Abstract
Low-volume sprint interval training (SIT), or repeated sessions of brief, intense intermittent exercise, elicits metabolic adaptations that resemble traditional high-volume endurance training (ET). The effects of these different forms of exercise training on vascular structure and function remain largely unexplored. To test the hypothesis that SIT and ET would similarly improve peripheral artery distensibility and endothelial function and central artery distensibility, we recruited 20 healthy untrained subjects (age: 23.3 ± 2.8 yr) and had them perform 6 wk of SIT or ET (n = 5 men and 5 women per group). The SIT group completed four to six 30-s “all-out” Wingate tests separated by 4.5 min of recovery 3 days/wk. The ET group completed 40–60 min of cycling at 65% of their peak oxygen uptake (V̇o2peak) 5 days/wk. Popliteal endothelial function, both relative and normalized to shear stimulus, was improved after training in both groups (main effect for time, P < 0.05). Carotid artery distensibility was not statistically altered by training (P = 0.29) in either group; however, popliteal artery distensibility was improved in both groups to the same degree (main effect, P < 0.05). We conclude that SIT is a time-efficient strategy to elicit improvements in peripheral vascular structure and function that are comparable to ET. However, alterations in central artery distensibility may require a longer training stimuli and/or greater initial vascular stiffness than observed in this group of healthy subjects.
Keywords: high-intensity exercise, intimal media thickness, arterial compliance
the distensibility of the arterial tree has an important regulatory impact on cardiac performance, perfusion, and homeostasis (23). A stiff arterial tree is also associated with adverse cardiovascular events (4). Also, decreased peripheral artery distensibility impacts coronary circulation through quicker pulse wave reflection, which augments systolic pressure while concomitantly lowering diastolic pressure and thus coronary perfusion pressure (39).
Traditional moderate-intensity exercise training improves central artery distensibility in populations with impaired vasculature (19, 43, 46), and most training studies showing improvements of artery distensibility have noted changes in the central arterial tree (aorta or carotid arteries) (19, 21, 43, 46, 49), whereas peripheral muscular arteries commonly show no exercise training-induced improvements (10, 19, 35, 45). However, these investigations of peripheral muscular artery distensibility were made in the relatively stiff common femoral artery (10, 19, 35, 45).
Brachial endothelial function is a surrogate indicator of coronary endothelial function and an independent measure of atherosclerotic disease risk (40, 44, 50). Also, coronary artery disease patients exhibit reduced popliteal artery flow-mediated dilation (2). Similar to artery distensibility, exercise training is a potent stimulus that improves brachial flow-mediated dilation and endothelial function in young healthy (9, 14) and diseased populations (28, 29). The popliteal artery, unlike the brachial artery, is a common site of peripheral vascular disease and displays unique elastic-like properties (13). To date, it has not been established whether exercise training can improve the structural and functional properties of this disease prone artery. In models of integrated vascular physiology, structure and function are tightly linked with decreased peripheral artery distensibility and endothelial function often occurring in concert, thereby creating an environment where disease progression accelerates. A recent review highlights the importance of exercise training in modifying traditional cardiovascular risk factors such as hypercholesterolemia and hypertension (17). However, 40% of the reduction of cardiovascular disease risk attributed to exercise cannot be explained by modifications of the mentioned risk factors (17). Other vascular indexes such as artery endothelial function and distensibility may provide useful information about the link between exercise stimuli and cardiovascular risk reduction (17).
Recently, there has been renewed interest in interval training models, particularly sprint interval (above 100% peak aerobic power) training, because of evidence that the ensuing metabolic adaptations mirror those observed after traditional endurance training (5, 6, 8, 26). High-intensity interval training has been shown to accelerate the kinetic responses of leg blood flow and oxygen uptake at the onset of high-intensity single leg-kicking exercise, indicating a more efficient cardiovascular response system (24). Also, studies in rodents reveal many alterations in vascular structure and function and endothelial nitric oxide synthase (eNOS) protein expression with this type of training (25). However, the mechanisms responsible for these adaptations have not been fully examined. We suspect that changes in vascular structure and function may impact the kinetic responses of skeletal muscle blood flow at the onset of exercise.
Therefore, the purpose of the current study was to evaluate whether 6 wk of high-intensity, low-volume, sprint interval training (SIT) improves central (carotid) artery distensibility and peripheral (popliteal) artery distensibility and endothelial function in the trained legs to the same extent as high-volume, moderate-intensity endurance training (ET). We hypothesized that central artery distensibility would increase to a similar degree with both training methods (ET and SIT). Furthermore, we hypothesized that popliteal artery distensibility would improve in concert with enhanced endothelial function, indicating improved peripheral vascular structure and function. Note that metabolic and performance adaptations to the training protocol have been previously described in a separate publication (7).
METHODS
Subjects.
