Abstract
The structure and biosynthesis of poly-N-acetyllactosamine display a dramatic change during development and oncogenesis. Poly-N-acetyllactosamines are also modified by various carbohydrate residues, forming functional oligosaccharides such as sialyl Lex. Herein we describe the isolation and functional expression of a cDNA encoding β-1,3-N-acetylglucosaminyltransferase (iGnT), an enzyme that is essential for the formation of poly-N-acetyllactosamine. For this expression cloning, Burkitt lymphoma Namalwa KJM-1 cells were transfected with cDNA libraries derived from human melanoma and colon carcinoma cells. Transfected Namalwa cells overexpressing the i antigen were continuously selected by fluorescence-activated cell sorting because introduced plasmids containing Epstein–Barr virus replication origin can be continuously amplified as episomes. Sibling selection of plasmids recovered after the third consecutive sorting resulted in a cDNA clone that directs the increased expression of i antigen on the cell surface. The deduced amino acid sequence indicates that this protein has a type II membrane protein topology found in almost all mammalian glycosyltransferases cloned to date. iGnT, however, differs in having the longest transmembrane domain among glycosyltransferases cloned so far. The iGnT transcript is highly expressed in fetal brain and kidney and adult brain but expressed ubiquitously in various adult tissues. The expression of the presumed catalytic domain as a fusion protein with the IgG binding domain of protein A enabled us to demonstrate that the cDNA encodes iGnT, the enzyme responsible for the formation of GlcNAcβ1 → 3Galβ1 → 4GlcNAc → R structure and poly-N-acetyllactosamine extension.
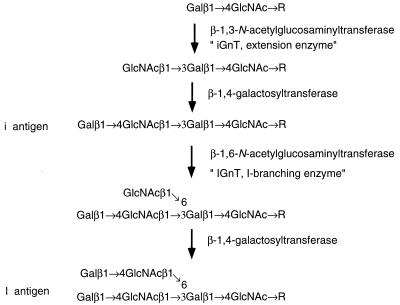
Poly-N-acetyllactosamine is a unique carbohydrate composed of N-acetyllactosamine repeats (Galβ1 → 4GlcNAcβ1 → 3)n. Poly-N-acetyllactosamines can be attached to N-glycans, O-glycans, or glycolipids and provide the backbone structure for additional modifications, which are very often cell-type-specific oligosaccharide structures (1–3). In humans, fetal erythrocytes express a linear poly-N-acetyllactosamine, Galβ1 → 4GlcNAcβ1 →3Galβ1 → 4GlcNAc → R, and adult erythrocytes express a branched poly-N-acetyllactosamine, Galβ1 → 4GlcNAcβ1 →3(Galβ1 → 4GlcNAcβ1 → 6)Galβ1 → 4GlcNAc → R (4, 5). These poly-N-acetyllactosamines are the determinants for the i and I antigen, which are the first human alloantigens shown to display developmental change (6, 7). For the synthesis of poly-N-acetyllactosamine, β-1,3-N-acetylglucosaminyltransferase (iGnT) is essential for the formation of the i antigen (Fig. 1). When I-forming β-1,6-N-acetylglucosaminyltransferase (IGnT) is also present with iGnT, the I antigen is synthesized instead of the i antigen (4, 8–14).
Figure 1.
Structure and biosynthesis of i and I antigens. The i antigen is synthesized by iGnT followed by β-1,4-galactosyltransferase. The i antigen is converted to the I antigen by the stepwise addition of a GlcNAc β1 → 6 and a Galβ1 → 4 residue (8). As an alternative pathway, another IGnT adds β-1,6-N-acetylglucosamine to GlcNAcβ1 → 3Galβ1 → 4GlcNAc → R precursor and this product, GlcNAcβ1 → 3(GlcNAcβ1 → 6)Galβ1 → 4GlcNAc → R, is converted to Galβ1 → 4GlcNAcβ1 → 3(Galβ1 → 4GlcNAcβ1 → 6)Galβ1 → 4GlcNAc → R, forming I antigen (9) (adapted from refs. 4 and 8–12).
In granulocytes, monocytes, and memory T lymphocytes, poly-N-acetyllactosamines carry sialyl Lex, NeuNAcα2 → 3Galβ1 → 4(Fucα1 → 3)GlcNAc → R, at their termini (15), which plays a critical role in recruiting leukocytes to inflammatory sites (16–19) and possibly when tumor cells adhere at metastatic sites (20–22). As an antigen specific to mouse embryo, stage specific embryonic antigen (SSEA)-1 antigen was identified as Galβ1 → 4(Fucα1 → 3)GlcNAc → R, which is present in poly-N-acetyllactosamines. By using anti-SSEA-1 antibody or oligosaccharides containing Galβ1 → 4(Fucα1 → 3)GlcNAc terminus as inhibitors, it was demonstrated that SSEA-1 might participate in adhesive events that are involved in compaction during embryogenesis (23, 24).
