Abstract
RhoA/Rho kinase (ROCK) signaling plays a key role in the pathogenesis of experimental pulmonary hypertension (PH). Dehydroepiandrosterone (DHEA), a naturally occurring steroid hormone, effectively inhibits chronic hypoxic PH, but the responsible mechanisms are unclear. This study tested whether DHEA was also effective in treating monocrotaline (MCT)-induced PH in left pneumonectomized rats and whether inhibition of RhoA/ROCK signaling was involved in the protective effect of DHEA. Three weeks after MCT injection, pneumonectomized rats developed PH with severe vascular remodeling, including occlusive neointimal lesions in pulmonary arterioles. In lungs from these animals, we detected cleaved (constitutively active) ROCK I as well as increases in activities of RhoA and ROCK and increases in ROCK II protein expression. Chronic DHEA treatment (1%, by food for 3 wk) markedly inhibited the MCT-induced PH (mean pulmonary artery pressures after treatment with 0% and 1% DHEA were 33 ± 5 and 16 ± 1 mmHg, respectively) and severe pulmonary vascular remodeling in pneumonectomized rats. The MCT-induced changes in RhoA/ROCK-related protein expression were nearly normalized by DHEA. A 3-wk DHEA treatment (1%) started 3 wk after MCT injection completely inhibited the progression of PH (mean pulmonary artery pressures after treatment with 0% and 1% DHEA were 47 ± 3 and 30 ± 3 mmHg, respectively), and this treatment also resulted in 100% survival in contrast to 30% in DHEA-untreated rats. These results suggest that inhibition of RhoA/ROCK signaling, including the cleavage and constitutive activation of ROCK I, is an important component of the impressive protection of DHEA against MCT-induced PH in pneumonectomized rats.
Keywords: hemodynamics, vascular remodeling, 3-hydroxy-3-methylglutaryl coenzyme A reductase, soluble guanylate cyclase, sex steroid hormones
pulmonary hypertension (PH) is a disease of diverse causes that is characterized by progressive narrowing of small pulmonary arteries (PAs), which, when severe, frequently results in right heart failure and death. Patients with severe PH have combinations of small PA adventitial and medial thickening, occlusive neointimal lesions, and obliterating thrombotic and plexiform lesions (49). Current therapy with prostacyclin analogs, endothelin-1 receptor blockers, and phosphodiesterase inhibitors improves symptoms and exercise tolerance (3), but persistent morbidity and mortality indicate that important pathogenic mechanisms are minimally affected. Thus more effective therapeutic approaches are urgently needed.
Dehydroepiandrosterone (DHEA) is a C-19 steroid that is produced mainly in the adrenal cortex from cholesterol and is a precursor for both estrogens and androgens. DHEA and its sulfated ester DHEA sulfate (DHEAS) are the most abundant steroid hormones in the human body, and their circulating levels decline progressively after reaching a peak around 25 yr of age. DHEA is a multifunctional bioactive substance that may have beneficial effects on cardiovascular diseases (5, 50). We (46) and others (7, 18) have reported that chronic DHEA treatment effectively inhibits hypoxic PH due at least partly to activation of potassium channels (7) and upregulation of soluble guanylate cyclase (sGC) (46). However, the mechanisms responsible for the impressive protective effect of DHEA against hypoxic PH have not been fully defined, and its effects in other animal models of PH are unknown.
Accumulating evidence from several laboratories, including our own, indicates that RhoA/Rho kinase (ROCK) signaling plays an important role in the pathogenesis of various experimental models of PH, including chronic hypoxia- (11, 16, 24, 35, 38, 40), monocrotaline (MCT)- (1, 38), bleomycin- (35), shunt- (31), and VEGF receptor inhibition and chronic hypoxia- (45) and mild hypoxia-induced PH in neonatal fawn-hooded rats (38, 39). Several studies show that this signaling pathway mediates sustained vasoconstriction, cell migration, proliferation, and survival, and inflammation (26, 52). In addition, the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, statins, which inhibit activation of RhoA and other small GTPases by preventing posttranslational isoprenylation of the protein and its translocation to the cell membrane (53), ameliorate PH in several different rat models (13–15, 30, 37, 42, 44, 57) and may also be effective in PH patients (29). Also, it has been reported that inhibition of RhoA activation (translocation to cell membrane) and of RhoA/ROCK signaling is involved in the protective effect of the phosphodiesterase-5 inhibitor, sildenafil, against hypoxic (16) and bleomycin-induced (20) PH. These observations indicate that, independent of the cause of PH, activation of the RhoA/ROCK pathway may serve as a point of convergence of various signaling cascades in the pathogenesis of PH and that drugs inhibiting this pathway might be very useful in the treatment of various forms of PH.
