Abstract
Daily exposure to particulate matter (PM) is known to adversely affect cardiac function and is also known to be exaggerated with senescence. This study tests the hypothesis that cardiac function is uniquely altered by PM exposure in senescent mice. A mechanism for PM-induced cardiac effects is also postulated by examining the activity of nitric oxide synthase (NOS) and the generation of reactive oxygen species (ROS) in heart tissue. Echocardiography is performed in awake 18- and 28-mo-old mice at baseline and immediately following 3-h exposures to either filtered air or carbon black (CB; ∼400 μg/m3) on 4 days. At 28 mo, left ventricular diameter at end-systole and end-diastole is significantly (P < 0.05) elevated, and fractional shortening is significantly reduced (49 ± 3% vs. 56 ± 3%) with CB exposure. In vivo hemodynamic measurements at 28 mo also demonstrate significant (P < 0.05) reductions in ejection fraction and increases in right ventricular and pulmonary vascular pressures following CB exposure. Functional changes at 28 mo are associated with increased ROS production as suggested by enhanced luminol activity. This elevated ROS production with aging and CB exposure is attributable to NOS uncoupling. Measurements of natriuretic peptide (atrial and brain) transcription and matrix metalloproteinase (MMP2 and MMP9) activity in heart tissue are significantly (P < 0.05) amplified with senescence and exposure to CB, pointing to increased cardiac stress and remodeling. These results demonstrate that acute PM exposure reduces cardiac contractility in senescent mice, and this decline in function is associated with increased ROS production linked to NOS uncoupling.
Keywords: aging, mouse echocardiography, cardiac effects of air pollution, reactive oxygen species, nitric oxide synthase uncoupling
epidemiological studies suggest that the association between daily mortality and levels of particulate matter (PM) is attributable to dysregulation of cardiac function; however, specific mechanisms of PM-induced cardiac effects remain uncertain (9, 14, 15, 19, 29, 30, 50). Several studies (15, 47, 48) show ECG changes consistent with myocardial ischemia (i.e., ST segment depression) and ventricular arrhythmias. For example, Peters et al., (47) suggested that acute exposures to increased levels of PM in air pollution are associated with an elevated incidence of angina pectoris and augmented risk of myocardial infarction. More recent epidemiology studies (46, 61, 62) have focused on cardiopulmonary dysregulation, including the role of PM exposure in provoking decompensated congestive heart failure (62). The generalized effects of acute PM exposure also incorporate elevated blood pressure mediated by increases in systemic arterial vascular constriction (8, 57). These effects are principally detected in very elderly subjects older than 65 yr of age (34, 62) and in subjects with severe lung disease (31).
Mechanistic studies demonstrating adverse cardiovascular effects of PM remain somewhat limited. Several investigators have suggested that ultrafine particles (i.e., a size fraction of PM < 0.18 μm) can reach the systemic circulation resulting in enhanced inflammatory and atherosclerotic processes (17, 38, 49). These studies among others (39, 40, 58) have led some to suggest that acute cardiac mortality risk is magnified in the face of elevated PM exposure due to hyperactive platelets and the formation of thrombosis. These events lead to an increased incidence of myocardial infarction (58) and can be abrogated by anti-inflammatory pretreatment. Other mechanistic studies focused on functional and structural changes in the systemic and pulmonary vasculature suggesting that vasomotor alterations occur in humans (7) and animal models (3, 41, 42), following PM exposure. Whereas growing evidence points to direct or indirect effects of PM on the vasculature, fewer mechanistic studies (60) have pointed to specific adverse effects of PM on heart function.
As suggested by Okayama et al. (43), one possible mechanism of PM-induced cardiac functional changes considers the role of reactive oxygen species (ROS). These investigators show that diesel exhaust particle exposures reduce the activity of antioxidant enzymes and lead to a greater superoxide generation in rat cardiac myocytes in a concentration and time-dependent manner. Furthermore, Bai et al. (2) suggested that nitric oxide synthase (NOS)-derived ROS formation is a potential mechanism leading to greater cytotoxicity in pulmonary endothelial cells exposed to diesel exhaust particles (DEP). Other studies suggest that the aging rodent heart is more susceptible to ROS generation (4, 10, 44, 63), particularly following ischemia/reperfusion (44). The age-dependent increase in ROS formation in cardiac myocytes is apparently associated with adverse changes in the aged hearts that reduce diastolic function (27, 63). Therefore, the primary purpose of the present study is to determine a potential mechanism for aged hearts to be more susceptible to acute PM exposure. As a corollary, a greater sensitivity to PM-induced ROS generation in aged cardiac myocytes is a likely basis for adverse cardiac functional changes observed in older individuals exposed to PM. Moreover, changes in NOS3 expression and ROS generation have been shown to be linked and influential to myocardial function (28, 55). For this reason, a secondary hypothesis of the present study is to evaluate the proposition that myocardial NOS3 regulation is altered by PM exposure to a greater extent in aged hearts.
