Abstract
Erectile dysfunction (ED) can be elicited by a variety of pathogenic factors, particularly impaired formation of and responsiveness to nitric oxide (NO) and the downstream effectors soluble guanylate cyclase (sGC) and cGMP-dependent protein kinase I (PKGI). One important target of PKGI in smooth muscle is the large-conductance, Ca2+-activated potassium (BKCa) channel. In our previous report (42), we demonstrated that deletion of the BKCa channel in mice induced force oscillations and led to reduced nerve-evoked relaxations and ED. In the current study, we used this ED model to explore the role of the BKCa channel in the NO/sGC/PKGI pathway. Electrical field stimulation (EFS)-induced contractions of corpus cavernosum smooth muscle strips were significantly enhanced in the absence of BKCa channel function. In strips precontracted with phenylephrine, EFS-induced relaxations were converted to contractions by inhibition of sGC, and this was further enhanced by loss of BK channel function. Sildenafil-induced relaxations were decreased to a similar extent by inhibition of sGC or BKCa channels. At concentrations >1 μM, sildenafil caused relaxations independent of inhibition of sGC or BKCa channels. Sildenafil did not affect the enhanced force oscillations that were induced by the loss of BKCa channel function. Yet, these oscillations could be completely eliminated by blocking L-type voltage-dependent Ca2+ channels (VDCCs). These results suggest that therapeutically relevant concentrations of sildenafil act through cGMP and BKCa channels, and loss of BKCa channel function leads to hypercontractility, which depends on VDCCs and cannot be modified by the cGMP pathway.
Keywords: smooth muscle, calcium-activated potassium channel, mouse
erectile dysfunction (ED) is described as the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance (44). It affects 30 million men in the United States (5), and its prevalence increases with age and diseases like diabetes and hypertension (4, 24, 31). Erectile function and a proper penile erection occurs in response to nerve stimulation and the release of nitric oxide (NO) from parasympathetic nonadrenergic-noncholinergic (NANC) nerves as well as from the vascular endothelium (2, 13, 16, 27). In mice, the NO-producing enzyme NO synthase has been found in the dorsal penile nerve and its branches in the mouse penis (9) as well as in intrinsic nerves of the erectile tissue (14). The majority of NO effects are mediated through the soluble guanylate cyclase (sGC) and its product, cGMP (2, 8, 16, 33). cGMP acts as a modifying agent on ion channels, phosphodiesterases, and protein kinases (19). One of the protein kinases, the cGMP-dependent protein kinase I (PKGI), phosphorylates various proteins, including ion channels and pumps, which are known to reduce intracellular Ca2+ concentration ([Ca2+]i) and smooth muscle contraction. The importance of this kinase in erectile tissue is supported by the observation that precontracted corpus cavernosum smooth muscle (CCSM) strips from mice lacking PKGI did not relax to nerve stimulation (14). It is known that PKGI activates large-conductance Ca2+-activated potassium (BKCa) channels (1, 32), which hyperpolarize smooth muscle cell membranes, causing muscle relaxation. Relaxation of arterial and CCSM is necessary to increase blood flow into the corpora cavernosa that leads to penile tumescence.
BKCa channels in CCSM clearly have an important role in the regulation of function. Blocking the BKCa channel with tetraethylammonium ions or charybdotoxin led to an increase of phenylephrine (PE)-induced contractions of CCSM strips in vitro (34). In aged or diabetic rats, intracavernous injection of cDNA encoding the human BKCa channel led to a reversal of ED (11, 25). Consistent with the significant role of BKCa channels to oppose smooth muscle contractility, precontracted CCSM strips from mice lacking the BKCa channel (Kcnma1−/−, also called Slo−/−) exhibited pronounced force fluctuations in the presence of the α-adrenergic receptor agonist PE and reduced relaxations in response to nerve stimulations compared with wild-type mice (Slo+/+) (42). The BKCa channel is an attractive target in the treatment of vascular and lower urinary tract disorders, including urinary incontinence and ED. Kcnma1 (Slo) gene therapy has been shown to ameliorate ED in rodents (11), and recently in humans (22).
Early pharmacological studies on erectile function demonstrated that direct injection of vasodilator drugs into the corpus cavernosum, such as the phosphodiesterase inhibitor papaverine, resulted in erection (41). Later, the importance of cGMP was reinforced by the development of drugs such as sildenafil, which inhibit the cGMP-specific type 5 phosphodiesterase (PDE5) (6, 39). Even though the positive effect of sildenafil on erectile function is well established, the downstream mechanisms through which cGMP and PKGI mediate relaxation have not been elucidated.
