Abstract
Epidemiologic and animal studies have shown that exposure to particulate matter air pollution (PM) is a risk factor for the development of atherosclerosis. Whether PM-induced lung and systemic inflammation is involved in this process is not clear. We hypothesized that PM exposure causes lung and systemic inflammation, which in turn leads to vascular endothelial dysfunction, a key step in the initiation and progression of atherosclerosis. New Zealand White rabbits were exposed for 5 days (acute, total dose 8 mg) and 4 wk (chronic, total dose 16 mg) to either PM smaller than 10 μm (PM10) or saline intratracheally. Lung inflammation was quantified by morphometry; systemic inflammation was assessed by white blood cell and platelet counts and serum interleukin (IL)-6, nitric oxide, and endothelin levels. Endothelial dysfunction was assessed by vascular response to acetylcholine (ACh) and sodium nitroprusside (SNP). PM10 exposure increased lung macrophages (P < 0.02), macrophages containing particles (P < 0.001), and activated macrophages (P < 0.006). PM10 increased serum IL-6 levels in the first 2 wk of exposure (P < 0.05) but not in weeks 3 or 4. PM10 exposure reduced ACh-related relaxation of the carotid artery with both acute and chronic exposure, with no effect on SNP-induced vasodilatation. Serum IL-6 levels correlated with macrophages containing particles (P = 0.043) and ACh-induced vasodilatation (P = 0.014 at week 1, P = 0.021 at week 2). Exposure to PM10 caused lung and systemic inflammation that were both associated with vascular endothelial dysfunction. This suggests that PM-induced lung and systemic inflammatory responses contribute to the adverse vascular events associated with exposure to air pollution.
Keywords: air pollution, alveolar macrophage, systemic inflammation, interleukin-6
over the past two decades, numerous epidemiologic studies have demonstrated a strong and consistent dose-dependent relationship between ambient air pollution exposure and morbidity and mortality (2, 4, 6). The most consistent association is between particles smaller than 10 μm (PM10) and mortality (6). Surprisingly, most of the events are not respiratory but cardiovascular in nature (13). The mechanisms linking PM10 exposure and cardiovascular disease have not been fully elucidated. Nemmar and colleagues (18) have postulated that inhaled fine particulate matter translocates directly into the systemic circulation through the pulmonary capillary bed, where it promotes atherothrombosis by breaching endothelial integrity and inciting a local inflammatory reaction. However, just a very small fraction of these fine and ultrafine particles accumulate in extrapulmonary organs such as the liver and the spleen (16), and currently there is no convincing evidence that these fine particles physically deposit in blood vessels. An alternate possibility is that the ambient particles elicit a strong inflammatory reaction in the lungs and proinflammatory mediators spill over into the systemic circulation (4). This systemic inflammation is known to contribute to endothelial dysfunction in the coronary vasculature, a pivotal step in the pathogenesis of acute coronary events (3). We postulate that the lung inflammation induced by exposure to ambient particulate matter air pollution relates to the processing of particles by alveolar macrophages, the downstream systemic inflammatory response, and endothelial dysfunction. The goal of the present study was to determine the relationship between lung inflammation, systemic inflammation, and endothelial function after exposure to ambient particulate matter, using a well-characterized rabbit model.
METHODS
Animal Model
Animals.
This study was approved by the Animal Experimentation Committee of the University of British Columbia. The acute study was based on 15 female New Zealand White rabbits with an average weight of 2.4 ± 0.2 kg (Charles River Laboratories, Montreal, QC, Canada), and the chronic study was based on 16 female New Zealand White rabbits with an average weight of 2.7 ± 0.3 kg (Covance Research Products, Denver, PA). All animals were 12 wk old at the start of the experimental protocol and were fed standard rabbit chow.
Urban air particles.
The animals were exposed to urban air PM10 (EHC-93), which was collected in 1993 over Ottawa, Canada, with filters with a nominal cutoff of 0.3 μm, from a single-pass air filtration system of the Environmental Health Centre in Ottawa (100% outdoor air) (29). The particles had a mean diameter of 0.8 ± 0.4 μm (mean ± SD), with 99% (in number, not mass) of particles <3.0 μm (29).
Experimental Protocol
The rabbits were anesthetized with 7 mg/kg ketamine and 5 mg/kg xylazine by intramuscular injection assisted by 2.5% isoflurane. For the acute experiments, animals (n = 9) were exposed to PM10 (2.6 mg/kg) suspended in saline (1 ml) or saline alone (n = 6) by intrapharyngeal instillation three times (1st, 3rd, and 5th days). For the chronic experiments, animals (n = 8) were exposed to PM10 (2 mg/kg) by intrapharyngeal instillation of particles suspended in saline (1 ml), while the control animals (n = 8) were exposed only to saline (1 ml) with the same method, twice weekly for 4 wk with a model we have previously described in detail (17). The rabbits were euthanized after the final instillations with an overdose of pentobarbital sodium.