By expression cloning, we have isolated a cDNA encoding IGnT by using Chinese hamster ovary (CHO) cells containing polyoma large tumor antigen because CHO cells lack the I antigen (12). In contrast, it has been extremely difficult to clone cDNA encoding iGnT because this enzyme is ubiquitously present in various cells, although its abundance is not as high as β-1,4-galactosyltransferase (10, 11, 13, 14).
Recently, we have demonstrated that it is possible to clone GD3/GT3 synthase by using COS-1⋅GD3 cells by transient expression cloning (25). COS-1⋅GD3 cells synthesized a substantial amount of GD3 but only a small amount of GT3. A cDNA encoding GD3/GT3 synthase could be isolated from COS-1⋅GD3 cells that were overexpressing GD3 and GT3 after transfection (25). This cloning taught us that it is possible to clone a cDNA encoding iGnT as long as transfected cells become highly positive for iGnT. This is important because cells entirely lacking the i antigen were not available. For this expression cloning strategy, we have selected Namalwa KJM-1 cells that express Epstein–Barr virus nuclear antigen 1 (EBNA-1) (26–28). In cells expressing EBNA-1, plasmids containing the replication origin of Epstein–Barr virus oriP can be continuously amplified as episomes (29), thus allowing continuous selection of those cells overexpressing the i antigen. Herein we report this cloning strategy, resulting in the isolation of a cDNA encoding iGnT, which is essential for poly-N-acetyllactosamine synthesis.
EXPERIMENTAL PROCEDURES
Isolation of a Human iGnT cDNA Clone.
All of the various cell lines in our hands, including COS-1 and CHO cells, were found to be positive for the i antigen. We thus decided to clone iGnT by overexpressing iGnT in cells where the i antigen was present. For this, Namalwa KJM-1, a human Burkitt lymphoma cell line, was used as recipient cells. cDNA libraries derived from poly(A)+ RNA of human melanoma WM266-4 (26) and colonic carcinoma SW1116 (27) cell lines were constructed in pAMo vector containing oriP of the Epstein–Barr virus. Namalwa KJM-1 cells were then transfected with a mixture of the above cDNA libraries and the transfected cells were first selected in the presence of G418, because pAMo also contains a neomycin-resistance gene. After 13 days, the transfected cells were incubated with human anti-i antigen serum (Den; ref. 7) followed by fluorescein isothiocyanate-conjugated goat anti-human IgM. The i-antigen-positive cells were isolated by fluorescence-activated cell sorting (EPICS Elite flow cytometer, Coulter) and cultured for an additional 18 days. The transfected cells were sorted two more times by using the same procedure.
After the third sorting, plasmids were recovered by the Hirt procedure (30) from those cells that were highly positive for the i antigen expression. Among 18 clones of 75 plasmids recovered, 1 clone, 16-2-12, was found to increase the expression of the i antigen by a factor of 7 compared with the cells transfected with pAMo containing no cDNA insert. The cDNA in this plasmid was sequenced by the dideoxynucleotide chain-termination method (31). The cDNA insert in 16-2-12 was digested with HindIII and XmnI and cloned into the HindIII and EcoRV sites of pcDNA3.1 (Invitrogen), resulting in pcDNA3.1-iGnT.
Northern Blot Analysis of Various Human Tissues.
Human multiple tissue Northern blots of poly(A)+ RNA were purchased from CLONTECH, and these blots were hybridized with a gel-purified cDNA insert of pcDNA3.1-iGnT or pc-DNAI-IGnT (12) after labeling with [α-32P]dCTP by random-oligonucleotide priming (Prime-IT II labeling kit, Stratagene).
Construction and Expression of the Protein A-iGnT Fusion Vector.