Although we are not aware of any reports of the effect of DHEA on RhoA/ROCK signaling, there are several possible mechanisms by which this steroid could impact the signaling pathway. These include inhibition of HMG-CoA reductase (48, 54) and upregulation of sGC (46), both of which prevent RhoA activation (membrane translocation; Refs. 16, 53), antioxidant/anti-inflammatory effects (2, 25), which inhibit activation and/or upregulation of ROCK (4, 21, 27, 28), and conversion of DHEA to estrogen, which inhibits ROCK expression and/or activity (10, 22, 23, 32). Based on this background, we investigated whether DHEA protected against MCT-induced occlusive neointimal lesions and severe PH in left pneumonectomized rats and whether inhibition of RhoA/ROCK signaling was involved in the protection. We used pneumonectomized rats in this study because of the evidence that MCT induces more severe PH and PA remodeling (i.e., neointimal lesions, a hallmark of human severe pulmonary arterial hypertension) in pneumonectomized than in normal rats (43, 47, 59).
METHODS
All experiments were approved by the University of Colorado at Denver and Health Sciences Center Institutional Animal Care and Use Committee.
Experimental Groups
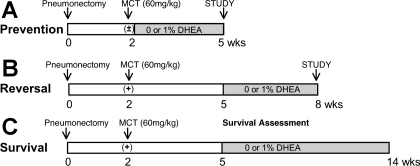
Experimental groups are summarized in Fig. 1 legend.
Fig. 1.
Experimental groups. MCT, monocrotaline; DHEA, dehydroepiandrosterone.
Prevention study.
Four groups of male left unilateral pneumonectomized rats (350–420 g, Zivic Laboratories; n = 6 each) were studied. Two weeks after the surgery, two groups were injected with MCT (60 mg/kg sc, Sigma), and the other two groups received vehicle (saline) injections. After the injection, one of the MCT- and saline-injected groups received rat food containing 0%, and the other received food containing 1% DHEA (Sigma) for 3 wk. The dose of DHEA (1%) was based on our previous study (46) that showed that chronic 0.3% and 1% DHEA treatment dose-dependently and completely inhibited hypoxia-induced PH. Food consumption and body weight were monitored. Three weeks after MCT or vehicle injection, hemodynamic measurements were performed in anesthetized rats. All rats were then euthanized by an overdose of pentobarbital sodium, and right lungs (for histology and morphometry), blood (for plasma hormone–DHEA, DHEAS, estrogen, and testosterone–level measurements), and hearts [for right ventricle (RV)-to-left ventricle (LV) + septum (S) ratio (RV/LV+S)] were collected.
Because protein expression and/or phosphorylation could be altered by the invasive procedure of catheterization, and available lung samples are limited in pneumonectomized rats, additional rats (n = 4) for each experimental group were used for lung protein expression/activity analyses. Three weeks after MCT or vehicle injection, rats were euthanized by an overdose of pentobarbital sodium, and right lungs were dissected, snap-frozen, and kept at −80°C until analyzed.
Reversal study.
Two groups of male pneumonectomized rats were studied. Two weeks after pneumonectomy, all rats were injected with MCT (60 mg/kg sc). Three weeks after MCT injection, one group was started on food containing 0%, and the other on food containing 1% DHEA. After the 3-wk treatment (from week 5 to 8), hemodynamic and morphometric analyses were performed.
Survival study.
Two weeks after pneumonectomy, two groups of male rats (n = 8 for 1% and n = 12 for 0% DHEA) were injected with MCT (60 mg/kg sc). Three weeks after MCT injection, seven rats were started on food containing 1% DHEA, and 10 rats were kept on food containing 0% DHEA (3 rats died before the treatment regimen was started). We then monitored their survival over the next 9 wk.
Catheterized Rats
After the 3-wk treatment with 0% or 1% DHEA (3 or 6 wk after MCT injection), rats were anesthetized with ketamine (100 mg/kg im) and xylazine (15 mg/kg im) for placement of three catheters: one in the right jugular vein, one in the pulmonary artery (via jugular vein and RV), and one in the right carotid artery (45). Mean pulmonary (MPAP) and systemic arterial pressures (MSAP) were measured with pressure transducers. Cardiac output was determined by a standard dye dilution method, and cardiac index (CI) was calculated by dividing cardiac output by the rat's body weight. Blood samples (for prevention study only) were then collected for measurement of plasma steroid levels. The heart was removed for assessment of right ventricular hypertrophy (RV/LV+S), and the lungs were prepared for morphometric analysis.
Western Blot Analysis
Frozen rat lung tissue was homogenized in lysis buffer (250 mM sucrose, 25 mM imidazole, 1 mM EDTA, 1% Nonidet P-40, protease inhibitor cocktail; 1:100 dilution; Pierce) and phosphatase inhibitor cocktail (1:100 dilution, Sigma). Homogenate was centrifuged at 10,000 g for 10 min. Protein concentration of the supernatant was determined by BCA Protein Assay Kit (Pierce). Equal amounts of protein were loaded on 4–12% gradient NuPAGE Bis-Tris gels (Invitrogen) and transferred to hydrophobic polyvinylidene fluoride transfer membrane (Millipore) in NuPAGE Transfer Buffer containing 10% methanol. Western blots were visualized using West Pico Chemiluminescence Substrate (Pierce) and estimated by densitometry (Bio-Rad). Antibodies used were anti-HMG-CoA reductase (Upstate), anti-sGC β1-subunit (Cayman), anti-cleaved caspase-3 (Cell Signaling), anti-RhoA (Santa Cruz), anti-ROCK I and II (BD Transduction Laboratories), anti-MYPT1 and phosphorylated MYPT1 (pMYPT1Thr696; Upstate), anti-IgG (Vector), and anti-β-actin (Sigma).