Twenty young, healthy men and women (n = 5 men and 5 women per group) volunteered for the study (Table 1). A preliminary screening process was employed to establish that subjects 1) were free of risk factors associated with cardiovascular, pulmonary, or metabolic disease; 2) were deemed safe to begin a physical activity program; and 3) other than activities of daily living, were not engaged in a regular training program (i.e., 2 sessions per week and 30 min per session, for at least 1 yr before the study, including recreational activity such as sport or leisure activities). Other exclusion criteria included cardiovascular disease, diabetes, obesity, hypertension (resting blood pressure >140/90 mmHg), medication use, and smoking as assessed through pretesting screening. The experimental procedures and potential risks were fully explained to the subjects before the study, and all subjects provided written, informed consent. Hamilton Health Sciences Research Ethics Board approved the experimental protocol.
Table 1.
Subject characteristics over the course of 6 wk of sprint interval or endurance training
|
Sprint |
Endurance
|
|||
|---|---|---|---|---|
| Pretraining | Posttraining | Pretraining | Posttraining | |
| Age, yr | 23.6±3.2 | 23.0±2.4 | ||
| Height, cm | 171.2±7.3 | 175.2±12.1 | ||
| Weight, kg | 69.1±9.4 | 68.3±8.9 | 75.4±13.3 | 74.9±12.7 |
| BMI, kg/m2 | 23.6±3.0 | 23.3±3.0 | 24.3±2.1 | 24.2±2.0 |
| Heart rate, beats/min | 57±8 | 56±5 | 65±10.0 | 62±8.0 |
| Brachial DBP, mmHg | 63±5 | 63±6 | 66±5 | 65±5 |
| Brachial SBP, mmHg | 112±9 | 114±10 | 124±14 | 121±13 |
| Brachial MAP, mmHg | 80±6 | 80±7 | 85±7 | 83±7 |
Data are means ± SD; n = 10 per group. BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; MAP, mean arterial pressure.
Preexperimental procedures.
Subjects initially performed a progressive exercise test (increasing 1 W every 2 s) on an electronically braked cycle ergometer (Excalibur Sport V2.0; Lode BV, Groningen, The Netherlands) to determine their peak oxygen uptake (V̇o2peak) using an online gas collection system (Moxus Modular V̇o2 System; AEI Technologies, Pittsburgh, PA). The value used for V̇o2peak corresponded to the highest value achieved over a 30-s collection period. All subjects also performed a 30-s test of all-out effort (Wingate test) on the same cycle ergometer against a resistance equivalent to 0.075 kg/kg body mass. After the familiarization procedures, subjects were randomly assigned to either a SIT or an ET group in a matched fashion based on sex and V̇o2peak.
Training protocol.
ET consisted of continuous cycling on an ergometer, 5 days per week (Monday–Friday) for 6 wk, at a power output corresponding to 65% V̇o2peak. Subjects performed 40 min of exercise per training session for the first 2 wk. Exercise time was increased to 50 min per session during weeks 3 and 4, and subjects performed 60 min of exercise per session during the final 2 wk. V̇o2peak tests were readministered after 3 wk of training, and training loads were adjusted to maintain a training intensity equivalent to 65% V̇o2peak. SIT consisted of repeated Wingate tests on an ergometer 3 days per week (Monday, Wednesday, and Friday) for 6 wk. The number of Wingate tests performed during each training session increased from four during weeks 1 and 2, to five during weeks 3 and 4, and finally, to six during weeks 5 and 6. For all training sessions, the recovery interval between Wingate tests was fixed at 4.5 min, during which time subjects cycled at a low cadence (<50 rpm) against a light resistance (30 W) to reduce venous pooling in the lower extremities and minimize feelings of light-headedness or nausea. The ET program was based on general guidelines recommended by a leading public health agency (1), whereas the SIT program was modeled on recent studies conducted in our laboratory that have examined metabolic and performance adaptations to low-volume, high-intensity interval training (5–8, 15). By design, the protocols differed substantially in terms of total training volume and time commitment to evaluate vascular adaptations to two diverse training programs.
Vascular assessment sessions.
All participants arrived at the laboratory at the same time of the day (at all testing sessions) for all vascular assessments. Time of testing was specific to each subject with some participants arriving in the morning and others in the afternoon. Female participants were tested in the same phase of their individual menstrual cycle to control for this potential confounding factor. Because of the pragmatic constraints of scheduling and the need to perform metabolic measurements (7) within a reasonable time, two participants were tested during the luteal phase, whereas the remaining eight were tested during the follicular phase. Furthermore, the two participants tested during the luteal phase were subsequently removed from the endothelial function data set because of poor image quality. Therefore, all female participants included in the endothelial function portion of the experiment were in the follicular phase at both pretraining and posttraining testing time points. Before arriving in the laboratory, participants were instructed to abstain from caffeine, and no participant was taking medication or using nicotine products for at least 12 h. Testing sessions were performed 4 h postprandial following the consumption of a commercially available standardized meal replacement drink (237 ml of BOOST; Mead Johnson Nutritionals, Ottawa, ON, Canada) to control for the acute effects of diet. Measurements were taken while subjects were in the supine position in a temperature-controlled (22–24°C) room. Vascular measurements were conducted twice before the initiation of training and at 48 and 72 h following their final exercise session. All measurements were taken following a 20-min supine rest period. Because no differences were noted between the two pretraining and two posttraining test sessions, the average of these tests was used for subsequent analysis.