The cDNA fragment encoding the stem region plus putative catalytic domain of iGnT was prepared by PCR using pcDNA3.1-iGnT as a template and fused with cDNA encoding a signal peptide sequence and the IgG binding domain of Staphylococcus aureus protein A (25–28). 5′ and 3′ primers for this PCR are 5′-CGGATCCACGGTCCGTGGACCAGGT-3′ and 5′-TCGCTCGAGGGCTCAGCAGCGTCGGG-3′ (BamHI and XhoI sites are underlined). The PCR product encoding amino acid residues 53–415 of iGnT was ligated into the BamHI and XhoI sites of pcDNAI-A (32), yielding plasmid pcDNAI-A⋅iGnTc. Plasmid pcDNAI-A and pcDNAI-A⋅iGnTc were separately transfected with LipofectAmine (GIBCO/BRL) into COS-1 cells as described (32), and 48 hr after transfection the medium was replaced with serum-free medium, macrophage-SFM (GIBCO/BRL) and cultured for an additional 24 hr. The chimeric iGnTc secreted into the culture medium was adsorbed to IgG-Sepharose 6FF (Pharmacia) and the enzyme bound to the beads was used as an enzyme source (33). Alternatively, the culture medium was concentrated by 10-fold and directly used as an enzyme source.
iGnT Assays and Product Characterization.
iGnT assays were performed essentially as described (34) except for a few modifications. In all assays, the reaction mixtures contained 0.1 mM UDP-[3H]GlcNAc (1 × 106 cpm/nmol), 20 mM MnCl2, 5 mM ATP, and 5 mM acceptor oligosaccharide, lacto-N-neotetraose, in a final volume of 100 μl of 100 mM cacodylate buffer (pH 7.0). In addition, 10 mM N-acetylglucosamino-1,5-lactone was included to inhibit breakdown of products by β-hexosaminidase(s) when the culture medium was used as an enzyme source. After a 10-hr incubation at 37°C, 0.3 ml of QAE-Sephadex was added to the reaction mixture. The supernatant recovered was again mixed with QAE-Sephadex, and its derived supernatant was applied to a column (1.0 × 120 cm) of Bio-Gel P-4 equilibrated with 0.1 M NH4HCO3. The above product, purified after Bio-Gel P-4 gel filtration, was incubated with β-1,4-galactosyltransferase (5 milliunits; Boehringer Mannheim) in the same reaction mixture as described above, except that UDP-[3H]GlcNAc was replaced with 0.5 mM UDP-[3H]Gal (1 × 106 cpm/5 nmol). α1-Acid glycoprotein (100 μg), desialylated by neuraminidase treatment, was also used as an acceptor. To analyze its product, α1-acid glycoprotein was precipitated by adding ethanol (90%, final concentration) to the reaction mixture and the centrifuged, and the precipitate after dissolving in 0.1 M NH4HCO3 was applied to a column (1.0 × 27 cm) of Sephadex G-50 (superfine). These biosynthetic products were digested with jack bean β-N-acetylglucosaminidase, β-galactosidase, or endo-β-galactosidase (35) and subjected to Bio-Gel P-2 gel filtration equilibrated with 0.1 M NH4HCO3.
RESULTS
Isolation of a cDNA Clone that Determines the Expression of the i Antigen.
Our preliminary results indicated that there is no cell line available that is deficient in poly-N-acetyllactosamine synthesis judging from the staining with human anti-i serum (Den; ref. 7) or tomato lectin, which reacts with poly-N-acetyllactosamine (36). By using tomato lectin, we also attempted to isolate CHO cells that are defective in iGnT without success. We thus decided to use Namalwa KJM-1 cells that express EBNA-1 as recipient cells for expression cloning. Namalwa KJM-1 cells have been used for cloning of α-2,3-sialyltransferase (ST3Gal IV), Fuc-TVII, and GD3 synthase (26–28). Besides, plasmids containing oriP continuously replicate as episomes in the presence of EBNA-1. This latter advantage allowed us to enrich Namalwa KJM-1 cells expressing an increased amount of iGnT without isolation of plasmids from sorted cells and retransfection of those plasmids into the recipient cells.
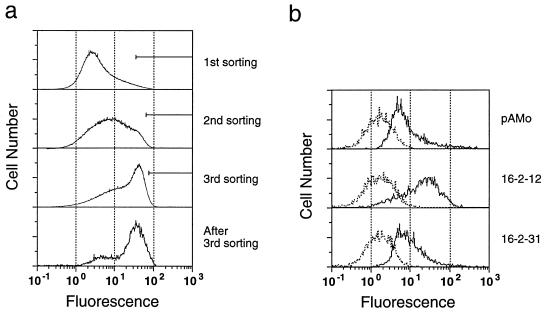
As a source of mRNAs for construction of a cDNA library, we tested HL-60, WM266-4, and SW1116 cells, which were known or shown to express the i antigen. As shown in Fig. 2, the expression of the i antigen on transfected Namalwa KJM-1 cells was substantially increased when the cells were transfected with a mixture of cDNA libraries derived from WM266-4 and SW1116. In contrast, no increase of the i antigen expression was achieved by using HL-60 cDNA library (data not shown). After the third sorting, more than 50% of the transfected cells showed increased expression of the i antigen (Fig. 2a). Plasmids were isolated from these Namalwa KJM-1 cells that were highly positive for the i antigen, and then each clone was tested for the ability to increase the expression of the i antigen. One of the plasmids, 16-2-12, directed 7 times-increased expression of the i antigen, and an unrelated plasmid, 16-2-31, did not (Fig. 2b). Because Namalwa KJM-1 cells endogeneously express i antigen, i antigen was detected in mock-transfected cells (Fig. 2b).