The primary goal of the current study was to explore the BKCa channel deletion model of ED and investigate the nature of its hypercontractile phenotype. Furthermore, we used this model to examine the effect of activating the cGMP signaling pathway with the PDE5 inhibitor sildenafil on the contractility of isolated CCSM strips in the absence and presence of the α-adrenergic receptor agonist PE.
METHODS
Animal handling and tissue preparation.
Slo−/− mice were generated as previously published (26). All procedures performed in the course of this study were approved by the Office of Animal Care Management at the University of Vermont. Adult male mice (10–20 wk old; ∼30 g body wt) were killed with intraperitoneal injection of pentobarbital sodium (150 mg/kg) followed by thoracotomy. For the in vitro contractility studies, the penis was removed and immediately placed in ice-cold dissection solution (in mM: 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, pH 7.3 adjusted with NaOH).
In vitro contractility studies.
Contractility studies were performed as described earlier (42). Briefly, the contractility of isolated CCSM strips was measured using a MyoMED myograph system (MED Associates, Georgia, VT). Each strip was mounted in a thermostatically controlled tissue bath and stretched to a resting tension of 0.1 mN. After a 1-h equilibration period, the force generation of the strips was analyzed by applying electrical field stimulation (EFS) in the absence or presence of 10 μM PE in the bath. EFS was delivered either continuously at 30 Hz every minute or at increasing frequencies (1, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz) every 3 min. Pulse amplitude was 20 V, and polarity was reversed for alternating pulses. Pulse width was 0.5 ms, and stimulus duration was 2 and/or 60 s (see Fig. 2). To apply stimuli, a model PHM-152V stimulator (MED Associates) was used.
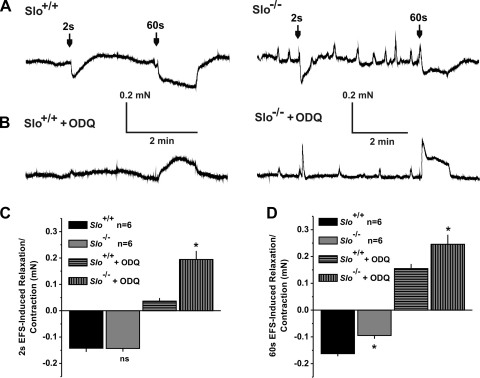
Fig. 2.
Inhibition of soluble guanylate cyclase (sGC) turns EFS-induced relaxations into contractions. A: sample recordings from a phenylephrine (PE)-precontracted Slo+/+ (left) and a Slo−/− (right) CCSM strip and EFS-induced relaxations lasting 2 and 60 s. B: same as in A but after adding ODQ to the bath. C and D: summary of relaxations and contractions induced by the 2-s (C) and 60-s (D) EFS shown in A and B; n, no. of CCSM strips from 3 mice; *P < 0.05 vs. Slo+/+.
Drugs and data analysis.
The compounds used were: iberiotoxin (IBTX; 300 nM; Peptides International), sildenafil (10 nM-10 μM; Pfizer), PE (10 μM), 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μM), and nisoldipine (100 nM; all from Sigma). Data were analyzed and presented using MyoMed (MED Associates), MiniAnalysis (Synaptosoft), Origin (OriginLab), Prism (GraphPad), and CorelDraw (Corel) software. Statistical comparisons were made using paired or unpaired t-tests, as applicable, and data are expressed with SEs. P < 0.05 was considered significant.
RESULTS
EFS-induced contractions are increased in CCSM strips from Slo−/− mice and from Slo+/+ mice in the presence of IBTX.
