Abstract
The mechanisms whereby maternal nutritional manipulation through pregnancy result in altered blood pressure in the offspring may include changes in fetal and newborn and adult renal prostaglandin (PG) synthesis, metabolism, and receptor expression. Since the postnatal effects of nutrient restriction on the renal PG synthesis and receptor system during nephrogenesis in conjunction with nephron numbers and blood pressure have not been evaluated in the rat, the present study examined the effect of reducing maternal food intake by 50% of ad libitum through pregnancy on young male rats. Six control-fed mothers and eight nutrient-restricted pregnant rats with single litter mates were used at each sampling time point, most of which occurred during nephrogenesis. Offspring of nutrient-restricted dams were lighter from birth to 3 days. This was accompanied by reduced PGE2, with smaller kidneys up to 14 days. Nutrient restriction also decreased mRNA expression of the PG synthesis enzyme, had little effect on the PG receptors, and increased mRNA expression of the degradation enzyme during nephrogenesis and the glucocorticoid receptor in the adult kidney. These mRNA changes were normally accompanied by similar changes in protein. Nephron number was also reduced from 7 days up to adulthood when blood pressure (measured by telemetry) did not increase as much as in control offspring during the dark, active period. In conclusion, maternal nutrient restriction suppressed renal PG concentrations in the offspring, and this was associated with suppressed kidney growth and development and decreased blood pressure.
Keywords: kidney, nutrient restriction, offspring, prostaglandins
maternal nutrient restriction during pregnancy has been previously shown to affect the renal development of the offspring. In the sheep, nutrient restriction targeted to the period of early kidney development subsequently increases organ size as well as affecting kidney shape and promotes glucocorticoid receptor (GCR) mRNA abundance (32). Global nutrient restriction (9) and protein restriction in particular can both reduce the total number of nephrons formed in developing rat kidneys, but this does not necessarily result in raised blood pressure in the offspring (11). The mechanisms by which maternal nutritional manipulation acts to compromise fetal development and ultimately adult health remain uncertain. It has been proposed that this is dependent in part on increased maternal corticosterone concentrations acting directly on the fetus (16). This proposal is supported by the finding that maternal administration of dexamethasone, which crosses the placenta to the fetus during pregnancy, can cause similar cardiovascular outcomes as observed with maternal food deprivation (40); indeed, dexamethasone administration also results in reduced maternal food consumption, suggesting a behavioural effect on appetite (37, 40). Recently, it has been shown that a transient increase in maternal corticosterone on days 14 and 15 of pregnancy in rats can impair development of the intra-renal renin-angiotensin system, thereby leading to reduced nephron number and raised blood pressure in the adult offspring (25). The overall dexamethasone effect, however, is dose dependent. At low doses, it reduces maternal food intake and weight gain, but there is no effect on blood pressure in their adult male offspring (37).
Nephron development in the kidney is dependent on the intra-renal renin-angiotensin system (38, 39) and on the synthesis of intra-renal prostaglandins (PGs). Indeed, studies examining the effects of knocking out or inhibiting PG synthesis indicate they are crucial for normal metanephric development (5, 14). Renal PG endoperoxide H synthase-2 (PGHS-2, COX-2) may also be responsible for renin mRNA expression (34). Interestingly, corticosterone is able to decrease the expression of PGHS-2 in the rat kidney cortex (42), but effects on the degradation enzyme PG dehydrogenase (PGDH) or blood pressure are not known.
The intriguing possibility therefore exists that maternal nutrient restriction decreases intra-renal PG synthesis, metabolism, and receptor levels, perhaps via elevated fetal or newborn plasma corticosterone levels. Should renal PG synthesis or action be suppressed during nephrogenesis, this could ultimately lead to decreased nephron numbers and consequently raised blood pressure in the young adult offspring. Surprisingly, the effects of nutrient restriction during pregnancy on the PG synthesis-receptor system in the kidney during development and into adulthood have not been evaluated in rodents. Our hypothesis was that maternal nutrient restriction through pregnancy would result in postnatal alterations in the renal PG synthesis and receptor system that may then persist into adulthood. These changes would be accompanied by increased plasma corticosterone, increased renal GCRs, and decreased nephron numbers that would be accompanied by a resetting of blood pressure profiles and responsiveness during early adulthood. In this first study, our objective was to examine closely the changes in the PG synthesis-receptor system and compare these with changes in the other parameters studied.
MATERIALS AND METHODS
Animals, diet, and tissue sampling.
All experimental animal work was approved by the University of Alberta Health Sciences Animal Policy and Welfare Committee and followed the Canadian Council on Animal Care guidelines. Fourteen female Long-Evans rats were received at the animal housing facility at 10 wk of age and allowed to acclimatize for 1 wk. Animals were then mated, and pregnancy was confirmed by the presence of sperm in a vaginal smear examined microscopically the following morning. This was considered day 0 of pregnancy, and dams were randomly separated into control (n = 6) or nutrient-restricted (NR; n = 8) groups. Food intake in the control group was measured daily, and 50% of this amount was given to the NR group for the duration of pregnancy. Control dams thus consumed 20 ± 1 g/day at the start of pregnancy and 25 ± 2 g/day near to term, whereas the NR group consumed half this amount. The laboratory rodent diet (LabDiet 5001, Richmond, IN; http://www.labdiet.com) was comprised of 23.4% protein, 10% total fat, 49.9% carbohydrates, and 6.9% ash plus water. All dams were weighed daily. After birth and for the remainder of the experiment, both groups had ad libitum access to food, which was recorded on a daily basis. Each pup was weighed daily for the first 3 wk after birth and then weekly after weaning, during which time food intake was measured daily. At weaning, all dams were euthanized by pentobarbital sodium overdose (100 mg/kg injected into the peritoneum) to enable the weights of hearts, livers, lungs, and kidneys to be assessed.