Resting heart rate and central and peripheral arterial blood pressure.
Electrocardiography was used to record ventricular depolarization via a three-lead setup, while simultaneous measurements of continuous brachial blood pressure were acquired by automated applanation tonometry (model CBM-7000; Colin Medical Instruments, San Antonio, TX). Both signals were acquired and recorded using commercially available hardware (Powerlab model ML795; ADInstruments, Colorado Springs, CO) and software (Chart 5; ADInstruments).
Direct arterial distensibility.
Measurements of vascular structure and artery distensibility were determined using two methods by the same investigator (M. Rakobowchuk), who has 6 yr of experience imaging the peripheral vasculature in similar research applications. Arterial distensibility in the vessel of interest (carotid or popliteal artery) was assessed directly using a combination of ultrasound imaging (System FiVe; GE Medical Systems, Horten, Norway) for the measurement of lumen diameter and vessel-specific blood pressure via applanation tonometry (model SPT-301; Millar Instruments, Houston, TX) or automated oscillatory cuff. These methods have been described previously (37) but have been modified slightly. Briefly, the same investigator throughout the protocol imaged the carotid artery and used baseline images as visual feedback to ensure similar ultrasound probe placement and imaging of the common carotid artery 2–3 cm proximal to the bifurcation. At the popliteal artery, the designated peripheral artery, measurements were made either proximal or distal to the branching of the middle genicular artery, yet consistent within each subject as verified by visualization of landmarks. This variation between subjects was needed to ensure the highest possible image quality.
Two ultrasound video clips of 10 heart cycles each were acquired at a frame rate of 15 frames/s, simultaneous to measurements of carotid or ankle pulse pressure. Simultaneous to imaging at the carotid artery, a handheld pressure transducer (model SPT-301; Millar Instruments), sensitive to hold-down pressure, was held against the carotid artery to acquire arterial blood pressure waveforms while simultaneous measurements of continuous absolute brachial blood pressure were obtained for the purpose of calibrating the carotid waveforms to diastolic and mean pressures (model CBM-7000; Colin Medical Instruments). The Colin model CBM-7000 device combines blood pressure from a brachial cuff and a wrist sensor to determine beat-to-beat brachial blood pressure. The device calibrates the radial blood pressure waveform to the brachial cuff-derived blood pressure, thus giving the equivalent of beat-to-beat brachial blood pressure. This modification of previous methods (22) simply provides brachial blood pressure for each beat so that carotid blood pressure calibration is beat specific. Briefly, it was assumed that both diastolic (DBP) and mean arterial blood pressure (MAP) are similar in all conduit arteries when an individual is in the supine position, whereas systolic blood pressure (SBP) is amplified through the arterial tree (33). The mean and minimum blood pressure values obtained from the carotid waveform were equated to the MAP and DBP of the radial artery. The maximum blood pressure waveform value recorded in the carotid artery was then used as an extrapolation point from the calibrated MAP and DBP. For popliteal measurements, ankle pulse pressure from two ankle cuff (model CBM-7000, Colin Medical Instruments)-derived measurements was used, because brachial cuff values do not correlate with posterior tibial pressures due to pulse wave amplification.
All video clips used to determine artery distensibility were analyzed by the same investigator using a semiautomated edge detection software program (AMS II; Chalmers University of Technology, Göteborg, Sweden). The 20 measurements of diameter change were subsequently used to calculate distensibility. The following equation was used to calculate distensibility (34):
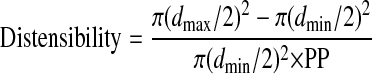 |
where dmax is maximum diameter, dmin is minimum diameter, and PP is pulse pressure.
Vascular structure measurements.
Arterial diameters acquired for arterial distensibility measurements were also used to determine resting vascular structure. Measurements of minimum, maximum, and mean arterial diameter were determined from carotid and popliteal arteries as described above. Mean arterial diameter was determined using a weighted average calculation [1/3(systolic diameter) + 2/3(diastolic diameter)]. Intima-media thickness (IMT) was also determined from carotid images. The average of 20 frames was used to determine IMT from images taken at end diastole, and each frame consisted of between 150 and 200 measures of IMT within a designated region of interest (AMS II; Chalmers University of Technology).
Vascular function of the popliteal artery.