RhoA Translocation to the Membrane
To assess membrane translocation of RhoA, the frozen lung tissues were homogenized in lysis buffer (10 mM HEPES, 2 mM EDTA, 1 mM MgCl2) containing protease inhibitors. Homogenate was centrifuged at 40,000 g for 30 min, and the supernatant was collected as the cytosolic fraction. The pellet was resuspended in lysis buffer containing 0.1% SDS and centrifuged again (40,000 g for 15 min) to generate the membrane fraction. Equal amounts of protein (15 μg) were loaded in gel. RhoA protein in membrane and cytosolic fractions was determined by standard Western blot analysis using mouse monoclonal anti-RhoA antibody (1:250 dilution) and a peroxidase-labeled anti-mouse IgG antibody (3:10,000 dilution). Relative density of membrane to cytosolic RhoA was determined using NIH Image software (National Institutes of Health).
Immunoprecipitation of MYPT1
To assess ROCK activity, we measured phosphorylation levels of one of the ROCK downstream effectors, MYPT1 (a regulatory subunit of myosin light chain phosphatase). Immunoprecipitation of MYPT1 was performed as described previously (45). Briefly, whole lung protein extracts (500 μl, protein concentration 10 μg/μl) were incubated with 7 μl of anti-MYPT1 antibody for 4 h at 4°C to allow antibody-antigen complexes to form. Twenty-five microliters of washed beads (EZview Red Protein A Affinity Gel, Sigma) were added to antigen-antibody complex and incubated overnight at 4°C with gentle mixing. Beads were pelleted by centrifugation and washed three times. Twenty-five microliters of 2× sample buffer were added to samples (Invitrogen), boiled for 10 min, and loaded in the gel. Western blots were then performed for phosphorylated MYPT1 and total MYPT1.
Morphological Analysis
Histological changes of PAs were quantified by morphometry as described below. Isolated lungs were inflated via trachea with 0.5% agarose + 1% formalin solution at 20 cmH2O pressure and fixed in 10% formalin. Paraffin sections of 5 μm were cut and stained with Verhoeff-Van Gieson and assessed microscopically for degree of arterial wall thickness. In each lung section, 30 small PAs (50–100 μm in diameter) were analyzed at ×400 in a blind manner. Medial wall thickness was expressed as the summation of two points of medial thickness/external diameter ×100 (%). Intra-acinar (precapillary) PAs (20–30 μm in diameter, 25 vessels each) were assessed for occlusive lesions as Grade 0 for no evidence of neointima lesion, Grade 1 for less than 50% luminal occlusion, and Grade 2 for more than 50% luminal occlusion (42). There was no evidence of neointimal lesion formation in any PAs from normal rats (all PAs were graded as 0).
Measurement of Plasma Steroid Hormone Levels
We measured plasma steroid hormone levels of DHEA, DHEAS, estradiol, and testosterone by ELISA kits (IBL-America).
Statistical Analysis
Values are expressed as means ± SE. Comparisons between groups were made with Student's t-test or ANOVA with Scheffé's post hoc test for multiple comparisons. Survival curves were analyzed by the Kaplan-Meier method. Differences were considered significant at P < 0.05.
RESULTS
Body Weight and Plasma Steroid Hormone Levels
Chronic DHEA treatment caused a decrease of body weight, which agrees with previous studies (19, 46, 60), in both MCT-uninjected and -injected rats in the prevention and reversal studies (Tables 1 and 2). Food consumption was not different between the 0% and 1% DHEA treated groups.
Table 1.
Body weight change in prevention study
| Group | BW Before | BW After |
|---|---|---|
| VEH | 405.7±12.0 | 502.2±16.5 |
| VEH + DHEA | 404.2±12.5 | 349.0±10.5*† |
| MCT | 405.0±9.6 | 475.0±10.7 |
| MCT + DHEA | 410.0±19.9 | 334.8±19.3*† |
Values are means ± SE of n = 5–6 in each group. VEH, vehicle-injected; MCT, monocrotaline (60 mg/kg)-injected; DHEA, 1% dehydroepiandrosterone-treated; BW Before, body weight before the treatment; BW After, body weight after the treatment.
P < 0.05 vs. VEH;
P < 0.05 vs. MCT.
Table 2.
Body weight change in reversal study
| Group | BW Before | BW After |
|---|---|---|
| MCT | 378.4±6.1 | 465.3±12.0 |
| MCT + DHEA | 390.1±11.1 | 419.1±10.0* |
Values are means ± SE of n = 9–10 in each group.
P < 0.05 vs. MCT.
The plasma levels of DHEA, DHEAS, estradiol, and testosterone in both MCT-injected and -uninjected rats were elevated similarly by DHEA treatment in the prevention study (Table 3).
Table 3.
