibitors. Routinely, cells were first incubated with inhibitors for 1 h before treatment with growth factors if inhibitors were applied. Control cell cultures received an equal amount of the solvent vehicle (i.e., PBS, DMSO, or ethanol) used with experimental cultures. The final concentration of DMSO or ethanol in the conditioned medium did not exceed 0.1% (vol/vol).
Cell culture and NE treatment.
Cells were seeded in six-well cluster plates at 2.5 × 105 cells per well in complete culture medium supplemented with 10% FBS. Cells were maintained for 4 days after seeding, with medium change every 2 days. Once they were in a postconfluent state, cells were placed in medium supplemented with 0.5% serum for 1 day and then in serum-free, antibiotic-free medium for another 2 days. For analysis of TGIF levels, serum-starved matrix-laden postconfluent RFL-6 cells (with 5 ml of serum-free medium per well) were treated for 1 h with or without specified inhibitors and then with or without 5 μg/ml NE for 1 h.
Preparation of cell lysate.
After treatment, cell cultures were quickly washed twice with PBS at room temperature and lysed by quick freezing and thawing (3 times) in ice-cold lysis buffer [50 mM Tris·HCl (pH 8.0), 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM DFP, and 0.2 mM sodium orthovanadate]. The lysates were cleared of cell debris by centrifugation at 4°C for 15 min at 15,000 g and kept at −80°C.
Preparation of nuclear extract and cytosolic fraction.
After treatment, cell cultures were quickly washed twice with PBS at room temperature. All the following steps were performed at 4°C. One milliliter of ice-cold hypotonic lysis buffer [20 mM HEPES-Na (pH 7.6), 20% glycerol, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 10 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 1 mM PMSF, and 0.5 mM DFP] was added per well of six-well cluster plate. Cells were allowed to swell on ice for 20 min before they were scraped and collected. Nuclei and associated cytoskeleton were pelleted by centrifugation at 600 g for 10 min in a table-top centrifuge. The supernatant (cytosolic fraction) was transferred to a new tube. The pellet was resuspended in 50 μl of nuclear extraction buffer (hypotonic lysis buffer + 500 mM NaCl). After incubation and rocking for 30 min, the nuclear extracts were cleared of debris by centrifugation and kept at −80°C.
Western blot analysis.
Equal amounts of samples were mixed with an appropriate volume of 3× SDS-PAGE sample buffer with 2-mercaptoethanol and heated for 10 min at 100°C, and all the samples were loaded on 4% stacking and 9% separating SDS-PAGE minigels. After electrophoresis, the proteins were electroblotted onto a 0.45-μm-pore nitrocellulose membrane (Schleicher and Schuell). For verification of even loading and transfer, transferred proteins were stained briefly with 0.1% (wt/vol) Ponceau S in 5% acetic acid (Sigma). Membranes were blocked in 5% (wt/vol) nonfat milk powder in Tris-buffered saline [10 mM Tris·HCl (pH 7.4) and 150 mM NaCl (TBS)] + 0.05% Tween 20 (TBST) for 1 h, washed three times for 10 min with TBST, incubated for 1 h with primary antibodies diluted in TBST, and then washed three times for 10 min with TBST and incubated for 1 h with peroxidase-conjugated secondary IgG diluted in TBST. The membranes were washed twice for 10 min with TBST and once for 5 min with TBS, and immunodetection of proteins was performed with enhanced chemiluminescence by using LumiGlo chemiluminescence detection kit (Kirkegaard and Perry Laboratories).
Northern blot analysis.
Total RNA was extracted from cell cultures by use of TRIzol reagent (Invitrogen). Equal amounts of total RNA (7 μg/lane) were electrophoresed through 1.0% agarose-formaldehyde gels, capillary transferred to nylon membranes (Osmonics, Minnetonka, MN), and cross-linked to filters by ultraviolet irradiation (Stratalinker, Stratagene). For comparison of integrity and correction of RNA loading, blots were stained with 0.04% methylene blue in 0.5 M sodium acetate. Membranes were prehybridized at 42°C for 2 h in a solution containing 50% formamide, 5× NaCl and sodium citrate (SSC; 1× SSC is 0.15 M NaCl and 0.015 M sodium citrate, pH 7.0), 5× Denhardt's reagent, 0.5% SDS, and 100 μg/ml denatured salmon sperm DNA and hybridized overnight in a solution containing 50% formamide, 5× SSC, 10% dextran sulfate, 0.5% SDS, and 32P-labeled cDNA fragments of desired genes. After hybridization, membranes were washed (twice in 1× SSC and 0.1% SDS at 55°C for 1 h) and exposed at −80°C in a cassette with double-intensifying screens to X-ray film (Abgene). The bands were quantified by densitometry within the linear range.
Cell culture and transient transfection of cells.
RFL-6 fibroblasts were seeded in complete growth medium supplemented with 10% FBS into 12-well cluster plates (1 × 105 cells per well). Cells at ∼90% confluence on the day after seeding were placed in 1 ml of serum-free, antibiotic-free medium. Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 5 h, transfection medium was aspirated, 2 ml of complete growth medium supplemented with 10% FBS were added per well, and the cells were maintained overnight. After 24 h, medium was replaced with 2 ml of serum-free, antibiotic-free medium. After 16 h, cell lysates were harvested.
Immunocytochemistry.
The intracellular localization of Smad2/3 was analyzed by indirect immunofluorescence. Cells were seeded on glass coverslips placed into 12-well cluster plates at a density of 5 × 104 cells per well and grown overnight in complete culture medium supplemented with 10% FBS. Cells were placed in serum-free, antibiotic-free medium on the day after seeding when cells were 50% confluent. After 4 h of starvation, cells were treated for 1 h with or without EGF, TGF-β1, and EGF + TGF-β1. Cells were quickly rinsed twice with PBS and then fixed with 3.7% paraformaldehyde in PBS for 5 min at room temperature, quickly washed three times with a large volume of PBS, permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature, quickly rinsed three times with a large volume of PBS, and blocked overnight at 4°C with blocking solution (3% normal donkey serum and 1% BSA in PBS). Cells were washed three times for 5 min with PBS + 0.1% Triton X-100 (PBST) and then incubated with a 1:250 dilution of anti-Smad2/3 in blocking solution for 1.5 h at room temperature. After the primary antibody incubation, cells were washed three times for 5 min in PBST and incubated for 1 h at room temperature with a 1:250 dilution of indocarbocyanine (Cy3)-conjugated secondary IgG (Jackson ImmunoResearch) in blocking solution. Intracellular distribution of Smad2/3 was examined using an inverted microscope (Zeiss 200/Axiovert 200M).