To test these hypotheses, we investigated the acute cardiac functional effects of PM exposure in senescent mice by using repeated echocardiographic and in vivo hemodynamic measurements. Although there are many investigations using these functional measurements in mouse models (e.g., see Refs. 35 and 55), studies on the acute cardiac effects of PM have not been explored. Since epidemiological studies (34, 62) have consistently shown that very elderly subjects are uniquely susceptible to PM-induced cardiac events, the present study is focused on the interactions of aging and PM exposure as mice approach the limits of their life span. Specifically, our experimental design focused on an age comparison between very old (28 mo) and middle-aged (18 mo) mice. These older age groups were chosen for repeated echocardiographic measurements before and immediately after acute PM exposures based on the principles of rodent use in gerontological research outlined by Miller and Nadon (36). In addition, we measured increased ROS production derived from altered NOS3 activity in association with adverse changes in cardiac function with aging and PM exposure. The basis for this aim is consistent with recent evidence suggesting that effects of ROS production on altered cardiac function is ameliorated by blocking NOS activity or knocking out the NOS3 gene (55). The results of the present study suggest that acute PM exposure leads to reductions in cardiac function in senescent mice, including lowered myocardial contractility. Likewise, these PM-induced cardiac functional changes in senescent mice are associated with increased ROS production derived from NOS3 uncoupling at the level of isolated cardiac myocytes. These functional and cellular changes induced by carbon black (CB) exposure were reversed in senescent animals with tetrahydrobiopterin (BH4) treatment.
METHODS
Animals.
Male mice from three different strains (i.e., C57BL/6, C3H/HeJ, and B6C3F1 mice) were purchased and housed at the Johns Hopkins Bloomberg School of Public Health. The C57BL/6 mice were obtained from the National Institute of Aging, and the other two strains were obtained from the Jackson Laboratory (Bar Harbor, ME). The environmental conditions (e.g., 12:12-h light-dark cycle, room temperature, etc.) before and during the following experiments and the animal handling were highly standardized. Water and mouse chow (Agway Pro-Lab RMH 1000) were provided ad libitum. All animal protocols were reviewed and approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutes.
Echocardiography.
The method described below was approved by the American Echocardiography Society. Before each echocardiography study, mice were habituated to handling on several occasions to reduce any stress that occurred during the experiment, allowing for the echocardiographic measurements to be performed in conscious, unanesthetized mice. Each mouse was gently held in the palm of the hand by securing the skin of the dorsal neck with the thumb and the index finger, and securing the tail between the handler's last two fingers. While in the supine position, the left hemithorax was shaved, and prewarmed hypoallergenic ultrasonic transmission gel was applied to the thorax. A 1- to 2-mm-thick layer of gel was applied to improve the ultrasonic penetration and the quality of images. Animal handling care was taken to minimize excessive pressure of the transducer on the chest wall and to avoid reflex bradycardia. The mouse was held on a steady platform to achieve an angle for the transducer consistent with the left lateral decubitus position for imaging.
Echocardiography was performed using a Sequoia C256, (Siemens, Mountain View, CA) with the 15-MHz linear array transducer. Generally, the mouse heart was first imaged in the two-dimensional mode in the parasternal short axis view at a sweep speed of 200 mm/s and a depth of 20 mm. From this view, an M-mode cursor was positioned perpendicular to the interventricular septum, and the left ventricular (LV) posterior wall thicknesses and chamber dimensions were measured. Three to five sample recordings were obtained from each mouse before (pre) and again, following 4 days of exposure (post) to either filtered air (FA) or CB. The same research technologist trained in cardiac echocardiography was devoted to performing the quantification of the images. The technologist was blinded to the experimental groups, and the data were retrieved from sample recordings and averaged for each measurement. The LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), and posterior wall thicknesses at end diastole (PWTED) were measured from a stable M-mode tracing. Fractional shortening (FS) and relative wall thickness (RWT) was computed as the percent change in LV cavity dimensions as shown in the following equations: FS (%) = [(LVEDD − LVESD)/LVEDD] × 100 and RWT (%) = [PWTED/ LVEDD] × 100.
