Abstract
Mycoplasma pne umoniae is an extracellular pathogen, residing on mucosal surfaces of the respiratory and genital tracts. The lack of cell walls in mycoplasmas facilitates the direct contact of the bacterial membrane with the host cell. The cell membrane of mycoplasma is the major inducer of the host pathogenic response. Airway diseases caused by M. pneumoniae include bronchiolitis, bronchitis, and rarely bronchiectasis. In such disorders, neutrophil infiltration of the airways predominates. More recently, M. pneumoniae has been implicated in the pathogenesis of asthma. Epithelial cells play an important role in recruiting inflammatory cells into the airways. Since M. pneumoniae infection of human epithelial cells induces expression of IL-8—a potent activator of neutrophils—we investigated the signaling and transcriptional mechanisms by which mycoplasma membrane induces expression of this chemokine. In BEAS-2B human bronchial epithelial cells, mycoplasma membrane fraction (MMF) increased IL-8 mRNA and protein production. Activation of the transcriptional elements activating protein-1, nuclear factor-interleukin-6, and particularly NF-κB are essential for optimal IL-8 production by MMF. The mitogen-activated protein kinases individually played a modest role in MMF-induced IL-8 production. Toll-like receptor-2 did not play a significant role in MMF-induction of IL-8. Antibiotics with microbicidal activity against M. pneumoniae are also known to have anti-inflammatory effects. Whereas clarithromycin, azithromycin, and moxifloxacin individually were able to inhibit TNF-α-induction of IL-8, each failed to inhibit MMF-induction of IL-8.
Keywords: mollicutes, chemokines, gene regulation, toll-like receptors
mycoplasma pneumoniae is a prokaryotic human pathogen belonging to the Mollicute class of organisms. Mycoplasmas are characterized by their small size (150–250 nm), absence of a cell wall, and the presence of a trilayered cell membrane. Several lines of evidence indicate that the cell membrane components of M. pneumoniae are not only major inducers of the host protective immune response but also significantly contribute to the pathogenic response: 1) M. pneumoniae is a surface parasite of the human respiratory tract. Via its cell membrane adhesin proteins, it attaches to sialoglycoprotein and sialoglycolipid receptors present on the airway epithelium (48). 2) Because mycoplasmas are very small, there is substantially larger surface area-to-volume ratio, and thus the membrane constitutes a greater proportion of the bacterial mass than the eubacteria (69). Mycoplasmal lipids are located exclusively in the cytoplasmic membrane and constitute 25–35% of the dry weight of the membrane (60). In addition to cholesterol, the membrane also contains di-galactosyldiacyl glycerol and tri-galactosyldiacyl glycerol and glucosylgalactosyldiacyl glycerol, and the phospholipids phosphatidyl glycerol and diphosphatidyl glycerol (60). Furthermore, a substantial fraction of the total cellular proteins are contained within the mycoplasmal cell membrane (39). 3) Mycoplasmal membrane molecules such as lipoproteins, glycolipids, and lipoglycans are the major antigenic determinants that induce the host pathogenic response (47, 58, 60, 69). At least 50 different membrane proteins have been isolated from M. pneumoniae by one-dimensional gel electrophoresis (15). 4) Several M. pneumoniae membrane proteins have been shown to have high affinity for various receptors on host cells (69). Via interaction with pattern recognition receptors such as the Toll-like receptors (TLRs)—particularly, TLR2 and TLR6—mycoplasma membrane lipoproteins are able to induce host immune responses (13, 41, 57, 62, 74). 5) Cell membrane glycolipids of M. pneumoniae may be responsible for induction of autoantibodies and development of disease distant from the respiratory tract (48).
M. pneumoniae is a frequent cause of upper and lower respiratory tract infections that typically manifest as pharyngitis, tracheobronchitis, and community-acquired pneumonia (3). Other less common pulmonary disorders attributed to M. pneumoniae include cellular bronchiolitis, constrictive bronchiolitis, bronchiolitis obliterans organizing pneumonia, pulmonary fibrosis, and exacerbation and pathogenesis of asthma (8, 10, 16, 25, 29, 34, 36, 59). In these disorders, the lungs are often infiltrated by neutrophils and lymphocytes. In mice infected with M. pneumoniae intranasally, airway lumina also demonstrate a predominance of neutrophils (14, 38). This neutrophilic response may be harmful to the host since the cells do not play a significant role in controlling mycoplasma infection, interfere with macrophage activity, and contribute to tissue damage (71). Neutrophils may also aid in the dissemination of mycoplasmal infection because these phagocytes can internalize the mycoplasma but are unable to kill the organisms (66).
The C-X-C chemokine IL-8 is a potent chemoattractant and activator for neutrophils (49), T-cells, basophils, and eosinophils (33, 42, 65). IL-8 is produced by a wide array of cell types, including epithelial cells, neutrophils, and macrophages in response to microbial infections (42). IL-8 and its murine equivalent KC have been shown to contribute to the pulmonary and extrapulmonary inflammation following M. pneumoniae infection (14, 18, 44, 45). Patients with M. pneumoniae lung infections have increased IL-8 in their bronchoalveolar lavages, and the levels of IL-8 correlate with higher bacterial loads and impairment of gas exchange (6).
Previous studies have shown that live M. pneumoniae infection induces IL-8 expression in human epithelial cells and macrophages (7, 75). Since the cell membrane components of M. pneumoniae are critical in the pathogenesis of neutrophilic lung injury, we examined the signaling and transcriptional mechanisms by which the bacterial membrane components of M. pneumoniae—mycoplasma membrane fraction (MMF)—induce IL-8 in the human bronchial epithelial cell line BEAS-2B. Our findings demonstrate the transcriptional activators NF-κB, activating protein-1 (AP-1), and nuclear factor-interleukin-6 (NF-IL-6) all enhanced MMF-induction of IL-8, whereas the family of MAPK played only a modest role in chemokine induction. The antibiotics azithromycin, clarithromycin, and moxifloxacin inhibited TNF-α-induction of IL-8 but had no effect on MMF induction of IL-8.
MATERIALS AND METHODS
Materials.
The human bronchial epithelial cell line BEAS-2B and the mouse macrophage cell line RAW 264.7 were purchased from American Type Culture Collection (Rockville, MD). Human TNF-α was obtained from R&D Systems (Minneapolis, MN). LPS was purchased from Sigma (St. Louis, MO). Rabbit polyclonal anti-p46 c-JNK, rabbit polyclonal anti-p42 ERK2, rabbit polyclonal anti-p38mapk (C-20), normal goat IgG polyclonal antibody, goat polyclonal anti-TLR2 antibody, rabbit polyclonal antibodies against p50-NF-κB, C/EBP-β, c-Jun, and c-Fos, and the C/EBP (NF-IL-6) consensus double-stranded oligonucleotide were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). γ-[32P]-ATP (>3,000 Ci/mmol) was purchased from MP Biomedicals (Irvine, CA). The MEK1 inhibitor PD98059 and phospho-specific p38mapkantibody were purchased from New England Biolabs (Beverly, MA). Pam3Cys-Ser-(Lys)4 (Pam3Cys lipopeptide), the p38mapk inhibitor SB203580, and JNK inhibitor II were purchased from Calbiochem (San Diego, CA). JNK inhibitor II is a small molecule (220 kDa) that competes with ATP for JNK. JNK inhibitor 1 was acquired from Alexis Biochemicals (San Diego, CA). In contrast to JNK inhibitor II, JNK inhibitor 1 is a peptide with a molecular mass of 3,823 kDa that competes with JNK to bind to its substrate c-Jun. Both JNK inhibitors can inhibit both p46 and p54 isoforms of JNK. BAY 11-7082, an inhibitor of Iκ-Bα kinase, was obtained from Biomol Research Laboratories (Plymouth Meeting, PA). Double-stranded oligonucleotides corresponding to the consensus-binding sites for NF-κB and AP-1, the phospho-specific ERK rabbit polyclonal antibody, and pRL-TK (a Renilla luciferase reporter vector) were purchased from Promega (Madison, WI). The wild-type IL-8 promoter-luciferase (IL-8-luc), mut-κB-IL-8-luc, mut-AP1-IL-8-luc, or mut-NF-IL-6-IL-8-luc plasmids were obtained from Dr. N. Mukaida (Kanazawa University, Kanazawa, Japan). TRIzol reagent and the Superscript First-Strand Synthesis System were purchased from Invitrogen (Carlsbad, CA). Products used for PCR amplification, including Taq polymerase, were purchased from Bio-Rad (Hercules, CA). The 28 base-pair single-stranded oligonucleotides corresponding to the NF-κB binding site on the IL-8 promoter: 5′-AGT TGC AAA TCG TGG AAT TTC CTC TGAC-3′ (sense) and 5′-GTCA GAG GAA ATT CCA CGA TTT GCA ACT-3′ (antisense), and all primers used for RT-PCR were ordered from Genelink (Hawthorne, NY). Azithromycin was obtained from Pfizer Pharmaceuticals (New York, NY). Clarithromycin was acquired from Abbott Laboratories (Abbott Park, IL). Moxifloxacin (BAY 12–8039) was provided by Bayer (Pittsburgh, PA).