Construction of TGIF small interfering RNA expression vectors.
To knock down TGIF expression, we used RNAi-Ready pSIREN-RetroQ vector (BD Biosciences) to establish TGIF small interfering RNA (siRNA) expression vectors. The vector contains a puromycin resistance gene for selection of stable transfectants. Two target sequences were picked up by using the Dharmacom web-based program: GAGTCCGTCCAGATTCTTCGA for small hairpin siRNA (shRNA) 226 (226-246) and CTTCATGCCAACTCTAGAA for shRNA 486 (486-504). We also designed another target sequence that is homologous to published shRNA to human TGIF (32): ATCTGGACCCAGTCCAAAT for shRNA 756 (756-774).
Hairpin sequence UUCAAGAGA was used to construct siRNA expression vectors as described elsewhere (4). A double-stranded oligonucleotide encoding an shRNA was inserted into linearized RNAi-Ready pSIREN-RetroQ vector. The sequences of plasmid were confirmed by automated DNA sequencing in sense and antisense directions at the DNA-Protein Core Facility at Boston University Medical Center.
Stable transfection.
For production of retroviruses, we used 293T cells. Cells were cotransfected with plasmids expressing retroviral proteins Gag-Pol, G (VSVG pseudotype; kindly provided by Dr. Michael Sherman, Boston University School of Medicine), and one of our constructs or pSIREN-RetroQ vector. At 48 h after transfection, supernatants containing the retrovirus were collected and frozen at −80°C if not used immediately. RFL-6 cells (30–40% confluent) were infected with two-times-diluted supernatant and 10 μg/ml polybrene overnight. Infected cells were placed in complete culture medium supplemented with 10% FBS. On reaching confluence (usually 2 days after infection), infected RFL-6 cells were reseeded and selected with 1 μg/ml puromycin. After 3 days in selection medium, cell lines stably transfected with shRNA constructs were established. The cells were passed and plated for experiments. Cells that were infected with RetroQ mock vector, shRNA-226, shRNA-486, and shRNA-756 are denoted RetroQ-, shRNA226-, shRNA486-, and shRNA756-RFL-6 cells, respectively. Stable transfectants were maintained or treated in medium containing 1 μg/ml puromycin.
RESULTS
EGF downregulates tropoelastin expression through abrogation of TGF-β signaling.
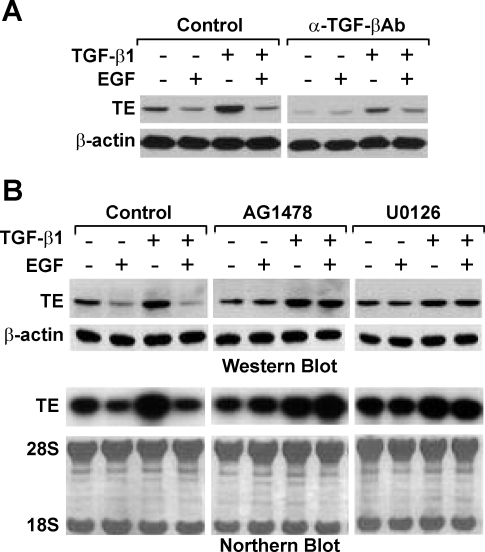
Since endogenous TGF-β has been shown to maintain tropoelastin expression in RFL-6 fibroblasts (10), it is plausible that EGF downregulates tropoelastin expression through inhibition of TGF-β signaling. Postconfluent serum-starved cells were treated for 1 h with or without TGF-β-neutralizing antibody and then treated for 24 h with or without EGF, TGF-β1, or EGF + TGF-β1. Whole cell lysates were prepared, and tropoelastin protein levels were analyzed by Western blot (Fig. 1A). In the absence of TGF-β-neutralizing antibody, tropoelastin protein levels were upregulated by TGF-β1 but downregulated by EGF. Interestingly, TGF-β1 treatment did not upregulate tropoelastin protein levels when cells were treated simultaneously with EGF, indicating that EGF abrogates the TGF-β-induced tropoelastin expression. Moreover, EGF did not downregulate tropoelastin protein levels further when the endogenous TGF-β signaling was inhibited by TGF-β-neutralizing antibody, but EGF was still able to downregulate tropoelastin protein levels when the TGF-β signaling and tropoelastin expression were rescued by the addition of TGF-β1. Together, these results indicate that EGF downregulates tropoelastin expression through the inhibition of TGF-β signaling.
Fig. 1.
Epidermal growth factor (EGF) downregulates transforming growth factor-β (TGF-β)-induced tropoelastin expression via the EGF receptor (EGFR)/Mek/Erk pathway in RFL-6 fibroblasts. A: postconfluent serum-starved cells were treated for 1 h with or without 20 μg/ml TGF-β-neutralizing antibody and then stimulated for 24 h with or without 10 ng/ml EGF, 2 ng/ml TGF-β1, or EGF + TGF-β1. Whole cell lysates were prepared, and 15 μg of total protein from each sample were analyzed by Western blot with antibodies (Ab) against tropoelastin (TE) or β-actin. β-Actin was probed as a loading control. Results are representative of an experiment that was repeated 3 times. B: postconfluent serum-starved cells were treated for 1 h with or without 10 μM AG1478 or 25 μM U0126 and then stimulated for 24 h with or without 10 ng/ml EGF, 2 ng/ml TGF-β1, or EGF + TGF-β1. Top: whole cell lysates were prepared, and 15 μg of each protein sample were analyzed by Western blot with antibodies against tropoelastin and β-actin. β-Actin was probed as a loading control. Bottom: total RNA was isolated, and 7 μg of each RNA sample were analyzed by Northern blot for tropoelastin mRNA. Methylene blue staining of 28S and 18S ribosomal RNAs is provided to ensure even RNA loading and integrity. Results are representative of experiments that were repeated 3 times.