Figure 2.
Expression cloning of i enzyme by flow cytometry. (a) Namalwa KJM-1 cells stably transfected with the WM266-4 and SW1116 cDNA libraries were stained with the anti-i antibody (Den) and subjected to three rounds of sorting with a fluorescence-activated cell sorter. The cells with strong fluorescence intensities indicated by the bars were collected and subjected to the subsequent sorting. (b) Flow cytometric analysis of the cells transfected with the empty vector pAMo (Top), a plasmid 16-2-12 harboring iGnT cDNA (Middle), or an unrelated plasmid 16-2-31 (Bottom) after staining with the anti-i antibody (solid lines) or PBS (dotted lines).
Predicted Amino Acid Sequence of iGnT.
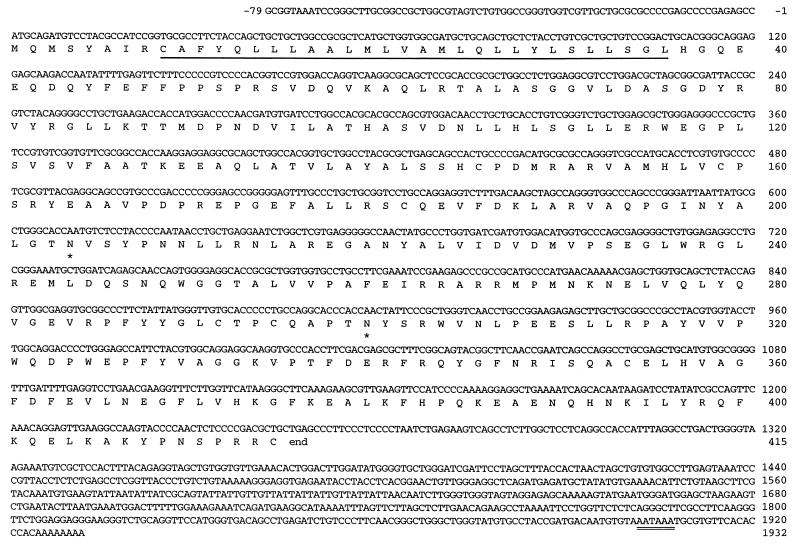
The cDNA insert in plasmid 16-2-12 encoding iGnT contains an ORF predicting a protein of 415 amino acid residues (Fig. 3). A hydropathy plot predicts that this protein has a type II transmembrane topology, with a short cytoplasmic sequence at the amino terminus, followed by the transmembrane domain, and then by the so-called stem region and a large catalytic domain, which presumably reside in the Golgi lumen. This topology has been found in almost all mammalian glycosyltransferases so far cloned (37). The iGnT is, however, characteristic in having a relatively long transmembrane domain that likely spans from residue 9 to residue 36, consisting of 28 residues. It is also characteristic in having only one basic amino acid at each end (Arg at residue 8 and His at residue 37) that flanks the transmembrane domain.
Figure 3.
DNA and translated amino acid sequences of iGnT. The full-length nucleotide and amino acid sequences of iGnT are shown. The signal/membrane-anchoring domain is underlined. Potential N-glycosylation sites are marked by asterisks. A polyadenylation signal is doubly underlined. The sequences are numbered relative to the translation initiation site.
There are two potential N-glycosylation sites (Fig. 3, asterisks). A consensus sequence for polyadenylation signal is present at nucleotides 1902–1907, which is probably used, judging from the size of mRNA (see below). No significant similarity was found between this protein sequence and other sequences reported in the Gene Data Bank.
Expression of iGnT and IGnT mRNAs in Human Tissues.