Isolation of mycoplasma membrane fraction and lipoprotein.
The lack of cell walls and intracytoplasmic membranes in M. pneumoniae facilitates isolation of MMF in a relatively pure form (39). Membrane fraction and lipoprotein from M. pneumoniae were prepared as previously described (12). In brief, M. pneumoniae (strain FH, American Type Culture Collection 15531) was grown in polystyrene flasks containing 100 ml of SP-4 medium at 37°C for 5 days. After 5 days of growth in SP-4 medium, M. pneumoniae culture was collected by scraping with a rubber policeman, and centrifugation at 8,000 g for 15 min at 4°C. The pellet was washed with PBS (pH 7.4) twice and centrifuged. The pellet was resuspended in PBS and layered on a discontinuous sucrose gradient (60%, 52%, 48%, and 40%) and centrifuged at 10,000 rpm (SW28) for 30 min at 4°C. The cells were recovered from 48–52% interface, mixed with PBS, and centrifuged at 8,000 g for 15 min at 4°C. The purified M. pneumoniae pellet was resuspended in PBS and mixed with 3 volumes of distilled water and incubated on ice for 30 min. The cells were lysed completely by probe sonication on ice for a total of 2 min, using 30-s sonication and 30-s cooling cycles. Polymyxin B (100 μg/ml) was added to the lysed M. pneumoniae cells and centrifuged at 100,000 g for 1 h at 4°C. The pellet was washed twice with polymyxin B and centrifuged at 100,000 g for 1 h at 4°C. The final M. pneumoniae membrane pellet was resuspended in PBS, homogenized, and used as MMF.
To isolate mycoplasmal lipoprotein, MMF was solubilized by the addition of 1% TritonX-114 and incubated at 4°C for 2 h with gentle shaking. TritonX-114-treated MMF was centrifuged at 12,000 g for 5 min at 4°C and the supernatant was collected and incubated at 37°C for 5 min. The cloudy solution was centrifuged at 8,000 g for 5 min at room temperature. The aqueous phase was incubated with 1% TritonX-114 at 37°C for 5 min and centrifuged. The last step was repeated once again. The saved TritonX-114 phase was pooled, methanol added, and incubated at −80°C overnight and centrifuged at 12,000 g for 5 min at 4°C. The pellet was dried and suspended in PBS and used as the mycoplasmal lipoprotein preparation (LPP).
Cell culture.
BEAS-2B cells, an adenovirus 12-SV40 hybrid of transformed human bronchial epithelial cells, were cultured in 100-cm2 tissue culture plates coated with LHC-9 medium containing fibronectin (Calbiochem, San Diego, CA), Vitrogen (Cohesion Technologies, Palo Alto, CA), and 0.1% BSA (Biosource, Rockville, MD). Cells were maintained in LHC-9 serum-free medium (Biosource) supplemented with penicillin-streptomycin (50 U/ml) in a 5% CO2 incubator at 37°C. Cells were utilized between passages 1 to 15 for all experiments. Cells plated and grown to 90% confluence were subjected to stimulating agents that included MMF, TNF-α, mycoplasma LPP, and LPS. RAW 264.7 cells were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, MD) supplemented with 10% FBS (Atlanta Biochemicals, Norcross, GA), 1% penicillin-streptomycin and L-glutamine (Irvine Scientific, Santa Ana, CA).
Electrochemiluminescence analysis of IL-8 protein.
Cells seeded at 5 × 106/plate were incubated with MMF with or without inhibitors for 24 or 48 h. Supernatants were collected and assayed for IL-8 concentration by electrochemiluminescence (ECL), as previously described (46b). In brief, a purified mouse anti-human IL-8 antibody (R&D Systems, Minneapolis, MN) was labeled with biotin (Igen, Gaithersburg, MD) and a goat anti-human IL-8 antibody (R&D Systems) was labeled with ruthenium (Igen), according to the manufacturer's instructions. The biotinylated antibody was diluted to a final concentration 1 μg/ml in PBS, pH 7.4, containing 0.25% BSA, 0.5% Tween-20, and 0.01% sodium azide (ECL buffer). Per assay tube, 25 μl of the biotinylated anti-IL-8 was incubated at RT with 25 μl of a 1 mg/ml solution of streptavidin-coated paramagnetic beads (Dynal, Lake Success, NY) for 30 min by vigorous shaking. Samples (25 μl) that had been diluted in RPMI 1640 were added to tubes followed by 25 μl of ruthenylated antibody (final concentration 1 μg/ml, diluted in ECL buffer). The tubes were then shaken for an additional 2 h. The reaction was quenched by the addition of 200 μl per tube of 1× PBS and the amount of chemiluminescence determined using an Origen Analyzer (Igen) and a standard curve.
Electrophoretic mobility shift assay.
BEAS-2B cells were grown in 6-well polystyrene tissue culture plates overnight. After incubation with MMF for 30 min to 4 h, the nuclear extracts were isolated as previously described (40). End-labeling of the oligonucleotides (listed in the materials section) with γ-[32P]-ATP, performance of binding reactions with 3 μg of the nuclear extract, and separation of the complexes by PAGE were as previously described (40).
Transfection and dual luciferase assay.
BEAS-2B cells were transfected with plasmids using Lipofectamine, according to manufacturer's protocol (Gibco BRL) and as previously described (9). Briefly, 10 × 106 cells/plate were cotransfected with 0.2 μg of pRL-TK plasmid—a Renilla luciferase reporter vector (Promega)—and 0.5 μg of plasmid DNA harboring either the wild-type IL-8 promoter-luciferase (WT-IL-8-luc) plasmid, mut-κB-IL-8-luc, mut-AP1-IL-8-luc, or mut-NF-IL-6-IL-8-luc. The DNA preparations were combined with 10 μl of Lipofectamine and LHC-9 media. After 5 h of incubation with the DNA-Lipofectamine mixture at 37°C in a 5% CO2 incubator, the wells were washed and replaced with 2 ml LHC-9 medium containing 10 U/ml DNAse I (Gibco BRL, Carlsbad, CA). The medium was changed 24 h after transfection, and after an additional 24 h, the cells were cultured with 10 μg/ml MMF for 6 h. The cells were washed, lysed, and assayed for luciferase activity, according to the Dual-Luciferase Reporter Assay System (Promega). The firefly luciferase activity was normalized to measured Renilla luciferase activity and reported as the relative fold increase in activity.
RT-PCR.
TRIzol Reagent (Invitrogen) was used to extract total RNA according to the manufacturer's protocol and cDNA was prepared using reverse transcriptase according to manufacturer's instructions (Invitrogen). Single-stranded cDNA products were used as templates for PCR. The primers used include IL-8 sense primer: 5′-CAT GAC TTC CAA GCT GGC CGTG-3′; IL-8 antisense primer: 5′-TCA CTG ATT CTT GGA TAC CAC AGAG-3′; GAPDH sense primer: 5′-TCC ATG ACA ACT TTG GTA TCG TG-3′; and the GAPDH antisense primer: 5′-TGT CGC TGT TGA AGT CAG AGGA-3′. The PCR reaction was carried out at 94°C for 4 min, followed by 30 cycles of amplification (94°C for 30 s, 55°C for 1 min, 72°C for 2 min), terminated at 72°C for 4 min, and kept at 4°C until use. PCR products were separated on 1.2% agarose gels containing ethidium bromide.
Northern blot analysis.