EGF abrogates TGF-β-induced tropoelastin expression via the EGFR/Mek/Erk pathway.
We next determined the pathway downstream of EGF signaling that is involved in downregulation of TGF-β-induced tropoelastin expression. Since EGF downregulates tropoelastin expression through the EGFR/Mek/Erk pathway (9, 10), we anticipated that EGF should also downregulate TGF-β-dependent tropoelastin expression through the same pathway. Postconfluent serum-starved cells were treated for 1 h with or without the EGFR tyrosine kinase-specific inhibitor AG1478 or the Mek/Erk uncoupler U0126 and then for an additional 24 h with or without EGF, TGF-β1, or EGF + TGF-β1. Whole cell lysates and total RNA were prepared, and tropoelastin protein and mRNA levels were assessed by Western and Northern blot, respectively (Fig. 1B). Consistent with previous results, EGF downregulated tropoelastin expression and abrogated TGF-β1-stimulated tropoelastin expression, but EGF could no longer downregulate tropoelastin expression or abrogate TGF-β1-stimulated tropoelastin expression in the presence of AG1478 or U0126, suggesting that the EGFR/Mek/Erk pathway is required for EGF-mediated TGF-β signaling blockade.
EGF does not attenuate TGF-β-induced nuclear accumulation of Smad2/3.
TGF-β signaling could be modulated by EGF at a variety of levels. Since the inhibitory effect of EGF on tropoelastin expression in RFL-6 fibroblasts was not rescued by exogenously added TGF-β1, it seems unlikely that EGF downregulates tropoelastin expression primarily through the inhibition of the secretion or activation of TGF-β. Alternatively, it is more likely that EGF acts to attenuate TGF-β-mediated signaling.
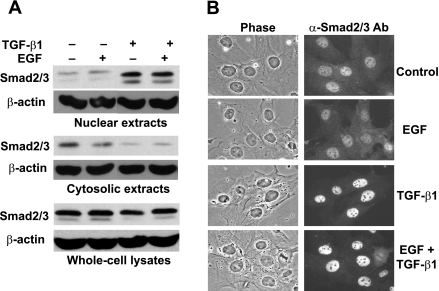
TGF-β/Smad-dependent signaling is transmitted to the nucleus through the nuclear translocation of Smad proteins on activation of TGF-β receptors by ligand binding (34). It has been suggested that EGF signaling can inhibit TGF-β signaling by blocking the nuclear accumulation of R-Smads (16, 17). We therefore examined whether EGF interferes with the TGF-β-induced Smad2/3 nuclear accumulation in RFL-6 fibroblasts. Postconfluent serum-starved cells were treated for 1 h with or without EGF, TGF-β1, or EGF + TGF-β1, and subcellular distribution of Smad2/3 was analyzed by Western blot (Fig. 2A). TGF-β1 treatment increased Smad2/3 nuclear levels and decreased their cytosolic levels accordingly. EGF did not change the subcellular distribution of Smad2/3 in cells treated with or without TGF-β1. The total amount of Smad2/3 was not changed by any of the treatments.
Fig. 2.
EGF does not decrease TGF-β-induced nuclear accumulation of Smad2/3 in RFL-6 fibroblasts. A: postconfluent serum-starved cells were treated for 1 h with or without 10 ng/ml EGF, 2 ng/ml TGF-β1, or EGF + TGF-β1. Cytosolic and nuclear extracts, as well as whole cell lysates, were prepared, and 30 μg of total protein from each sample were analyzed by Western blot with antibodies against Smad2/3 and β-actin. β-Actin was probed as a loading control. Results are representative of an experiment that was repeated 3 times. B: cells were grown on coverslips and to 50% confluence, serum-starved for 4 h, and then treated for 1 h with or without 10 ng/ml EGF, 2 ng/ml TGF-β1, or EGF + TGF-β1. Cells were fixed and stained with anti-Smad2/3 primary and Cy3-conjugated secondary antibodies, and their phase and fluorescent images were obtained (original magnification ×250). Results are representative of an experiment that was repeated 3 times.
Subcellular distribution of Smad2/3 was also analyzed by immunocytochemistry. Cells were treated for 1 h with or without EGF, TGF-β1, or EGF + TGF-β1, and endogenous Smad2/3 was visualized by indirect immunofluorescence (Fig. 2B). The Smad2/3 signal was dispersed throughout untreated cells but localized predominantly in the nucleus after TGF-β1 treatment. EGF did not alter the subcellular distribution pattern of Smad2/3 in cells treated with or without TGF-β1. Taken together, these results demonstrate that EGF does not attenuate TGF-β-induced nuclear accumulation of Smad2/3 in RFL-6 fibroblasts.
EGF stabilizes Smad corepressor TGIF through the EGFR/Mek/Erk pathway.
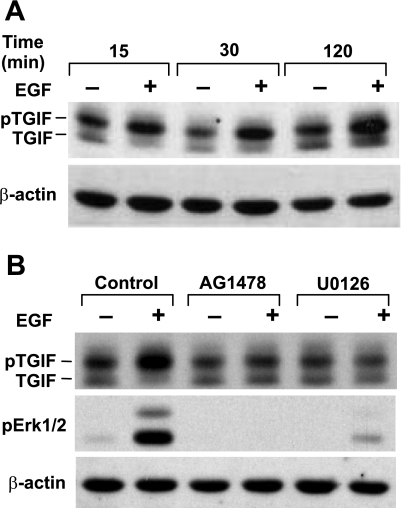
Since EGF does not decrease TGF-β-induced nuclear accumulation of Smad2/3, it is likely that EGF attenuates Smad activity in the nucleus. On translocation to the nucleus, activated Smad complexes interact with coactivators such as CBP/P300 or with corepressors such as TGIF and, thus, activate or suppress target gene transcription (34). Formation of these mutually exclusive complexes is determined by the relative levels of Smad coactivators and corepressors within the nucleus. The levels of TGIF, a short-lived protein, are normally very low. EGF has been shown to stabilize TGIF via EGFR/Mek/Erk-mediated phosphorylation of TGIF at its COOH terminus (22), thus favoring the formation of Smad-TGIF complexes. This, in turn, results in the suppression of gene transcription, which is normally upregulated by TGF-β. We therefore assessed whether EGF could stabilize TGIF in RFL-6 fibroblasts. We treated cells with or without EGF for different periods of time and analyzed TGIF levels in nuclear extracts by Western blot (Fig. 3A). TGIF is recognized as double protein bands, with the higher- and lower-mobility bands corresponding to nonphosphorylated and phosphorylated TGIF, respectively (22). The phosphorylated form is more abundant. EGF stimulation led to a rapid increase in the relative amount of phosphorylated TGIF at 15 min. Phosphorylated TGIF levels remained elevated at 30 min and 2 h of treatment, and total TGIF levels were increased at these time points.