Flow-mediated dilation (FMD) was used to assess vascular function in the legs at the popliteal artery by using a combination of Doppler ultrasound and B-mode imaging. Participants were positioned prone throughout the FMD protocol. Briefly, a pneumatic cuff connected to a rapid inflation system (model E20 and AG101; Hokanson, Bellevue, WA) was placed around the leg 2–3 cm distal to the popliteal fossa. The cuff was inflated to a pressure of at least 250 mmHg to ensure complete occlusion of the popliteal artery. Occlusion was maintained for a period of 5 min. Longitudinal popliteal artery images and blood velocity measurements were made using a 10-MHz (18 participants) or 5-MHz (2 participants) linear array pulse Doppler ultrasound probe (System FiVe; GE Medical Systems) which was positioned ∼3–5 cm proximal to the popliteal fossa, either 2 cm proximal or distal to the branching of the middle genicular artery. This was consistent between testing days within each subject to ensure maximal image quality. All pretesting images were available to the ultrasonographer and displayed on an additional monitor throughout subsequent testing to ensure identical probe placement. Continuous video recording of the image of the popliteal artery was obtained from 15 s before cuff deflation until 4 min after cuff deflation. In addition, a single heart cycle digital video clip was stored at 15-s intervals from 30 s to 4 min after cuff deflation. This digital video clip contained images acquired at a rate of 15 Hz. Simultaneous to the imaging of the popliteal artery, mean blood velocity (MBV) was obtained using the duplex function of the previously described linear array probe from 15 s before cuff deflation until 25 s after cuff release to determine peak and mean postocclusion blood flow and shear rates. The raw audio signal corresponding to blood velocity was output from the Doppler ultrasound system to an external spectral analysis system (model Neurovision 500M TCD; Multigon Industries, Yonkers, NY), which applies a fast Fourier transform (FFT) to the raw audio signal to determine MBV continuously. Blood velocity was corrected for insonation angle during postacquisition analysis. MBV, like all other physiological signals, was acquired and recorded using the previously described Powerlab system.
Image analysis of relative flow-mediated dilation.
With the use of semiautomated analysis software (AMS II; Chalmers University of Technology), diameters at end diastole were acquired from leading edge to leading edge from all images acquired for 4 min postocclusion. The maximal postocclusion end-diastolic value was compared with resting end-diastolic diameters and expressed as a relative change. The following equation was used to calculate relative FMD (12):
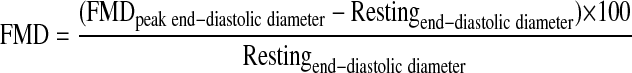 |
Postocclusion reactive hyperemia.
As previously described, blood velocity measurements were acquired 15 s before until 25 s after cuff release. Mean blood flow was calculated (vessel cross-sectional area × MBV) and used to quantify the hyperemic response. Also, mean wall shear rate (MWSR) was determined as:
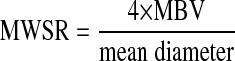 |
Normalized FMD.
Resultant measurements of FMD were normalized to the average MWSR during the first 25 s after cuff release, since the amount of dilation is dependent on the resultant hyperemic flow stimulus as represented by MWSR (36). The following equation was used:
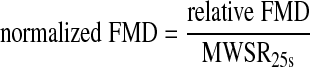 |
where MWSR25s is the mean wall shear rate during the first 25 s after cuff release.
Reproducibility of measurements.
The reproducibility of the measurements in our laboratory was determined in the 20 participants of this study through evaluation of all measures at two time points before training, separated by 5–7 days. All participants underwent identical procedures to those outlined above on both of these testing days. Carotid diameter, pulse pressure, and distensibility showed very good reproducibility with coefficients of variation (CV) of 2, 8, and 8%, respectively. Popliteal diameter, pulse pressure, and distensibility also showed good reproducibility with CV of 2, 8, and 18%, respectively. Measurements of IMT also showed very good reproducibility with a CV of 5%. Relative popliteal FMD also showed reproducibility with a CV of 28% in 16 participants.
Data analysis and statistics.
Data are means ± SD. Measurements acquired twice before and twice after training were averaged. All variables were analyzed using a two-way mixed analysis of variance, with the repeated factor “time” (pretraining vs. posttraining) and the between factor “group” (SIT vs. ET), using commercially available software (SPSS 11.0 for Mac OS X; SPSS, Chicago, IL). Significance for all analysis was set at P ≤ 0.05. Analyses of popliteal parameters were performed on 18 rather than 20 participants because of image quality issues with one participant from each group, whose measurements were removed from the data set. Analysis of FMD was performed on 16 participants rather than 20 because of image quality issues with two participants from each of the groups, whose measurements were removed from the data set.
RESULTS
Evidence of a training effect and average weekly work.