Measurement of Vascular Tone
After euthanasia, the carotid arteries were harvested and isolated from the rabbits and placed in ice-cold physiological salt solution (PSS). Without damaging the endothelium, the vessels were carefully cleaned of connective tissue, sectioned into 2-mm rings, and mounted on a wire myograph (model 610M; Danish Myo Technology, Aarhus, Denmark). Each vessel was bathed in oxygenated PSS at 37°C for an hour, during which time the resting tension was gradually increased to 10 mN with three changes of PSS at 10-min intervals followed by stabilization of the vessels at resting tension (10 mN) for 30 min. Thereafter the vessels were twice stimulated with 80 mM KCl. The relaxant responses to acetylcholine (ACh) and sodium nitroprusside (SNP) were determined after precontraction with phenylephrine (PE, 10 μM). Once sustained contraction was evoked, cumulative concentrations of ACh (10 nM–0.1 mM) were applied to evaluate endothelium-dependent nitric oxide (NO)-mediated vasorelaxation. An identical protocol was used to study the effects of SNP (10 nM-0.1 mM) to determine endothelium-independent (NO mediated) relaxation.
Solutions and Chemicals
PSS consisted of the following (in mM): 119 NaCl, 4.7 KCl, 1.18 KH2PO4, 24 NaHCO3, 1.17 MgSO4·7H2O, 1.6 CaCl2, 5.5 glucose, and 0.026 EDTA. All reagents were purchased from Sigma (St. Louis, MO).
Histological Studies of the Lung
After euthanasia, the lungs were harvested from the rabbits. The left lung was inflated by instilling 10% phosphate-buffered formalin through the trachea at 25 cmH2O. After full inflation, the trachea was ligated and the lung was suspended in 10% phosphate-buffered formalin for 24 h. The next day, the lung was divided into four equal parts and embedded in paraffin. The right lung was inflated by instilling OCT (Tissue-Tek, Sakura Finetek, Torrance, CA) diluted 1:1 with saline through the trachea at 25 cmH2O and suspended over liquid nitrogen until frozen. These tissue samples were then stored in −80°C conditions.
Morphometric Studies of the Lung
Paraffin-embedded lung tissue was sectioned 4 μm thick, mounted on glass slides, and stained with hematoxylin and eosin (H & E). On each slide, the lung tissue was visually divided into four equal parts. Two images were then randomly taken from each section under ×100 magnification. The images were captured with a spot digital camera (Microspot; Nikon, Tokyo, Japan) and were examined by a single investigator, who was blinded to the experimental condition used in the rabbit in question.
Quantitative Analysis of Lung Inflammation
Volume fractions of macrophages and neutrophils were determined with a modified stereological analysis described by Cruz-Orive and Weibel (5). The coded images were analyzed with a point-counting grid that was superimposed onto the captured images (Image Pro Plus; Media Cybernetics, Silver Spring, MD). The volume fractions of alveolar macrophages, tissue macrophages, alveolar polymorphonuclear leukocytes (PMN), and tissue PMN were calculated as the number of grid points that superimposed on the target cell divided by the total number of grid points on the screen. As for positive and activated alveolar macrophages, we counted 400 macrophages on each slide at ×400 magnification and calculated the proportion of alveolar macrophages that contained particulate matter. The latter were defined as positive macrophages. Activated macrophages were defined as macrophages that stained positively with CD11b antibodies.
Immunohistochemical Staining of the Lung
Paraffin-embedded lung tissue.
The tissue was sectioned 4 μm thick, and the sections were mounted on glass slides. Serial sections were cut and then stained: one section was stained with H & E, one with mouse anti-rabbit monoclonal antibody RAM11 (DAKO, Mississauga, ON, Canada) to identify macrophages, one with mouse anti-rabbit monoclonal antibody NP-5 (Abcam, Cambridge, MA) to identify PMN, and one with mouse monoclonal antibody to CD11b (clone 198; Serotec, Raleigh, NC) to identify activated macrophages. The three-step avidin-biotin complex (ABC) staining method was used for immunohistochemical labeling of the cells according to the method outlined by Hsu et al. (11). Briefly, the slides were deparaffinized and then autoclaved in Citra Antigen Retrieval Buffer, pH 6.0 (BioGenex, San Ramon, CA) for 22 min at 121°C. Sections were then treated with Protein Serum Free Block (DAKO) for 15 min. All antibodies were diluted in Tris-buffered saline (TBS), pH 7.6 with 1% bovine serum albumin, and all incubations were carried out at room temperature. Primary antibodies were used at 3, 5, and 20 μg/ml, respectively, and applied to sections for 1 h. Sections were washed with TBS and then incubated in biotinylated goat anti-mouse (Abcam) at a concentration of 20 μg/ml for 30 min. After washing, the sections were incubated in ABC-AP (Dako) for 30 min. Sections were then washed and incubated for 20 min with New Fuchsin/AS-BI Naphthol as a substrate to detect binding of the antibodies. resulting in a pink/red deposit. Sections were counterstained with Gill hematoxylin.