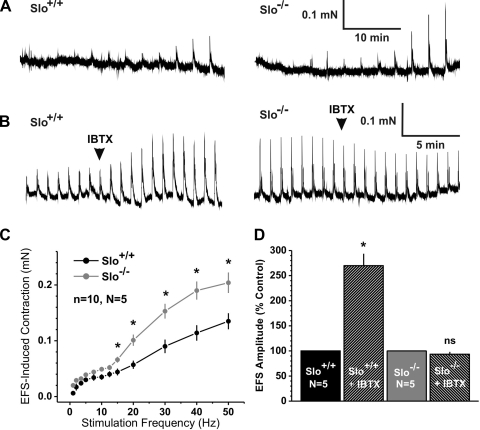
Transmural EFS has been used to stimulate all autonomic nerves that innervate the smooth muscle of the corpus cavernosum (2, 14, 27). Depending on the contractile state of the CCSM, EFS can induce smooth muscle contraction through the stimulation of sympathetic nerves present in the CCSM strips, or it can induce relaxation through the stimulation of NANC nerves and the release of NO. We analyzed the contractile response of isolated CCSM strips from Slo+/+ and Slo−/− mice to the application of EFS with increasing stimulation frequencies from 1 Hz to 50 Hz. (Fig. 1, A and C). In both genotypes, contractions were observed at the lowest frequencies. Above 12.5 Hz, EFS-induced force was up to 1.6 times greater in strips from Slo−/− than Slo+/+. The results with Slo−/− mice suggest that blocking BKCa channels with IBTX should also elevate nerve-evoked force above 12.5 Hz. Indeed, in Slo+/+ strips, IBTX lead to a 170% increase of the 30 Hz-induced contractions, an effect that was absent in Slo−/− strips, supporting the role of BKCa channel to oppose sympathetic nerve-mediated contractions (Fig. 1, B and D). These results are consistent with BKCa channels opposing CCSM excitability.
Fig. 1.
Electrical field stimulation (EFS)-induced contractions are increased in corpus cavernosum smooth muscle (CCSM) strips from Slo−/− mice. A: representative recordings from a Slo+/+ (left) and a Slo−/− (right) CCSM strip of EFS-induced contractions with increasing frequencies (1, 2, 3.5, 5, 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz) every 3 min. B: representative recordings from Slo+/+ (left) and Slo−/− (right) CCSM strips of EFS-induced contractions with continuous 30-Hz stimulation every minute before and after adding iberiotoxin (IBTX). C: relationship between stimulation frequency and force in CCSM strips from Slo+/+ and a Slo−/− mice. D: effect of IBTX on continuous 30-Hz EFS-induced contractions, normalized to values before IBTX; n, no. of CCSM strips; N, no. of mice. *P < 0.05 vs. Slo+/+; ns, Not significant.
Inhibition of sGC converts EFS-induced relaxations into contractions that are increased in Slo−/−.
In the flaccid state, the corpus cavernosum is under tonic sympathetic influence to keep the smooth muscle contracted. As in our previous study, this condition was simulated by adding the α-adrenergic receptor agonist PE (10 μM) to induce contraction of isolated CCSM strips (42). Electrical stimulation would stimulate both sympathetic and NANC nerves. However, under these conditions, the α-adrenergic receptors are already activated by the presence of the externally added PE, and the NO release from NANC nerves then leads to relaxation of the CCSM strips.
In precontracted CCSM strips, EFS has been widely used to analyze the activation of NANC nerves and the subsequent relaxation of the cavernous tissue (2, 14). In our previous study (42), the following two stimulation protocols were used: one lasting for 2 s to induce a brief and transient relaxation and the other for 60 s to induce a prolonged and more physiological activation of nerves. Here we used these stimulation protocols to analyze the importance of cGMP for EFS-induced relaxations by inhibiting the cGMP-producing enzyme sGC. We applied electrical stimulation before and after incubating the CCSM strips from Slo+/+ and Slo−/− mice with the sGC inhibitor ODQ (1 μM; Fig. 2). In CCSM strips, ODQ transiently increased the PE-induced tonic force, but without a significant difference between Slo+/+ (0.14 ± 0.014 mN) and Slo−/− (0.13 ± 0.012 mN; P = 0.8). As we have shown previously, CCSM strips exhibit PE-induced force oscillations in the absence of the BKCa channel or in the presence of its blocker, IBTX (42). In Slo−/− strips, ODQ did not affect the amplitude (absence: 0.075 ± 0.007 mN and presence: 0.075 ± 0.035 mN; P = 0.5) or the frequency (absence: 0.12 ± 0.085/min and presence: 0.12 ± 0.0/min; P = 1.0) of these force oscillations. Due to the very small amplitude of the force oscillations in Slo+/+ (see Ref. 42), no effects could be observed here.
In the absence of ODQ, the 2-s EFS relaxed the precontracted strips of both genotypes without any difference, as previously reported (42). Inhibition of sGC led to complete block of the relaxing effect of EFS, indicating that sGC is necessary for nerve-evoked relaxations in both genotypes. In fact, EFS-induced relaxations were converted into contractions for both Slo+/+ and in Slo−/− strips. However, this transient contraction was 5.3 times greater in the absence of the BKCa channel during the 2-s stimulation (Fig. 2, A–C).