Training increased V̇o2peak, with no difference between groups (SIT: pretraining, 41 ± 2 vs. posttraining, 44 ± 2 ml·kg−1·min−1; ET: pretraining, 41 ± 2 vs. posttraining, 45 ± 2 ml·kg−1·min−1) (7). As previously described, training reduced steady-state exercising heart rate and improved Wingate peak power with no differences between groups (7). The SIT group performed on average 225 kJ of work per week, whereas the ET group performed on average 2,250 kJ of work per week. The average workload for the sprint intervals was ∼500 W, whereas that for the ET workload was ∼150 W.
Heart rate and resting arterial blood pressure.
Resting heart rate (P = 0.16) and brachial blood pressure were not significantly altered (SBP, P = 0.69; DBP, P = 0.38) with training in either group (Table 1).
Arterial distensibility and structure.
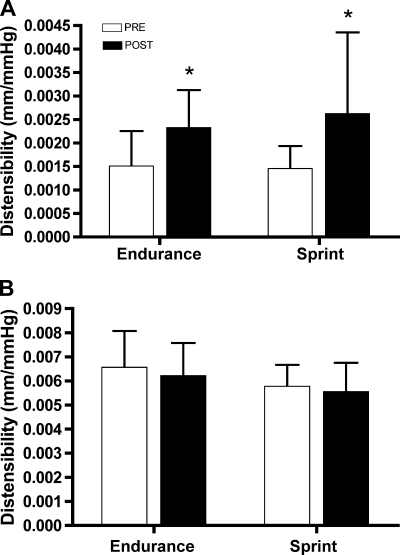
Popliteal artery distensibility was increased after training in both groups (P < 0.01, main effect for time), whereas differences in carotid artery distensibility were not statistically significant (Fig. 1, P = 0.29). Ankle pulse pressure was not statistically altered with training in either group (Table 2, P = 0.41). However, the change in popliteal cross-sectional area within each heart cycle increased after training in both groups (Table 2, P < 0.01). Resting arterial structure, as estimated by mean diameter, was not statistically different with training in either the carotid (P = 0.10) or popliteal arteries (Table 2, P = 0.10). IMT was not statistically different after training (Table 2, P = 0.69).
Fig. 1.
Arterial distensibility of the popliteal (A) and carotid arteries (B) before (PRE) and after (POST) 6 wk of either sprint interval training (SIT) or endurance training (ET). Popliteal artery distensibility was higher after training in both groups, whereas carotid artery distensibility was unchanged. Values are means ± SD; n = 10 per group. *P < 0.05 vs. PRE; main effect for time.
Table 2.
Vascular structure changes with sprint interval or endurance training
|
Sprint |
Endurance
|
|||
|---|---|---|---|---|
| Pretraining | Posttraining | Pretraining | Posttraining | |
| Carotid IMT, mm | 0.43±0.04 | 0.42±0.04 | 0.46±0.06 | 0.46±0.06 |
| Carotid mean diameter, mm | 6.3±0.3 | 6.3±0.3 | 6.2±0.5 | 6.1±0.5 |
| Carotid ΔCSA within heart cycle, mm2 | 7.0±0.4 | 6.6±0.4 | 6.5±0.4 | 6.4±0.4 |
| Carotid PP, mmHg | 39±8 | 40±9 | 42±8 | 43±7 |
| Popliteal mean diameter, mm | 5.5±0.5 | 5.3±0.6 | 5.6±0.8 | 5.4±0.8 |
| Popliteal ΔCSA within heart cycle, mm2 | 2.0±0.5 | 3.0±1.2* | 2.4±1.1 | 3.4±1.4* |
| Ankle PP, mmHg | 60±8 | 61±6 | 64±5 | 66±6 |
Data are means ± SD; n = 10 per group.
P ≤ 0.05 vs. pretraining; main effect for time. IMT, intima-media thickness; CSA, cross-sectional area; PP, pulse pressure.
Vascular function assessed by FMD.
Absolute popliteal artery FMD was improved with training (pretraining: 0.28 ± 0.03 vs. posttraining: 0.36 ± 0.03 mm); however, it did not reach statistically significant levels (P = 0.06). When normalized to resting end-diastolic diameter, popliteal relative FMD increased after training in both groups (Fig. 2A, P = 0.05). The enhanced endothelium-dependent dilation was also apparent in both groups after normalization to postocclusion MWSR (Fig. 2B, P = 0.047).
Fig. 2.
Relative (A) and normalized (B) flow-mediated dilation (FMD) of the popliteal artery before and after 6 wk of either SIT or ET. Relative and normalized popliteal FMD was higher after training in both groups. Values are means ± SD; n = 8 per group. *P < 0.05 vs. PRE; main effect for time.
Resting blood flow in the popliteal artery was not statistically altered with training (Table 3). Postocclusion blood flow and MWSR following cuff release evaluated during the FMD test were also not statistically different (Table 3, P = 0.23).
Table 3.