Total RNA was extracted using the TRIzol Reagent, according to manufacturer's instructions. Fifteen micrograms of RNA was lyophilized and resuspended in a 1× sample buffer [formamide, 37% (neat) formaldehyde, and 10× MOPS buffer], heated to 68°C for 5 min, then cooled on ice for 5 min. Loading buffer was added, and the samples were electrophoresed on a 1% formaldehyde-agarose gel and subsequently transferred to a Nytran membrane, UV crosslinked, and subjected to prehybridization in ExpressHyb Hybridization Solution (BD Biosciences-Clontech, Palo Alto, CA) containing 100 μl denatured salmon sperm DNA (Gibco BRL) overnight at 42°C. Blots were then hybridized overnight at 42°C with either an IL-8 or GAPDH denatured probe. Probes were constructed from RT-PCR products and purified using a gel extraction kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Probes were labeled with α-[32P] dCTP using the Random Primers DNA Labeling System (Gibco BRL). After hybridization, the blots were washed twice with 2× SSC/SDS at 42°C for 30 min each, washed with 0.2× SSC/SDS at 65°C for 30 min, washed with 0.2× SSC at 65°C for 2 min, and then subjected to autoradiography.
Western blot analysis.
Cells were processed with α lysis buffer [50 mM Tris·HCl buffer, pH 8.0, containing 137 mM NaCl, 10% glycerol, 1% (vol/vol) NP-40, 1 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 10 μg/ml leupeptin, and 5 μg/ml aprotinin] and centrifuged at 21,000 g for 10 min at 4°C. Protein concentrations of the supernatants prepared from the lysates were determined by the bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL), 20-μg aliquots were separated by SDS-PAGE and subsequently transferred to nitrocellulose membranes, blocked overnight at 4°C with 5% (wt/vol) nonfat dry milk, and immunoblotted with phospho-specific antibodies to p42/p44 ERK, p46/p54 JNK, or p38mapk in 5% (wt/vol) BSA. To verify equal loading of proteins between samples, the corresponding membranes were probed with rabbit polyclonal p42/p44 ERK, p46/p54 JNK, or p38mapkantibody.
p38mapk and JNK activities.
The p38mapk MAP kinase assay kit (Cell Signaling Technology, Beverly, MA) was used according to the manufacturer's instructions. Briefly, cell lysates were immunoprecipitated with an immobilized phospho-p38mapkmonoclonal antibody overnight at 4°C. Washed pellets were then suspended in 1× kinase buffer containing 200 μM ATP and 2 μg ATF-2/GST fusion protein and incubated for 30 min at 30°C. The reaction was terminated with 2× Laemilli/DTT. The samples were boiled for 5 min at 100°C, separated by SDS-PAGE, and subsequently, the proteins were transferred onto nitrocellulose membrane and immunoblotted with phospho-ATF-2 antibody.
To measure JNK activity, BEAS-2B cells were incubated with MMF (10 μg/ml) for 30 min and then lysed at 4°C with 500 μl of an ice-cold lysis buffer as described previously (9). JNK activity in each sample of lysate was bound to 15 μl of a 1:1 slurry mixture of lysis buffer and c-Jun1–79-GST-Sepharose beads and incubated at 4°C for 2 h. The beads were then washed, and JNK activity was determined by an in vitro kinase assay that detects the level of c-Jun-GST phosphorylation (11). In appropriate samples, the cells were pretreated with either 10 μM “JNK inhibitor 1” or 20 μM “JNK inhibitor II” for 1 h prior to stimulation with MMF. Both JNK inhibitors are able to inhibit both p46 and p54 isoforms of JNK through different mechanisms.
Statistical analysis.
Replicate experiments were independent, and summary results presented as means (SD). Differences were considered significant for P < 0.05. Group means were compared by repeated-measures ANOVA using Fisher's least significant difference test.
RESULTS
BEAS-2B cells produce IL-8 in response to MMF.
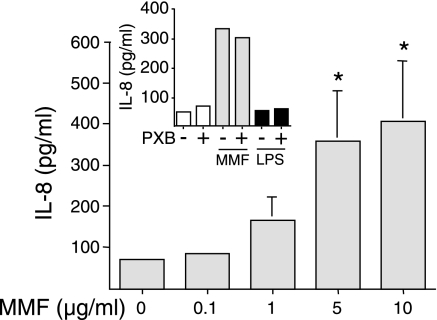
To determine whether MMF induces IL-8 protein expression, BEAS-2B cells were incubated with 0.1 to 10 μg/ml of MMF for 24 h and the supernatants assayed for IL-8. As seen in Fig. 1, MMF induced IL-8 production in a concentration-dependent fashion. Essentially, all IL-8 that was produced was secreted with little or no intracellular detection (data not shown). To exclude the possibility that this effect may be due to LPS contamination, 10 μM polymyxin B (PXB) was added to cells incubated with either 10 μg/ml MMF or 10 ng/ml LPS. As shown in Fig. 1 (inset), 10 μM polymyxin B did not affect MMF-induced IL-8 production; as also shown, BEAS-2B cells in the LHC-9 serum-free medium are hyporesponsive to LPS, consistent with a previous study, which showed that BEAS-2B cells cultured in the absence of normal human serum are unresponsive to LPS (55).
Fig. 1.
Mycoplasma membrane fraction (MMF) induces IL-8 production in a concentration-dependent fashion. BEAS-2B cells were incubated with MMF at concentrations of 0.1 to 10 μg/ml for 24 h. Supernatants were collected and assayed for IL-8 levels by electrochemiluminescence (ECL). Data shown are the means (SD) of three independent experiments, *P < 0.05 compared with unstimulated cells. Inset shows BEAS-2B cells incubated with 10 μg/ml MMF or 10 ng/ml LPS with and without the addition of 10 μM polymyxin B (PXB). Data shown are representative of two experiments.
MMF-induction of IL-8 is transcriptionally regulated.
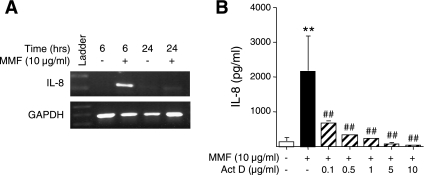
IL-8 is a CXC chemokine that is primarily regulated at the transcriptional level (22). To determine whether IL-8 is transcriptionally regulated by mycoplasma challenge, BEAS-2B cells were incubated with 10 μg/ml MMF for either 6 h or 24 h, and relative IL-8 mRNA levels were estimated by RT-PCR using total RNA for the template. As shown in Fig. 2A, MMF induced expression of IL-8 mRNA after 6 h of stimulation, and the transcript levels returned to near basal values after 24 h. To validate the transcriptional regulation of IL-8 protein, BEAS-2B cells were pretreated for 1 h with the transcriptional inhibitor actinomycin D, prior to incubation with 10 μg/ml MMF for 48 h. As can be seen in Fig. 2B, actinomycin D decreased MMF-induced IL-8 protein production in a concentration-dependent fashion.
Fig. 2.
IL-8 induction by MMF is transcriptionally regulated. A: BEAS-2B cells were incubated with MMF for 6 or 24 h, and expression of IL-8 mRNA and GAPDH mRNA was determined by RT-PCR. Data shown are representative of three independent experiments. B: BEAS-2B cells were pretreated for 1 h with 0.1 to 10 μg/ml of actinomycin D, then coincubated with 10 μg/ml MMF for an additional 48 h, followed by assay for IL-8 of the supernatants. Data shown are the means (SD) of three independent experiments. **P < 0.01 compared with bar 1 and ##P < 0.01 compared with bar 2.
MMF activates NF-κB, AP-1, and NF-IL-6.
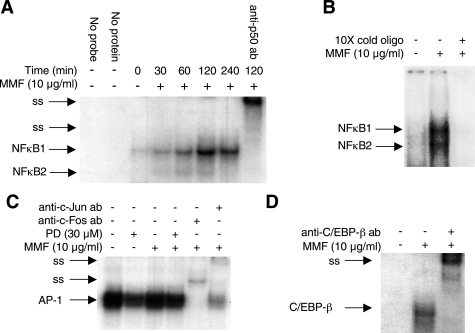
Since IL-8 is transcriptionally induced by MMF, we next examined potential transcriptional regulatory elements that may be involved in this process. Potential cis-regulatory elements for the transcription factors NF-κB, AP-1, and NF-IL-6 (also known as CCAAT/enhancer-binding protein—C/EBP) are present on the 5′-flanking region of the IL-8 promoter (32, 43, 50, 51, 73). To assess whether these sites are important for MMF-induction of IL-8, we first determined whether MMF could enhance the binding of these transcription factors to their corresponding cis-regulatory elements. BEAS-2B cells were incubated with MMF from 30 min to 4 h, and EMSAs were performed using γ-[32P]-labeled oligonucleotides that contain the consensus binding sites for NF-κB, AP-1, or NF-IL-6. As shown in Fig. 3A, MMF increased NF-κB binding to the oligonucleotide that contains the consensus binding site for NF-κB. Peak binding occurred 2 h after incubation with MMF. Two NF-κB-oligonucleotide complexes were observed, and labeled NF-κB1 and NF-κB2. The specificity of the oligonucleotide-NF-κB complexes was confirmed by retarded migration of the complexes with the anti-p50 antibody. Since both complexes supershifted with the p50-NF-κB antibody, both contain the p50 subunit of NF-κB. On the basis of previous work with the p65-NF-κB antibody, the NF-κB1 complex comprises the p50- p65 heterodimer and the NF-κB2 complex comprises the p50-p50 homodimer (76). A 28-bp oligonucleotide containing the precise NF-κB-binding cis-element on the 5′-flanking region of the IL-8 promoter was synthesized and also used as a probe. As shown in Fig. 3B, MMF-induced NF-κB also binds to this IL-8-specific NF-κB binding site.