Frozen tissue.
Frozen tissue was sectioned at 8 μm and mounted on glass slides. Sections were then fixed in a −70°C solution of methanol-acetone (1:1) for 10 min, followed by blocking with Protein Block as above. These sections were incubated with goat anti-mouse monoclonal antibody to IL-6 (AF406NA; R&D Systems, Minneapolis, MN) at a concentration of 0.1 mg/ml. Subsequent labeling was performed with ABC as previously described. For confocal microscopy, frozen sections were labeled with RAM11 (same concentration as above) and monoclonal rat CD11b (Abcam; concentration 20 μg/ml) to colocalize these two antigens. After blocking and primary antigen incubation, the sections were incubated with Alexa Fluor 594-tagged goat anti-mouse IgG and Alexa Fluor 488-tagged goat anti-rat IgG (Molecular Probes, Eugene, OR) for 30 min. Slides were then incubated in DAPI for 2 min as counterstain for the nuclei.
Blood Count and Measurements of IL-6, NO, and Endothelin
Blood samples were obtained weekly from the central ear artery before each PM10 or saline instillation. Each sample was separated into two components. One was for the blood count for white blood cells (WBC) and PMN. Another was centrifuged, and the sera were stored at −80°C for measurement of circulating IL-6, NO, and endothelin.
WBC and differential leukocyte counts were determined on an Abbott Diagnostics Cell-Dyn 3700 instrument (Abbott Park, IL) calibrated for analysis for rabbit blood cell counts.
From the serum samples, IL-6 was measured with a human IL-6 ELISA Kit (12-MKL6-1; Alpco Diagnostics, Salem, NH), NO was measured with a Total NO/Nitrite/Nitrate Assay Kit (KGE001; R&D Systems), and endothelin was measured with an Endothelin ELISA Kit (04-BI-20082; Alpco Diagnostics).
Statistical Analysis
All results are expressed as means ± SE. Data on inflammation were analyzed with SAS 9.1 and R 2.3, using t-tests, linear regression, or a mixed linear effects model as appropriate. Owing to the longitudinal nature of the serum measurements, we applied a mixed effects model for serum IL-6 analysis. Data on vascular function were analyzed with NCSS 2000 and PASS 2000 software, using analysis of variance (ANOVA) and/or repeated-measures ANOVA. Bonferroni's correction factor was applied to adjust for multiple comparisons. All results of statistical tests were considered statistically significant at P < 0.05 (2-tailed).
RESULTS
PM10 and Lung Inflammation
Particles were observed in alveolar macrophages in both the PM10-exposed and control groups. However, there were more macrophages (P = 0.024), alveolar macrophages (P = 0.011), positive alveolar macrophages (i.e., macrophages that contained a particulate matter in the cytoplasm) (Fig. 1A; P = 0.001), and activated alveolar macrophages (i.e., macrophages that had CD11b expression) (Fig. 1, E and F; P = 0.006) in the PM10-exposed group than in the control group (see Table 1). There were fewer tissue macrophages in the PM10-exposed group than in the control group (P = 0.024). There were no significant differences in the PMN count in either the tissue or the air spaces between the two groups.
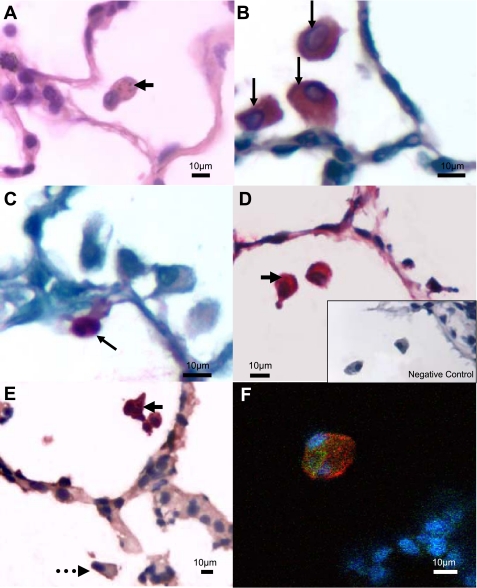
Fig. 1.