From groups of 18- and 28-mo-old mice (n = 15 mice/age group) subjected to repeated echocardiography measurements, reproducibility tests were performed to evaluate intraobserver, interobserver, and test-retest variability as described elsewhere (52, 64). In Table 1, the intraobserver, interobserver, and test-retest variabilities were comparable to those previously reported for echocardiography (64). The smallest errors (i.e., % SD of the error) were seen in the measurements of LVEDD and HR among the three reproducibility tests. The largest errors were seen in the measurements of PWTED and RWT for the intraobserver and interobserver variabilities. In addition, the correlation coefficients were generally very robust.
Table 1.
Reproducibility in echocardiography among 18- and 28-month old mice
| r-Value | Mean ± SD | Mean ± SD Difference | Mean ± SD Difference, % | |||||
|---|---|---|---|---|---|---|---|---|
| Intraobserver Variability, n = 24 | ||||||||
| LVEDD, mm | 0.95 | 3.48±0.38 | −0.001±0.13 | 0.08±3.8 | ||||
| LVESD, mm | 0.90 | 1.50±0.29 | −0.02±0.14 | −1.93±9.9 | ||||
| PWTED, mm | 0.66 | 1.00±0.13 | 0.01±0.10 | 0.73±10.1 | ||||
| FS, % | 0.73 | 57.0±6.3 | 0.83±4.7 | 1.40±8.3 | ||||
| RWT, % | 0.72 | 28.1±4.6 | 0.15±3.5 | 0.65±12.3 | ||||
| HR, beats/min | 0.81 | 628±44 | 1.1±28 | 0.23±4.6 | ||||
| Interobserver Variability, n = 24 | ||||||||
| LVEDD, mm | 0.93 | 3.55±0.41 | −0.07±0.15 | −1.98±4.6 | ||||
| LVESD, mm | 0.91 | 1.53±0.34 | −0.02±0.15 | −1.22±9.4 | ||||
| PWTED, mm | 0.71 | 0.97±0.11 | 0.01±0.09 | 0.82±9.2 | ||||
| FS, % | 0.84 | 57.1±6.9 | −0.18±4.2 | 0.14±8.9 | ||||
| RWT, % | 0.79 | 27.7±4.2 | 0.90±2.9 | 2.79±10.0 | ||||
| HR, beats/min | 0.93 | 632±41 | −1.9±16 | −0.27±2.4 | ||||
| Test-Retest Variability, n = 15 | ||||||||
| LVEDD, mm | 0.99 | 3.51±0.24 | −0.002±0.03 | −0.03±0.9 | ||||
| LVESD, mm | 0.98 | 1.50±0.24 | −0.02±0.05 | −1.11±3.5 | ||||
| PWTED, mm | 0.96 | 0.97±0.09 | 0.004±0.03 | 0.37±2.9 | ||||
| FS, % | 0.98 | 57.5±6.1 | 0.67±1.4 | 1.25±2.4 | ||||
| RWT, % | 0.98 | 27.8±3.7 | 0.13±0.7 | 0.40±2.8 | ||||
| HR, beats/min | 0.20 | 632±25 | −20.2±30 | −3.23±4.8 | ||||
Data are means ± SD. The variability among the types of reproducibility tests was determined by averaging the differences between paired observations, and by dividing the differences by the average of the paired observations expressed as a percentage. LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; PWTED, posterior wall thickness at end diastole; FS, fractional shortening; RWT, relative wall thickness; HR, heart rate.
CB exposure.