Flow and shear rate at rest and after cuff release (FMD protocol) before and after sprint interval or endurance training
|
Sprint |
Endurance
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pretraining | Posttraining | Pretraining | Posttraining | |||||
| Rest | ||||||||
| Popliteal MBV, cm/s | 1.2±0.4 | 1.4±0.4 | 1.6±0.8 | 1.6±1.1 | ||||
| Popliteal MBF, ml/min | 17±6 | 18±7 | 25±16 | 24±24 | ||||
| Popliteal MWSR, s−1 | 0.9±0.3 | 1.0±0.4 | 1.2±0.7 | 1.1±0.6 | ||||
| Reactive hyperemia | ||||||||
| Popliteal MBV25s, cm/s | 17.9±1.8 | 16.7±1.5 | 18.4±1.7 | 16.5±1.4 | ||||
| Popliteal MBF25s, ml/min | 244±48 | 226±76 | 250±100 | 216±52 | ||||
| Popliteal MWSR25s, s−1 | 13.5±3.9 | 12.5±2.7 | 13.9±4.8 | 12.8±4.2 | ||||
Data are means ± SD, n = 10 per group. FMD, flow-mediated dilation; MBV, mean blood velocity; MBF, mean blood flow; MWSR, mean wall shear rate. Reactive hyperemic variables are averages during the 25 s following cuff release.
DISCUSSION
To our knowledge, this is the first study to show that both low-volume SIT and traditional high-volume ET improve popliteal artery distensibility and endothelial function to the same extent in young, healthy men and women. As previously reported, the time commitment and total training volumes were much lower with SIT (7) compared with ET. Also, contrary to recent studies of high-intensity training (3, 16), endothelial function improved with SIT. Finally, the increases in V̇o2peak of ∼10% show the effectiveness of both ET and SIT training programs.
Alterations of peripheral arterial distensibility.
The current observation of improved popliteal artery distensibility is unique, because peripheral distensibility is not often measured, and in cases when it has been examined, there was usually no indication of change with exercise training (10, 19, 35). Only one study that evaluated stiffness in the superficial femoral artery showed improvements with training in older participants (48). Possible reasons for the contrasting findings may include differences in exercise modes (rowers) (10, 35) and populations (elderly) (19). Recent evidence suggests the popliteal artery has some structural and functional characteristics of an elastic artery rather than a muscular artery, since it exhibits age-related stiffening (13). The popliteal artery may therefore be a site of significant vascular disease and may be a prime target for exercise intervention.
The mechanisms responsible for training-induced improvements in popliteal artery distensibility may be local alterations in vessel wall structural and/or alterations in vascular tone. Substances such as endothelin-1 (ET-1), nitric oxide (NO), prostaglandins, or reactive oxygen species (ROS) regulate resting vascular tone along side sympathetic output and are influenced by training (16, 27, 49). Specifically, ET has been shown to decrease ET-1 (27), increase basal NO levels (49), and reduce basal ROS levels (16), which combine to improve vascular tone and potentially artery stiffness. Structural alterations such as collagen/elastin ratios, reductions of uncoiled collagen fibers, and fewer frayed elastic fibers may contribute to an altered extracellular matrix and a less stiff artery (53). Finally, basal sympathetic tone, which has been shown to acutely regulate distensibility (42), may be altered. Resting diameters of both the popliteal and carotid artery and resting brachial blood pressure were not statistically different with either exercise training program, limiting the likelihood that basal sympathetic nervous tone accounts for the observed improvements in popliteal distensibility.
Unaltered central artery distensibility.
In central arteries such as the carotid artery, age-associated stiffening is often reversed or attenuated with ET (19, 43, 46, 49) and combined endurance-strength training (10). However, similar to findings of the current study, young sedentary participants do not exhibit differences compared with endurance-trained athletes (46). Our participants had relatively high levels of baseline carotid distensibility compared with older cohorts, leaving little room for improvement with training (46). This is evident from our carotid distensibility measures, which were quite high and similar to those of healthy young subjects previously reported by Miyachi et al. (32) (∼0.007 mm/mmHg).
Structural adaptations: IMT and artery diameter size.
Contrary to our hypothesis, resting central and peripheral artery structure estimated using end-diastolic diameters and carotid IMT were not statistically altered with either training program in the current study. Similar to previous findings, IMT in our group was low at baseline, which likely explains why there was no reduction (47). ET lasting 6 wk was shown previously to result in increased cross-sectional area of the common femoral artery (31). Contrary to these results, our study did not show an increased popliteal artery diameter, which may be related to our observation of improved distensibility or a different time course of adaptation specific to this artery (20).
Endothelial function in the exercised limb.
Popliteal artery endothelial function, another measure associated with cardiovascular disease, was improved equally with both training methods. Relative and normalized FMD increased, whereas the mean hyperemic blood flow response to occlusion was not statistically different with training. Enhanced FMD accompanied by no change in postocclusion blood flow points specifically to a training-induced improvement. Had there been an increase of the shear stimulus following training, the stimulus may have been the cause of increased FMD, rather than exercise training. Mechanistically, improved endothelial function likely relates to reduced oxidative stress (16), an improved antioxidant defense system (38) (circulating and ROS scavenging enzyme capacity), or an upregulation of eNOS gene expression (18). All of these factors would improve NO bioavailability upon shear-induced endothelial stimulation and have been noted previously with training.