Shortly after birth, litter sizes were reduced to 10 pups/dam, and the remaining animals were all euthanized by decapitation. These pups were selected depending on their size, with the smallest and largest pups being selected as well as up to three medium-sized animals, depending on litter size, with only one medium sized offspring being used in any kidney analyses. Then, at postnatal day 7, a further two medium-sized pups from each litter were euthanized by pentobarbital sodium overdose. Only male pups from each litter were sampled at postnatal days 14, 21, and 28 since this is coincident with maturation of the hypothalamic-pituitary axis and to avoid any potential for a confounding influence of gender. Then, following blood pressure assessments as described below, each adult male was euthanized at 12 wk of age. For all animals, the brain, heart, liver, lung, and kidney were removed, weighed, and snap frozen in liquid nitrogen then stored at −80°C.
Heparinized blood samples were also taken from selected pups at every sampling point. In the neonatal pups killed by decapitation, this was achieved by collecting trunk blood (∼1 ml) of the medium-sized pups. At all other time points, blood was taken by cardiac puncture once the animals were anesthetized by pentobarbital sodium. Blood samples were immediately centrifuged at 3,000 rpm for 10 min, and the plasma was removed and stored at −20°C. To obtain sufficient plasma for later analyses, plasma from the three pups at day 0 in each litter were combined.
Radiotelemetry monitoring of offspring blood pressure and heart rate.
Twenty-four-hour measurements of systolic, diastolic, and mean blood pressure together with heart rate were made by radiotelemetry (PA-C40 Data Sciences International, St. Paul, MN). This was undertaken in one male animal from each litter that was moved to the surgical facility between 9 and 10 wk of age. They were then allowed at least 7 days to acclimatize before undergoing surgery to enable implantation of a catheter into the aorta and the transmitter attached to the abdominal wall as described by Khan et al. (12). Recordings were then made at least 1 wk after surgery, by which time feed intake of each animal was the same as before surgery. Each animal was placed in a cage adjacent to one of the receiving devices, and, following 12-h acclimatization, continuous recordings were taken over a period of 24 h using WINDAQ acquisition software (Dataq Instruments, Akron, OH). In addition, to measure the animals’ responses to an acute stress exposure, their cages were connected to an air pipe. This pipe was then switched on for a period of 10 s, producing a loud noise and a blast of air into the cage. This test was always carried out between 1300 and 1400 while the animals were resting.
Messenger RNA detection.
Total RNA was extracted from ∼0.1 g of kidney using TRIzol (Invitrogen, Ontario, Canada). Reverse transcription was then performed by adding 2 μg of RNA to an initial mixture (1 μl random primers, 2 μl dNTP mix, 19 μl water) and heating at 65°C for 5 min then chilling on ice. A second mixture was then added (8 μl 5 × RT buffer, 4 μl 0.1 M DTT, 2 μl RNase Out) with either 2 μl Superscript II reverse transcriptase enzyme or 2 μl water and incubated accordingly.
Oligonucleotide primers specific for each gene were optimized for annealing temperature (Table 1). Correct product formation was confirmed by determining size by agarose electrophoresis. PCR reactions of 25 μl were prepared containing 12.5 μl of 2 × SYBR Green, 0.25 μl Amp Erase, 0.5 μl each of 10 mM forward and reverse primers and 10.25 μl water. Briefly, the protocol used for RT-PCR was initial denaturation step (95°C for 10 min) followed by 40 cycles of a denaturation step (95°C for 20 s) and an amplification and quantification step (annealing temperature for 1 min). This was followed by a melt curve step, which involved the incremental (0.5°C/12 s) heating from 55 to 95°C to establish that every product melted at the same temperature, thus confirming that they were all the same. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, was tested as a standard comparator and found to be unaffected by the animal's age and dietary history (e.g., day 0: control 16.9 ± 0.2; NR 16.5 ± 0.4; adult - 16.1 ± 0.4; NR 16.9 ± 0.7 AU). In addition, single samples were run on each plate to assess interassay variation, and all samples were run in triplicate. Both the inter- and intra-assay variations were <10%. Threshold cycle results were normalized to GAPDH expression for each sample and expressed as a percentage of the reference sample run on all plates.
Table 1.
Primer sequences and annealing temperatures used for the detection of mRNA expression
| Primer Set | Product Size (Base Pairs) | Primer Sequence | Annealing Temp., °C |
|---|---|---|---|
| PGHS-1 | 117 | For: 5′-GGA ATT CAA CCA CCT CTA TGA CTC-3′ | 61 |
| Rev: 5′-GAC ACC GTA GTC CAC CAG CAT-3′ | |||
| PGHS-2 | 130 | For: 5′-CCT TGA ACA CGG ACT TGC TCA C-3′ | 62 |
| Rev: 5′-TCT CTC TGC TCT GGT CAA TGG A-3′ | |||
| PGDH | 283 | For: 5′-ATG CAC GTG AAC GGC AAA GTG-3′ | 62 |
| Rev: 5′-TTC ACT CCT GCG TTG TTG ACC-3′ | |||
| EP1 | 98 | For: 5′-AAC TGC TTC GCC TCC TAC-3′ | 60 |
| Rev: 5′-AAC TAC GCA GTG AAC TGG-3′ | |||
| EP2 | 301 | For: 5′-TTC GGA GCA AAA GAA GCC-3′ | 62 |
| Rev: 5′-GAG CGC ATT AGT CTC AGG-3′ | |||
| EP3 | 310 | For: 5′-GCT GTC TGT GCT CGC CTT-3′ | 59.2 |
| Rev: 5′-CCA TAA GCT GGA TAG-3′ | |||
| EP4 | 240 | For: 5′-GGA AGA CTG TGC TCA GTA-3′ | 59 |
| Rev: 5′-GAA GCA AAT TCT TGC CTC-3′ | |||
| GCR | 210 | For: 5′- AGG GAT TCA GCA AGC CAC-3′ | 60 |
| Rev: 5′-CGC CCA CCT AAC ATG TTG-3′ | |||
| GAPDH | 110 | For: 5′-GGC AAG TTC AAT GGC ACA GT-3′ | 60 |
| Rev: 5′-TGG TGA AGA CGC CAG TAG ACT C-3′ |
Primer sequences and annealing temperatures used for the detection of mRNA expression for prostaglandin endoperoxide H synthase-2 (PGHS-2), prostaglandin dehydrogenase (PGDH), prostaglandin receptors EP1-4, and the glucocorticoid receptor (GCR). For, forward; Rev, reverse.