Fig. 3.
MMF activates NF-κB, activating protein-1 (AP-1), and NF-IL-6. A: BEAS-2B cells were incubated with 10 μg/ml MMF for 30 min to 4 h, and the nuclear extracts were prepared. A binding reaction was then carried out between the nuclear extracts and an oligonucleotide that corresponds to a consensus binding sequence for NF-κB. The binding reactions were detected using EMSA. B: BEAS-2B cells were incubated with MMF for 120 min, and the nuclear extracts were probed for binding interactions by EMSA with a 28-bp oligonucleotide that corresponds to the NF-κB-binding site located in the 5′-flanking region of the IL-8 promoter. C: BEAS-2B cells were either left unstimulated or stimulated with 10 μg/ml MMF for 120 min with or without the MEK1-ERK inhibitor PD98059. An EMSA was performed with a consensus oligonucleotide for AP-1. D: cells were either left unstimulated or stimulated with 10 μg/ml MMF for 120 min. An EMSA was performed with a consensus oligonucleotide for NF-IL-6 (C/EBP-β). ss, supershifted complexes. Data shown are representative of three independent experiments.
In BEAS-2B cells, the transcription factor AP-1 was found to be bound constitutively to the oligonucleotide that contains its cis-regulatory element, and MMF stimulation did not further increase binding (Fig. 3C). The addition of a MEK1-ERK inhibitor (PD98059) modestly inhibited AP-1 binding. Specificity of the AP-1-oligonucleotide complex was confirmed by incubation of the binding reaction with anti-c-Fos or anti-c-Jun antibody. Figure 3D shows that MMF increased NF-IL-6 (C/EBP-β) binding to its corresponding cis-regulatory element in BEAS-2B cells. The specificity of the NF-IL-6-oligonucleotide complex was confirmed by incubation of the binding reaction with anti-C/EBP-β antibody, which resulted in supershifting of the complex.
NF-κB, AP-1, and NF-IL-6 are required for optimal induction of IL-8 by MMF.
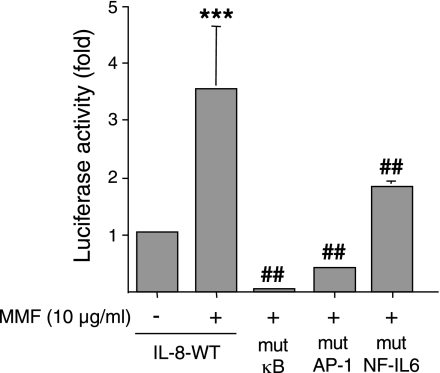
To determine whether the potential cis-regulatory sites for NF-κB, AP-1, and NF-IL-6 are required for MMF-induction of IL-8, an assay was performed using a luciferase reporter gene in which the firefly luciferase gene (luc) was cloned downstream of either a full-length wild-type (WT) IL-8 promoter (WT-IL-8-luc), or one that contained a loss-of-function mutation in the binding site for NF-κB (mut-κB-IL-8-luc), AP-1 (mut-AP1-IL-8-luc), or NF-IL-6 (mut-NFIL-6-IL-8-luc). To normalize transfection efficiency, a Renilla (sea pansy) luciferase reporter vector (pRL-TK) was cotransfected with each of the firefly luciferase constructs. Following transfection, the cells were incubated with MMF for 6 h, lysed, sequentially assayed for both firefly and sea pansy luciferase activities, and reported as a fold increase above baseline after normalization for transfection efficiency. In cells transfected with WT-IL-8-luc, MMF increased luciferase activity 3.5-fold above basal levels (Fig. 4). In contrast, cells transfected with mut-κB-IL-8-luc had profoundly reduced IL-8 promoter activity with MMF stimulation. Similarly, MMF incubation of BEAS-2B cells transfected with either mut-AP1-IL-8-luc or mut-NFIL-6-IL-8-luc also exhibited significantly less luciferase activity than WT-IL-8-luc, although the decrease was not as complete as that seen with the mut-κB-IL-8-luc. Thus, maximal induction of the IL-8 promoter by MMF requires activation of at least three transactivating factors.
Fig. 4.
Binding sites for NF-κB, AP-1, and NF-IL-6 are important cis-regulatory elements for MMF-induction of IL-8. Each of four plasmids that contained either the full-length wild-type IL-8 promoter-luciferase reporter construct or a mutation in the binding sites for NF-κB, AP-1, or NF-IL-6 (WT-IL-8-luc, mut-κB-IL-8-luc, mut-AP1-IL-8-luc, and mut-NFIL-6-IL-8-luc, respectively) were cotransfected into BEAS-2B cells along with pRL-TK plasmid, which encodes for a Renilla luciferase used for normalization of luciferase activity. Following incubation with MMF, luciferase activity was measured, normalized against Renilla luciferase activity, and reported as fold increase above baseline. Data shown are the means (SD) for three independent experiments, ***P < 0.001 compared with bar 1, ##P < 0.001 compared with bar 2.
Inhibition of NF-κB activation abrogates MMF-induced IL-8 production.
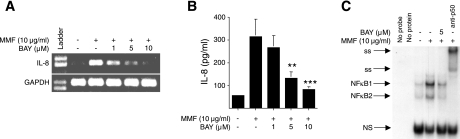
To specifically test for the requirement of NF-κB activation as a necessary step in MMF-induced IL-8 protein expression, an Iκ-Bα kinase inhibitor (BAY 11-7082) was used to block NF-κB activation. By inhibiting the phosphorylation of the regulatory molecule Iκ-Bα, NF-κB remains bound to Iκ-Bα and thus remains sequestered in the cytoplasm (46). In these experiments, BEAS-2B cells were pretreated with increasing concentrations of BAY 11-7082 for 1 h and subsequently incubated with 10 μg/ml MMF for 6 h. The production of IL-8 mRNA was determined by RT-PCR. As shown in Fig. 5A, MMF-induced IL-8 mRNA production was inhibited by BAY 11-7082 in a concentration-dependent response. In additional experiments, BEAS-2B cells were incubated with 1, 5, and 10 μM of BAY 11-7082 for 1 h, followed by a culture with 10 μg/ml MMF for 24 h, and the culture supernatant was assayed for IL-8 protein. As shown in Fig. 5B, BAY 11-7082 also inhibited MMF-induced IL-8 synthesis and secretion. The data in Fig. 5C also show that BAY 11-7082 inhibited translocation of NF-κB to the nucleus, as assessed by MMF-induced NF-κB binding to its promoter sequence.
Fig. 5.
Inhibition of NF-κB activation blocks MMF-induction of IL-8. A: BEAS-2B cells were preincubated with the Iκ-Bα kinase inhibitor (BAY 11-7082) at 1 to 10 μM for 1 h, then cultured with 10 μg/ml MMF for an additional 6 h, and expression of mRNA for IL-8 and GAPDH was determined by RT-PCR. Data shown are representative of two independent experiments. B: BEAS-2B cells were pretreated with 1 to 10 μM BAY11-7082 for 1 h and then incubated with 10 μg/ml MMF for an additional 24 h. Supernatants were collected and assayed for IL-8 by electrochemiluminescence, **P < 0.01, ***P < 0.001 compared with bar 2. Data shown are the means (SD) of three independent experiments. C: BEAS-2B cells were incubated with 10 μg/ml MMF for 120 min with or without pretreatment with BAY 11-7082 and an EMSA performed to determine NF-κB binding. Data shown are representative of three independent experiments.