Microphotographs of lung tissues of exposed rabbits. A: lung section stained with hematoxylin and eosin, showing an alveolar macrophage containing PM10 particles (arrow). B: macrophage-specific staining with a RAM11 antibody (arrows). C: neutrophil-specific staining with an NP5 antibody (arrow). D: alveolar macrophages produce interleukin (IL)-6-containing PM10 particles (arrow); inset, negative control. E: activated macrophage labeled with CD11b antibody that contains a PM10 particle (solid arrow); dotted arrow represents a macrophage that does not contain a PM10 particle. F: confocal image illustrating colocalization of RAM11 (red) and CD11b (green) antibodies in the same cell; blue represents nuclei stained with DAPI.
Table 1.
Volume fraction of inflammatory cells in lungs of rabbits exposed to PM10 for 4 wk
| Group | Control | PM10 | P Value |
|---|---|---|---|
| Alveolar macrophage | 0.0246±0.0034 | 0.0475±0.0063 | 0.011 |
| Positive alveolar macrophage | 0.0091±0.0013 | 0.0213±0.0023 | 0.001 |
| Activated alveolar macrophage | 0.0158±0.0022 | 0.0314±0.0042 | 0.006 |
| Tissue macrophage | 0.0070±0.0005 | 0.0027±0.0010 | 0.003 |
| Total macrophage | 0.0316±0.0036 | 0.0502±0.0060 | 0.024 |
| Alveolar PMN | 0.0037±0.0006 | 0.0045±0.0002 | 0.264 |
| Tissue PMN | 0.0143±0.0029 | 0.0095±0.0017 | 0.187 |
| Total PMN | 0.0180±0.0035 | 0.0140±0.0018 | 0.339 |
Data are means ± SE. Rabbits were exposed to EHC-93 [particulate matter smaller than 10 μm (PM10)] twice a week for 4 wk; total 16 mg/kg. PMN, polymorphonuclear leukocyte. Volume fraction of positive alveolar macrophages was calculated by multiplying the volume fraction of alveolar macrophages by the proportion of positive macrophages that contained particulate matter in cytoplasm by ×400 magnification. Volume fraction of activated alveolar macrophages (positive immunohistochemical stain for CD11b) was calculated by multiplying the volume fraction of alveolar macrophages by the proportion of activated macrophages. P values are given for the row comparison between control and PM10 groups.
Circulating Biomarkers for Systemic Inflammation
Circulating WBC and PMN were determined over the 4 wk of the experimental protocol and are summarized in Supplemental Fig. S1.1 In the first week of the experiment relative to baseline, WBC and PMN were significantly higher in the PM10-exposed group than in the control group. However, by weeks 2, 3, and 4, no significant differences were noted. The increase in counts in the first week correlates with the number of activated alveolar macrophages (R2 = 0.254; P = 0.079) and impaired maximal vasodilatory responses in the carotid arteries to ACh (R2 = 0.259; P = 0.076).
Serum IL-6 levels over the 4 wk of the experimental protocol are summarized in Table 2. The lower limit of detection for the assay was 1.2 pg/ml. In the first 2 wk of the experiment, serum IL-6 levels were significantly higher in the PM10-exposed group than in the control group. However, by weeks 3 and 4, no significant differences were noted. There were no significant differences in those two groups in serum NO and endothelin levels across the 4 wk. The notable exception was at week 1, when serum NO levels were higher in the PM10-exposed group (see Supplemental Table S1).
Table 2.
Serum circulating IL-6 levels induced by exposure to PM10 for 4 wk
| Group |
Control |
PM10
|
P Value | ||
|---|---|---|---|---|---|
| Absolute | Relative to Baseline | Absolute | Relative to Baseline | ||
| Baseline | 4.49±0.71 | 0 | 2.89±0.12 | 0 | |
| IL-6 week 1 | 3.51±0.42 | −0.97±0.31 | 3.07±0.23 | 0.19±0.14 | 0.009 |
| IL-6 week 2 | 3.60±0.44 | −0.89±0.31 | 2.90±2.81 | 0.01±0.15 | 0.028 |
| IL-6 week 3 | 4.49±0.51 | 0.00±0.35 | 2.81±0.22 | −0.07±0.21 | 0.863 |
| IL-6 week 4 | 3.30±0.37 | −1.19±0.42 | 2.19±0.18 | −0.70±0.21 | 0.331 |
| Integrated | 34.33±4.07 | −6.04±2.49 | 25.29±1.19 | −0.69±0.51 | 0.076 |
All interleukin (IL)-6 data (×10−2 ng/ml)are means ± SE. Rabbits were exposed to EHC-93 (PM10) twice a week for 4 wk; total 16 mg/kg. Integrated, sum of all measurements of IL-6 across the 4 wk. P values comparing the relative difference in IL-6 levels from baseline between control and PM10 groups are given.