Protein detection.
Protein was extracted from ∼0.1 g of kidney, and Western blotting was then used to determine the abundance of each protein using the antibodies detailed in Table 2. Densitometric analysis was performed using the Flour-S Max software (Bio-Rad), and data was expressed as a percentage of a single reference sample that was run on all gels, except for PGHS-2 and GCR proteins where commercially available standards were run on each gel and used for quantification [Cayman Chemicals, Ontario, Canada (no. 360120) and Santa Cruz, CA (no. 121-420)]. Where standards were not available, antibody specificity was established by comparing band patterns produced with those produced when the antibody was incubated with a specific blocking peptide.
Table 2.
Summary of antibody suppliers and dilutions used for the detection of protein abundance
| Antibody | Company | Catalog Number | Dilution |
|---|---|---|---|
| PGHS-1 | Santa Cruz | H-62 | 1:100 |
| PGHS-2 | Cayman | 160126 | 1:1000 |
| PGDH | Cayman | 160615 | 1:250 |
| EP1 | Cayman | 101740 | 1:250 |
| EP2 | Cayman | 101750 | 1:250 |
| EP3 | Cayman | 101760 | 1:250 |
| EP4 | Cayman | 101770 | 1:500 |
| GCR | Santa Cruz | H-300 | 1:200 |
Summary of antibody suppliers and dilutions used for the detection of protein abundance for PGHS-2, PGDH, EP1-4, and GCR.
Measurement of glomeruli number.
The number of glomeruli was determined using the mild acid-hydrolysis method (31), which enables the number of individual glomeruli to be counted (1). For the kidneys sampled at postnatal days 0 and 7, the entire kidney was incubated in 1 ml of 1 M hydrochloric acid for 30 min at 37°C, whereas for the older animals 1 g of tissue (obtained from the same region in all animals) was used. The acid was then removed, and the tissue was blotted dry, then placed into 1 ml of 50 mM PBS pH 7.4, and homogenized, after which three 20-μl aliquots of the sample were placed on slides, and the number of glomeruli were counted under a ×10 objective lens. All counting was undertaken by the same individual, who was blinded to both the age and nutritional group of the sample. Mean results were averaged and then used to calculate the number of glomeruli for each animal.
PGE2 assay.
PGs were extracted from adult kidney samples by homogenizing 0.1 g tissue in 500 μl of ethanol to which 4 ml of 50 mM citrate buffer (pH 3.5) were then added to reduce the ethanol concentration to <15%. The samples were then centrifuged at 1,250 g for 10 min and then purified using C-18 solid-phase extraction (SPE) Sep-Pak cartridges. The protein content of the pellet was then determined using the Micro BCA protein assay kit, and following homogenization, the PGE2 content was determined using EIA assay (Cayman Chemicals, Ontario, Canada, no. 514010). The intra-assay coefficient of variation was 9%.
Statistical analyses.
All data were analyzed by two-way analysis of variance (SigmaStat, Systat, Point Richmond, CA) with diet and age as the two variables. In addition, for the tissue data at 0 days of age, the additional effect of birth weight was assessed. When a significant F value was obtained, this was followed by a Holm-Sidak post hoc test to differentiate treatment effects. PGE2 assay data were analyzed using the Student's t-test. A P value of <0.05 was accepted as significant.
For the cardiovascular measurements, hourly means from each animal were combined to obtain a 24-h mean of the effects of nutrient restriction on blood pressure and heart rate. The effect of time of day and diet were then determined. These data were analyzed to compare measurements from the light (0600–1800) to dark periods (1800–0600) using a Mann-Whitney U test because of unequal variances. To assess the effect of maternal diet on the circadian variability of blood pressure, the Z score was calculated for each animal during the light and dark periods using the following equation:
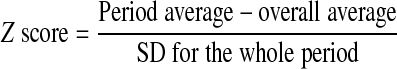 |
RESULTS
Effects of nutrient restriction on maternal body weight, offspring birth weight, and later growth.
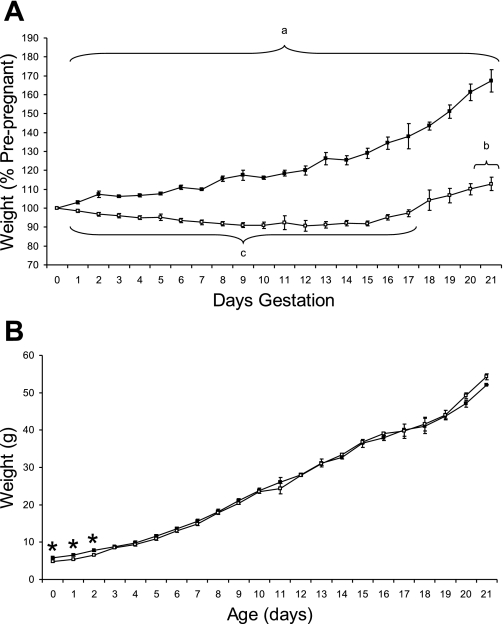
NR dams immediately lost weight and remained lighter than controls throughout pregnancy (Fig. 1A). Controls gained weight through pregnancy, whereas NR dams lost weight until ∼15–16 days gestation. Although all dams gained weight through lactation, NR dams remained lighter, but the weights of all major body organs were similar between groups (data not shown). There was no difference in litter size between groups [control 14.9 ± 0.6 pups/dam (n = 6); NR 14.8 ± 0.3 pups/dam (n = 8)].