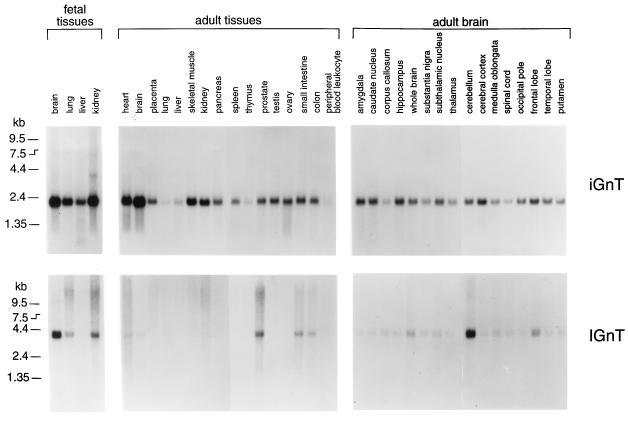
To determine the expression profile of iGnT and IGnT, Northern blots of poly(A)+ RNA derived from various human tissues were hybridized with iGnT probe followed by IGnT probe (12). A band of 2.2 kb for iGnT transcript was detected in all poly(A)+ RNA isolated from various tissues (Fig. 4). In fetal tissues, the signal was more prominent for poly(A)+ RNA derived from brain and kidney than from lung or liver. In adult tissues, the signal was weaker from thymus, peripheral blood leukocytes, lung, and liver than the other tissues.
Figure 4.
Northern blot analysis of iGnT and IGnT in various human fetal and adult tissues. Each lane contained 2 μg of poly(A)+ RNA. The same blots were probed by 32P-labeled iGnT cDNA (iGnT) followed by IGnT cDNA (IGnT).
In general, however, all of the tissues examined contained the transcript for iGnT. In contrast, the signal for IGnT was found only in certain tissues (Fig. 4). In fetal tissues, the transcript for IGnT was substantially expressed in brain and moderately expressed in kidney and lung but was almost undetectable in liver. In adult tissue, the transcript for IGnT was strongest in prostate, moderate in small intestine and colon, and barely detected in heart, brain, kidney, and pancreas. In adult brain, the IGnT transcript is much more prominent in cerebellum than the other parts of brain. Thus, these results indicate that iGnT is ubiquitously expressed in various tissues, although its amount varies according to tissues, and IGnT is clearly expressed in a tissue-specific manner.
Expression of Catalytically Active iGnT.
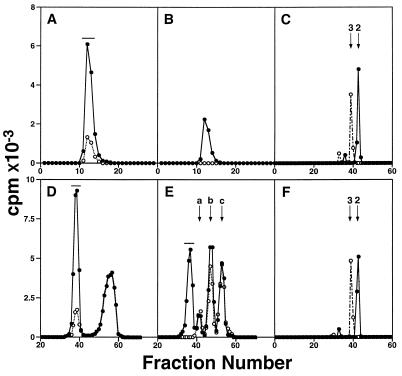
To confirm that the cDNA isolated encodes iGnT, the sequence corresponding to the putative stem region plus catalytic domain, iGnTc, was fused in-frame with cDNA encoding a signal peptide and the IgG binding domain of S. aureus protein A (25–28). As shown in Fig. 5A, the spent medium derived from COS-1 cells transfected with pcDNAI-A⋅iGnTc produced [3H]GlcNAc-labeled α1-acid glycoprotein. Less than one-fifth of the product was obtained by using the supernatant from COS-1 cells transfected with pcDNAI-A lacking cDNA insert. The labeled product was digested by endo-β-galactosidase, resulting in the release of [3H]GlcNAcβ1 → 3Gal (Fig. 5C). The activity in the supernatant from mock-transfected COS-1 cells was most likely due to iGnT endogenously expressed in COS-1 cells (38). In parallel, the labeled α1-acid glycoprotein was incubated with UDP-[3H]Gal and β-1,4-galactosyltransferase, and the resultant product was digested by endo-β-galactosidase, releasing [3H]Galβ1 → 4[3H]GlcNAcβ1 → 3Gal (Fig. 5C). The structures of these released oligosaccharides were deduced based on the standard oligosaccharides and the substrate specificity of endo-β-galactosidase (35). To confirm that the above activity was derived from the expressed enzyme, the chimeric enzyme adsorbed to IgG-Sepharose was used as an enzyme source. Fig. 5B demonstrates that the same labeled product was obtained by this enzyme but the IgG-Sepharose beads, adsorbed by the mock-transfected culture medium, had no activity.
Figure 5.