Influence on Vascular Endothelial Function
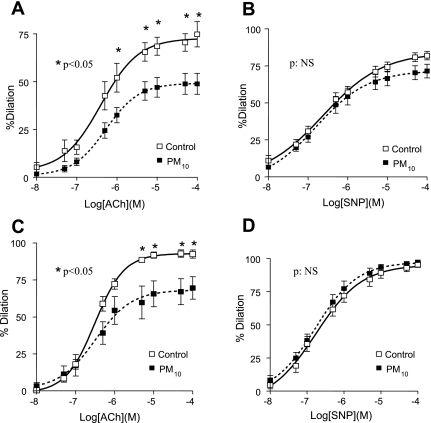
In the acute model, PM10 exposure significantly reduced ACh-stimulated relaxation (74.6 ± 16.0% vs. 48.9 ± 16.3%, control group vs. PM10 group, P < 0.05; Fig. 2A). Similarly, ACh-stimulated relaxation was also significantly reduced in the acute model (92.5 ± 3.4% vs. 69.6 ± 7.5%, control group vs. PM10 group, P < 0.05; Fig. 2C). To determine whether the impairment of ACh relaxation was due to the reduction of smooth muscle sensitivity to NO and/or to the impairment of smooth muscle function, we examined SNP-induced endothelium-independent vasorelaxation and smooth muscle contractility in response to 80 mM KCl and PE, respectively. PM10 exposure did not alter SNP-stimulated relaxation in either the acute model (81.9 ± 7.0% vs. 71.5 ± 13.8%, control vs. PM10; Fig. 2B) or the chronic model (95.2 ± 3.6% vs. 97.3 ± 2.9%, control vs. PM10; Fig. 2D) and also did not affect PE- and KCl-elicited vasocontraction (data not shown).
Fig. 2.
Relationship between PM10 exposure and acetylcholine (ACh)- and sodium nitroprusside (SNP)-stimulated vasodilatory responses in carotid arteries. A and B: carotid arterial endothelial dysfunction of a model of PM10, total dose 16 mg/kg over 4 wks (2 mg/kg twice a week). C and D: model of PM10; total dose 8 mg/kg over 5 days (2.67 mg/kg, 1st, 3rd, and 5th days). A: after PM10 exposure, the maximal ACh-stimulated vasodilatation was significantly attenuated (92.5 ± 3.4% vs. 69.6 ± 7.5%, control group vs. PM10 group; P < 0.05). B: maximal SNP-induced endothelium-independent vasodilatation was not affected by PM10 exposure [95.2 ± 3.6% vs. 97.3 ± 2.9%, control vs. PM10 group; not significant (NS)]. C: in acute model, which is designed to show earlier vessel function, ACh-stimulated relaxation was significantly reduced (74.6 ± 16.0% vs. 48.9 ± 16.3%, control group vs. PM10 group; P < 0.05). D: maximal SNP-induced endothelium-independent vasodilatation was not affected by PM10 exposure (81.9 ± 7.0% vs. 71.5 ± 13.8%, control vs. PM10 group).
Association Between Lung and Circulating Biomarkers
Overall, there was a significant relationship between the volume fraction of positive alveolar macrophages (R2 = 0.322; P = 0.034) as well as activated alveolar macrophages (R2 = 0.318; P = 0.036) and changes in serum IL-6 levels over the 4 wk. The relationship was strongest at week 1 (Supplemental Fig. S2) and then dissipated over the ensuing weeks. There was no statistically significant correlation between lung cells and the other biomarkers (data not shown).
Association Between Circulating Biomarkers and Vascular Endothelial Dysfunction
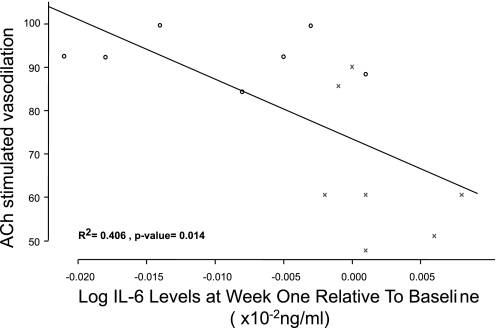
In the acute model, there was a significant inverse relationship between IL-6 levels and maximal relaxation responses to ACh (R2 = 0.477, P = 0.0269) supported by a significant inverse relationship between changes in serum IL-6 level both at week 1 (R2 = 0.406; P = 0.014; Fig. 3) and at week 2 (R2 = 0.372; P = 0.021) to the maximal relaxation responses to ACh in the chronic model. Together these data highlight the possible importance of systemic inflammation on endothelial function persisting for a prolonged period of time. There was no statistically significant correlation between vascular endothelial dysfunction and the other biomarkers (data not shown).