Fig. 1.
Effect of maternal nutrient restriction through pregnancy on maternal weight during pregnancy and pup growth over the first 21 days of lactation. A: pregnant rats were either fed ad libitum [control, closed symbols (n = 6)] or 50% of this amount [nutrient restricted (NR), open symbols (n = 8)]. Data are means ± SE. aSignificant difference between control and nutrient restricted. bTime points when nutrient-restricted maternal weight is significantly higher than at start. cTime points when nutrient-restricted maternal weight is significantly lower than at start. B: offspring were born to mothers that were fed either ad libitum [control, closed symbols (n = 6)] or 50% of this amount [NR, open symbols (n = 8)]. Data are means ± SE. *Significant differences between groups (P < 0.05).
Pups born to the NR dams were significantly lighter than controls and remained so until 3 days of age (Fig. 1B). By 21 days of age, the male NR rats tended to weigh more than control rats, a pattern that continued to the end of the study when they remained heavier than controls [controls 348 ± 8 g; NR 396 ± 10 g (P = 0.004)]. The NR males ate significantly more than control males from 6 wk of age until the end of the study [e.g., week 10: control 190 ± 13 g/wk; NR 230 ± 8 g/wk (P = 0.04)].
Effect of NR on offspring organ development.
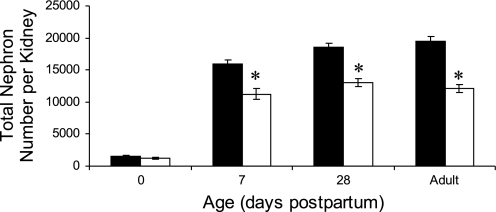
Absolute and relative kidney weights were significantly reduced in NR offspring at birth and 7 and 14 days of age [e.g., 14 days: control 0.37 ± 0.01 g; NR 0.30 ± 0.01 g (P < 0.05): control 1.16 ± 0.02; NR 0.95 ± 0.02% body weight (P < 0.05)] but were not accompanied by any difference in the weights of other organs with the exception of the liver that was only reduced at birth [control 282 ± 23; NR 194 ± 12 mg (P = 0.003)] but not later in development. Furthermore, this decrease in NR kidney weight was found in small, medium, and large offspring (e.g., smallest pup per litter: control 59 ± 4 mg; NR 42 ± 1 mg; P < 0.001). The reduced rate of kidney growth for the first 2 wk after birth was accompanied by a pronounced reduction in nephron number per kidney that persisted into adulthood (Fig. 2).
Fig. 2.
Effect of maternal nutrient restriction through pregnancy on total nephron number in the resulting offspring. Offspring were born to mothers that were fed either ad libitum [control, closed bars (n = 6)] or 50% of this amount [NR, open bars (n = 8)]. Data are means ± SE. *Significant differences between groups (P < 0.05).
Radiotelemetry monitoring of offspring blood pressure and heart rate.
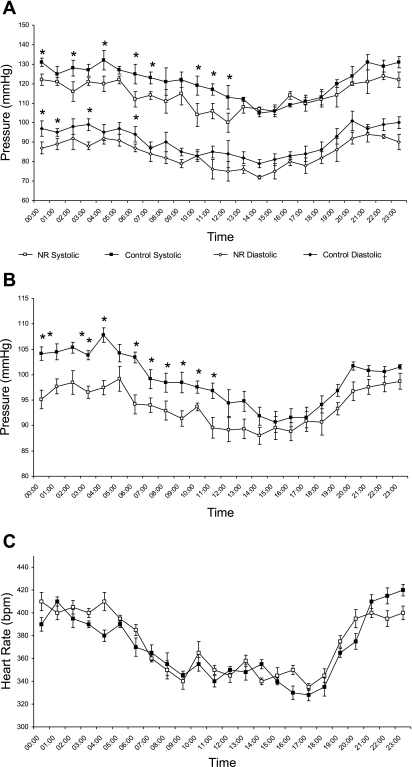
Systolic, diastolic, and mean arterial blood pressures in young male adult offspring were higher at night than during the day and were reduced in NR offspring, largely as a result of the nocturnal rise being blunted (Fig. 3). There was no significant difference in Z score for blood pressure between groups (control 0.6 ± 0.05, NR 0.6 ± 0.04), indicating no effect of maternal diet on circadian variation. Although heart rate was higher at night than during the day, this was not different between nutritional groups (Fig. 3). Measurement of blood pressure response to acute stress between 1300 and 1400 showed no significant difference between nutritional groups in either maximal pressure (control 127 ± 5 mmHg; NR 119 ± 6 mmHg) or magnitude of response (control 38 ± 2 mmHg; NR 33 ± 2 mmHg). Unfortunately, because of the acute nature of this challenge, consistent values for heart rate were not measurable.
Fig. 3.
Effect of maternal nutrient restriction through pregnancy on 24-h systolic and diastolic blood pressure (A), mean blood pressure (B), and heart rate profiles (C) in the resulting male offspring as measured between 11 and 12 wk of age. Offspring were born to mothers that were fed either ad libitum [control, closed symbols (n = 6)] or 50% of this amount [nutrient restricted (NR), open symbols (n = 8)]. Data are means ± SE. *Significant differences between groups (P < 0.05).
PGHS and PGDH expression in the kidney and its PGE2 content.