Gel filtration analysis of reaction products by iGnT. COS-1 cells were transfected with pcDNAI-A⋅iGnT (solid line) or pcDNAI-A (dotted line), and the derived spent medium (A and D) or the enzyme adsorbed to IgG-Sepharose (B) was used as an enzyme source. (A and B) The desialylated α1-acid glycoprotein was used as an acceptor and the product was analyzed by Sephadex G-50 gel filtration. (C) Bio-Gel P-2 gel filtration after endo-β-galactosidase digestion of product A obtained in A (solid line) or the galactosylated product A (dotted line). (D) Lacto-N-neotetraose was used as an acceptor and the product was analyzed by Bio-Gel P-4 gel filtration. (E) The product shown in D (denoted by horizontal bar) was incubated with β-1,4-galactosyltransferase and UDP-[3H]Gal and subjected to Bio-Gel P-4 gel filtration. Peaks a, b, and c denote contaminated radioactivity derived from UDP-[3H]galactose present in both experiments with (solid line) or without (dotted line) the acceptor. (F) Bio-Gel P-2 gel filtration after endo-β-galactosidase treatment of the product obtained in D (solid line) or the product obtained in E (dotted line). Arrows 2 and 3 in C and F denote the elution positions of GlcNAcβ1 → 3Gal and Galβ1 → 4GlcNAcβ1 → 3Gal, respectively. The products subjected to additional analysis are shown by horizontal bars.
To determine whether iGnT adds GlcNAc to N-acetyllactosamine repeats, lacto-N-neotetraose was used as an acceptor. As shown in Fig. 5D, iGnT efficiently added GlcNAc to lacto-N-neotetraose (0.7 nmol formed from 500 nmol of the acceptor) and this product, [3H]GlcNAcβ1 → 3Galβ1 → 4GlcNAcβ1 → 3Galβ1 → 4Glc, was converted to [3H]Galβ1 →4[3H]GlcNAcβ1 → 3Galβ1 → 4GlcNAcβ1 → 3Galβ1 → 4Glc by incubation with β-1,4-galactosyltransferase and UDP-[3H]Gal (Fig. 5E). The same results were obtained with the chimeric enzyme adsorbed to IgG-Sepharose beads (data not shown). From these labeled products, [3H]GlcNAcβ1 → 3Gal and [3H]Galβ1 → 4[3H]GlcNAcβ1 → 3Gal were respectively released by endo-β-galactosidase treatments (Fig. 5F).
In addition, the chimeric enzyme adsorbed to IgG-Sepharose transferred β-1,3-linked GlcNAc to Galβ1 → 4Glc, but the IgG-Sepharose beads, adsorbed by the mock-transfected culture medium, had no activity. No transfer of [3H]GlcNAc was observed toward Galβ1 → 3GlcNAcβ1 → 3Galβ1 → 4Glc (data not shown). Thus, these results indicate that iGnT cloned in the present study can add N-acetylglucosamine to lactose and both N-acetyllactosamines attached to N-glycans of α1-acid glycoprotein and lacto-N-neotetraose.
DISCUSSION
In the present study, we report the isolation of a human cDNA clone encoding iGnT, determination of its expression in various tissues, and demonstration of its in vitro activity. For this cloning, we used Namalwa KJM-1 cell line as recipient cells for transfection and a vector containing the replication origin of Epstein–Barr virus oriP. Because Namalwa KJM-1 cells synthesize EBNA-1, amplification of a vector containing oriP is possible in an episomal manner. Because of this advantage, it was possible to continuously enrich the transfected cells expressing an increased amount of the i antigen without isolation of plasmids and reintroduction of the plasmids to recipient cells. This is an improved method and combination of two previous methods for cloning GD3/GT3 synthase and Fuc-TVII by using an overexpression protocol (25, 27) and cloning ST3Gal IV and GD3 synthase (26, 28) by using Namalwa KJM-1 cells and pAMo vector.
The predicted amino acid sequence of iGnT has several characteristics. In particular, iGnT apparently has a longer transmembrane domain consisting of 28 amino acid residues than other glycosyltransferases so far cloned. Most other glycosyltransferases contain a transmembrane domain consisting of 14–24 amino acid residues (37). As shown previously, the size of poly-N-acetyllactosamines is longer in those attached to membrane glycoproteins than those attached to secretory glycoproteins (1). Moreover, human chorionic gonadotropin-α glycoprotein acquired poly-N-acetyllactosamine once it became a membrane glycoprotein by fusing with the transmembrane and cytoplasmic portions of vesicular stomatitis virus G protein (38). These results strongly suggest that iGnT has a unique characteristic in binding to acceptor substrates, preferentially adding poly-N-acetyllactosamine to membrane glycoproteins. Such a unique property may be related to its long transmembrane domain. The availability of iGnT cDNA will allow us to determine whether or not changes in its transmembrane domain alter the property of iGnT in this aspect.