Fig. 3.
Relationship between systemic inflammation at week 1 and ACh-stimulated vasodilatory responses in carotid arteries. There was a significant negative correlation between the circulating serum IL-6 level at week 1 (relative to baseline) and carotid endothelial dysfunction (R2 = 0.406; P = 0.014). ○, data from control rabbits; ×, data from PM10-exposed rabbits.
Association Between Lung and Vascular Endothelial Dysfunction
There was a significant inverse relationship between both the volume fraction of positive alveolar macrophages (R2 = 0.363, P = 0.023; Supplemental Fig. S3A) and activated alveolar macrophages (R2 = 0.389; P = 0.017; Supplemental Fig. S3B) and the maximal response to ACh.
DISCUSSION
Although the relationship between PM10 exposure and cardiovascular events has been shown in numerous epidemiologic studies, the mechanism(s) by which this occurs remains largely unknown and an area of active investigation. We found that repeated instillation of PM10 particles over 4 wk induced lung inflammation, characterized mostly by infiltration and activation of macrophages in the air spaces, with concomitant reduction in tissue monocytes/macrophages. The magnitude of this macrophage-related inflammatory response in the lungs was associated with both systemic inflammation and endothelial dysfunction in the carotid arteries. Collectively, these data highlight the importance of lung inflammation in mediating adverse cardiovascular events following increase in ambient PM10 levels.
The model we have used to test our hypothesis has been established in our laboratory and is well characterized with regard to both to the local and the systemic response (17, 26). The particulate matter we used (EHC-93), ambient particles collected over Ottawa in 1993, has also been well characterized and chemically analyzed, is complex in nature, and contains >26 metals (30). Several components of particulate matter have been implicated in the adverse vascular effects of air pollution, including inorganic (e.g., sulfates and nitrates) and carbonaceous components of PM10 and metal components of PM10 (31, 32). This suggests that it is unlikely that a single component (e.g., metals such as zinc) is responsible for all its downstream biological/vascular effects. Our study was not designed to identify the key constituent(s) of PM10 responsible for the cardiopulmonary effects observed, and future studies are needed to address this critical question.
We have used an instillation method because it provides more accurate dosing, given that rabbits are nose breathers that filter most inhaled particles. With an estimated 5.9-m2 alveolar surface for a 2.5-kg rabbit, the calculated alveolar exposure was 4.3 ng/cm2 for each dose or 34.4 ng/cm2 over the entire experimental period in the chronic model and ∼50% of this exposure in the acute model. This chronic exposure is similar to that of a human exposed to 150 μg/m3 for ∼27 days, which occurred during the Southeast Asian forest fires of 1997 (28). For the acute exposure model we elected to expose rabbits to the same dose of EHC-93 that rabbits received during the first 2 wk of the chronic model (when IL-6 levels were elevated) spread over three exposures within a week. This exposure simulates an acute episode of exposure and is similar to exposures used previously by other investigators (1, 9, 10, 27).
We have used morphometric methods to quantify lung inflammation and enumerate inflammatory cells in the lung. We showed that PM10 produced a predominant macrophage/monocytic inflammatory response in the lung (Fig. 1). We previously showed (28) that macrophages exposed to the ambient particles (EHC-93) ex vivo produced a variety of proinflammatory cytokines including tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6, and IL-1β in a dose-dependent fashion. We suspect that these cytokines may in turn spill into the systemic circulation, inducing a state of systemic inflammation. In the same model, we showed previously that EHC-93 exposure produces a brisk bone marrow response, causing a rapid and persistent elevation in blood leukocyte and platelet counts. The intensity of the bone marrow response correlated with the amount of particles phagocytosed by alveolar macrophages in the lung, indicating a strong link between lung and systemic inflammation. These data are consistent with findings by Fukushima and colleagues (8). Using a guinea pig model, they found that TNF levels were over two times higher in the aorta compared with the right atrium after intratracheal instillation of Escherichia coli organisms caused by a significant spillage of TNF from the lungs into the systemic circulation. An alternate explanation is that PM10 particles translocate directly from the lung compartments into the systemic circulation, inducing systemic effects. A study from Bagate and colleagues (1) reported that particulate matter (EHC-93) decreased vasorelaxation of male spontaneously hypertensive rat (SHR) aorta, with the peak effect time at 4 h after instillation, which coincides with peak blood levels of soluble components such as transition metals (zinc and vanadium). This suggests that ultrafine particulate matter or at least soluble components of particles could enter the bloodstream and influence vessel function directly. Interestingly, they also reported that pulmonary inflammation reached a maximum at 24 h, and this coincides with impairment of vasorelaxation, suggesting that different mechanisms could operate at different times after exposure. Mills and colleagues (16) demonstrated that 95.6 ± 1.7% of 99mTc-radiolabeled carbon nanoparticles inhaled by 10 healthy volunteers were detected in the lungs at 6 h after inhalation, representing <3% of aerosolized nanoparticles translocated from the lungs into the systemic circulation. There are currently no in vivo data demonstrating that translocated particles themselves impact either blood cells or vessels in vivo. We suspect that either inflammatory mediators (such as IL-6 and TNF) translocate from the lung into the bloodstream or mediators released from circulating leukocytes (such as monocytes) interacting with translocated particles impact blood vessels, resulting in vascular events.