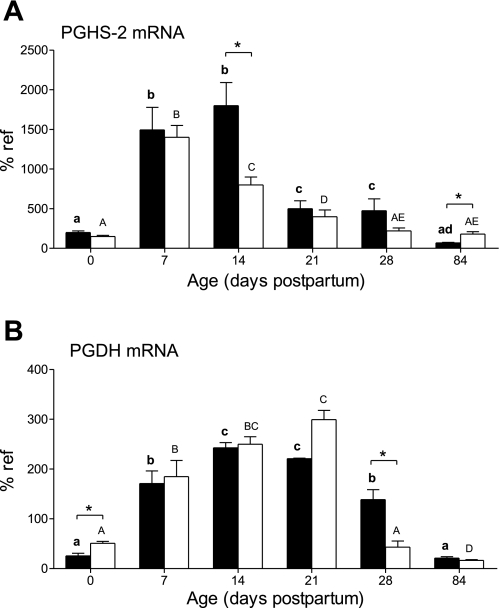
PGHS-2 mRNA (Fig. 4A) and protein (data not shown) increased after birth in the control group, peaked at postnatal day 14, and then decreased to adulthood. In NR animals, the peak in mRNA abundance was earlier, occurring on day 7; then levels significantly decreased (P < 0.05) on postnatal day 14 and thereafter to adulthood. Furthermore, these levels in NR offspring were considerably lower (P < 0.05) than in control offspring on day 14. Both mRNA and protein expression in NR offspring were slightly, but significantly (P < 0.05), higher than in control offspring in adult animals [protein: control 10 ± 2%; NR 18 ± 1% of reference (P < 0.05)]. When cortical and medullary protein expression were analyzed separately in the adult kidney, it was found that they were both significantly increased by maternal nutrient restriction. In contrast, there were no significant differences in PGHS-1 protein expression with age or between nutritional groups (data not shown), whereas from birth and postnatal day 7, the mRNA expression of PGHS-1 significantly increased threefold and then maintained a steady state.
Fig. 4.
Effect of maternal nutrient restriction on expression of prostaglandin endoperoxide H synthase-2 (PGHS-2) (A) and prostaglandin dehydrogenase (PGDH) (B) mRNA. Pregnant rats were fed either ad libitum [control, closed bars (n = 6)] or 50% of this amount [NR, open bars (n = 8)]. Bar graphs illustrate means with their standard errors. Significant differences between control and NR groups: *P < 0.05, and significant differences between ages in the same nutritional group of animals indicated by different superscripts: P < 0.05 (control, bold lowercase letters; NR, capital letters).
Expression of PGDH mRNA (Fig. 4B) and protein (data not shown) increased after birth and then decreased to adult levels. NR animals had raised mRNA abundance at birth and then showed an accelerated decrease in both mRNA and protein expression after 21 days of age so that it was significantly lower at postnatal day 28. Protein abundance then remained unchanged in offspring born to NR mothers so that as adults this was significantly raised [control 70 ± 30%; NR 144 ± 28% of reference (P < 0.05)]. The tissue content of PGE2 in the adult NR animals was significantly lower compared with controls [control 4,630 ± 380 pg PGE2/mg protein; NR 3,120 ± 95 pg PGE2/mg protein (P < 0.05)].
EP expression.
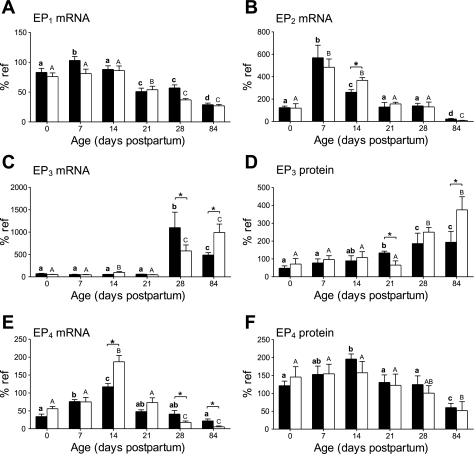
Expression of EP1 mRNA (Fig. 5A) and protein (data not shown) increased after birth and then decreased to significantly lower adult values with no differences between control and NR animals at any time. Messenger RNA (Fig. 5B) and protein (data not shown) abundance for EP2 also increased after birth to then decrease by day 14. This response was delayed in NR animals in which EP2 expression was significantly higher at 14 days of age. Then, as adults, NR animals showed decreased protein expression [control 190 ± 50%; NR 35 ± 14% of reference (P < 0.05)]. Expression of EP3 mRNA (Fig. 5C) did not rise immediately after birth but showed a sudden increase between postnatal days 21 and 28, followed by a decrease to adult levels in control but not NR offspring. EP3 protein abundance (Fig. 5D) showed a more gradual increase in expression, reaching the adult level by day 28. NR animals had a delayed increase in protein expression with blunted increase in mRNA expression and as adults exhibited significantly raised mRNA and protein abundance. Expression of EP4 mRNA (Fig. 5E) and protein (Fig. 5F) increased after birth, then decreased to adult levels. NR animals showed a greater increase in mRNA expression that was significantly higher at day 14 and was followed by a greater decrease in expression so that the adults had significantly lower abundance of mRNA but not protein.
Fig. 5.
Effect of maternal nutrient restriction on expression of the prostaglandin receptors (A), EP1 mRNA (B), EP2 mRNA (C), EP3 mRNA (D), EP3 protein (E), EP4 mRNA (F), and EP4 protein (H). Pregnant rats were fed either ad libitum [control, closed bars (n = 6)] or 50% of this amount [NR, open bars (n = 8)]. Bar graphs illustrate means with their standard errors. Significant differences between control and NR groups: *P < 0.05; and significant differences between ages in the same nutritional group of animals indicated by different superscripts: P < 0.05 (control: bold lowercase letters; NR: capital letters).
GCR expression.