In previous studies, iGnT was detected and partially purified from human serum (10), Novikoff ascites tumor cells (13), and calf serum (14). The substrate specificity of these enzymes are very similar to those of the cloned iGnT, and all the enzymes add N-acetylglucosamine to Galβ1 → 4GlcNAc, Galβ1 → 4Glc, and Galβ1 → 4GlcNAcβ1 → 3Galβ1 → 4Glc but not to Galβ1 → 3GlcNAcβ1 → 3Galβ1 → 4Glc. These results strongly suggest that the enzyme cloned in the present study can initiate the synthesis of poly-N-acetyllactosamine and also elongate poly-N-acetyllactosamine. On the other hand, the iGnT cloned in the present study has a predicted molecular weight of 47,125 for its polypeptide, whereas the molecular mass of a iGnT partially purified from calf serum was found to be 70 kDa (14). If two N-glycans are attached to the cloned iGnT, then the mature iGnT may have an approximate mass of 53 kDa. These results suggest that the calf serum iGnT may be different from the human iGnT cloned in the present study. Alternatively, iGnT may contain a large amount of O-glycans in addition to N-glycans, although this possibility is unlikely judging from the deduced amino acid sequence.
It has been shown that tumor cells express more poly-N-acetyllactosamine than normal counterparts (39–42). It has been also demonstrated that the activity of GnTV is also increased in those tumor cells (39–41). In parallel, iGnT was shown to prefer Galβ1 → 4GlcNAcβ1 → 6Manα1 → 6Manβ1 → R, which is formed by GnTV, as an acceptor over other side chains in N-glycans (13, 43, 44). These combined results suggest that the tumorigenic phenotype acquired by the introduction of GnTV cDNA (45) could be due to the increase of poly-N-acetyllactosamine built on the side chain synthesized by GnTV. Further studies using the cloned iGnT will be of significance to determine whether the increase of poly-N-acetyllactosamine, but not the increase in the side chain formed by GnTV, is the primary cause for transformed phenotype displayed by tumor cells.
Acknowledgments
We thank Dr. Michiko N. Fukuda for useful discussion, Ms. Sachiko Kodama and Ayumi Natsume for excellent technical assistance, Dr. Edgar Ong for critical reading of the manuscript, and Ms. Susan Greaney for organizing the manuscript. The work was supported by Grant CA48737 from the National Cancer Institute (to M.F.) and a Toyobo Biotechnology Fellowship (to M.U.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: iGnT, β-1,3-N-acetylglucosaminyltransferase; IGnT, I-branch forming β-1,6-N-acetylglucosaminyltransferase; R, aglycon; EBNA-1, the nuclear antigen-1 of Epstein–Barr virus; GnTV, N-acetylglucosaminyltransferase ∇.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF029893).
References
- 1.Fukuda M, Fukuda M N. In: The Biology of Glycoproteins. Ivatt R J, editor. New York: Plenum; 1984. pp. 183–234. [Google Scholar]
- 2.Feizi T. Nature (London) 1985;314:53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M, Fukuda M N, Hakomori S. J Biol Chem. 1979;254:3700–3703. [PubMed] [Google Scholar]
- 5.Watanabe K, Hakomori S, Childs R A, Feizi T. J Biol Chem. 1979;254:3221–3228. [PubMed] [Google Scholar]
- 6.Wiener A S, Unger L H, Cohen L, Feldman J. Ann Intern Med. 1956;44:221–240. doi: 10.7326/0003-4819-44-2-221. [DOI] [PubMed] [Google Scholar]
- 7.Feizi T, Childs R A, Watanabe K, Hakomori S-I. J Exp Med. 1979;149:975–980. doi: 10.1084/jem.149.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu J, Nishikawa A, Fujii S, Gasa S, Taniguchi N. J Biol Chem. 1992;267:2994–2999. [PubMed] [Google Scholar]
- 9.Piller F, Cartron J P, Maranduba A, Veyrieres A, Leroy Y, Fournet B. J Biol Chem. 1984;259:13385–13390. [PubMed] [Google Scholar]
- 10.Piller F, Cartron J P. J Biol Chem. 1983;258:12293–12299. [PubMed] [Google Scholar]
- 11.Koenderman A H, Koppen P L, Van den Eijnden D H. Eur J Biochem. 1987;166:199–208. doi: 10.1111/j.1432-1033.1987.tb13502.x. [DOI] [PubMed] [Google Scholar]