The relaxation induced by the 60-s stimulation was significantly less in Slo−/− (42). ODQ converted the relaxations to contractions during the 60-s stimulation (Fig. 2, B and D), which were more robust than during the 2-s pulse. The EFS-induced force was significantly higher in CCSM strips from Slo−/− mice, particularly the immediate response at the beginning of the pulse (Fig. 2, A, B, and D).
Involvement of BKCa channels in sildenafil-mediated relaxations of CCSM.
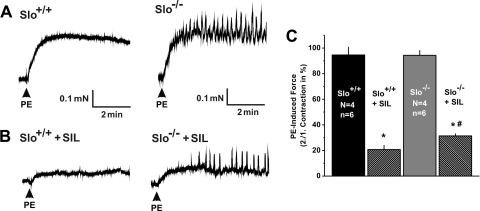
It has been well established in animals and in humans that the PDE5-specific inhibitor sildenafil relaxes CCSM through elevating cGMP levels (6, 35, 40). One of the possible effectors of the elevated cGMP levels is the BKCa channel (3), but functional data supporting a role of BKCa channels in the actions of sildenafil have been lacking. We used Slo+/+ and Slo−/− mice to investigate the role of BKCa channel in sildenafil-mediated relaxations of CCSM strips. Two consecutive contractions with PE (10 μM) were induced, and sildenafil (10 μM) was added after the first contraction (Fig. 3). In the absence of sildenafil, there was no difference between first and second contractions (data not shown). In Slo+/+ strips, sildenafil (10 μM) reduced the PE-induced contractile force to 20.7 ± 3.5% of the control (compared with a second contraction in the absence of sildenafil). In strips from Slo−/− mice, sildenafil was less effective (Fig. 3, B and C). Furthermore, force oscillations were observed in Slo−/− strips, even in the presence of sildenafil (Fig. 3B, right).
Fig. 3.
Sildenafil-mediated suppression of PE-induced contractions is diminished in Slo−/−. A and B: representative PE-induced contractions recorded from a Slo+/+ (left) and a Slo−/− (right) CCSM strip before (A) and after (B) adding sildenafil (SIL). C: summary of the SIL-mediated reduction of PE-induced contractions as shown in A and B; n, no. of CCSM strips; N, no. of mice; P < 0.01 vs. untreated (*) and vs. Slo+/+ + SIL (#).
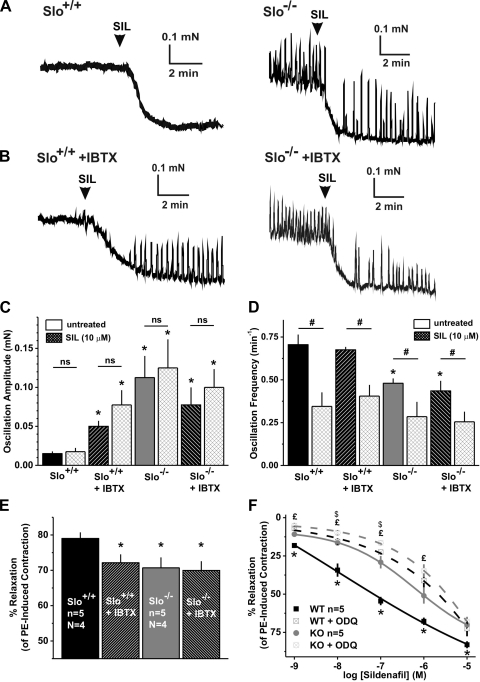
Consistent with the effects of sildenafil on PE-induced force (Fig. 3), the application of sildenafil (10 μM) to precontracted Slo+/+ strips led to a 79.0 ± 1.7% relaxation (Fig. 4, A and E). Blocking the BKCa channel with IBTX before the sildenafil treatment had a small, but significant, effect, reducing the relaxation by ∼7% (72.1 ± 2.4% relaxation of the PE-induced force) (Fig. 4, B and E). A similar reduction was observed in Slo−/− strips (70.7 ± 2.9% relaxation), whereas IBTX had no effect. The amplitudes of the force oscillations in the presence of IBTX or in Slo−/− strips were not altered by 10 μM sildenafil (Fig. 4C). However, in the absence of BK channel function, sildenafil was able to reduce the frequency of the force oscillations (Fig. 4D).
Fig. 4.