Plasma steroid hormone levels
| Group | DHEA, ng/ml | DHEAS, μg/ml | Estradiol, pg/ml | Testosterone, ng/ml |
|---|---|---|---|---|
| VEH | 6.5±2.4 | 0.029±0.008 | 2.8±1.2 | 3.4±0.7 |
| VEH + DHEA | 353.5±58.5*† | 6.0±1.6*† | 15.0±2.0*† | 18.2±3.7*† |
| MCT | 2.0±0.294 | 0.012±0.004 | 3.7±1.0 | 2.4±0.6 |
| MCT + DHEA | 306.2±40.5*† | 4.6±0.818*† | 13.0±1.0*† | 16.9±1.1*† |
Values are means ± SE of n = 8–9 in each group. DHEAS, DHEA sulfate.
P < 0.05 vs. VEH;
P < 0.05 vs. MCT.
Effects of DHEA on MCT-induced PH in Pneumonectomized Rats
Prevention study.
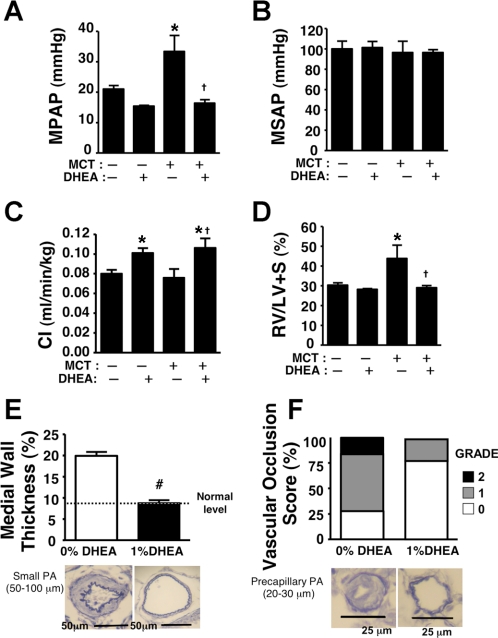
Consistent with previous reports, a single MCT injection caused increases in MPAP and RV/LV+S ratio (42, 44) but no changes in CI and MSAP. In left pneumonectomized rats at 3 wk, the PH was accompanied by severe pulmonary vascular remodeling, i.e., small PA medial wall thickening and occlusive arteriole neointimal lesions (Fig. 2). DHEA treatment almost completely prevented the increases in MPAP and RV/LV+S ratio (Fig. 2, A and D). There was no effect on MSAP, and CI was increased (Fig. 2, B and C). The treatment also markedly inhibited the MCT-induced severe pulmonary vascular remodeling (Fig. 2, E and F). Except for a slight increase in CI, DHEA had no effect in MCT-uninjected rats.
Fig. 2.
Effects of chronic preventive DHEA (1%) treatment on mean pulmonary (MPAP; A) and systemic artery pressure (MSAP; B), cardiac index (C; C), right ventricle to left ventricle + septum weight ratio (RV/LV+S; D), and pulmonary artery (PA) remodeling (E and F). Bottom are representative Verhoeff-Van Gieson-stained light micrographs of PAs. Values are means ± SE of n = 4–6 in each group. *P < 0.05 vs. MCT-DHEA-; †P < 0.05 vs. MCT+DHEA-; #P < 0.05 vs. 0% DHEA.
Reversal study.
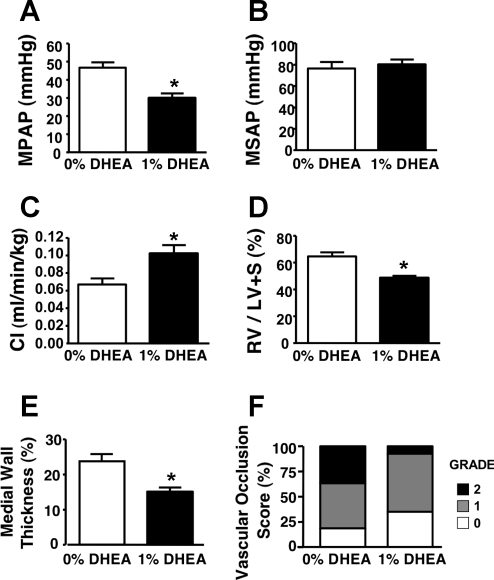
Compared with those at 3 wk after MCT injection, pneumonectomized rats at 6 wk after MCT had developed more severe PH (MPAP: 47 ± 3 vs. 33 ± 5 mmHg), RV hypertrophy (RV/LV+S ratio: 65% ± 3% vs. 44% ± 7%), and vascular remodeling (medial wall thickness: 24% ± 2% vs. 20% ± 1%; and Grade I and II occlusion: 37% and 45% vs. 17% and 56%). A 3-wk DHEA treatment started 3 wk after MCT injection prevented the progression of PH, RV hypertrophy, and vascular remodeling (Figs. 2 and 3). There was an increase in CI but no effect on MSAP.
Fig. 3.
Effects of chronic therapeutic DHEA (1%) treatment on MPAP (A), MSAP (B), CI (C), RV/LV+S (D), and PA remodeling (E and F). Values are means ± SE of n = 9–10 for hemodynamic and heart weight data and n = 5 for histological data. *P < 0.05 vs. 0% DHEA.
Survival study.