- 12.Bierhuizen M F A, Mattei M G, Fukuda M. Genes Dev. 1993;7:468–478. doi: 10.1101/gad.7.3.468. [DOI] [PubMed] [Google Scholar]
- 13.Van den Eijnden D H, Koenderman A H, Schiphorst W E. J Biol Chem. 1988;263:12461–12471. [PubMed] [Google Scholar]
- 14.Kawashima H, Yamamoto K, Osawa T, Irimura T. J Biol Chem. 1993;268:27118–27126. [PubMed] [Google Scholar]
- 15.Fukuda M, Spooncer E, Oates J E, Dell A, Klock J C. J Biol Chem. 1984;259:10925–10935. [PubMed] [Google Scholar]
- 16.Lowe J B, Stoolman L M, Nair R P, Larsen R D, Berhend T L, Marks R M. Cell. 1990;63:475–484. doi: 10.1016/0092-8674(90)90444-j. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M L, Nudelman E, Gaeta F C A, Perez M, Singhai A K, Hakomori S-I, Paulson J C. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- 18.Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Science. 1990;250:1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- 19.Lowe B J. In: Molecular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford: Oxford Univ. Press; 1994. pp. 163–205. [Google Scholar]
- 20.Fukushima K, Hirota M, Terasaki P I, Wakisaka A, Togashi H, Chia D, Suyama N, Fukushi Y, Nudelman E, Hakomori S. Cancer Res. 1984;44:5279–5285. [PubMed] [Google Scholar]
- 21.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 22.Sawada R, Tsuboi S, Fukuda M. J Biol Chem. 1994;269:1425–1431. [PubMed] [Google Scholar]
- 23.Bird J M, Kimber S J. Dev Biol. 1984;104:449–460. doi: 10.1016/0012-1606(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 24.Fenderson B A, Zehavi U, Hakomori S. J Exp Med. 1984;160:1591–1596. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakayama J, Fukuda M N, Hirabayashi Y, Kanamori A, Sasaki K, Nishi T, Fukuda M. J Biol Chem. 1996;271:3684–3691. [PubMed] [Google Scholar]
- 26.Sasaki K, Watanabe E, Kawashima K, Sekine S, Dohi T, Oshima M, Hanai N, Nishi T, Hasegawa M. J Biol Chem. 1993;268:22782–22787. [PubMed] [Google Scholar]
- 27.Sasaki K, Kurata K, Funayama K, Nagata M, Watanabe E, Ohta S, Hanai N, Nishi T. J Biol Chem. 1994;269:14730–14737. [PubMed] [Google Scholar]
- 28.Sasaki K, Kurata K, Kojima N, Kurosawa N, Ohta S, Hanai N, Tsuji S, Nishi T. J Biol Chem. 1994;269:15950–15956. [PubMed] [Google Scholar]
- 29.Margolskee R F, Kavathas P, Berg P. Mol Cell Biol. 1988;8:2837–2847. doi: 10.1128/mcb.8.7.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirt B. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 31.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama J, Fukuda M. J Biol Chem. 1996;271:1829–1832. doi: 10.1074/jbc.271.4.1829. [DOI] [PubMed] [Google Scholar]
- 33.Bierhuizen M F A, Fukuda M. Proc Natl Acad SciUSA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee N, Wang W-C, Fukuda M. J Biol Chem. 1990;265:20476–20487. [PubMed] [Google Scholar]
- 35.Fukuda M N. J Biol Chem. 1981;256:3900–3905. [PubMed] [Google Scholar]
- 36.Merkle R K, Cummings R D. J Biol Chem. 1987;262:8179–8189. [PubMed] [Google Scholar]
- 37.Schachter H. In: Molecular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford: IRL Press; 1994. pp. 88–162. [Google Scholar]
- 38.Fukuda M, Guan J-L, Rose J K. J Biol Chem. 1988;263:5314–5318. [PubMed] [Google Scholar]
- 39.Yamashita K, Ohkura T, Tachibana Y, Takasaki S, Kobata A. J Biol Chem. 1984;259:10834–10840. [PubMed] [Google Scholar]
- 40.Pierce M, Arango J. J Biol Chem. 1986;261:10772–10777. [PubMed] [Google Scholar]
- 41.Dennis J W, Laferté S, Waghorn C, Breitman M L, Kerbel R S. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 42.Saitoh O, Wang W-C, Lotan R, Fukuda M. J Biol Chem. 1992;267:5700–5711. [PubMed] [Google Scholar]
- 43.Cummings R D, Kornfeld S. J Biol Chem. 1984;259:6253–6260. [PubMed] [Google Scholar]
- 44.Sasaki H, Bothner B, Dell A, Fukuda M. J Biol Chem. 1987;262:12059–12076. [PubMed] [Google Scholar]
- 45.Demetriou M, Nabi I R, Coppolino M, Dedhar S, Dennis J W. J Cell Biol. 1995;130:383–392. doi: 10.1083/jcb.130.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]