Fig. 3.
EGF stabilizes TGIF via EGFR/Mek/Erk-mediated phosphorylation of TGIF. Postconfluent serum-starved cells were treated with or without 10 ng/ml EGF for 15–30 min (A) or for 1 h with or without 10 μM AG1478 or 25 μM U0126 and then with or without 10 ng/ml EGF for 30 min (B). Nuclear extracts were prepared, and 30 μg of total protein from each sample were analyzed by Western blot with antibodies against TGIF or β-actin. β-Actin was probed as a loading control. Results are representative of an experiment that was repeated ≥3 times. pTGIF, phosphorylated TGIF.
We determined the pathway downstream of EGF signaling that is involved in the EGF-mediated stabilization of TGIF. Since EGF prevents TGF-β-induced tropoelastin expression through the EGFR/Mek/Erk pathway, we anticipated that EGF might stabilize TGIF through the same pathway. Indeed, AG1478 or U0126 treatment prevented the EGF-mediated increase in phosphorylated and total TGIF levels (Fig. 3B), demonstrating that the EGFR/Mek/Erk pathway is required for this action.
Elevated TGIF levels are sufficient to downregulate tropoelastin expression.
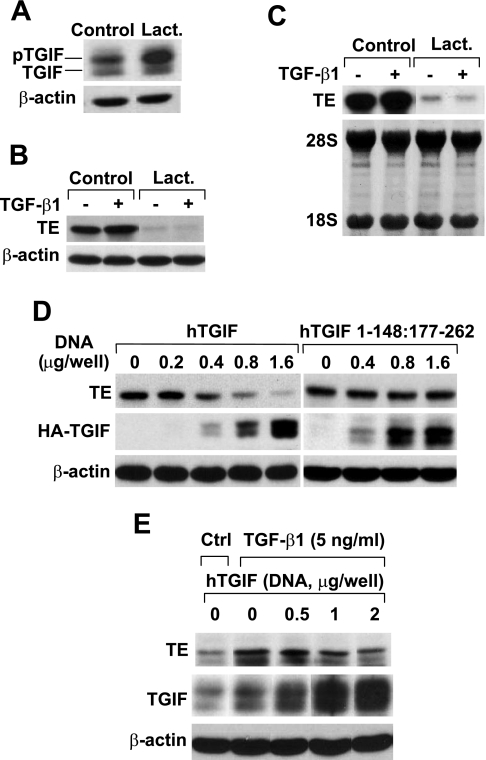
If EGF downregulates tropoelastin expression through stabilization of TGIF, tropoelastin expression should be downregulated when the TGIF levels are increased by prevention of its degradation or by overexpression of TGIF. For prevention of TGIF degradation, cells were treated for 2 h with or without the proteasome inhibitor lactacystin. Nuclear extracts were prepared, and TGIF levels were analyzed by Western blot. Lactacystin caused a dramatic increase in TGIF levels (Fig. 4A). Thus TGF-β-mediated tropoelastin expression was investigated under these conditions. Cells were treated for 1 h with or without lactacystin and then stimulated for an additional 24 h with or without TGF-β1. Treatment of cells with lactacystin dramatically reduced tropoelastin protein and mRNA levels (Fig. 4, B and C). Moreover, TGF-β1 treatment did not induce tropoelastin expression in the presence of lactacystin, suggesting that lactacystin interfered with the TGF-β1-mediated tropoelastin expression. Thus lactacystin, similar to EGF, leads to increased TGIF levels and decreased tropoelastin expression and prevents TGF-β-induced tropoelastin expression in RFL-6 fibroblasts.
Fig. 4.
Elevated TG-interacting factor (TGIF) levels are sufficient to downregulate tropoelastin expression. A: postconfluent serum-starved cells were treated with or without 20 μM lactacystin (Lact) for 2 h. Nuclear extracts were prepared, and 30 μg of total protein from each sample were analyzed by Western blot with antibodies against TGIF or β-actin. β-Actin was probed as a loading control. B and C: postconfluent serum-starved cells were treated for 1 h with or without 20 μM lactacystin and then stimulated with or without 2 ng/ml TGF-β1 for 24 h. Total RNA and whole cell lysates were isolated. B: 15 μg of total protein from each sample were analyzed by Western blot with antibodies against tropoelastin and β-actin. β-Actin was probed as a loading control. C: 7 μg of total RNA from each sample were analyzed by Northern blot for tropoelastin mRNA. Methylene blue staining of 28S and 18S ribosomal RNAs is provided to ensure even loading and RNA integrity. D: cells were transfected with increasing amounts of hemagglutinin (HA) epitope-tagged human TGIF wild-type (hTGIF WT) construct (left) or HA epitope-tagged human TGIF Smad2/3 interaction domain delta mutant (hTGIF 1–148:177–262) construct (right). Total amounts of DNA (1.6 μg) were kept constant by refilling with empty vector where needed. After 40 h, whole cell lysates were harvested, and 15 μg of total protein from each sample were analyzed by Western blot with antibodies against tropoelastin, HA epitope, or β-actin. Expression levels of hTGIF WT and mutant were visualized with anti-HA epitope antibody. E: cells were transfected with increasing amounts of hTGIF WT construct. Total amount of DNA (2.0 μg) was kept constant by refilling with empty vector where needed. After 36 h, cells were serum starved for 4 h and then treated for 24 h with or without 5 ng/ml TGF-β1. Whole cell lysates were harvested, and 15 μg of total protein from each sample were analyzed by Western blot with antibodies against tropoelastin, TGIF, or β-actin. Ctrl, control. Results are representative of experiments that were repeated ≥3 times.