A CB (Regal 660; density of 1.95 g/cm3; specific surface area of 112 m2/g) aerosol was generated using a Wright dust feed particle generator (BGI, Waltham, MA). Animals were exposed to either CB or FA for 3 h on four separate exposure days; two weekly sessions of two consecutive days were conducted to allow for intermediate echocardiography measurements. During each 3-h exposure, animals were individually placed in a 200-ml cylindrical Plexiglas holding chamber. The mass median aerodynamic diameter of the CB aerosol was measured using an aerodynamic particle sizer (APS; TSI, Atlanta, GA). While the CB aerosol generated by this method was polydispersed in terms of particle size, the majority of particles generated (i.e., number concentration) were in the respirable range (fine mode: 0.1 to 1.0 μm). Prior to the exposure studies, gravimetric sampling was used to determine the CB concentration as a time-weighted average. Personal environmental monitors (SKC, Eighty Four, PA) were placed in individual exposure chambers and CB particles were collected on 37-mm filters (2-μm pore size membrane filters) for 3 h. The flow rate through the filter was 4 l/min, and impactors were used with cut points for 2.5 μm and 10 μm. The weight of the filters before and after sampling was determined using a Mettler Toledo balance. From a collection of 47 gravimetric samples, the average concentration was 401 ± 46 μg/m3 for PM2.5 concentrations and 553 ± 49 μg/m3 for PM10 concentrations. Average particle distribution results, obtained using the APS and showing mass and number concentration as a function of particle size, have been reported elsewhere (56). Subsequent studies to determine the effects of PM on in vivo hemodynamics and molecular parameters used an identical exposure dose and duration as described above. All outcome measurements were performed within 12 h following the final exposure.
In vivo hemodynamics.
Mice 28 mo of age were exposed to either FA or CB (n = 4 mice per treatment) for 4 days following the same sequences as described above. Immediately following exposure, in vivo right ventricular (RV) and LV function and vascular pressures were measured using a pressure-volume (PV) catheter as described previously (5, 18, 54). Mice were anesthetized with 1–2% isoflurane, urethane (0.8–1.0 mg/g ip), etomidate (5–10 μg/g ip), and morphine (1–2 μg/g ip) before undergoing tracheostomy. Mice were ventilated at a respiratory rate of 130 breaths/min and a tidal volume of 6–7 μl/g. Blood volume was maintained using 12.5% albumin (50–100 μl in 5 min) delivered through a 30-gauge cannula inserted in the right external jugular vein. The ventricular wall was exposed through an incision between the seventh and eighth ribs. A 0.42-mm-diameter PV catheter was advanced through the apex to lie along the longitudinal axis. The absolute volume was calibrated, and PV data were measured at steady state.
Determination of NOS3 activity.
LV tissue was obtained from five mice of each age group exposed to either CB or FA exposure to conduct the following assays. NOS3 activity was immunoprecipitated as previously described (32), and samples were added to Laemmli buffer on a nonreducing gel to identify dimer dissociation due to reduced disulfide bonds. Denatured control lanes were generated by boiling samples for 15 min prior to loading. Electrophoresis was performed using Tris glycine 6% gels, which were maintained at 4°C. Ca2+-dependent and -independent NOS activity was assayed by [3H]-l-arginine to [3H]-l-citrulline conversion (Sigma-Aldrich) by using myocardial homogenates. The Ca2+-dependent NOS activity principally reflects NOS3 activity.
Adult cardiac myocytes were isolated as previously described (54). From these isolated myocytes, SDS-resistant NOS3 dimers and monomers were assayed using low-temperature SDS-PAGE under nondenaturing conditions, NOS3 was immunoprecipitated, and the resulting samples were added to fivefold Laemmli buffer (0.32 mol/l Tris·HCl, pH 6.8, 0.5 mol/l glycine, 10% SDS, 50% glycerol, and 0.03% bromophenol blue) in nonreducing gel (without 2-mercaptoethanol) to identify dimer dissociation due to reduced disulfide bridges.
ROS production and nitrotyrosine determination.
Several methods were performed to measure ROS production. The LV samples were also assayed by luminol-enhanced chemiluminescence (EMD Biosciences). Tissue was flash-frozen and homogenized in iced PBS buffer and centrifuged. The precipitate was resuspended in an assay buffer with 100 μM of luminol following the manufacturer's instructions. To evaluate the role of NOS-uncoupling in ROS generation, the tissue extracts subjected to luminol chemiluminescence assay were done with or without coincubation with the NOS inhibitor, NG-nitro-l-arginine methyl ester (l-NAME) (1 mM). A similar assay was performed in which LV tissue obtained from 28-mo-old mice exposed to CB was coincubated with 1 μM of the selective NOS2 inhibitor, GW274150 (Alexis Biochemicals, San Diego, CA) (1). Data were normalized by sample weight.