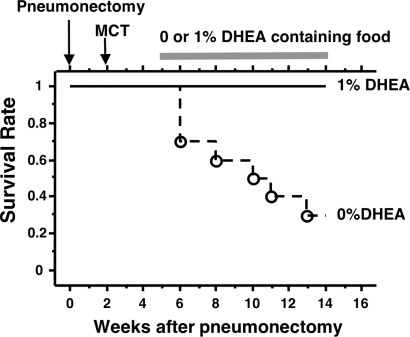
The survival rate of MCT-injected pneumonectomized rats not treated with DHEA was 30% (7 out of 10 rats died), whereas all DHEA-treated rats (n = 7) survived over the 9 wk of observation (Fig. 4).
Fig. 4.
Kaplan-Meier survival plot. Three weeks after MCT injection, pneumonectomized rats were started on 1% DHEA containing food (n = 7) or kept on food containing 0% DHEA (n = 10).
Effects of DHEA on RhoA/ROCK Signaling
RhoA activity.
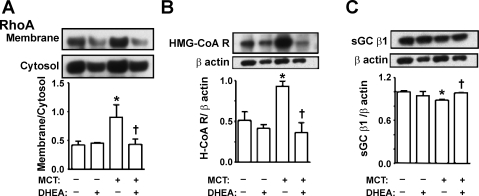
Three weeks after injection of MCT in pneumonectomized rats, the hypertensive lungs had higher RhoA activity (as reflected by an increase in its membrane-cytosol ratio), and DHEA treatment prevented the increase (Fig. 5A). Since it has been reported that DHEA inhibits expression of HMG-CoA reductase (48) and increases that of sGC (46), which could potentially inhibit RhoA activity, we next measured the levels of these two proteins. HMG-CoA reductase expression was markedly increased in lungs from MCT-injected rats, and the increase was prevented by DHEA treatment (Fig. 5B). MCT caused a slight decrease in sGCβ1 expression, which was restored by DHEA (Fig. 5C).
Fig. 5.
Effects of chronic preventive DHEA (1%) treatment on lung protein expression. A: RhoA (membrane/cytosol). B: 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG- CoA R, H-CoA R). C: soluble guanylate cyclase (sGC) β1-subunit. Top shows representative immunoblots, and bottom shows densitometric assessment. Values are means ± SE of n = 4. *P < 0.05 vs. MCT-DHEA-; †P < 0.05 vs. MCT+DHEA-.
ROCK Expression and Activity
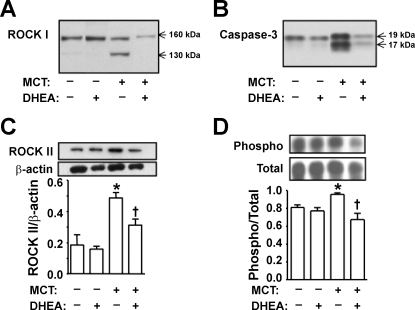
Cleaved ROCK I (constitutively active, 130 kDa; Refs. 9, 55) was detected in lungs from MCT-injected pneumonectomized rats, and DHEA treatment prevented the MCT-induced ROCK I cleavage (Fig. 6A). The cleavage of ROCK I was associated with increased caspase-3 activity (cleaved caspase-3 expression), which was also prevented by DHEA treatment (Fig. 6B). ROCK II protein expression was increased in MCT-injected rat lungs, and DHEA treatment markedly inhibited the increase (Fig. 6C). The ratio of phosphorylated-to-total MYPT1, which reflects ROCK activity, was also increased in the MCT-injected pneumonectomized rat lungs, and DHEA treatment inhibited ROCK activation (Fig. 6D).
Fig. 6.
Effects of chronic preventive DHEA (1%) treatment on lung protein expression. A: RhoA/Rho kinase (ROCK) I. B: cleaved caspase-3. C: ROCK II. D: MYPT1 (phospho/total), a regulatory subunit of myosin light chain phosphatase. For C and D, bottom shows densitometric assessment. Values are means ± SE of n = 4–6 in each group. *P < 0.05 vs. MCT-DHEA-; †P < 0.05 vs. MCT+DHEA-.
DISCUSSION
This study demonstrated that chronic dietary treatment of MCT-injected pneumonectomized rats with 1% DHEA prevented the development of PH, RV hypertrophy, and PA remodeling (medial wall thickening and occlusive neointimal formation) with no adverse systemic hemodynamic effects. DHEA, when started 3 wk after MCT injection, also completely inhibited the progression of MCT-induced severe PH and, more importantly, strikingly improved survival. Protein analyses of lungs from pneumonectomized rats revealed that MCT injection caused: 1) increased RhoA activity accompanied by markedly increased HMG-CoA reductase and slightly decreased sGC expression; 2) constitutive activation (cleavage) of ROCK I associated with caspase-3 activation; 3) increased ROCK II expression; and 4) increased ROCK activity. Chronic DHEA treatment nearly normalized the increased RhoA/ROCK signaling pathway activity, which was associated with inhibition of caspase-3 activation and HMG-CoA reductase overexpression and preservation of sGC expression.