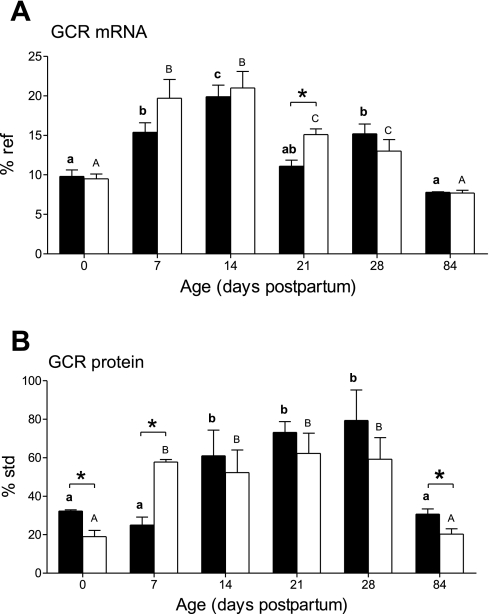
Expression of GCR mRNA (Fig. 6A) increased by day 7, peaked at postnatal day 14, and then decreased to neonatal levels in the adults. The increase in GCR protein (Fig. 6B) occurred later and did not decrease until after postnatal day 28. NR animals had significantly lower GCR protein levels at birth, which then increased to become significantly higher than controls at postnatal day 7 only. They also showed a transiently delayed decrease in mRNA, whereas protein abundance was again significantly raised in adulthood.
Fig. 6.
Effect of maternal nutrient restriction on expression of glucocorticoid receptor (GCR) mRNA (A) and GCR protein (B). Bar graphs illustrate means with their standard errors. Pregnant rats were fed either ad libitum [control, closed bars (n = 6)] or 50% of this amount [NR, open bars (n = 8)]. Significant differences between control and nutrient restricted groups: *P < 0.05; and significant differences between ages in the same nutritional group of animals indicated by different superscripts: P < 0.05 (control: bold lowercase letters; NR: capital letters).
DISCUSSION
The major finding of the present study is that maternal nutrient restriction leads to maternal weight loss and fetal growth restriction, decreased newborn PG synthetic capacity, and decreased blood pressure in young adult males. These changes are accompanied by increased newborn plasma corticosterone (2) that is followed by decreased renal weight and nephron numbers from 7 days of age in conjunction with lower PGE2 concentrations in adult kidneys. These offspring did not then achieve the same elevation of blood pressure relative to the control offspring during the nocturnal (i.e., active period) hours. The elevated plasma concentrations of corticosterone are expected, and the sensitivity of the growing kidney to maternal nutrient restriction is in accord with a number of other studies, but this does not necessarily equate with raised blood pressure (11). We achieved our principal goal of associating changes in NR offspring renal PG synthesis capacity and concentrations with nutrition restriction together with a reduction in blood pressure. Such data suggest the possibility that renal PGs mediate, in part, the effect of NR on blood pressure.
A global reduction in maternal food intake is expected to result in an immediate weight loss in the dam (15, 30). It is therefore not until day 15 of gestation that NR dams start to increase their body weight in conjunction with the rapid rise in feto-placental mass and coincident with the time at which maternal food intake in the control dams increased significantly above non-pregnant intake. Our study is the first to report that NR dams remain significantly lighter than controls throughout lactation despite their food intake being similar. The apparent failure to restore maternal body weight is in contrast to the catch-up growth in the offspring whose weight is the same as controls by 3 days after birth. It is interesting to note that, as with all other studies of this type, litter size is unaffected (30). Taken together, these findings suggest the intriguing possibility that rats have evolved to adapt to adverse nutritional conditions by maintaining the maximum number of pups at the expense of both pup and dam size, with any deficit in fetal growth being overcome during lactation (21), which is the time at which maturation of the hypothalamic-pituitary axis in the offspring occurs. This could explain the critical importance of this stage of development with regard to amplifying later adult outcomes (26).
The reduction in pup birth weight seen in the present study is less marked than other studies, which have used a more severe nutritional challenge, i.e., fed a 70% rather than 50% reduction of ad libitum nutrition (17, 20, 36). Previous studies using a 70% nutrition reduction have demonstrated that both hyperphagia and sedentary behavior patterns can be programmed by maternal nutrient restriction (17, 30). We also observed an increase in food intake of NR offspring and greater weight as adults. Our 50% nutrient restriction diet caused a small but statistically significant reduction in kidney size (both absolute and relative to body weight) at birth, and this observation is consistent with the findings of previous studies of the effect of both low protein (11) and global nutrient restriction in rats (17, 30). Furthermore, we observed that primarily the kidney was affected for the first 2 wk of life, since most other organs were normal during this period. The liver was smaller only at birth but caught up to control weights by day 7. This supports the hypothesis that the kidneys are particularly susceptible to the effects of maternal nutrient restriction and is an adaptation that occurs irrespective of birth weight (32).
In association with their smaller size in the newborn period, the kidneys of the NR animals had significantly fewer nephrons from 7 days after birth to adulthood but not at birth, which was coincident with the time at which plasma corticosterone remained at the relatively high postpartum concentrations in NR offspring, rather than falling as in controls (2). Interestingly, a similar magnitude of maternal nutrient restriction but commencing on day 13 of gestation in Wistar rats led to elevated fetal plasma corticosterone levels on day 21 of gestation and lower placental levels of 11β-hydroxysteroid dehydrogenase type 2 mRNA (16). However, by 2 h after birth, plasma corticosterone was lower in NR offspring compared with controls, although no further determinations were made in older animals.
In the present study, plasma corticosterone concentrations were the same in control and NR offspring at birth (2), indicating they were not obviously higher in NR fetuses compared with controls during late gestation. At day 14, higher plasma corticosterone in postnatal NR offspring was observed (2). It is established that nephrogenesis in the fetal rat begins at 12 days of gestation and is largely complete by postnatal day 14 (35), so elevated plasma corticosterone would only inhibit this process if this occurred before this date. In other species, such as sheep, that have comparable numbers (and between animal variation) of glomeruli to the human (27), are born after a long gestation, and have nephron number set in utero, maternal dexamethasone administration around the time of development of the mesonephros leads to impaired kidney function in later life (6). This can lead to an increase in blood pressure in castrated offspring (6). Interestingly, when maternal nutrient restriction is targeted over a similar period of development although nephron number is reduced, blood pressure is not raised when the offspring remain intact (10), and the kidney is actually protected from the adverse structural and molecular responses to later obesity (33).