We have elected to use IL-6 and circulating leukocytes as biomarkers of the systemic response induced by PM10 exposure. Elevated circulating levels of IL-6 have been associated with the atherosclerosis burden (14) and acute vascular events (19, 21), and IL-6 is also expressed in walls of diseased blood vessels (24, 25), implicating a role for IL-6 in the pathogenesis of atherosclerosis. We took serial blood measurements over the 4 wk of PM10 exposure and showed IL-6 and PMN response in the first 2 wk after exposure, signifying an acute systemic inflammatory response. We previously showed (28) that the alveolar macrophages are potent producers of IL-6 when exposed ex vivo to EHC-93, supported by immunohistochemical staining for IL-6 in alveolar macrophages of rabbit lungs (Fig. 1D). The local lung inflammation as well as the impaired carotid endothelial function correlated best with circulating IL-6 in the first 2 wk (both acute and chronic models). These data suggest that the vascular dysfunction persists after IL-6 levels return back to baseline, supporting the notion of a more prolonged effect of PM10 exposure on blood vessels. The mechanism(s) of this prolonged vascular effect is unclear. Circulating endothelin and NO metabolites were not elevated at the 4 wk time point (Supplemental Table S1), and we speculate that persistent lung inflammation with the production of secondary mediators could be responsible for this prolonged vascular effect. Future studies are needed to address this important and novel observation.
Abnormal ACh-induced endothelium-dependent relaxation is an early manifestation of endothelial injury (20) and signals an abnormality in NO-mediated pathways and predicts increased risk for myocardial infarction and stroke (7, 15). Salient to our findings, Saura et al. (23) found that IL-6 has direct effects on endothelial cells, inhibiting endothelial nitric oxide synthase expression by modulating signal transducer and transactivator-3. Furthermore, Schieffer and colleagues (24) also showed IL-6 sequestration in diseased coronary blood vessel walls, and we postulate that via these mechanisms PM10 exposure reduced local expression of NO in the vascular endothelium, resulting in endothelial dysfunction. The positive relationship between IL-6 levels and endothelial dysfunction supports this notion. However, further studies are needed to establish a cause-and-effect relationship between PM10-induced increases in IL-6 and endothelial dysfunction.
We previously showed (26), using a similar exposure protocol in Watanabe heritable hyperlipidemic rabbits (which develop atherosclerosis naturally), that lung inflammation (notably infiltration of positive alveolar macrophages) was associated with an increased atherosclerotic burden, increased cellular content of the atherosclerotic lesions, and thinning of atherosclerotic caps. These data in conjunction with the present findings suggest that the lung and systemic inflammatory responses to PM10 are important contributors to vascular dysfunction and atherosclerosis. Numerous epidemiologic studies have shown an increase in cardiac events within days after an increase in ambient particulate matter (12, 22), and our study extended this observation by showing a prolonged downstream vascular effect following exposure to ambient particles, supporting our data on the contribution of chronic PM10 exposure to the progression of atherosclerosis (26).
In summary, repeated exposure to PM10 caused lung and systemic inflammation as well as endothelial dysfunction. The inflammatory responses in the lung were characterized predominantly by activation and infiltration of alveolar macrophages, and the systemic inflammatory response was characterized by an increase in circulating leukocytes and IL-6. These inflammatory responses were associated with endothelial dysfunction. We conclude that exposure to ambient particulate matter has a prolonged impact on blood vessels, and we speculate that this contributes to the vascular events associated with exposure to air pollution.