Sildenafil-induced relaxation of PE-precontracted CCSM strips is reduced in Slo−/−. A and B: sample recordings from a PE-precontracted Slo+/+ (left) and a Slo−/− (right) CCSM strip in the absence (A) and presence (B) of IBTX that represent the SIL-induced relaxation. C and D: average oscillation amplitudes and frequency, respectively, of Slo+/+ and a Slo−/− CCSM strip in the absence and presence of IBTX, before and after adding SIL. E, average %SIL-induced relaxation of PE-precontracted strips shown in A and B. F: dose-response curves of Slo+/+ and Slo−/− CCSM strips to SIL from 1 nM to 10 μM in the absence and presence of ODQ; n, no. of CCSM strips; N, no. of mice. P < 0.05, Slo+/+ vs. all others (*), untreated vs. sildenafil treated (#), Slo−/− vs. Slo−/− + ODQ (£), and Slo+/+ + ODQ vs. Slo−/− + ODQ ($).
Although sildenafil is commonly used at concentrations 10 μM and greater in studies on rabbit and human tissue (35, 40), this concentration is high relative to inhibition of PDE5. (35, 40). At very high concentrations, sildenafil is thought to act on other pathways independent of the NO pathway (17, 21). Moreover, since we observed only a small difference between the two genotypes at a relatively high concentration of sildenafil (10 μM), the relaxing effects of sildenafil from 1 nM to 10 μM were examined, using precontracted Slo+/+ and Slo−/− CCSM strips. At all concentrations, the relaxing effect of sildenafil was significantly reduced in Slo−/−, with the largest difference at 100 nM (Slo+/+ 54.5 ± 2.8% and Slo−/− 29.1 ± 4.1%; Fig. 4F) along with a right shift of the dose-response curve to higher concentrations in the absence of the BKCa channel (Slo+/+: logEC50 = −7.60 ± 0.79; Slo−/−: logEC50 = −5.75 ± 0.22; P < 0.05).
cGMP is synthesized by guanylate cyclases, and broken down by PDE5, which is inhibited by sildenafil. Inhibition of the sGC with ODQ increased smooth muscle tone and attenuated but did not prevent sildenafil-induced relaxation entirely (21) (Fig. 4F). ODQ reduced the relaxation to sildenafil (100 nM) from 54.5 ± 2.8 to 22.5 ± 1.5% in Slo+/+ strips and from 29.1 ± 4.1 to 16.4 ± 1.6% in Slo−/− strips. In the presence of ODQ, the sildenafil response was altered by the loss of the BKCa channel at 10 and 100 nM (Fig. 4F), but without a significant shift of the dose-response curve (Slo+/+ + ODQ: logEC50 = −3.99 ± 0.67; Slo−/− + ODQ: logEC50 = −3.51 ± 1.09; P = 0.31). These results indicate that blocking sGC or loss of the BKCa channel caused a similar reduction in sildenafil's action. This is consistent with the idea that BKCa channel is one of the major downstream targets of the elevation of cGMP by sildenafil.
Blocking of voltage-dependent Ca2+ channels inhibits force oscillations in CCSM strips from Slo−/− and from Slo+/+ plus IBTX.
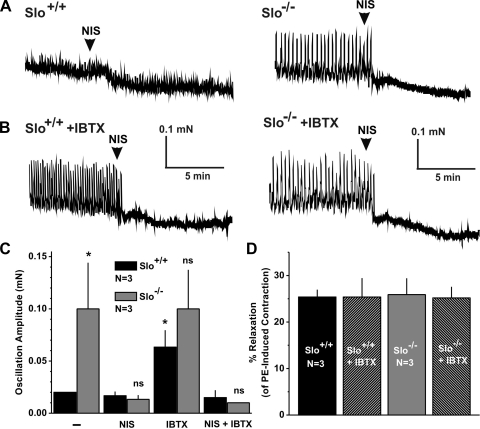
Loss of BKCa channel function by IBTX or in the Slo−/− mice leads to prominent force oscillations in the presence of PE, and sildenafil does not prevent these oscillations. It is likely that these force oscillations reflect an unstable membrane potential, and thus modulation of Ca2+ influx through voltage-dependent Ca2+ channels (VDCC). To examine this possibility, the effects of the VDCC blocker nisoldipine (100 nM) on PE-induced oscillations were tested. In Slo+/+ strips, the small-amplitude oscillations were unaffected by nisoldipine (Fig. 5, A and C). However, the larger oscillations observed in strips from Slo−/− mice or from Slo+/+ mice in the presence of IBTX were completely abolished by blocking VDCCs (Fig. 5, A-C). In addition to the block of force oscillations, nisoldipine relaxed CCSM strips up to 25%, irrespective of genotypes or the presence of IBTX (Fig. 5C).