In the present study, we only measured blood pressure at one age, i.e., between 10 and 11 wk of age. It is thus possible that the lower values we find in NR offspring from between 0000 and 1200 could increase with age. A majority of studies in the rat, however, show little change in blood pressure after this age under a range of genetic and nutritional programming models (13, 28). One notable exception is found in female offspring born to mothers fed a high-fat diet (i.e., 4 times greater fat content compared with controls) through pregnancy and lactation in which there is no difference in blood pressure at 10 wk of age, but it is higher in these offspring at 6 and 12 mo of age (12). Interestingly, this adaptation is accompanied by an increased body mass with age despite no difference in food intake and has not been shown to date to have any effect on the kidney.
To further establish whether blood pressure responsiveness may have been reset in NR offspring, we subjected each male to a stress challenge and found no difference between groups. This is of interest since it contrasts with the effect of intra-uterine growth retardation induced by uterine artery ligation 4 days before term (22) and may be indicative that blood pressure remains lower in the longer term in NR offspring. One alternative explanation is that they are developing a gradual rise in blood volume, thereby promoting peripheral vasodilatation. Ultimately, this may lead to higher blood pressure. An adaptation of this type is partly in accord with the enhanced rise in blood volume seen in pregnant sheep following maternal nutrient restriction between early and mid gestation followed by normal dietary intake up to term (4). Clearly, additional studies are required to test this hypothesis with regard to the impact of the kidney on blood volume regulation (7) and how they may potentially be reset in utero.
Despite the smaller birth size, elevated corticosterone levels, and appreciably lower number of nephrons, these NR young adult males displayed a lower increase in blood pressure at night than controls as measured by radiotelemetry. Importantly, there was no time point over the 24-h recording period in which blood pressure was raised and is thus in accord with most recent findings in the offspring that are maintained on a low-protein diet through their life cycle that similarly have fewer nephrons (11). Indeed, the magnitude of reduction in nephron number observed in this earlier study as determined using morphometric methods is very similar to our results in offspring born to mothers subjected to a global reduction in food intake through pregnancy. Our additional analyses of the kidney with regard to PGs, EP, and GCR receptors provide some novel insights into the potential mechanisms by which maternal nutrient restriction through pregnancy can impact its development from birth up to adulthood.
The observation that maternal nutrient restriction changes the expression of PGHS-2 is novel. In this regard, the reduction in mRNA expression at day 14 was not matched by protein and could thus be indicative of a slower turnover of protein following maternal nutrient restriction. Any adaptation of this type is transient since by 28 days both mRNA and protein were similarly reduced in NR offspring. By adulthood, however, both these indexes of renal PGHS-2 were raised in the renal cortex and medulla. Similar adaptations were found for PGDH at 28 days and in the adult that was accompanied by a lower PGE2 content in the adult kidney. Taken together, these findings suggest enhanced degradation of PGs in the kidney as seen in offspring born to dams fed a low-protein diet (24).
One potential mechanism by which PGHS-2 abundance was raised in the adult offspring born to NR dams is through elevated plasma corticosterone, which persisted from 14 days of postnatal life through to adulthood. Corticosterone inhibits cortical PGHS-2 (18, 42) but promotes medullary PGHS-2 expression (43), although it is only thought to be significantly expressed in the medulla after weaning (41). An adaptation of this type may therefore have been mediated by the increased GCR in NR adults that may have similarly impacted PGDH expression (8, 19). Interestingly, it was protein and not mRNA abundance for the GCR that was specifically upregulated in the adult NR offspring, which may relate to a slower rate of loss of this receptor with aging in these animals. To further examine the role of corticosterone on kidney development following maternal nutrient restriction through pregnancy, the impact of GCR antagonists could now be explored.
Nutrient restriction had differential effects on EP expression in the developing and adult kidney, with EP1 being unaffected, suggesting it is not involved in the nutritional programming of renal development. Interestingly, the ontogenic decrease in EP2 expression is delayed with nutrient restriction and could reflect a delay in nephron maturation or altered blood flow to the vasa recta (3). With respect to the reduced EP2 protein abundance in adulthood following maternal nutrient restriction, this may be reflective of differences in PGE2 action between the two groups or decreased nephron number as demonstrated in earlier studies (9). In contrast, EP3 abundance was raised in these offspring, and since activation of this receptor concentrates urine, NR offspring could potentially retain more water than their control counterparts. An adaptation of this type has previously been shown to lead to hypertension (17) but is clearly not the case in our study and may relate to the differential effects of raised GCR. The extent to which the accelerated rise in EP4 mRNA abundance and subsequent decline contributed to any functional changes in the kidney of NR offspring is unclear since there were no accompanying effects on protein content. One possible outcome is reduced renin production (23), which is in accord with adaptations found in offspring born to dams fed a low-protein diet through pregnancy (29) that also have fewer nephrons (11).
Perspectives and Significance
A global reduction in maternal food intake through pregnancy does alter growth of the fetus and resulting offspring, with kidney development being specifically compromised. PG synthesis, metabolism, and possibly receptors are associated with renal developmental changes, which may lead to other downstream physiological changes, such as with blood pressure. This nutrition restriction does not, however, contribute to raised blood pressure and indicates that the adaptive capacity of the fetus and newborn to resist later disease is greater than previously appreciated. Future experiments should confirm the mechanisms whereby nutrition restriction alters renal PGs and their consequent actions on renal development and other physiological outcomes.