MMF activates the MAPKs.
To determine whether other signaling pathways play a role in MMF-induction of IL-8, we examined the MAPKs because they are known to affect the activation of a number of transcriptional elements including NF-κB, AP-1, and NF-IL-6. Another reason for examining the MAPKs is that distinct transcriptional elements that are activated by the MAPKs may cooperate with NF-κB to enhance transcription; e.g., AP-1 (c-Jun/c-Fos) can synergize with p65-NF-κB to enhance transcription (61a). The MAPKs can also activate ATF-2, which can also associate and synergize with members of the AP-1 family (1a).
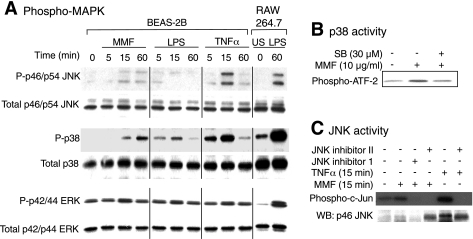
MAPKs comprise three main family members—p42/p44 ERK, p38mapk, and p46/p54 JNK—and mediate cellular responses to an array of extracellular stimuli such as bacterial products, cytokines, and osmotic shock (23). Initially, we determined which MAPKs are activated by MMF. Since MAPKs are early signal transduction events whose activation is often apparent within 30 min of stimulating the cells, BEAS-2B cells were incubated with 10 μg/ml MMF for 5 min to 60 min and examined for phosphorylation and activation of the MAPKs. As positive controls, BEAS-2B cells and RAW 264.7 macrophages were also stimulated with TNF-α and LPS, respectively. Phosphorylated MAPKs were identified by Western blot analysis using antibodies directed against phosphorylated-p42/p44 ERK, phosphorylated-p38mapk, or phosphorylated-p46/p54 JNK. As shown in Fig. 6A, peak phosphorylation of p46/p54 JNK in response to MMF occurred after 15 min. Peak phosphorylation of p38mapk occurred at 60 min after incubation with MMF. In contrast, p42/p44 ERK were basally phosphorylated in BEAS-2B cells, and there was minimal increase in p42/p44 ERK phosphorylation after 15 min of incubation with MMF. Immunoblotting with an antibody directed against total-p46/p54 JNK, total-p38mapk, and total-p42/p44 ERK demonstrated equal loading of the total MAPKs among different experimental conditions. TNF-α stimulation of BEAS-2B cells also induced JNK and p38mapk activation, although peak phosphorylation of p38mapk occurred earlier than observed with MMF stimulation. As with MMF, the basal increase in p42/p44 ERK phosphorylation was not significantly altered by TNF-α stimulation. LPS did not induce phosphorylation of either JNK or ERK and induced a minimal increase in p38mapk phosphorylation in the BEAS-2B cells. In contrast, LPS induced robust phosphorylation of the three MAPKs in RAW 264.7 macrophages.
Fig. 6.
Activation of ERK, p38mapk, and JNK signaling in response to MMF. A: BEAS-2B cells were cultured with 10 μg/ml MMF, 10 ng/ml LPS, or 10 ng/ml TNF-α for 5, 15, or 60 min. As controls for MAPK activation, RAW 264.7 cells were either left unstimulated (US) or stimulated with LPS and their cell lysates were immunoblotted for phospho- and total-MAPKs. Cells were lysed and for each condition; 20 μg of protein were separated by 10% SDS-PAGE, transferred onto a nitrocellulose membrane and immunoblotted with a phospho-specific antibody to p46/p54 JNK, p38mapk, or p42/p44 ERK. Equal loading among experimental conditions was confirmed with an antibody against total-p46/p54 JNK, total-p38mapk, or total-p42/p44 ERK, respectively. B: in vitro kinase assay for p38mapk activity was measured using ATF-2 as the substrate. The samples were prepared from BEAS-2B cells that were either untreated or challenged with MMF with or without SB203580 as indicated. C: Solid phase in vitro kinase assays for JNK activity were performed using c-Jun GST as the substrate. The BEAS-2B cells were either untreated, or stimulated with MMF or TNF-α as indicated in either the presence or absence of JNK inhibitor 1 or JNK inhibitor II. WB, Western blot. Data shown are representative of three independent experiments.
To determine the effectiveness of the p38mapk inhibitor (SB203580), BEAS-2B cells were pretreated with 30 μM of SB203580 for 1 h, followed by incubation with 10 μg/ml MMF. The cell extracts were then subjected to the p38mapk in vitro kinase assay using ATF-2 as the substrate (70). As shown in Fig. 6B, MMF increased ATF-2 phosphorylation, whereas SB203580 inhibited p38mapk activity back down to basal levels. To determine the potency of the JNK inhibitors, a solid phase in vitro kinase assay was performed using c-Jun-GST-sepharose beads as the substrate for JNK. Two types of JNK inhibitors are commercially available: “JNK inhibitor 1” is a peptide (3,823 kDa) that competes with JNK for its substrate; “JNK inhibitor II” is a small molecule (220 kDa) that competes with ATP for JNK. Both are able to inhibit both p46 and p54 JNK isoforms. As shown in Fig. 6C, incubation of the cells with either JNK inhibitor abolished MMF-induced c-Jun phosphorylation. A sequential immunoblot with a p46/p54 JNK antibody showed increased binding of JNK to the c-Jun-GST bead substrate with MMF incubation, which was inhibited by the small peptide JNK inhibitor 1. Interestingly, although JNK inhibitor II was able to inhibit JNK activity, it did not prevent JNK binding to its substrate c-Jun (Fig. 6C). TNF-α potently induced JNK activity and was inhibited by JNK inhibitor II (Fig. 6C).
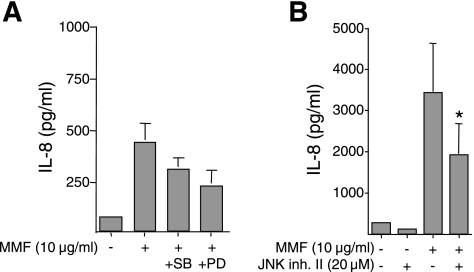
To determine the role of MAPKs in MMF-induction of IL-8 synthesis and secretion, BEAS-2B cells were pretreated with 30 μM SB203580, 30 μM PD98059, or 20 μM JNK inhibitor II for 1 h, followed by stimulation with 10 μg/ml MMF for an additional 24 or 48 h. As shown in Fig. 7, inhibition of each of the MAPK had only a modest effect on MMF-induced IL-8 production; e.g., inhibition of MAPK-ERK kinase (MEK), the upstream kinase of ERK, with PD98055, or of p38mapk with SB203580 partially decreased the amount of MMF-induced IL-8 to levels that were not statistically significant (Fig. 7A). Inhibition of JNK decreased IL-8 production by ∼40%, and this effect was statistically significant at 24 h (data not shown) and at 48 h (Fig. 7B).
Fig. 7.
MAPK inhibitors partially reduce IL-8 production induced by MMF. A: BEAS-2B cells were pretreated with p38mapk inhibitor SB203580 (30 μM) or MEK1-ERK inhibitor PD98059 (30 μM) for 1 h, then cultured with 10 μg/ml MMF for an additional 24 h. Supernatants were collected and IL-8 concentrations measured. Data shown are the means (SD) of four independent experiments. B: BEAS-2B cells were preincubated with 20 μM JNK inhibitor II for 1 h, and then coincubated with 10 μg/ml MMF for 48 h. Supernatants were collected and assayed for IL-8 by electrochemiluminescence. *P < 0.05 compared with bar 3. Data shown are the means (SD) of three independent experiments.
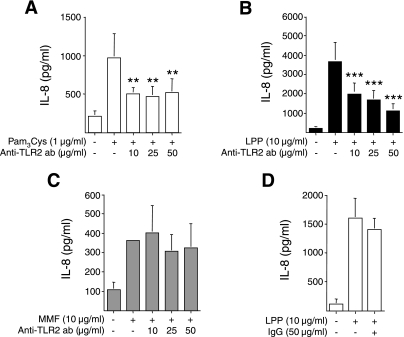
TLR2 neutralization inhibits mycoplasma lipoprotein induction of IL-8.