A number of in vivo studies from several laboratories, including our own, indicate that activation of RhoA/ROCK signaling contributes significantly to the pathogenesis of various experimental models of PH by mediating sustained abnormal vasoconstriction and promoting vascular inflammation and remodeling (11, 14–17, 24, 35, 38, 40). We found in this study that DHEA markedly attenuated PH in pneumonectomized rats injected with MCT, a rat model of PH that more closely mimics the pathology of human severe PH than conventional models (42, 44, 47, 59), and the attenuation was associated with an inhibition of RhoA/ROCK activity. These results suggest that inhibition of RhoA/ROCK signaling pathway activity may be a major component of the striking protective effect of DHEA against PH, although other mechanisms may also be involved (7, 18, 46).
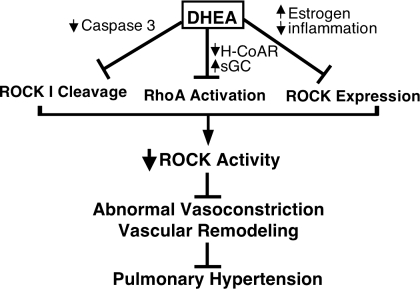
A novel, pharmacologically interesting, and potentially clinically important finding of this study is that DHEA prevented development of MCT-induced severe PH in pneumonectomized rats at least partly via inhibition of the RhoA/ROCK signaling pathway, which was apparently due to multiple different mechanisms (Fig. 7). First, DHEA treatment inhibited MCT-induced activation of caspase-3 (15, 61) and ROCK I cleavage (constitutive activation). It was recently reported that DHEA inhibits activation of caspase-3 in endothelial cells (33), and ROCK I has been shown to be cleaved and activated by caspase-3, i.e., active caspase-3 removes the COOH-terminal inhibitory domain of ROCK I, resulting in constitutive kinase activity that is independent of RhoA activation, in cardiac myocytes and Jurkat cells (9, 55). Since caspase-3-mediated constitutive activation of ROCK I is associated with membrane blebbing and apoptosis (9, 55), and since PA endothelial cell apoptosis may be an early key step in the pathogenesis of severe PH (36), including MCT-induced PH in rats (61), it is tempting to speculate that constitutive activation of PA endothelial cell ROCK I may play a significant role in the early stages of the disease and could be a new therapeutic target.
Fig. 7.
Possible mechanisms of protection by DHEA against pulmonary hypertension via inhibition of RhoA/ROCK signaling pathway.
Second, DHEA treatment prevented activation of RhoA (membrane RhoA expression), which might have involved both inhibition of HMG-CoA reductase expression and upregulation of sGC expression. It is known that inhibition of HMG-CoA reductase, by statins for instance, reduces the isoprenylation of RhoA and thereby prevents its translocation from cytosol to cell membrane (53). Similarly, the nitric oxide/sGC/cGMP/protein kinase G pathway phosphorylates RhoA, which also inhibits the membrane translocation of GTP-bound RhoA (16). DHEA has been observed to inhibit HMG-CoA reductase activity and gene expression (48, 54), and we (46) have recently reported that it increases sGC protein expression in rat PAs. We found in this study that MCT caused a marked increase in HMG-CoA reductase and a slight decrease in sGC protein expression, and DHEA prevented these changes. Although little is known about the regulation of HMG-CoA reductase expression, there is evidence for its upregulation by inflammatory cytokines, especially IL-6 (41). Since the expression of IL-6 is increased in MCT-induced hypertensive rat lungs (6), we speculate that inflammation might account for the increased HMG-CoA reductase in the hypertensive lungs of MCT-injected pneumonectomized rats.
Third, DHEA treatment reduced the MCT-induced increased ROCK II expression. The increased ROCK II expression in hypertensive lungs from MCT-injected pneumonectomized rats is possibly due to MCT-induced inflammation, since recent studies suggest that reactive oxygen species and NF-κB are involved in the activation and expression of ROCK (4, 21, 27, 28). We speculate that DHEA treatment inhibited the ROCK II upregulation by its antioxidant/anti-inflammatory (including inhibition of NF-κB) effects (2, 25).
Consistent with earlier studies (19, 46, 60), chronic DHEA treatment caused a loss of body weight. Whereas some studies observe decreased food consumption during chronic DHEA treatment (8), others do not and suggest that an alteration in fat metabolism may be the cause of the weight loss (19). A previous study has shown that severe dietary restriction (reduction of total caloric intake) and resultant decreases in polyamines and DNA syntheses protect against the development of MCT-induced PH (17). However, this mechanism is unlikely to be involved in the protective effect or DHEA because we did not find any difference in food consumption between the DHEA-treated and -untreated groups.
DHEA can be metabolized to estrogens and androgens. In fact, we detected higher plasma levels of estradiol and testosterone in DHEA-treated than in DHEA-untreated animals. Estradiol has been shown to protect against MCT-induced PH (12). Our study did not exclude a possible role of estradiol in the protective effects of DHEA. For example, the increased levels of estradiol in DHEA-treated rats may have played a role in inhibiting the MCT-induced ROCK II upregulation and/or ROCK activation, since recent studies indicate that estrogen inhibits ROCK expression/activity (10, 22, 23, 32). However, two lines of indirect evidence suggest it is unlikely the increased levels of estradiol had a major impact. First, although the levels of estradiol in DHEA-treated rats were increased (∼13.0 to ∼15.0 pg/ml), they were still far below the levels achieved in the study by Farhat and colleagues (∼8.1 to ∼14.6 ng/ml; Ref. 12). Second, in our previous study (46), we tested effects of a lower DHEA dose (0.3%) on hypoxic PH and found that the treatment inhibited the development of hypoxic PH without increasing the plasma estradiol levels.