Fresh-frozen LV tissue was also assayed for nitrotyrosine (NT) expression (polyclonal NT Ab; 1:100: Upstate Biotechnology), which reflects the ROS production associated with the formation of peroxynitrite (ONOO−) species. NT was quantitatively determined by ELISA assay (Oxford International).
Cardiac gelatinase assay.
Gelatin lysis by matrix metalloproteinase 2 and 9 (MMP2 and MMP9) was also measured in heart tissue lysates by zymography. Modified Laemmli buffer was added to lysed samples and loaded on a 10% gelatin gel (Invitrogen). After electrophoresis, gels were washed with renaturing buffer at room temperature followed by a developing buffer and then stained to visualize lytic bands (SimplyBlue; Invitrogen). This procedure was repeated on LV tissue homogenates as well as isolated cardiac myocytes.
Atrial and brain natriuretic peptides quantitative PCR analysis.
RNA samples were prepared from snap-frozen hearts using Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA was extracted per manufacturer's instructions, and each sample was subsequently treated with RNase-free DNase I (Roche, Indianapolis, IN) to remove traces of genomic DNA. Real-time quantitative PCR (qPCR) was performed using a 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Duplicates of each sample were subjected to reverse transcription (48°C for 30 min) and standard multiplex real-time qPCR (95°C for 10 min followed by 50 cycles of 95°C for 15 s and 60°C for 60 s) using TaqMan One-Step RT-PCR Master Mix Reagents (Applied Biosystems). Levels of atrial and brain natriuretic peptides (ANP and BNP) expression were normalized to GAPDH levels measured for each respective sample and expressed graphically as the percent of younger control values. To substantiate whether or not changes in expression levels corresponded to increased peptide concentration in the blood, plasma levels of BNP were determined using ELISA (Phoenix Pharmaceuticals, Burlingame, CA).
Determination of PKG-1 activity.
Whole heart tissues lysates were analyzed for PKG-1 activity using colorimetric methods (CycLex) according to the manufacturer's specifications and read on a Spectramax M5 reader (Molecular Devices). Activation of PKG-1 reflects the primary downstream effector kinase for cGMP in cardiac myocytes, and is activated in the presence of NO and natriuretic peptides.
BH4 treatment.
An additional group of 28-mo-old mice were treated with oral BH4 (1 mg/g food; Sigma-Aldrich) mixed with their rodent chow providing 5 mg/day based on 4–6 g daily diet, as described previously (55). Treated (n = 5) and control (n = 5) mice were then exposed to CB using a similar protocol, and in vivo hemodynamics were performed following exposure. Heart tissue and isolated myocytes were tested for ROS generation, NOS and PKG-1 activity and NOS dimerization.
Data analysis.
The values reported in the tables and figures are means ± SE. A comprehensive analysis of detectable differences between strains of mice showed no statistical significance. Therefore, two-way analyses of variance were performed using age groups (18 vs. 28 mo of age), and exposure (CB vs. FA) as main effects to evaluate statistically significant changes in cardiac molecular assays and functional parameters obtained by echocardiography. Post hoc mean comparisons between average data obtained within each exposure were compared using paired (echocardiograph results) and unpaired (molecular results) t-tests. Unpaired t-tests were performed to analyze the in vivo hemodynamic results. Statistical significance was established at an α-level of 0.05.
RESULTS
Echocardiographic results.
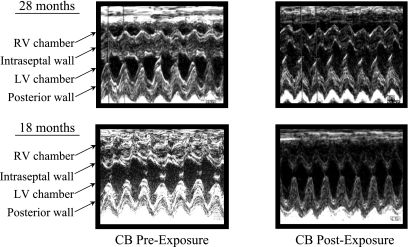
In Fig. 1, representative echocardiographic images are shown in mice at 18 and 28 mo of age prior to and following CB exposure for 4 days. In the 28-mo-old mice, there is a visible RV dilation that is not apparent in the 18-mo-old mice prior to CB exposure. Also, the interventricular septal wall appears more flattened and desynchronized during LV contraction with the posterior wall at 28 mo compared with 18-mo-old mice. These same characteristics appear to be accentuated following CB exposure. The interventricular septal wall (IVSW) thicknesses at end systole supports the visual observations. There is a significant (P < 0.05) age-dependent depression in IVSW following FA exposure at 28 (1.75 ± 0.07 mm) vs. 18 mo (2.00 ± 0.10 mm) old, which is significantly (P < 0.05) exacerbated following CB exposure in only 28-mo-old mice (1.61 ± 0.07 vs. 1.83 ± 0.06 mm).