TLRs are pattern recognition proteins present on innate immune cells that bind to motifs common to many invading pathogens (27). On the basis of the lack of response of BEAS-2B cells to LPS in the absence of normal human serum (55) and corroborated by us with our experimental conditions (Fig. 1, inset), it is logical to conclude that mycoplasma ligands do not use TLR4. To determine whether MMF-induction of IL-8 production is via TLR2, BEAS-2B cells were incubated with 10 μg/ml, 25 μg/ml, or 50 μg/ml of an anti-TLR2 neutralizing antibody for 24 h, then costimulated with either 10 μg/ml MMF, 1 μg/ml Pam3Cys lipopeptide, or 10 μg/ml mycoplasma lipoprotein (LPP) for 48 h. Pam3Cys lipopeptide and LPP are known to signal via TLR2 (13). As shown in Fig. 8, A and B, anti-TLR2 antibody significantly inhibited Pam3Cys lipopeptide- or LPP-induced IL-8 production but not MMF-induced IL-8 expression (Fig. 8C). Pretreatment with 50 μg/ml of a nonimmune goat polyclonal IgG antibody had no effect on LPP induction of IL-8 (Fig. 8D). From these data, we conclude that LPP acts primarily through TLR2, but MMF uses additional cell surface molecules to stimulate signaling and production of IL-8.
Fig. 8.
Anti-TLR2 inhibits mycoplasma LPP but not MMF induction of IL-8. BEAS-2B cells were pretreated with an anti-TLR2 antibody overnight prior to incubation with either 1 μg/ml Pam3Cys lipopeptide (A), 10 μg/ml M. pneumoniae lipoprotein (LPP) (B), or 10 μg/ml MMF (C), for an additional 48 h, followed by assay for IL-8 by electrochemiluminescence. **P < 0.01 ***P < 0.001 compared with their respective bar 2. Data shown are the means (SD) for three independent experiments. D: BEAS-2B cells pretreated with 50 μg/ml of nonimmune goat IgG antibody, followed by stimulation with LPP, and assay for IL-8. Data shown are representative of two independent experiments.
Azithromycin, clarithromycin, or moxifloxacin failed to inhibit MMF-induction of IL-8.
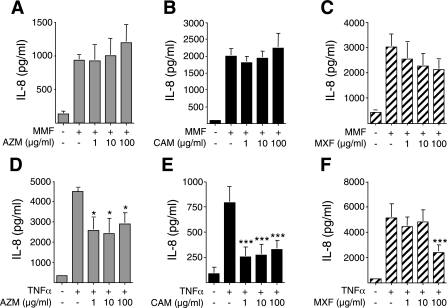
While effective antibiotics are available against M. pneumoniae, in many instances, the ensuing inflammatory response to the infection perpetuates the disease process. In this context, macrolide antibiotics also possess anti-inflammatory activities and have been used in the treatment of several inflammatory airway diseases, such as diffuse panbronchiolitis, cystic fibrosis, and recalcitrant asthma (5, 20, 53, 77). Moxifloxacin is a fluoroquinolone antibiotic shown to inhibit IL-8 and TNF-α production in THP-1 macrophages (67). Thus, antibiotics have the potential not only to treat the infection but also to inhibit the inflammatory response. To determine whether azithromycin, clarithromycin, or moxifloxacin could inhibit MMF-induced IL-8 production, BEAS-2B cells were pretreated with 1 to 100 μg/ml of azithromycin, clarithromycin, or moxifloxacin for 24 h, followed by coincubation with 10 μg/ml MMF for an additional 48 h. The range of antibiotic concentrations chosen for these in vitro experiments was based on peak serum levels typically seen in patients with standard dosing; that is, the peak serum levels for azithromycin is 0.2 to 0.7 μg/ml, clarithromycin is 2 to 7 μg/ml, and moxifloxacin is 3 to 5 μg/ml. As shown in Fig. 9, A–C, increasing concentrations of either azithromycin, clarithromycin, or moxifloxacin did not inhibit MMF-induced IL-8 production. To determine whether these antibiotics exhibit anti-inflammatory activity to another stimulus, cells were pretreated with each of these antibiotics for 24 h, stimulated with 10 ng/ml TNF-α, and then assayed for IL-8. As shown in Fig. 9, D and E, azithromycin (AZM) or clarithromycin (CAM) at ≥ 1 μg/ml significantly reduced TNF-α-induction of IL-8. Moxifloxacin also inhibited TNF-α-induced IL-8 production but only in cells treated with 100 μg/ml moxifloxacin (MXF; Fig. 9F). From these experiments, we conclude that although azithromycin, clarithromycin, and moxifloxacin are effective at suppressing mycoplasmal growth, they are ineffective at suppressing inflammation induced by mycoplasmal membrane components in vitro.
Fig. 9.
Antibiotics do not suppress MMF induction of IL-8. BEAS-2B cells were pretreated with 1 to 100 μg/ml of azithromycin (A; AZM), clarithromycin (B; CAM), or moxifloxacin (C; MXF) for 24 h, then costimulated with MMF for an additional 48 h, followed by assay for IL-8 by electrochemiluminescence. In separate wells, BEAS-2B cells were also pretreated with 1 to 100 μg/ml AZM (D), CAM (E), or MXF (F) for 24 h and then costimulated with TNF-α for an additional 48 h, followed by IL-8 measurement. Data shown are the means (SD) for three or more independent experiments. *P < 0.05 and ***P < 0.001 compared with their respective bar 2.
DISCUSSION
M. pneumoniae is a well-known cause of community-acquired pneumonia, acute bronchitis, and asthma exacerbations. Less commonly, it may contribute to the pathogenesis of more chronic lung diseases, such as bronchiolitis, bronchiectasis, and asthma (8, 28). In many of these conditions, neutrophils are the predominant inflammatory cell type and are considered to be an important contributor to the pathogenesis. Through its ability to attract and activate neutrophils from the bloodstream, IL-8 likely contributes to the pathogenesis of various lung diseases caused by M. pneumoniae (45). Neutrophils amplify the inflammation and injure the airways through the release of inflammatory cytokines, proteases, and reactive oxygen and nitrogen intermediates (54, 72). In an in vivo study of C57BL/6 and BALB/c mice infected with M. pneumoniae, the BALB/c mice exhibited higher concentrations of KC (the mouse equivalent of IL-8), larger neutrophilic infiltration of the airspaces, and greater airway hyperresponsiveness (14).
Previous studies have shown that in human lung epithelial cell lines, infection with live M. pneumoniae or stimulation with a sonicated whole cell lysate of M. pneumoniae increased IL-8 production (61, 75). In this study, we found that MMF, which comprises various lipoproteins and glycolipids, is capable of inducing IL-8 synthesis and secretion in human bronchial epithelial cells. Variations in the amount of IL-8 measured in the culture supernatant between experiments is likely related to the time of stimulation as IL-8 accumulates in the culture supernatant, the volume of culture medium used, and differences among passages 1–15 of the BEAS-2B cells. Mechanistically, MMF induces IL-8 gene expression by activating the transcriptional elements NF-κB, AP-1, and NF-IL-6. Although AP-1 was found to be constitutively active in the BEAS-2B cells, this activation alone was insufficient for induction of IL-8 gene expression. However, AP-1 binding was necessary for maximal induction of IL-8 promoter activity by MMF, indicating that activated AP-1 is a necessary but, in itself, insufficient for optimal induction of IL-8 by MMF. Although AP-1 family comprises many different forms, including homodimer of c-Jun, and heterodimers of c-Jun/c-Fos, c-Jun/JunD, c-Jun/JunB, c-Jun/Fra-1, c-Jun/FosB, and c-Jun with various ATF families (1a, 46a), many of which are induced or activated by JNK but also by ERK and p38mapk, it appears that most of the AP-1 complexes in BEAS-2B cells comprise the c-Jun/c-Fos heterodimer (Fig. 3). Whereas the MAPK signaling pathways played only a modest role in MMF-induction of IL-8, the IKK-NF-κB signaling pathway played a major and essential regulatory step for increasing IL-8 expression.
BEAS-2B cells contain TLR1 through TLR6 but lack TLR7, TLR8, and TLR9 (55). The induction of IL-8 expression in response to the TLR2 agonists Pam3Cys lipopeptide, and LPP also corroborates the presence of TLR2 in these cells. Schulz et al. (55) also showed that BEAS-2B cells can respond to LPS but only in the presence of normal human serum, a component that was absent in our studies. Interestingly, the necessary components supplied by normal human serum in their study was not LPS-binding protein (LBP), since these cells still did not respond to LPS even in the presence of added LBP. Although our in vitro experiments showed that MMF-induction of IL-8 by BEAS-2B cells is not mediated by TLR4, it is not known whether this holds true in vivo. While TLR2 is an important receptor for mycoplasma LPP induction of IL-8, it appears that MMF act through other pattern recognition proteins for induction of IL-8.