DHEA has been approved by the United States Food and Drug Administration as a food supplement and used extensively in human studies without any major side effects. Although several clinical trials have provided evidence that even pharmacological doses (up to 2,250 mg/day for 12 wk) of DHEA have no serious adverse effects (58), there is a concern that long-term high-dose DHEA treatments may promote hormone-dependent cancer (such as breast cancer in women and prostate cancer in men), because DHEA can be metabolized to both estrogens and androgens. On the other hand, there are reports that suggest DHEA is a potent inhibitor of cancer induction in experimental models, including prostate and breast cancer (51, 56). Therefore, it is presently unknown whether high effective doses of DHEA would actually promote such cancer. Nevertheless, careful monitoring for plasma sex steroid hormone levels as well as sex steroid-dependent cancer development will be required in long-term high-dose DHEA treatments. Alternatively, future studies should evaluate the effectiveness of the synthetic analogs of DHEA such as 16α-fluoro-5-androsten-17-one, which have minimal sex steroid activity but retain the antiproliferative and cancer preventive activity of DHEA (34).
A limitation of this study is that since whole lung protein expression was measured, we are uncertain of where, which lung and/or vascular cells, the various changes in protein expression occurred. Future studies should clarify this point by using techniques such as laser capture microdissection and immunohistochemistry.
In summary, this study showed that chronic DHEA treatment effectively prevented development of MCT-induced severe PH and that inhibition of RhoA/ROCK signaling, apparently by multiple mechanisms, including inhibition of ROCK I cleavage, was involved in the impressive protective effects of DHEA. DHEA treatment also prevented the progression of MCT-induced severe PH and dramatically improved survival. These results suggest that DHEA may be a useful oral treatment for severe PH. Our novel finding that the cleavage and constitutive activation of ROCK I is involved in activation of ROCK in the hypertensive lungs of MCT-injected pneumonectomized rats may lead to identification of new mechanisms of ROCK activation in human severe PH.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-14985 and HL-07171 and the American Heart Association Grants SDG-0335208N and BGIA-0765477Z.
Acknowledgments
We thank Kenneth G. Morris for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94: 385–393, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Aragno M, Mastrocola R, Brignardello E, Catalano M, Robino G, Manti R, Parola M, Danni O, Boccuzzi G. Dehydroepiandrosterone modulates nuclear factor-kappaB activation in hippocampus of diabetic rats. Endocrinology 143: 3250–3258, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest 131: 1917–1928, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E, Goodman-Gruen D. The epidemiology of DHEAS and cardiovascular disease. Ann NY Acad Sci 774: 259–270, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava A, Kumar A, Yuan N, Gewitz MH, Mathew R. Monocrotaline induces interleukin-6 mRNA expression in rat lungs. Heart Dis 1: 126–132, 1999. [PubMed] [Google Scholar]
- 7.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 100: 9488–9493, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalina F, Milewich L, Kumar V, Bennett M. Dietary dehydroepiandrosterone inhibits bone marrow and leukemia cell transplants: role of food restriction. Exp Biol Med (Maywood) 228: 1303–1320, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA 103: 14495–14500, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrissobolis S, Budzyn K, Marley PD, Sobey CG. Evidence that estrogen suppresses rho-kinase function in the cerebral circulation in vivo. Stroke 35: 2200–2205, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol 110: 719–723, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girgis RE, Li D, Zhan X, Garcia JG, Tuder RM, Hassoun PM, Johns RA. Attenuation of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Heart Circ Physiol 285: H938–H945, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimoda L, Tuder RM, Johns RA, Hassoun PM. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol 292: L1105–L1110, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Guerard P, Rakotoniaina Z, Goirand F, Rochette L, Dumas M, Lirussi F, Bardou M. The HMG-CoA reductase inhibitor, pravastatin, prevents the development of monocrotaline-induced pulmonary hypertension in the rat through reduction of endothelial cell apoptosis and overexpression of eNOS. Naunyn Schmiedebergs Arch Pharmacol 373: 401–414, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol 146: 1010–1018, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacker AD Dietary restriction, polyamines and monocrotaline-induced pulmonary hypertension. Biochem Pharmacol 45: 2475–2481, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Hampl V, Bibova J, Povysilova V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J 21: 862–865, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hansen PA, Han DH, Nolte LA, Chen M, Holloszy JO. DHEA protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am J Physiol Regul Integr Comp Physiol 273: R1704–R1708, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294: L24–L33, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Hiroki J, Shimokawa H, Higashi M, Morikawa K, Kandabashi T, Kawamura N, Kubota T, Ichiki T, Amano M, Kaibuchi K, Takeshita A. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Cell Cardiol 37: 537–546, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hiroki J, Shimokawa H, Mukai Y, Ichiki T, Takeshita A. Divergent effects of estrogen and nicotine on Rho-kinase expression in human coronary vascular smooth muscle cells. Biochem Biophys Res Commun 326: 154–159, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Hong SK, Yang JH, Kim TB, Kim SW, Paick JS. Effects of ovariectomy and oestrogen replacement on the function and expression of Rho-kinase in rat bladder smooth muscle. BJU Int 98: 1114–1117, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Hyvelin JM, Howell K, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N. Dehydroepiandrosterone-sulfate inhibits nuclear factor-kappaB-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin Endocrinol Metab 89: 3449–3454, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Jin L, Ying Z, Hilgers RH, Yin J, Zhao X, Imig JD, Webb RC. Increased RhoA/Rho-kinase signaling mediates spontaneous tone in aorta from angiotensin II-induced hypertensive rats. J Pharmacol Exp Ther 318: 288–295, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Kao PN Simvastatin treatment of pulmonary hypertension: an observational case series. Chest 127: 1446–1452, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Laudi S, Trump S, Schmitz V, West J, McMurtry IF, Mutlak H, Christians U, Weimann J, Kaisers U, Steudel W. Serotonin transporter protein in pulmonary hypertensive rats treated with atorvastatin. Am J Physiol Lung Cell Mol Physiol 293: L630–L638, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Xia W, Li A, Zhao C, Sun R. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res 55: 64–71, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Lin AD, Levin RM, Kogan BA, Whitbeck C, Leggett RE, Kearns C, Mannikarottu A. Alteration of contractile and regulatory proteins in estrogen-induced hypertrophy of female rabbit bladder. Urology 68: 1139–1143, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Gαi protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology 148: 3068–3076, 2007. [DOI] [PubMed] [Google Scholar]
- 34.McCormick DL, Johnson WD, Kozub NM, Rao KV, Lubet RA, Steele VE, Bosland MC. Chemoprevention of rat prostate carcinogenesis by dietary 16alpha-fluoro-5-androsten-17-one (fluasterone), a minimally androgenic analog of dehydroepiandrosterone. Carcinogenesis 28: 398–403, 2007. [DOI] [PubMed] [Google Scholar]
- 35.McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, Van Vliet T, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol 294: L205–L213, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Michelakis ED Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: heterogeneous BMP signaling may have therapeutic implications. Circ Res 98: 172–175, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, Ozaki H. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol 25: 2335–2342, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol 100: 996–1002, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, Oka M. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287: L665–L672, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura F, Taniguchi A, Yamaguchi-Morimoto M, Soga Y, Iwamoto Y, Kokeguchi S, Kuroe A, Fukushima M, Nakai Y, Seino Y. Periodontal infection and dyslipidemia in type 2 diabetics: association with increased HMG-CoA reductase expression. Horm Metab Res 38: 530–535, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med 166: 1403–1408, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura T, Faul JL, Berry GJ, Veve I, Pearl RG, Kao PN. 40-O-(2-hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med 163: 498–502, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 108: 1640–1645, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res 74: 377–387, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 151: 1019–1025, 1997. [PMC free article] [PubMed] [Google Scholar]
- 48.Pascale RM, Simile MM, De Miglio MR, Nufris A, Seddaiu MA, Muroni MR, Danni O, Rao KN, Feo F. Inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase activity and gene expression by dehydroepiandrosterone in preneoplastic liver nodules. Carcinogenesis 16: 1537–1542, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43: 25S–32S, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Porsova-Dutoit I, Sulcova J, Starka L. Do DHEA/DHEAS play a protective role in coronary heart disease? Physiol Res 49, Suppl 1: S43–S56, 2000. [PubMed] [Google Scholar]
- 51.Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE, Kelloff GJ, McCormick DL. Chemoprevention of rat prostate carcinogenesis by early and delayed administration of dehydroepiandrosterone. Cancer Res 59: 3084–3089, 1999. [PubMed] [Google Scholar]
- 52.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4: 446–456, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res 97: 1232–1235, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulz S, Nyce JW. Inhibition of protein isoprenylation and p21ras membrane association by dehydroepiandrosterone in human colonic adenocarcinoma cells in vitro. Cancer Res 51: 6563–6567, 1991. [PubMed] [Google Scholar]
- 55.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol 3: 346–352, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Shilkaitis A, Green A, Punj V, Steele V, Lubet R, Christov K. Dehydroepiandrosterone inhibits the progression phase of mammary carcinogenesis by inducing cellular senescence via a p16-dependent but p53-independent mechanism. Breast Cancer Res 7: R1132–R1140, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 291: L668–L676, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Tummala S, Svec F. Correlation between the administered dose of DHEA and serum levels of DHEA and DHEA-S in human volunteers: analysis of published data. Clin Biochem 32: 355–361, 1999. [DOI] [PubMed] [Google Scholar]
- 59.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L583–L590, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Yen TT, Allan JA, Pearson DV, Acton JM, Greenberg MM. Prevention of obesity in Avy/a mice by dehydroepiandrosterone. Lipids 12: 409–413, 1977. [DOI] [PubMed] [Google Scholar]
- 61.Zhao YD, Campbell AI, Robb M, Ng D, Stewart DJ. Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ Res 92: 984–991, 2003. [DOI] [PubMed] [Google Scholar]