Fig. 1.
Representative echocardiographic images for 18- and 28-mo-old mice prior to and following 4 days of carbon black (CB) exposure for 3 h/day. The image at 28 mo shows noticeable right ventricular (RV) dilation that is not apparent in the 18-mo-old mice prior to CB exposure. Also, the intraventricular septal wall appears to be flattened in the older mice before exposure and appears desynchronized during left ventricular (LV) contraction with the posterior wall following CB exposure.
The average echocardiographic parameters for the two age groups prior to exposure (preexposure) are reported in Table 2. With the exception of a significantly (P < 0.01) greater LVEDD at 18 mo compared with 28-mo-old mice, there are no detectable age-dependent differences in cardiac function before exposure.
Table 2.
Preexposure echocardiographic parameters between 18- and 28-mo-old mice
| Parameters | 18 mo | 28 mo | %Difference | t-test (P values) |
|---|---|---|---|---|
| BW, g | 33.9±2.0 | 30.9±1.4 | −8.9 | 0.22 |
| LVEDD, mm | 3.62±0.1 | 3.23±0.1 | −10.8 | 0.01† |
| LVESD, mm | 1.62±0.13 | 1.45±0.07 | −10.5 | 0.25 |
| PWTED, mm | 0.97±0.02 | 0.97±0.03 | 0.0 | 0.86 |
| FS, % | 55.7±3.0 | 55.1±1.5 | −1.1 | 0.86 |
| RWT, % | 27.1±1.0 | 30.3±1.4 | 11.8 | 0.07 |
| HR, beats/min | 625±14 | 613±10 | −1.9 | 0.46 |
Data are means ± SE; n = 15 mice/age group.
P < 0.05, 28 mo vs. 18 mo.
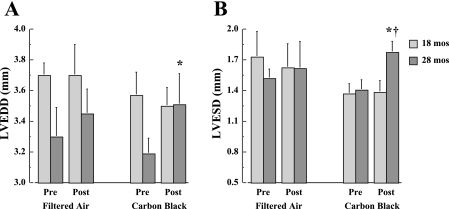
In Fig. 2, LVEDD and LVESD results are shown before and after FA or CB exposure in 18- and 28-mo-old mice. There are significant (P < 0.05) increases in both LVEDD and LVESD following CB exposure in 28-mo-old mice. This change is not apparent following FA exposure, and is not observed in the 18-mo-old groups.
Fig. 2.
Average values for LV end-diastolic (LVEDD; A) and end-systolic (LVESD; B) diameters are depicted for 18- and 28-mo-old mice (n = 15 mice/age group) before and after 4 exposure days each for 3 h/day to either filtered air (FA) or CB. *P < 0.05, vs. preexposure; †P < 0.05, 28 mo vs. 18 mo.
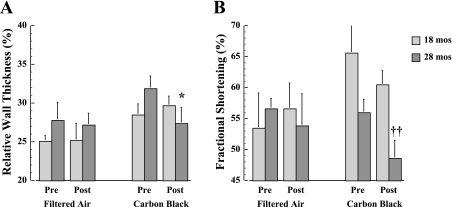
In Fig. 3, RWT and FS results are shown before and after FA or CB exposure in 18- and 28-mo-old mice. There is a significant (P < 0.05) decrease in RWT unique to the 28-mo-old mice following CB exposure (Fig. 3A). This CB-induced change is attributable to the significant increase in LVEDD without a corresponding thinning of the PWTED (data not shown). The results in Fig. 3B demonstrate that FS is significantly (P < 0.01) lower at 28-mo-old compared with 18-mo-old mice following CB exposure. There are no detectable (P > 0.05) age-dependent differences in FS prior to CB exposure or with FA exposure.
Fig. 3.
Average values for relative wall thickness (A) and fractional shortening (B) are depicted for 18- and 28-mo-old mice (n = 15 mice per age group) before and after 4 exposure days each for 3 h/day to either FA or CB. *P < 0.05, vs. preexposure; ††P < 0.01, 28 mo vs. 18 mo.
In vivo hemodynamic results.