The host inflammatory response to M. pneumoniae infection is considered to play an important role in the pathogenesis of the ensuing clinical disease. Macrolide antibiotics not only possess antimicrobial activity against M. pneumoniae, but they also have anti-inflammatory properties (77). This anti-inflammatory effect of macrolides is used in diffuse panbronchiolitis in which low-dose erythromycin has been shown to be efficacious (31). Chronic daily use of macrolides also appear promising for controlling respiratory inflammation associated with cystic fibrosis, chronic sinusitis, and possibly asthma (5, 17, 20, 24, 30, 35, 37, 52, 53). The mechanism(s) by which macrolides exert their anti-inflammatory effect is not clearly defined, although it may occur, in part, through their ability to inhibit binding of transcription factors necessary for induction of proinflammatory cytokines (1, 21, 26, 63, 64). Although clarithromycin or azithromycin did not inhibit MMF-induced IL-8 expression, TNF-α-induced IL-8 production was inhibited by each of these antibiotics at a concentration (1 μg/ml) that is commensurate with serum concentrations that are achieved with therapeutic doses of the antibiotics in humans. Similarly, Abe et al. (1) showed that in the BET-1A human bronchial epithelial cell line, clarithromycin inhibited TNF-α-induced IL-8 expression. Since many acute and chronic inflammatory disease processes are mediated by TNF-α, including asthma (4), perhaps some of the anti-inflammatory effects of azithromycin or clarithromycin occur through inhibition of TNF-α function. Interestingly, in a murine model of M. pneumoniae-induced pneumonia, clarithromycin attenuated lung cytokine response to live M. pneumoniae infection but had no effect with dead M. pneumoniae infection (19). Although the TELICAST study found that telithromycin helped ameliorate asthma exacerbations, there was no relationship between infection with C. pneumoniae or M. pneumoniae and response to telithromycin treatment (24). The fluoroquinolone antibiotics also have immunomodulatory capabilities (2, 67, 68). As seen with the macrolides, moxifloxacin also inhibited TNF-α-induced but not MMF-induced IL-8 production, but only at levels that far exceeded therapeutic serum concentrations in humans with standard dosing. Nevertheless, in the context of the anti-inflammatory effects of antibiotics, it is noteworthy that severe mycoplasma-associated lung diseases, such as bronchiolitis may improve dramatically with corticosteroid treatment (8, 10). Whether more targeted immunosuppression with a neutralizing IL-8 antibody would ameliorate the deleterious inflammatory component of mycoplasma-associated lung diseases remains to be determined.
In summary, we found that MMF-induced IL-8 expression in BEAS-2B human bronchial epithelial cells by a transcriptional mechanism that is largely dependent on NF-κB activation, with lesser contributions by AP-1, NF-IL-6, and the MAPKs. MMF induction of IL-8 in these cells is largely independent of either TLR2 or TLR4 and was not inhibited by clarithromycin, azithromycin, or moxifloxacin.
GRANTS
This work was supported by National Institutes of Health Grant HL-66112 (E. D. Chan) and NIH Grant PHL 073907 (D. R. Voelker).
Present address for K. Kuronuma and H. Mitsuzawa: Department of Biochemistry, Sapporo Medical University School of Medicine, S1W17, Chuo-ku, Sapporo, Japan.
Acknowledgments
We thank Dr. Naofumi Mukaida at the Kanazawa University in Kanazawa, Japan, for the IL-8-WT and IL-8-mutant luciferase plasmids. We also thank Dr. Charles Dinarello at the University of Colorado Health Sciences Center in Denver, Colorado, for use of reagents and the Origen Analyzer for IL-8 detection. We thank Dr. Richard J. Martin for critical review of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe S, Nakamura H, Inoue S, Takeda H, Saito H, Kato S, Mukaida N, Matsushima K, Tomoike H. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding site in human bronchial epithelial cells. Am J Respir Cell Mol Biol 22: 51–60, 2000. [DOI] [PubMed] [Google Scholar]
- 1a.Ameyar-Zazoua M, Wisniewska MB, Bakiri L, Wagner EF, Yaniv M, Weitzman JB. AP-1 dimers regulate transcription of the p14/p19ARF tumor suppressor gene. Oncogene 24: 2298–2306, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Araujo FG, Slifer TL, Remington JS. Effect of moxifloxacin on secretion of cytokines by human monocytes stimulated with lipopolysaccharide. Clin Microbiol Infect 8: 26–30, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Baum SG Mycoplasma Pneumoniae and Atypical Pneumonia. New York: Churchill Livingstone, 2000.
- 4.Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, Bradding P, Brightling CE, Wardlow AJ, Pavord ID. Evidence of a role of tumor necrosis factor α in refractory asthma. N Engl J Med 354: 697–708, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Beuther DA, Martin RJ. Antibiotics in asthma. Curr Allergy Asthma Rep 4: 132–138, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bohnet S, Kotschau U, Braun J, Dalhoff K. Role of interleukin-8 in community-acquired pneumonia: relation to microbial load and pulmonary infection. Infection 25: 95–100, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broaders SA, Hooper WC, Phillips DJ, Talkington DF. Mycoplasma pneumoniae subtype-independent induction of proinflammatory cytokines in THP-1 cells. Microb Pathog 40: 286–292, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Chan ED, Kalayanamit T, Lynch DA, Tuder R, Arndt P, Winn R, Schwarz MI. Mycoplasma pneumoniae-associated bronchiolitis causing severe restrictive lung disease in adults. Chest 115: 1188–1194, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DWH. Induction of inducible nitric oxide synthase-NO by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect Immun 69: 2001–2010, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan ED, Welsh CH. Fulminant Mycoplasma pneumoniae pneumonia. West J Med 162: 133–142, 1995. [PMC free article] [PubMed] [Google Scholar]
- 11.Chan ED, Winston BW, Jarpe MB, Wynes MW, Riches DWH. Preferential activation of the p46 isoform of JNK/SAPK in mouse macrophages by TNF-α. Proc Natl Acad Sci USA 94: 13169–13174, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba H, Pattanajitvilai S, Evans AJ, Harbeck RJ, Voelker DR. Human surfactant protein D (SP-D) binds Mycoplasma pneumoniae by high affinity interactions with lipids. J Biol Chem 277: 20379–20385, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, Campbell K, Harbeck RJ, Martin RJ. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J Immunol 174: 5713–5719, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca-Aten M, Rios AM, Mejias A, Chavez-Bueno S, Katz K, Gomez AM, McCracken GH, Hardy RD. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am J Respir Cell Mol Biol 32: 201–210, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Gabridge MG, Singer SE, Esposito RA. Gradient, polyacrylamide gel electrophoresis of proteins from cytotoxic mycoplasma membranes. Biochem Biophys Res Commun 70: 271–279, 1976. [DOI] [PubMed] [Google Scholar]
- 16.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy Asthma Immunol 70: 23–25, 1993. [PubMed] [Google Scholar]
- 17.Gotfried MH Macrolides for the treatment of chronic sinusitis, asthma, and COPD. Chest 125: 52S–61S, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hardy RD, Jafri HS, Olsen K, Wordemann M, Hatfield J, Rogers BB, Patel P, Duffy L, Cassell G, McCracken GH, Ramilo O. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun 69: 3869–3876, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy RD, Rios AM, Chavez-Bueno S, Jafri HS, Hatfield J, Rogers BB, McCracken GH, Ramilo O. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob Agents Chemother 47: 1614–1620, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatipoglu U, Rubinstein I. Low-dose, long-term macrolide therapy in asthma: An overview [Online]. Clin Mol Allergy 2: 4, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichiyama T, Nishikawa M, Yoshitomi T, Hasegawa S, Matsubara T, Hayashi T, Furukawa S. Clarithromycin inhibits NF-κB activation in human peripheral blood mononuclear cells and pulmonary epithelial cells. Antimicrob Agents Chemother 45: 44–47, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iizasa H, Matsushima KIL8. Cytokine Reference, vol. 1, Ligands, edited by Oppenheim JJ and Feldmann M. San Diego: Academic Press, 2001, p. 1061–1067.