Fig. 5.
Blocking of voltage-dependent Ca2+ channels (VDCC) inhibits force oscillations in Slo−/− CCSM strips and in Slo+/+ with IBTX. A and B: representative recordings from a PE-precontracted Slo+/+ (left) and a Slo−/− (right) CCSM strip in the absence (A) and presence (B) of IBTX that demonstrate the effect of blocking VDCC with nisoldipine. C: average oscillation amplitudes of Slo+/+ and a Slo−/− CCSM strip in the absence and presence of IBTX, before and after adding nisoldipine. D: summary of total relaxation of PE-precontracted CCSM strips induced by nisoldipine as shown in A and B. NIS, nisoldipine; N, no. of mice; *P < 0.05 vs. Slo+/+ untreated.
DISCUSSION
The role of BKCa channels in controlling CCSM tone.
The two principal findings of this study are that, in the absence or blockade of the BKCa channel, CCSM exhibit enhanced contractility to stimulations of sympathetic nerves present in CCSM strips (Fig. 1) and, when precontracted with PE, display a reduced ability to relax in response to sildenafil (Fig. 4). Indeed, we found that relaxation of CCSM strips by sildenafil was blocked to a similar extent by loss of BK channel function or inhibition of sGC (Fig. 4), suggesting a major role of BK channels as a downstream target of sildenafil's actions. In particular, the reduced relaxation is accompanied by continuous oscillatory contractions, which could not be prevented by sildenafil. In a very significant way, this study extends our previous findings (42), which demonstrated that a lack of BKCa channels leads to hypercontractility and reduced relaxation abilities to nerve stimulation in precontracted CCSM.
The enhancement of nerve-evoked contractions by IBTX and in Slo−/− mice in this study could be explained by inhibition and absence of the BKCa channel in the smooth muscle, respectively. Nerve-evoked contractions are mediated by the release of norepinephrine from sympathetic nerves, are blocked by the α-adrenergic receptor blocker prazosin (13), and, without the BKCa channel to counteract these contractions, they are very likely to be enhanced (Fig. 1). Furthermore, BKCa channels were only found in the smooth muscle of the penis (42).
In our previous study, loss of BK channel function reduced the relaxation to 60 s stimulation but had no effect on 2 s stimulation (42). Therefore, it is possible that the longer-duration stimulation engages additional relaxation pathways. However, inhibition of sGC in WT strips dramatically affected both the 2-s and the 60-s relaxations, converting relaxation to contraction. These results indicate that the 2- and 60-s nerve stimulations cause relaxation through the NO/sGC/cGMP pathway; however, the 2-s stimulation seems to recruit different downstream effector mechanisms of this pathway of which BKCa is only one (for review, see Ref. 15). Moreover, although inhibiting this pathway, EFS still activates sympathetic pathways, which leads to further contractions in precontracted strips (14). Under these conditions, the EFS-induced contractions again are enhanced in Slo−/− comparable to the previous experiment in nonprecontracted strips (compare Figs. 1 and 2).
NO activates sGC to synthesize cGMP, and sildenafil inhibits the breakdown of it. Thus sildenafil acts on the NO/sGC/cGMP pathway and amplifies its relaxing effect in smooth muscle. cGMP-dependent protein kinase directly activates the BKCa channel (32, 36), and indirectly through phosphorylation of phospholamban and elevation of local Ca2+ release events through ryanodine receptors (30). We found that the relaxing effect of sildenafil (10 μM) was reduced by ∼10% in Slo−/− CCSM. However, loss of BKCa channel function has a very substantial effect on nerve-evoked relaxations (Fig. 2) and increases in intracavernous pressure (37). Furthermore, sildenafil's relaxing effects were almost equally affected by the loss of BKCa channel function or inhibition of sGC (Fig. 4), suggesting that sildenafil largely acts through cGMP and activation of BKCa channel. ODQ and loss of BKCa channel function had an identical, but small, effect (10%) on sildenafil-induced relaxation at 10 μM sildenafil (Fig. 4F). This result suggests that sildenafil has additional effects (non-cGMP, non-BKCa channel) at 1 μM and greater. Indeed, sildenafil has been shown to inhibit VDCCs in cardiac muscle >1 μM (10). In CCSM, inhibition of VDCCs reduced smooth muscle contraction; particularly, it entirely eliminated the increased phasic contractions induced by the absence of BKCa function (Fig. 5). Sildenafil, however, did not affect the phasic contraction amplitude (Fig. 4), suggesting that, in CCSM, high sildenafil concentrations act through mechanisms other than inhibition of VDCCs. These results indicate that therapeutically relevant concentrations (<1 μM) of sildenafil act through cGMP and BKCa channels and that higher concentrations act through other, yet unknown mechanisms.