To further explore the role of TGIF in EGF-mediated inhibition of tropoelastin expression, we also analyzed tropoelastin expression in cells that overexpress human TGIF (hTGIF) wild-type (hTGIF WT) or hTGIF mutant (hTGIF 1-148:177-262), which is devoid of the Smad interaction domain and is not able to interact with Smad2/3 (35). As shown in Fig. 4D, tropoelastin expression was inhibited by overexpression of hTGIF WT in a dose-dependent manner but was not affected by overexpression of hTGIF 1-148:177-262, suggesting that the interaction between TGIF and Smad is required for TGIF-mediated downregulation of tropoelastin expression.
The inhibitory effect of TGIF on TGF-β-induced tropoelastin expression was also confirmed by overexpression of TGIF. As shown in Fig. 4E, the TGF-β1-mediated upregulation of tropoelastin expression was inhibited by overexpression of hTGIF.
Taken together, these results suggest that elevated TGIF levels are sufficient to suppress tropoelastin expression in RFL-6 fibroblasts.
Elevated TGIF levels are essential for EGF-mediated downregulation of tropoelastin expression.
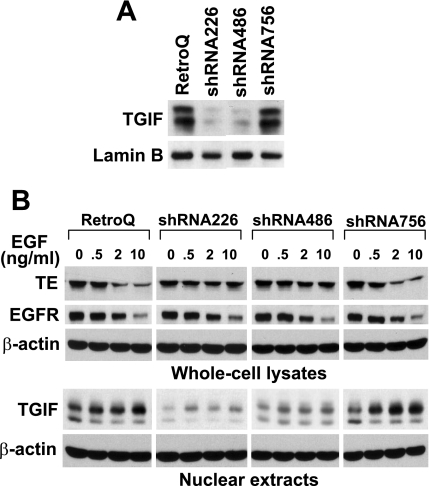
To further confirm the role of TGIF in EGF downregulation of tropoelastin expression, we generated RFL-6 cell lines with reduced levels of TGIF. To knock down TGIF, we constructed three pSIREN-RetroQ retroviral vectors that target three different regions in rat TGIF mRNA. RFL-6 fibroblasts were infected with retroviruses encoding TGIF siRNA, and stable transfectants were achieved after selection with puromycin. The TGIF levels in stable transfectants were analyzed by Western blot (Fig. 5A). TGIF levels were knocked down by ∼80% in shRNA226- and shRNA486-RFL-6 cells compared with RetroQ- and shRNA756-RFL-6 cells.
Fig. 5.
Elevated TGIF levels are essential for EGF-mediated downregulation of tropoelastin expression. A: generation of RFL-6 fibroblast lines with reduced levels of TGIF. To knock down endogenous TGIF protein levels, TGIF small interfering RNA (siRNA) expression vectors were constructed, and stable transfectants with siRNA expression vector were established after infection with retroviruses encoding small hairpin siRNA (shRNA) and selection with puromycin. RFL-6 fibroblasts stably transfected with RetroQ empty vector, shRNA226, shRNA486, and shRNA756 are denoted RetroQ-, shRNA226-, shRNA486-, and shRNA756-RFL-6 cells, respectively. Nuclear extracts were prepared from postconfluent cells, and 30 μg of total protein from each sample were analyzed by Western blot with antibodies against TGIF or lamin B. Lamin B was probed as loading control. Results are representative of an experiment that was repeated 3 times. B: postconfluent cells were placed in complete culture medium supplemented with 5% FBS and treated for 24 h with or without EGF (0, 0.5, 2, or 10 ng/ml). Whole cell lysates and nuclear extracts were prepared in parallel, and 15 μg of total protein from each whole cell lysate were analyzed by Western blot with antibodies against tropoelastin, EGFR, or β-actin, and 30 μg of total protein from each nuclear extract were analyzed by Western blot with antibodies against TGIF or β-actin. Results are representative of an experiment that was repeated 3 times.
The inhibitory effect of EGF on tropoelastin expression was then analyzed in cells with reduced levels of TGIF. As shown in Fig. 5B, tropoelastin protein levels were downregulated by EGF in a dose-dependent manner in RetroQ- and shRNA756-RFL-6 cells that have normal TGIF levels and responded to EGF treatment with an increase in TGIF levels. However, the inhibitory effect of EGF on tropoelastin protein was dramatically diminished in shRNA226- and shRNA486-RFL-6 cells in which TGIF levels are knocked down by RNAi. Similar results were observed in TGF-β-treated cells (data not shown). To investigate whether EGF signaling was functioning properly in those cells, EGFR levels were also probed. EGFR levels were similar and were downregulated by EGF in a dose-dependent manner in all four RFL-6 cell lines, suggesting that the reduced ability of EGF to downregulate tropoelastin in the TGIF-knocked-down cells was not the result of altered EGFR signaling. These data indicate that TGIF is required for EGF-mediated inhibition of tropoelastin expression.
NE-initiated EGFR/Mek/Erk signaling leads to stabilization of TGIF.