The current observation of improved popliteal FMD contrasts with the results of two previous studies in healthy young populations (3, 16) that have shown no improvement (16) or reduced endothelial function (3). In the current study, vascular assessment sessions were conducted at 48 and 72 h following the last training session. This delay before measurement was designed to limit the effects of oxidative stress induced by the final training session on FMD, which has been noted by other researchers following ischemia inducing exercise (30, 41). The stimulus used to evaluate endothelial function was different. Previous studies used invasive measures (intra-arterial ACh infusion) specific to endothelium-derived NO release (3, 16), whereas the current study used flow-mediated shear, which may cause the release of vasoactive substances other than NO in the popliteal artery. Finally, we evaluated endothelial function in the vasculature of the trained limb rather than a nontrained limb, and specifically, a conduit artery rather than the resistance vessels, as was the case with both previous studies (3, 16). It is likely that SIT training in the current study resulted in eNOS protein upregulation specific to this vessel that also contributed to greater local NO bioavailability beyond improved or maintained antioxidant defenses.
Mechanistic insight into improved aerobic performance.
As described previously, V̇o2peak was improved in the current study to the same degree with SIT and ET (7). Structural and functional vascular improvements may contribute to better performance noted previously with high-intensity training (8, 24). Greater peripheral artery distensibility and enhanced endothelium-dependent vasodilation at the onset of exercise may facilitate greater oxygen availability through more efficient blood delivery; however, further study is needed to determine cause and effect.
Study limitations and interpretations.
Although the magnitudes of the adaptations in vascular structure and function were similar between SIT and ET, the required dose of ET needed to improve popliteal artery endothelial function and distensibility is unknown and requires specific dose-response research. We also acknowledge that SIT is not practical for some populations, since it requires high levels of motivation and possibly supervised training facilities. Therefore, further studies that determine ideal interval exercise intensities specific for each disease condition are warranted. In fact, initial studies in patients with coronary artery disease and chronic heart failure have shown that the effectiveness of a high-intensity training regime may be greater compared with traditional ET methods (51, 52), and high-intensity aerobic interval training is now being recommended for several disease populations.
Also, we acknowledge that normalization of FMD to the area under the flow curve until the time of maximal dilation is the optimal method (36). We were unable to capture the full postocclusion blood flow response because of technical limitations; however, given the lack of difference between our pre- and posttraining hyperemic responses (MWSR25s), we believe it is reasonable to assume we have accounted for most of the stimulus through normalization to the shear stimulus for the first 25 s after cuff release.
Perspectives and Significance
Both training protocols improved V̇o2peak, yet the actual total work performed over the period of 6 wk was much different, highlighting the time efficiency of SIT. From a vascular health perspective, this study shows that the beneficial effects of exercise on prognostic indicators of cardiovascular disease, such as peripheral artery distensibility and endothelial function, are modified effectively with either ET or SIT. Extension of interval training has already begun in populations with compromised health, such as those with coronary artery disease (51), chronic obstructive pulmonary disease (11), and chronic heart failure (52). Whether the vascular benefits outlined in the present study are apparent in these populations awaits further attention.
GRANTS
This work was supported by operating grants from the National Sciences and Engineering Research Council of Canada (NSERC; to M. J. MacDonald and M. J. Gibala). M. Rakobowchuk is the recipient of a Canadian Graduate Scholarship from the Canadian Institutes of Health Research. K. A. Burgomaster was supported by an NSERC Canada Graduate Scholarship. K. R. Howarth was supported by an Ontario Graduate Scholarship.
Acknowledgments
We acknowledge the assistance of Lindsay Gurr and Alicia Jury with testing and training.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.ACSM. ASCM's Resource Manual for Guidelines for Exercise Testing and Prescription. New York: Lippincott Williams & Wilkins, 2001.