GRANTS
K. A. Brennan was supported by postgraduate studentships from the University of Nottingham and the Canadian Institutes of Health Research (CIHR) Strategic Training Program in Maternal-Fetal-Newborn Health from the CIHR Institute for Human Development, Child and Youth Health. The work in the laboratory of D. M. Olson is supported by the Canadian Institutes of Health Research.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 48: 2240–2245, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Brennan KA, Olson DM, Symonds ME. Maternal nutrient restriction alters renal development and blood pressure regulation of the offspring. Proc Nutr Soc 65: 116–124, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Dandrea J, Cooper S, Ramsay MM, Keller-Woods M, Broughton Pipkin F, Symonds ME, Stephenson T. The effects of pregnancy and maternal nutrition on the maternal renin-angiotensin system in sheep. Exp Physiol 87: 353–359, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng Vm Collins RJ, Czerniak PM, Gorry S, Trzakos J. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J 16: 1017–1026, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Dunn A, Lo V, Donnelly S. The role of the kidney in blood volume regulation: the kidney as a regulator of the hematocrit. Am J Med Sci 334: 65–71, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Erman A, Pitcock JA, Liston T, Brown P, Baer PG, Nasjletti A. Biphasic effect of dexamethasone on urinary prostaglandins in rats: relation to alterations in renal medulla triglycerides and prostaglandin metabolism. Endocrinology 121: 1853–1861, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Franco MCP, Arruda RMMP, Fortes ZB, Oliveira SF, Carvalho MHC, Tostes RCA, Nigro D. Severe nutritional restriction in pregnant rats aggravates hypertension, altered vascular reactivity, and renal development in spontaneously hypertensive rats offspring. J Cardiovasc Pharmacol 39: 369–377, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishnan G, Gardner DS, Dandrea J, Langley-Evans SC, Pearce S, Kurlak LO, Walker RM, Sweetho I, Keisler DH, Ramsay MM, Stephenson T, Symonds ME. Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. Br J Nutr 94: 938–947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoppe CC, Evans RG, Moritz KM, Cullen-McEwen LA, Fitzgerald SM, Dowling J, Bertram JF. Combined prenatal and postnatal protein restriction influences adult kidney structure, function, and arterial pressure. Am J Physiol Regul Integr Comp Physiol 292: R462–R469, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41: 168–175, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Koeners MP, van Faassen EE, Wesseling S, de Sain-van der Velden M, Koomans HA, Braam B, Joles JA. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension 50: 1077–1084, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Komhoff M, Wang JL, Cheng HF, Langenbach R, McKanna JA, Harris RC, Breyer MD. Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int 57: 414–422, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Leizea JP, Gonzalez CG, Garcia FD, Patterson AM, Fernandez SF. The effects of food restriction on maternal endocrine adaptations in pregnant rats. J Endocrinol Invest 22: 327–332, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 142: 1692–1702, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Lucas SR, Costa SVL, Miraglia SM, FZG. Functional and morphometric evaluation of offspring kidney after intrauterine undernutrition. Pediatr Nephrol 11: 719–723, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Madsen K, Stubbe J, Yang T, Skott O, Bachmann S, Jensen BL. Low endogenous glucocorticoid allows induction of kidney cortical cyclooxygenase-2 during postnatal rat development. Am J Physiol Renal Physiol 286: F26–F37, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Nasjletti A, Erman A, Cagen LM, Baer PG. Plasma concentrations, renal excretion, and tissue release of prostaglandins in the rat with dexamethasone-induced hypertension. Endocrinology 114: 1033–1040, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol 530: 141–152, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature 427: 411–412, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Schreuder MF, Fodor M, Van Wijk JAE, Delemarre-Van De Waal, AH. Association of birth weight with cardiovascular parameters in adult rats during baseline and stressed conditions. Pediatr Res 59: 126–130, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–F433, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Sherman RC, Jackson AA, Langley-Evans SC. Long-term modification of the excretion of prostaglandin E-2 by fetal exposure to a maternal low protein diet in the rat. Ann Nutr Metab 43: 98–106, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, Moritz KM. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol 579: 503–513, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Symonds ME Integration of physiological and molecular mechanisms of the developmental origins of adult disease: new concepts and insights. Proc Nutr Soc 66: 442–450, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Symonds ME, Budge H, Mostyn A, Stephenson T, Gardner DS. Nutritional programming of foetal development: endocrine mediators and long-term outcomes for cardiovascular health. Curr Nutr Food Sci 2: 389–398, 2006. [Google Scholar]
- 28.Thone-Reineke C, Kalk P, Dorn M, Klaus S, Simon K, Pfab T, Godes M, Persson P, Unger T, Hocher B. High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am J Physiol Regul Integr Comp Physiol 291: R1025–R1030, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279: E83–E87, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Welham SJ, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int 61: 1231–1242, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11 beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology 142: 2854–2864, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Williams P, Kurlak LO, Perkins A, Budge H, Stephenson T, Keisler DH, Symonds ME, Gardner DS. Impaired renal function and hypertension accompany juvenile obesity: effect of prenatal diet. Kidney Int 72: 279–289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams SJ, McMillen IC, Zaragoza DB, Olson DM. Placental restriction increases the expression of prostaglandin endoperoxide G/H synthase-2 and EP2 mRNA in the fetal sheep kidney during late gestation. Pediatr Res 52: 879–885, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Wintour EM, Moritz KM. Comparative aspects of fetal renal development. Equine Vet J 24: 51–58, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res 40: 438–443, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Woods LL Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 291: R1069–R1075, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Woods LL, Rasch R. Perinatal ANG II programs adult blood pressure, glomerular number, and renal function in rats. Am J Physiol Regul Integr Comp Physiol 275: R1593–R1599, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol 289: R955–R962, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Yao B, Harris RC, Zhang MZ. Interactions between 11β-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol 288: R1767–R1773, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Zhang MH, Harris RC, McKanna JA. Regulation of cyclooxygenase-2 (COX-2) in rat renal cortex by adrenal glucocorticoids and mineralocorticoids. PNaS 96: 15280–15285, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang MZ, Hao CM, Breyer MD, Harris RC, McKanna JA. Mineralocorticoid regulation of cyclooxygenase-2 expression in rat renal medulla. Am J Physiol Renal Physiol 283: F509–F516, 2002. [DOI] [PubMed] [Google Scholar]