GRANTS
D. D. Sin and S. F. van Eeden are Senior Scholars with the Michael Smith Foundation for Medical Research. D. D. Sin is also supported by a Canada Research Chair, and S. F. van Eeden is an American Lung Association Career Investigator. N. Bai is a recipient of a scholarship from the Natural Sciences and Engineering Research Council of Canada and a studentship from the Michael Smith Foundation for Health Research. This work was supported by the British Columbia Lung Association, the Heart and Stroke Foundation of Canada, and the Canadian Institutes of Health Research.
Supplementary Material
Acknowledgments
The authors thank Gary Chiang, Reena Wei, Leanna Loy, and Mark Elliott for technical support. We also thank Dr. Renaud Vincent from Health Canada, who provided the EHC-93.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Bagate K, Meiring JJ, Gerlofs-Nijland ME, Vincent R, Cassee FR, Borm PJ. Vascular effects of ambient particulate matter instillation in spontaneous hypertensive rats. Toxicol Appl Pharmacol 197: 29–39, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bates DV Health indices of the adverse effects of air pollution: the question of coherence. Environ Res 59: 336–349, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Orive LM, Weibel ER. Sampling designs for stereology. J Microsc 122: 235–257, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Dockery DW, Pope CA 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med 329: 1753–1759, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol 31: 51–60, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima R, Alexander JW, Gianotti L, Ogle CK. Isolated pulmonary infection acts as a source of systemic tumor necrosis factor. Crit Care Med 22: 114–120, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Gerlofs-Nijland ME, Boere AJ, Leseman DL, Dormans JA, Sandstrom T, Salonen RO, van Bree L, Cassee FR. Effects of particulate matter on the pulmonary and vascular system: time course in spontaneously hypertensive rats. Part Fibre Toxicol 2: 2, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happo MS, Salonen RO, Halinen AI, Jalava PI, Pennanen AS, Kosma VM, Sillanpaa M, Hillamo R, Brunekreef B, Katsouyanni K, Sunyer J, Hirvonen MR. Dose and time dependency of inflammatory responses in the mouse lung to urban air coarse, fine, and ultrafine particles from six European cities. Inhal Toxicol 19: 227–246, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SM, Raine L, Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol 75: 734–738, 1981. [DOI] [PubMed] [Google Scholar]
- 12.Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, Rossi G, Zmirou D, Ballester F, Boumghar A, Anderson HR, Wojtyniak B, Paldy A, Braunstein R, Pekkanen J, Schindler C, Schwartz J. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology 12: 521–531, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med 173: 667–672, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WY, Allison MA, Kim DJ, Song CH, Barrett-Connor E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo Study). Am J Cardiol 99: 99–102, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315: 1046–1051, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, de la Fuente JM, Cassee FR, Boon NA, Macnee W, Millar AM, Donaldson K, Newby DE. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med 173: 426–431, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Mukae H, Vincent R, Quinlan K, English D, Hards J, Hogg JC, van Eeden SF. The effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med 163: 201–209, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med 164: 1665–1668, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 351: 2599–2610, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Quyyumi AA Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 105: 32S-39S, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767–1772, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med 343: 1742–1749, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Saura M, Zaragoza C, Bao C, Herranz B, Rodriguez-Puyol M, Lowenstein CJ. Stat3 mediates interleukin-6 inhibition of human endothelial nitric-oxide synthase expression. J Biol Chem 281: 30057–30062, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, Nussberger J, Harringer W, Drexler H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation 101: 1372–1378, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Sukovich DA, Kauser K, Shirley FD, DelVecchio V, Halks-Miller M, Rubanyi GM. Expression of interleukin-6 in atherosclerotic lesions of male ApoE-knockout mice: inhibition by 17beta-estradiol. Arterioscler Thromb Vasc Biol 18: 1498–1505, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol 39: 935–942, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med 176: 395–400, 2007. [DOI] [PubMed] [Google Scholar]
- 28.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM10). Am J Respir Crit Care Med 164: 826–830, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Vincent R, Bjarnason SG, Adamson IY, Hedgecock C, Kumarathasan P, Guenette J, Potvin M, Goegan P, Bouthillier L. Acute pulmonary toxicity of urban particulate matter and ozone. Am J Pathol 151: 1563–1570, 1997. [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent R, Goegan P, Johnson G, Brook JR, Kumarathasan P, Bouthillier L, Burnett RT. Regulation of promoter-CAT stress genes in HepG2 cells by suspensions of particles from ambient air. Fundam Appl Toxicol 39: 18–32, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol Lung Cell Mol Physiol 277: L1026–L1033, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Yamada H, Damiano VV, Tsang AL, Meranze DR, Glasgow J, Abrams WR, Weinbaum G. Neutrophil degranulation in cadmium-chloride-induced acute lung inflammation. Am J Pathol 109: 145–156, 1982. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.