- 2.Angerer P, Negut C, Stork S, von Schacky C. Endothelial function of the popliteal artery in patients with coronary artery disease. Atherosclerosis 155: 187–193, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bergholm R, Makimattila S, Valkonen M, Liu ML, Lahdenpera S, Taskinen MR, Sovijarvi A, Malmberg P, Yki-Jarvinen H. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis 145: 341–349, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 39: 10–15, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Burgomaster KA, Cermak NM, Phillips SM, Benton CR, Bonen A, Gibala MJ. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol 292: R1970–R1976, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Burgomaster KA, Heigenhauser GJ, Gibala MJ. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol 100: 2041–2047, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586: 151–160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgomaster KA, Hughes SC, Heigenhauser GJ, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol 98: 1985–1990, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol 33: 1379–1385, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Cook JN, DeVan AE, Schleifer JL, Anton MM, Cortez-Cooper MY, Tanaka H. Arterial compliance of rowers: implications for combined aerobic and strength training on arterial elasticity. Am J Physiol Heart Circ Physiol 290: H1596–H1600, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Coppoolse R, Schols AM, Baarends EM, Mostert R, Akkermans MA, Janssen PP, Wouters EF. Interval versus continuous training in patients with severe COPD: a randomized clinical trial. Eur Respir J 14: 258–263, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Debasso R, Astrand H, Bjarnegard N, Ryden Ahlgren A, Sandgren T, Lanne T. The popliteal artery, an unusual muscular artery with wall properties similar to the aorta: implications for susceptibility to aneurysm formation? J Vasc Surg 39: 836–842, 2004. [DOI] [PubMed] [Google Scholar]
- 14.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575: 901–911, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation 108: 530–535, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. First published January 3, 2008; doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed]
- 18.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55: 235–239, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc 38: 445–454, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawano H, Tanaka H, Miyachi M. Resistance training and arterial compliance: keeping the benefits while minimizing the stiffening. J Hypertens 24: 1753–1759, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 80: 1652–1659, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Kingwell BA Large artery stiffness: implications for exercise capacity and cardiovascular risk. Clin Exp Pharmacol Physiol 29: 214–217, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol 559: 335–345, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol 96: 233–244, 2004. [DOI] [PubMed] [Google Scholar]
- 26.MacDougall JD, Hicks AL, MacDonald JR, McKelvie RS, Green HJ, Smith KM. Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol 84: 2138–2142, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci 69: 1005–1016, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Maiorana A, O'Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol 279: H1999–H2005, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Maiorana A, O'Driscoll G, Dembo L, Goodman C, Taylor R, Green D. Exercise training, vascular function, and functional capacity in middle-aged subjects. Med Sci Sports Exerc 33: 2022–2028, 2001. [DOI] [PubMed] [Google Scholar]
- 30.McGowan CL, Levy AS, Millar PJ, Guzman JC, Morillo CA, McCartney N, Macdonald MJ. Acute vascular responses to isometric handgrip exercise and effects of training in persons medicated for hypertension. Am J Physiol Heart Circ Physiol 291: H1797–H1802, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Miyachi M, Iemitsu M, Okutsu M, Onodera S. Effects of endurance training on the size and blood flow of the arterial conductance vessels in humans. Acta Physiol Scand 163: 13–16, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 110: 2858–2863, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Nichols WW, Hartley C, McDonald DA, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretic, Experimental, and Clinical Principles. London: Oxford University Press, 1998.
- 34.O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 15: 426–444, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Petersen SE, Wiesmann F, Hudsmith LE, Robson MD, Francis JM, Selvanayagam JB, Neubauer S, Channon KM. Functional and structural vascular remodeling in elite rowers assessed by cardiovascular magnetic resonance. J Am Coll Cardiol 48: 790–797, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol 102: 1510–1519, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Rakobowchuk M, McGowan CL, de Groot PC, Bruinsma D, Hartman JW, Phillips SM, Macdonald MJ. Effect of whole body resistance training on arterial compliance in young healthy males. Exp Physiol 2005. [DOI] [PubMed]
- 38.Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068–H2076, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Safar ME, Lacolley P. Disturbance of macro- and microcirculation: relations with pulse pressure and cardiac organ damage. Am J Physiol Heart Circ Physiol 293: H1–H7, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 101: 1899–1906, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Silvestro A, Scopacasa F, Oliva G, de Cristofaro T, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis 165: 277–283, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Sonesson B, Vernersson E, Hansen F, Lanne T. Influence of sympathetic stimulation on the mechanical properties of the aorta in humans. Acta Physiol Scand 159: 139–145, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Sugawara J, Otsuki T, Tanabe T, Hayashi K, Maeda S, Matsuda M. Physical activity duration, intensity, and arterial stiffening in postmenopausal women. Am J Hypertens 19: 1032–1036, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 18: 127–132, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol 92: 1458–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Thijssen DH, de Groot PC, Smits P, Hopman MT. Vascular adaptations to 8-week cycling training in older men. Acta Physiol (Oxf) 190: 221–228, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Tordi N, Colin E, Mourot L, Bouhaddi M, Regnard J, Laurant P. Effects of resuming endurance training on arterial stiffness and nitric oxide production during exercise in elite cyclists. Appl Physiol Nutr Metab 31: 244–249, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Vita JA Endothelial function and clinical outcome. Heart 91: 1278–1279, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warburton DE, McKenzie DC, Haykowsky MJ, Taylor A, Shoemaker P, Ignaszewski AP, Chan SY. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol 95: 1080–1084, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086–3094, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 25: 932–943, 2005. [DOI] [PubMed] [Google Scholar]