- 23.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med 354: 1589–1600, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med 172: 1078–1089, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi T, Hagiwara K, Honda Y, Gomi K, Kobayashi T, Takahashi H, Tokue Y, Watanabe A, Nukiwa T. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-κB transcription factors. J Antimicrob Chemother 49: 745–755, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol 11: 13–18, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Kraft M The role of bacterial infections in asthma. Clin Chest Med 21: 301–313, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, Gaydos CA, Martn RJ. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med 158: 998–1001, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest 121: 1782–1788, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kudoh S, Uetake K, Hagiwara K, Hirayama M, Hus LH, Kimura H, Sugiyama Y. Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis. Jpn J Thorac Dis: 632–642, 1987. [PubMed]
- 32.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol 153: 153–164, 1994. [PubMed] [Google Scholar]
- 33.Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy 59: 793–805, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman D, Lieberman D, Printz S, Ben-Yaakov M, Lazarovivh Z, Ohana B, Friedman MG, Dvoskin B, Leinonen M, Boldur I. Atypical pathogen infection in adults with acute exacerbation of bronchial asthma. Am J Respir Crit Care Med 167: 406–410, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Little FF Treating acute asthma with antibiotics-not quite yet, editorial. N Engl J Med 354: 1632–1634, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Llibre JM, Urban A, Garcia E, Carrasco MA, Murcia C. Bronchiolitis obliterans organizing pneumonia associated with acute Mycoplasma pneumoniae infection. Clin Infect Dis 25: 1340–1342, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Martin RJ Infections and asthma. Clin Chest Med 27: 87–98, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol 24: 557–582, 2001. [DOI] [PubMed] [Google Scholar]
- 39.McElhaney Membrane structure RN. Mycoplasmas: Molecular biology and pathogenesis, edited by Maniloff J, McElhaney RN, Finch LR and Baseman JB. Washington, D.C.: American Society for Microbiology, 1992, p. 113–155.
- 40.Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-κB signaling pathway and κB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun 71: 1442–1452, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mu HH, Pennock ND, Humphreys J, Kirschning CJ, Cole BC. Engagement of Toll-like receptors by mycoplasmal superantigen: downregulation of TLR2 by MAM/TLR4 interaction. Cell Microbiol 7: 789–797, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Mukaida N Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 284: L566–L577, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem 265: 21128–21133, 1990. [PubMed] [Google Scholar]
- 44.Narita M, Tanaka H, Togashi T, Abe S. Cytokines involved in CNS manifestations caused by Mycoplasma pneumoniae. Pediatr Neurol 33: 105–109, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Narita M, Tanaka H, Yamada S, Abe S, Ariga T, Sakiyama Y. Significant role of interleukin-8 in pathogenesis of pulmonary disease due to Mycoplasma pneumoniae infection. Clin Diagn Lab Immunol 8: 1028–1030, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 272: 21096–21103, 1997. [DOI] [PubMed] [Google Scholar]
- 46a.Powers C, Krutzsch H, Gardner K. Modulation of JunD-AP-1 DNA binding activity by AP-1-associated factor 1 (AF-1). J Biol Chem 271: 30089–30095, 1996. [DOI] [PubMed] [Google Scholar]
- 46b.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNF-α production from non-CD14+ human blood mononuclear cells. J Clin Invest 101: 711–721, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induce proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun 64: 637–643, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Razin Mycoplasmas S Medical Microbiology, edited by Baron S. Galveston, TX: The University of Texas Medical Branch at Galveston, 1996, p. 475–485. [PubMed]
- 49.Richman-Eisenstat JBY, Jorens PG, Hebert CA, Ueki I, Nadel JA. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol Lung Cell Mol Physiol 264: L413–L418, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Roebuck KA Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 19: 429–438, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Roger T, Out TA, Mukaida N, Matsushima K, Jansen HM, Lutter R. Enhanced AP-1 and NF-κB activities and stability of interleukin-8 (IL-8) transcripts are implicated in IL-8 mRNA superinduction in lung epithelial H292 cells. Biochem J 330: 429–435, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest 125: 70S–78S, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Cambell PW. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. JAMA 290: 1749–1756, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Sampson AP The role of eosinophils and neutrophils in inflammation. Clin Exp Allergy 30: 22–27, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Schulz C, Farkas L, Wolf K, Kratzel K, Eissner G, Pfeifer M. Differences in LPS-induced activation of bronchial epithelial cells (BEAS-2B) and type II-like pneumocytes (A-549). Scand J Immunol 56: 294–302, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA 92: 12230–12234, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu T, Kida Y, Kuwano K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-κB through toll-like receptors 1 and 2. Immunology 121: 473–483, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simecka JW Immune responses following mycoplasma infection. In: Mycoplasmas: Molecular Biology, Pathogenicity, and Strategies for Control, edited by Blanchard A and Browning G. Norfolk, U. K.: Horizon Bioscience, 2005, p. 485–534.
- 59.Singh AM, Busse WW. Asthma exacerbations: Aetiology. Thorax 61: 809–816, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith PF Membrane lipid and lipopolysaccharide structures. In: Mycoplasmas: Molecular Biology and Pathogenesis, edited by Maniloff J, McElhaney RN, Finch LR, and Baseman JB. Washington, D.C.: American Society for Microbiology, 1992, p. 79–91.
- 61.Sohn MH, Lee KE, Choi SY, Kwon BC, Chang MW, Kim KE. Effect of Mycoplasma pneumoniae lysate on interleukin-8 gene expression in human respiratory epithelial cells. Chest 128: 322–326, 2005. [DOI] [PubMed] [Google Scholar]
- 61a.Stein B, Baldwin AS, Ballard DW, Greene WC, Angel P, Herrlich P. Cross-coupling of the NF-κB p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J 12: 3879–3891, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res 8: 459–463, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, Tanaka M, Kasama T, Kobayashi K, Nakajima J, Ito K. Erthromycin modulates IL-8 expression in normal and inflammed human bronchial epithelial cells. Am J Respir Crit Care Med 156: 266–271, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Takizawa H, Desaki M, Ohtoshi T, Kitamura T, Okazaki H, Sato M, Akiyama N, Shoji S, Hiramatsu K, Ito K. Erythromycin suppresses interleukin 6 expression by human bronchial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun 210: 781–786, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Tanimoto Y, Takahashi K, Kimura I. Effects of cytokines on human basophil chemotaxis. Clin Exp Allergy 22: 1020–1025, 1992. [DOI] [PubMed] [Google Scholar]
- 66.Webster AD, Furr PM, Hughes-Jones NC, Gorick BD, and Taylor-Robinson D. Critical dependence on antibody for defence against mycoplasmas. Clin Exp Immunol 71: 383–387, 1988. [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss T, Shalit I, Blau H, Werber S, Halperin D, Levitov A, Fabian I. Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-κB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines. Antimicrob Agents Chemother 48: 1974–1982, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werber S, Shalit I, Fabian I, Steuer G, Weiss T, Blau H. Moxifloxacin inhibits cytokine-induced MAP kinase and NK-κB activation as well as nitric oxide synthesis in a human respiratory epithelial cell line. J Antimicrob Chemother 55: 293–300, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Wieslander A, Boyer MJ, Wroblewski H. Membrane protein structure. In: Mycoplasmas: Molecular Biology and Pathogenesis, edited by Maniloff J, McElhaney RN, Finch LR, and Baseman JB. Washington, D. C.: American Society for Microbiology, 1992, p. 93–112.
- 70.Winston BW, Chan ED, Johnson GL, Riches DWH. Activation of p38mapk, MKK3, and MKK4 by TNF-α in mouse bone marrow-derived macrophages. J Immunol 159: 4491–4497, 1997. [PubMed] [Google Scholar]
- 71.Winterbourn CC Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 181–182: 223–227, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Woodruff PG, Fahy JV. A role for neutrophils in asthma? Am J Med 112: 498–500, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. J Biol Chem 272: 2396–2403, 1997. [PubMed] [Google Scholar]
- 74.Wu Q, Martin RJ, Lafasto S, Efaw BJ, Rino JG, Harbeck RJ, Chu HW. Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. Am J Respir Crit Care Med 177: 720–729, 2008. [DOI] [PubMed] [Google Scholar]
- 75.Yang J, Hooper WC, Phillips DJ, Talkington DF. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect Immun 70: 3649–3655, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger GR, Henson P, Johnson GL. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation. Proc Natl Acad Sci USA 97: 7272–7277, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zalewska-Kaszubska J, Gorska D. Anti-inflammatory capabilities of macrolides. Pharmacol Res 44: 451–454, 2001. [DOI] [PubMed] [Google Scholar]