The lack of effects of sildenafil on the force oscillations when the BKCa channel is blocked or absent is noteworthy. These results indicate that, in a situation of compromised or diminished BKCa channel function, the CCSM cannot be kept in a relaxed state, and consequently the penis in an erect state, even in the presence of the potent ED drug sildenafil. BKCa channel expression has been shown to decrease with age in coronary arteries (38), and, if this occurs in the CCSM, it may diminish the efficacy of a PDE5 inhibitor therapy.
BKCa channel and intracellular Ca2+.
Previous investigations established that the BKCa channel has a central role in the modulation of nonvascular (26, 37) and vascular contractility (18, 28), as well as in the corpus cavernosum (11, 12, 25). The suggested mechanism of BKCa channel function is linked to the hyperpolarization of smooth muscle cells and a concomitant decrease in transmembrane Ca2+ flux through L-type VDCC (7, 18). These observations indicate the importance of membrane potential in the control of smooth muscle tone and [Ca2+]i. Therefore, ablation of the BKCa channel should lead to an unstable membrane potential, increased Ca2+ influx, and finally to a reduced ability of the smooth muscle to oppose contraction. Indeed, CCSM strips from Slo−/− mice showed an increased contractile response to sympathetic nerve stimulations (Fig. 1). Furthermore, in the presence of the α-adrenergic receptor agonist PE, deficiency or blockade of the BKCa channel induced continuous force oscillations that were not present in Slo+/+ mice. We provide evidence that these oscillations are caused by increased Ca2+ influx through VDCCs because a specific inhibition of the Ca2+ channels completely suppressed force oscillations (Fig. 5). No difference was observed in the overall relaxation through the VDCC inhibition. The small total relaxation of ∼25% is consistent with other reports where transient PE-induced Ca2+ influx was not reduced by removing extracellular Ca2+ but dependent on release from intracellular Ca2+ stores (43). Thus the observed increase of force oscillations is very likely due to membrane potential instability and increased opening of VDCCs but not due to a change in Ca2+ channel expression in CCSM strips from Slo−/− mice or from Slo+/+ mice in the presence of IBTX.
Perspectives and Significance
In vascular smooth muscle of aged rats (20, 29) as well as of humans (38), the BKCa channel α- and β1-subunit expression is decreased. Correlated with this downregulation are increased risks of cardiovascular diseases such as coronary artery vasospasm, myocardial ischemia, and infarct (20, 38). Recently, gene therapy using the gene for the pore-forming BKCa α-subunit (Kcnma or Slo1) has been shown to restore age-related erectile function in vivo in rats (23, 25). Moreover, ED is suggested to be one of the early symptoms of cardiovascular diseases (4); however, it is a complex disease, and its interconnections are not completely understood. Our results support the idea that the BKCa channel could be a very important target in treating patients with ED and other cardiovascular dysfunctions and may help patients who do not respond to the currently available drug therapy.
GRANTS
The work was supported by National Institutes of Health Grants DK-5R01DK053832, 1R01DK-065947, HL-44455, and HL-077378, the Howard Hughes Medical Institute, and the British Heart Foundation (PG/07/115).