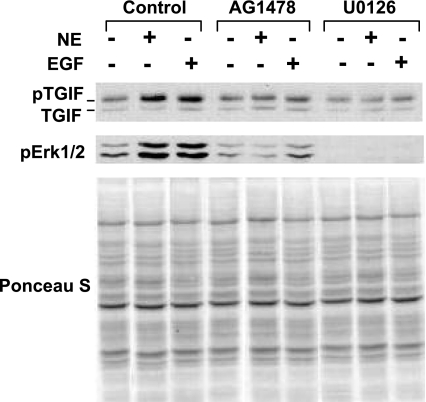
NE downregulates tropoelastin mRNA levels through transactivation of the EGFR/Mek/Erk pathway, dependent on the release of EGF-like growth factors (9, 10, 21). Since EGF acts via TGIF, we examined whether NE treatment results in stabilization of TGIF. Cells were treated for 1 h with or without AG1478 or U0126 and then treated for an additional 1 h with or without EGF or NE, and TGIF levels were measured. Similar to EGF, NE led to phosphorylation and stabilization of TGIF, which coincided with Erk activation (Fig. 6). TGIF phosphorylation and stabilization were prevented by incubation with AG1478 and U0126, suggesting that NE stabilizes TGIF via the EGFR/Mek/Erk pathway. Importantly, NE treatment does not result in direct inactivation of endogenous TGF-β; instead, NE treatment stimulated the release of active TGF-β from RFL-6 cells, indicating that the ability to interfere with TGF-β signaling through EGFR/Mek/Erk-mediated TGIF stabilization is the basis of NE downregulation of tropoelastin mRNA.
Fig. 6.
Neutrophil elastase (NE) stabilizes TGIF via the EGFR/Mek/Erk pathway in RFL-6 fibroblasts. Postconfluent serum-starved cells were treated for 1 h with or without 10 μM AG1478 or 25 μM U0126 and then for an additional 1 h with or without 10 ng/ml EGF or 5 μg/ml NE. Nuclear extracts were prepared, and 30 μg of total protein from each sample were analyzed by Western blot with antibodies against TGIF or phosphorylated Erk (pErk). Ponceau S staining is provided as loading control. Results are representative of an experiment that was repeated 3 times.
DISCUSSION
We demonstrated previously that treatment of neonatal rat lung fibroblasts and RFL-6 fibroblasts with NE downregulates tropoelastin mRNA levels through the release of EGF-like growth factors and activation of the EGFR/Mek/Erk pathway (9, 10). In the present study, we examined the mechanism by which EGF downregulates tropoelastin expression in RFL-6 cells. In this system, tropoelastin is maintained by endogenous TGF-β and can be induced by exogenous TGF-β. Our data indicate that EGF downregulates tropoelastin expression by abrogation of TGF-β signaling. In this regard, we observed that EGF treatment leads to the stabilization of the Smad corepressor TGIF, indicating a mechanism whereby EGF interferes with TGF-β-mediated tropoelastin expression. These results suggest a potentially important process whereby EGF-like ligands and TGF-β act to control tropoelastin in the lung.
EGF has been shown to downregulate tropoelastin expression (9, 10) and to prevent TGF-β-induced tropoelastin expression (present study) through the EGFR/Mek/Erk pathway. Consistently, we demonstrate here that EGF stabilizes TGIF through the EGFR/Mek/Erk pathway, suggesting that EGF might downregulate tropoelastin expression through stabilization of TGIF. In the present study, we also demonstrate that elevated levels of TGIF, either by preventing its degradation or by overexpressing TGIF, are sufficient to downregulate tropoelastin expression. Moreover, shRNA knockdown of TGIF made cells refractory to EGF-mediated downregulation of tropoelastin. These loss-of-function and gain-of-function experiments, together with AG1478 and U0126 inhibitor assays, establish that EGF inhibits TGF-β-induced tropoelastin expression through the EGFR/Mek/Erk-mediated stabilization of Smad corepressor TGIF.
TGIF could inhibit TGF-β signaling through a variety of mechanisms. It has been shown that the interaction between Smad2 and TGIF can be stabilized by c-Jun, which is a downstream effector of the JNK pathway (28). This mechanism is probably not involved in the RFL-6 cells, inasmuch as the JNK-specific inhibitor SP600125 did not affect EGF downregulation of tropoelastin expression in these cells (unpublished observations). TGIF has also been implicated in the degradation of Smad2 (32). However, we did not observe any changes in total Smad2 levels with EGF treatment (Fig. 3A), suggesting that Smad2 degradation is not likely the major mechanism involved. TGIF has also been suggested to inhibit TGF-β signaling by sequestering the cytoplasmic promyelocytic leukemia protein in the nucleus (31), thus preventing its association with Smad anchor for receptor activation in the cytoplasm, which is required for the activation and nuclear translocation of Smad2/3 (20). This mechanism also does not fit the data from our system, because the TGF-β-induced nuclear translocation of Smad2/3 was not affected by EGF in RFL-6 fibroblasts (Fig. 2). TGIF is also suggested to inhibit TGF-β signaling through recruitment of histone deacetylases (35), but treatment of RFL-6 cells with the histone deacetylase inhibitor trichostatin A neither prevented EGF-mediated downregulation of tropoelastin expression nor enhanced the TGF-β-induced tropoelastin expression (unpublished observations). TGIF is also suggested to inhibit TGF-β signaling through the replacement of coactivators, such as CBP/p300, from the Smad transcription complex (35); thus additional studies are required to carefully evaluate this possibility in the RFL-6 cell system.
Since tropoelastin expression is primarily maintained by TGF-β, other negative modulators of tropoelastin expression might work through inhibition of TGF-β signaling. Phorbol ester has been shown to downregulate tropoelastin expression (14, 27), but the mechanism is unknown. Phorbol ester is known to transactivate EGFR signaling in an ADAM (a disintegrin and metalloproteinase)-dependent manner (2), so it is possible that phorbol ester inhibits tropoelastin expression through stabilization of TGIF. Basic fibroblast growth factor has been shown to downregulate tropoelastin expression (3, 8, 29) in an Erk-dependent manner (6) and to antagonize TGF-β-induced tropoelastin expression (8). Therefore, it is possible that basic fibroblast growth factor might also inhibit tropoelastin expression through Erk-mediated stabilization of TGIF. Thus the ability to stabilize TGIF through Erk-dependent phosphorylation might be a consistent feature of tropoelastin downregulation mechanisms.