The measurements of in vivo hemodynamics in 28-mo-old mice exposed to either FA or CB are reported in Table 3. There are no significant (P > 0.05) differences in systemic arterial pressures between exposure groups. In contrast, the right atrial and ventricular pressures are significantly (P < 0.05) elevated in 28-mo-old mice exposed to CB relative to FA. Likewise, the pulmonary arterial pressure and vascular resistance are also significantly (P < 0.05) elevated in CB-exposed mice. The results also show that LV and RV contractility is significantly (P < 0.05) reduced in CB-exposed senescent mice. Specifically, the ejection fraction (EF) and maximun change in pressure over time (dP/dtmax) are attenuated following CB exposure in 28-mo-old mice.
Table 3.
In vivo hemodynamics in 28-mo-old mice exposed to filtered air (FA) or carbon black (CB)
| FA | CB | |||
|---|---|---|---|---|
| LV Pressures and Volumes | ||||
| Systolic pressure, mmHg | 105±3 | 102±8 | ||
| Diastolic pressure, mmHg | 77±5 | 70±9 | ||
| Mean systemic arterial pressure, mmHg | 84±6 | 75±7 | ||
| End-systolic volume, ml | 16.8±1.7 | 20.1±1.4* | ||
| End-diastolic volume, ml | 31.0±1.8 | 38.2±1.9* | ||
| PWRmax/EDV, mmHg/s | 28.6±0.5 | 22.0±1.1* | ||
| dP/dtmax, ms | 13429±488 | 10231±508* | ||
| Ejection fraction, % | 64.5±2.7 | 56.0±3.1* | ||
| Tau, ms | 6.5±0.4 | 7.0±0.8 | ||
| Heart rate, beats/min | 602±22 | 631±26 | ||
| Cardiac output, ml/min | 13.9±1.3 | 14.6±2.7 | ||
| Total peripheral resistance, mmHg·ml−1·min−1 | 5.96±0.6 | 5.18±0.7 | ||
| Heart weight, mg | 147±4 | 149±5 | ||
| RV Pressure-Volume Loop Results | ||||
| RV systolic pressure, mmHg | 14.3±0.5 | 22.4±0.6* | ||
| RV diastolic pressure, mmHg | 3.1±0.3 | 5.4±0.3* | ||
| Mean right atrial pressure, mmHg | 4.0±0.4 | 4.9±0.2* | ||
| dP/dtmax, ms | 2943±124 | 2486±97* | ||
| dP/dt/IP | 254±14 | 208±20* | ||
| Tau, ms | 7.1±0.2 | 9.6±0.4* | ||
| Pulmonary Pressures | ||||
| Systolic pulmonary arterial pressure, mmHg | 13.3±0.5 | 23.6±0.6* | ||
| Diastolic pulmonary arterial pressure, mmHg | 8.0±0.4 | 14.6±0.5* | ||
| Mean pulmonary arterial pressure, mmHg | 10.1±0.4 | 17.4±0.6* | ||
| Mean pulmonary arterial wedge pressure, mmHg | 4.0±0.4 | 5.7±0.3* | ||
| Pulmonary vascular resistance, mmHg·ml−1·min−1 | 0.43±0.05 | 0.80±0.04* | ||
Data are means ± SE; n = 4 mice/exposure group. RV, right ventricular; IP, instantaneous pressure.
P < 0.05, CB vs. FA.
Molecular results from heart tissue.
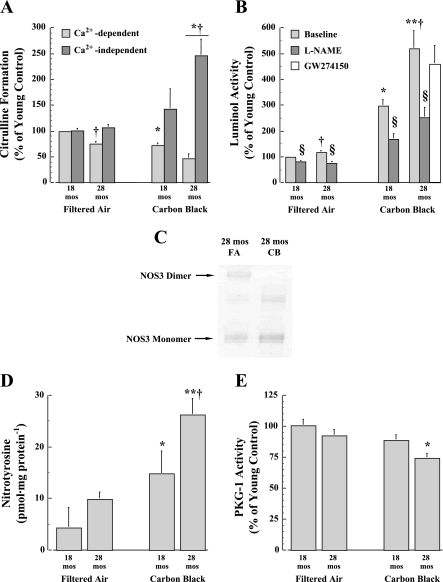
To test the possibility that decreased ventricular function is associated with greater uncoupling of NOS3 activity and an enhanced ROS production, NOS activity and ROS formation are evaluated in heart samples from 18- and 28-mo-old mice exposed to either FA or CB. The uncoupling of NOS (NOS3 and NOS1) is evident by a significant (P < 0.05) decline in Ca2+-dependent NOS activity in both age groups following CB exposure (Fig. 4A). That is, a significant (P < 0.05) reduction in NOS activity occurs in 28-mo-old hearts compared with 18 mo after FA, and CB exposure further exacerbated this age-dependent difference. In addition, Ca2+-independent activity (NOS2) is concomitantly upregulated in only 28-mo-old mice exposed to CB (Fig. 4A).