Acknowledgments
We thank Anna-Maria Knorn and Denise Bradley for mouse breeding and genotyping.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 273: 32950–32956, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev 75: 191–236, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Archer SL Potassium channels and erectile dysfunction. Vascul Pharmacol 38: 61–71, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Behr-Roussel D, Gorny D, Mevel K, Compagnie S, Kern P, Sivan V, Bernabe J, Bedigian MP, Alexandre L, Giuliano F. Erectile dysfunction: an early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 288: R276–R283, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Benet AE, Melman A. The epidemiology of erectile dysfunction. Urol Clin North Am 22: 699–709, 1995. [PubMed] [Google Scholar]
- 6.Boolell M, Gepi-Attee S, Gingell JC, Allen MJ. Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br J Urol 78: 257–261, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 532–535, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science 257: 401–403, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Burnett AL, Nelson RJ, Calvin DC, Liu JX, Demas GE, Klein SL, Kriegsfeld LJ, Dawson VL, Dawson TM, Snyder SH. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol Med 2: 288–296, 1996. [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang CE, Luk HN, Wang TM, Ding PY. Effects of sildenafil on cardiac repolarization. Cardiovasc Res 55: 290–299, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Christ GJ, Day N, Santizo C, Sato Y, Zhao W, Sclafani T, Bakal R, Salman M, Davies K, Melman A. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol 287: H1544–H1553, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Christ GJ, Spray DC, Brink PR. Characterization of K currents in cultured human corporal smooth muscle cells. J Androl 14: 319–328, 1993. [PubMed] [Google Scholar]
- 13.Hedlund P, Alm P, Andersson KE. NO synthase in cholinergic nerves and NO-induced relaxation in the rat isolated corpus cavernosum. Br J Pharmacol 127: 349–360, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, Fassler R, Andersson KE. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci USA 97: 2349–2354, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Holmquist F, Stief CG, Jonas U, Andersson KE. Effects of the nitric oxide synthase inhibitor NG-nitro-l-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol Scand 143: 299–304, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int 96: 423–427, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J 7: 328–338, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res 88: 210–216, 2001. [DOI] [PubMed] [Google Scholar]
- 21.McAuley IW, Kim NN, Min K, Goldstein I, Traish AM. Intracavernosal sildenafil facilitates penile erection independent of the nitric oxide pathway. J Androl 22: 623–628, 2001. [PubMed] [Google Scholar]
- 22.Melman A, Bar-Chama N, McCullough A, Davies K, Christ G. The first human trial for gene transfer therapy for the treatment of erectile dysfunction: preliminary results. Eur Urol 48: 314–318, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Melman A, Biggs G, Davies K, Zhao W, Tar MT, Christ GJ. Gene transfer with a vector expressing Maxi-K from a smooth muscle-specific promoter restores erectile function in the aging rat. Gene Ther 15: 364–370, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Melman A, Christ GJ. Integrative erectile biology. The effects of age and disease on gap junctions and ion channels and their potential value to the treatment of erectile dysfunction. Urol Clin North Am 28: 217–31, vii, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol 170: 285–290, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Mizusawa H, Hedlund P, Hakansson A, Alm P, Andersson KE. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br J Pharmacol 132: 1333–1341, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol 559: 849–862, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol Cell Physiol 274: C1346–C1355, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Rehman J, Chenven E, Brink P, Peterson B, Walcott B, Wen YP, Melman A, Christ G. Diminished neurogenic but not pharmacological erections in the 2- to 3-month experimentally diabetic F-344 rat. Am J Physiol Heart Circ Physiol 272: H1960–H1971, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 265: C299–C303, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta 1178: 153–175, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Spektor M, Rodriguez R, Rosenbaum RS, Wang HZ, Melman A, Christ GJ. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J Urol 167: 2628–2635, 2002. [PubMed] [Google Scholar]
- 35.Stief CG, Uckert S, Becker AJ, Truss MC, Jonas U. The effect of the specific phosphodiesterase (PDE) inhibitors on human and rabbit cavernous tissue in vitro and in vivo. J Urol 159: 1390–1393, 1998. [PubMed] [Google Scholar]
- 36.Swayze RD, Braun AP. A catalytically inactive mutant of type I cGMP-dependent protein kinase prevents enhancement of large conductance, calcium-sensitive K+ channels by sodium nitroprusside and cGMP. J Biol Chem 276: 19729–19737, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Toro L, Marijic J, Nishimaru K, Tanaka Y, Song M, Stefani E. Aging, ion channel expression, and vascular function. Vascul Pharmacol 38: 73–80, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Turko IV, Ballard SA, Francis SH, Corbin JD. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (type 5) by sildenafil and related compounds. Mol Pharmacol 56: 124–130, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Uckert S, Hedlund P, Waldkirch E, Sohn M, Jonas U, Andersson KE, Stief CG. Interactions between cGMP- and cAMP-pathways are involved in the regulation of penile smooth muscle tone. World J Urol 22: 261–266, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Virag R Intracavernous injection of papaverine for erectile failure (Abstract). Lancet 2: 938, 1982. [DOI] [PubMed] [Google Scholar]
- 42.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J Physiol 567: 545–556, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams BA, Liu C, DeYoung L, Brock GB, Sims SM. Regulation of intracellular Ca2+ release in corpus cavernosum smooth muscle: synergism between nitric oxide and cGMP. Am J Physiol Cell Physiol 288: C650–C658, 2005. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems. Geneva, Switzerland: World Health Organization 1992.