Pulmonary emphysema is associated with unlimited elastolysis and insufficient repair of interstitial elastin (1, 33). The mechanism underlying the insufficient repair of elastin is still under investigation. It has been shown that NE downregulates tropoelastin mRNA levels in lung fibroblasts through the transactivation of the EGFR/Mek/Erk signaling pathway, which is dependent on release of the EGF-like growth factors (9, 10). Interestingly, in different animal models of emphysema, it was demonstrated that NE is colocalized with EGF-like growth factors and TGF-β in the obstructively remodeling alveolar interstitium (23). Since we demonstrated that tropoelastin expression in RFL-6 fibroblasts is maintained by TGF-β and that EGF downregulates tropoelastin expression through inhibition of TGF-β signaling, it is possible that the insufficient repair of elastin in the emphysematous lung could involve EGF-like growth factors released by elastase.
It is well established that TGF-β can upregulate tropoelastin expression through stabilization of tropoelastin mRNA (15, 18, 24). We have also demonstrated that EGF can downregulate tropoelastin expression by destabilizing tropoelastin mRNA (10). Most of the mechanisms that control mRNA stability involve a specific interaction between trans-acting factors and cis-acting elements (30). Two distinct cis-acting elements have been identified within the primary structure of tropoelastin mRNA that are involved in regulating its stability (12, 13, 36). One element is localized to a GA-rich sequence of the 3′-untranslated region (3′-UTR) (35), and another is localized to exon 30 of tropoelastin mRNA (34). It was previously shown that postnatal destabilization of tropoelastin mRNA in aorta is correlated with decreased binding of cytosolic protein(s) to a GA-rich sequence in the 3′-UTR of tropoelastin mRNA. This motif is present in the 3′-UTR of tropoelastin mRNA from several species. Binding protein(s) is present in cytoplasmic extracts, and the abundance of binding protein is associated with tissues producing elastin and correlated with circumstances in which tropoelastin mRNA is stable (35). Thus TGF-β might stabilize tropoelastin mRNA by increasing the production of these 3′-UTR binding proteins. It has also been suggested that TGF-β signaling stabilizes tropoelastin mRNA in lung fibroblasts by interfering with the binding of an unidentified cytosolic 50-kDa destabilizing factor to exon 30 (34).
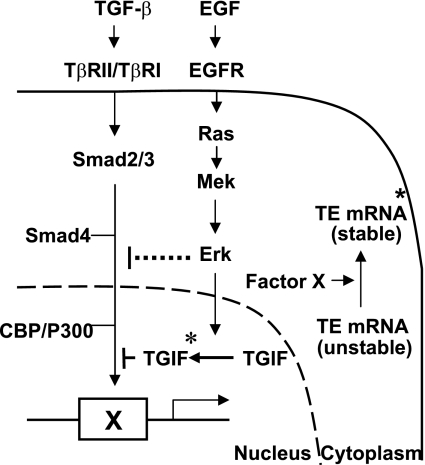
On the basis of these previous findings and our present results, we suggest a model by which EGF suppresses TGF-β-induced tropoelastin expression (Fig. 7 ). Tropoelastin protein levels correlate with tropoelastin mRNA levels, which are primarily determined by the turnover rate of the message in the mature lung. Our data suggest that a yet unspecified TGF-β-inducible cytosolic factor X potentially functions to stabilize tropoelastin mRNA. Smad corepressor TGIF levels are normally very low because of a high turnover rate, such that TGF-β signaling can lead to transcriptional transactivation of factor X, which in turn stabilizes tropoelastin mRNA, for instance, by binding to the GA-rich sequence in the 3′-UTR and/or interfering with the binding of a destabilizing factor(s) to exon 30 of tropoelastin mRNA. Both mechanistic scenarios would lead to upregulation of tropoelastin expression. EGF signaling could interfere with this process by repressing the expression or activity of factor X by stabilizing the Smad corepressor TGIF, resulting in destabilization of tropoelastin mRNA and decreased tropoelastin expression. It will be important to continue to evaluate the interactions of the EGF and TGF-β signaling pathways with respect to the regulation of tropoelastin expression to gain a more complete appreciation of how elastin production is controlled in vivo normally, after injury, and in disease states.
Fig. 7.
Model of EGF downregulation of tropoelastin expression in RFL-6 fibroblasts. Data demonstrate that EGF does not prevent TGF-β-induced nuclear accumulation of Smad2/3 but does stabilize the Smad corepressor TGIF via EGR/Mek/Erk-mediated phosphorylation of TGIF. Smad corepressor TGIF levels are normally very low because of the high turnover rate of TGIF protein. TGF-β signaling leads to transcription transactivation of a putative cytosolic factor X, which stabilizes tropoelastin mRNA by binding to tropoelastin mRNA and/or disrupting the interaction of tropoelastin mRNA and its degradation machinery. On EGF stimulation, activated Erk translocates to the nucleus, phosphorylates, and stabilizes Smad corepressor TGIF. Elevated levels of TGIF shut off transcription of factor X through competition with coactivator CBP/p300 for the activated Smad complex, leading to destabilization of tropoelastin mRNA and downregulation of tropoelastin expression. TβRI and TβRII, TGF-β type I and II receptors. *Indicates TGIF that has been phosphorylated by Erk.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants P01 HL-046902 and R01 HL-088572.
Acknowledgments
We thank Dr. Joan Massague (Memorial Sloan-Kettering Cancer Center) for generously providing human TGIF expression constructs.
Present address of M. P. Panchenko: Department of Pathology, Massachusetts General Hospital and Harvard Medical School, 185 Cambridge St., CPZN 8400 Simches Research Bldg., Boston, MA 02114 (e-mail: mpanchenko@partners.org).
This study was presented in part at the Experimental Biology Annual Meeting of the American Physiological Society and American Society for Biochemistry and Molecular Biology in 2005.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672–688, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Blobel CP ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6: 32–43, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Brettell LM, McGowan SE. Basic fibroblast growth factor decreases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol 10: 306–315, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Burri PHWE Ultrastructure and morphometry of the developing lung. In: Development of the Lung. 1. Structural Development, edited by Wa H. New York: Dekker, 1977, p. 215–268.
- 6.Carreras I, Rich CB, Jaworski JA, Dicamillo SJ, Panchenko MP, Goldstein R, Foster JA. Functional components of basic fibroblast growth factor signaling that inhibit lung elastin gene expression. Am J Physiol Lung Cell Mol Physiol 281: L766–L775, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Sun J, Buckley S, Chen C, Warburton D, Wang XF, Shi W. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol 288: L683–L691, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Davidson JM, Zoia O, Liu JM. Modulation of transforming growth factor-β1 stimulated elastin and collagen production and proliferation in porcine vascular smooth muscle cells and skin fibroblasts by basic fibroblast growth factor, transforming growth factor-α, and insulin-like growth factor-I. J Cell Physiol 155: 149–156, 1993. [DOI] [PubMed] [Google Scholar]
- 9.DiCamillo SJ, Carreras I, Panchenko MV, Stone PJ, Nugent MA, Foster JA, Panchenko MP. Elastase-released epidermal growth factor recruits epidermal growth factor receptor and extracellular signal-regulated kinases to down-regulate tropoelastin mRNA in lung fibroblasts. J Biol Chem 277: 18938–18946, 2002. [DOI] [PubMed] [Google Scholar]
- 10.DiCamillo SJ, Yang S, Panchenko MV, Toselli PA, Naggar EF, Rich CB, Stone PJ, Nugent MA, Panchenko MP. Neutrophil elastase-initiated EGFR/MEK/ERK signaling counteracts stabilizing effect of autocrine TGF-β on tropoelastin mRNA in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 291: L232–L243, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hew Y, Grzelczak Z, Lau C, Keeley FW. Identification of a large region of secondary structure in the 3′-untranslated region of chicken elastin mRNA with implications for the regulation of mRNA stability. J Biol Chem 274: 14415–14421, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Hew Y, Lau C, Grzelczak Z, Keeley FW. Identification of a GA-rich sequence as a protein-binding site in the 3′-untranslated region of chicken elastin mRNA with a potential role in the developmental regulation of elastin mRNA stability. J Biol Chem 275: 24857–24864, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Kahari VM, Chen YQ, Bashir MM, Rosenbloom J, Uitto J. Tumor necrosis factor-α down-regulates human elastin gene expression. Evidence for the role of AP-1 in the suppression of promoter activity. J Biol Chem 267: 26134–26141, 1992. [PubMed] [Google Scholar]
- 15.Kahari VM, Olsen DR, Rhudy RW, Carrillo P, Chen YQ, Uitto J. Transforming growth factor-β up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation. Lab Invest 66: 580–588, 1992. [PubMed] [Google Scholar]
- 16.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature 389: 618–622, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev 13: 804–816, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucich U, Rosenbloom JC, Abrams WR, Bashir MM, Rosenbloom J. Stabilization of elastin mRNA by TGF-β: initial characterization of signaling pathway. Am J Respir Cell Mol Biol 17: 10–16, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Kucich U, Rosenbloom JC, Abrams WR, Rosenbloom J. Transforming growth factor-β stabilizes elastin mRNA by a pathway requiring active Smads, protein kinase C-δ, and p38. Am J Respir Cell Mol Biol 26: 183–188, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-β signalling. Nature 431: 205–211, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Rich CB, Buczek-Thomas JA, Nugent MA, Panchenko MP, Foster JA. Heparin-binding EGF-like growth factor regulates elastin and FGF-2 expression in pulmonary fibroblasts. Am J Physiol Lung Cell Mol Physiol 285: L1106–L1115, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lo RS, Wotton D, Massague J. Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J 20: 128–136, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucattelli M, Bartalesi B, Cavarra E, Fineschi S, Lunghi B, Martorana PA, Lungarella G. Is neutrophil elastase the missing link between emphysema and fibrosis? Evidence from two mouse models. Respir Res 6: 83, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan SE, Jackson SK, Olson PJ, Parekh T, Gold LI. Exogenous and endogenous transforming growth factors-β influence elastin gene expression in cultured lung fibroblasts. Am J Respir Cell Mol Biol 17: 25–35, 1997. [DOI] [PubMed] [Google Scholar]
- 25.McGowan SE, McNamer R. Transforming growth factor-β increases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol 3: 369–376, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Mecham R Elastic fibers. In: The Lung: Scientific Foundations, edited by Crystal RG, West JB. Philadelphia, PA: Lippincott-Raven, 1997, p. 729–736.
- 27.Parks WC, Kolodziej ME, Pierce RA. Phorbol ester-mediated downregulation of tropoelastin expression is controlled by a posttranscriptional mechanism. Biochemistry 31: 6639–6645, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Pessah M, Prunier C, Marais J, Ferrand N, Mazars A, Lallemand F, Gauthier JM, Atfi A. c-Jun interacts with the corepressor TG-interacting factor (TGIF) to suppress Smad2 transcriptional activity. Proc Natl Acad Sci USA 98: 6198–6203, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich CB, Nugent MA, Stone P, Foster JA. Elastase release of basic fibroblast growth factor in pulmonary fibroblast cultures results in down-regulation of elastin gene transcription. A role for basic fibroblast growth factor in regulating lung repair. J Biol Chem 271: 23043–23048, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Ross J mRNA stability in mammalian cells. Microbiol Rev 59: 423–450, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo SR, Ferrand N, Faresse N, Prunier C, Abecassis L, Pessah M, Bourgeade MF, Atfi A. Nuclear retention of the tumor suppressor cPML by the homeodomain protein TGIF restricts TGF-β signaling. Mol Cell 23: 547–559, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Seo SR, Lallemand F, Ferrand N, Pessah M, L'Hoste S, Camonis J, Atfi A. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J 23: 3780–3792, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro SD, Ingenito EP. The pathogenesis of chronic obstructive pulmonary disease: advances in the past 100 years. Am J Respir Cell Mol Biol 32: 367–372, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Wotton D, Lo RS, Lee S, Massague J. A Smad transcriptional corepressor. Cell 97: 29–39, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Pierce RA, Wachi H, Mecham RP, Parks WC. An open reading frame element mediates posttranscriptional regulation of tropoelastin and responsiveness to transforming growth factor β1. Mol Cell Biol 19: 7314–7326, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]