Fig. 4.
A: Ca2+-dependent and Ca2+-independent NOS activity as measured by the rate of citrulline formation from heart tissue samples obtained from 18- and 28-mo-old mice exposed to either FA or CB for 4 days each for 3 h. Left: nitric oxide synthase (NOS) uncoupling was evident by a decline in Ca2+-dependent NOS activity (NOS3 and NOS1) in both age groups following CB exposure. Right: Ca2+-independent activity (NOS2) was upregulated in only the 28-mo-old mice exposed to CB. B: luminol activity at baseline and during NG-nitro-l-arginine methyl ester (l-NAME) coincubation indicates an increase in reactive oxygen species (ROS) generation with CB exposure in both age groups, and the origin of ROS formation is associated with an increase in NOS activity. The coincubation with the selective NOS2 inhibitor GW274150 was not significantly (P > 0.05) different from baseline. C: representative blot comparing NOS3 dimer and monomer from isolated cardiac myocytes from 28-mo-old mice exposed to either FA or CB demonstrating a reduction of NOS3 dimer with CB exposure. D: increase in nitrotyrosine concentration as an indicator of peroxynitrite (ONOO− species) formation further suggest that greater ROS generation occurs in hearts of 28-mo-old animals following CB exposure. E: significant reduction in PKG activity in 28-mo-old mice exposed to FA or CB indicates lower second messenger activation by NOS or natriuretic peptide release (n = 10 mice per age group). *P < 0.05 and **P < 0.01, CB vs. FA exposure; †P < 0.05, 28 mo vs. 18 mo; §P < 0.05, l-NAME vs. baseline.
As reported in Table 4, a similar shift in NOS activity in the 28-mo-old mice exposed to CB was observed in isolated cardiac myocytes. That is, a significant (P < 0.05) decrease in Ca2+-dependent NOS activity accompanies a significant (P < 0.05) increase to Ca2+-independent activity in cardiac myocytes from CB-exposed 28-mo-old mice.
Table 4.
Citrulline formation and MMP activity in isolated myocytes
| 18 mo FA | 28 mo FA | 28 mo CB | |
|---|---|---|---|
| Ca2+-dependent NOS activity | 9.16±0.88 | 7.01±0.54† | 4.27±1.25* |
| Ca2+-independent NOS activity | 2.97±0.83 | 4.18±1.01 | 6.62±1.16* |
| MMP activity (relative units) | |||
| MMP2 | 1.00±0.18 | 1.68±0.42† | 3.42±0.73* |
| MMP9 | 1.00±0.22 | 1.67±0.31† | 5.12±0.93* |
Values are in pmol·mg protein−1·h−1. Data expressed as means ± SE; n = 4. MMP, matrix metelloproteinase; NOS, nitric oxide synthase.
P < 0.05, 28 mo vs. 18 mo;
P < 0.05, CB versus FA.
Given the decline in Ca2+-dependent NOS activity, we next tested whether NOS-derived ROS generation increased with CB by luminol activity with or without the NOS inhibitor, l-NAME (Fig. 4B). After FA exposure, the relative contribution of NOS in ROS generation is modest, but significant (P < 0.05) in 18-mo-old mice, and there is a significantly (P < 0.05) greater contribution of NOS-uncoupling at 28 mo. While both age groups demonstrate a significant (P < 0.05) increase in ROS generation following acute CB exposure, the 28-mo-old mice show a significantly greater CB-induced ROS production compared with the 18-mo-old mice. Moreover, the relative contribution of ROS production attributable to NOS-uncoupling is twofold greater (P < 0.05) in the 28-mo-old vs. 18-mo-old mice after CB exposure.
To test the relative contribution of NOS2 in the uncoupling observed in the 28-mo-old mice exposed to CB, the same tissue samples are coincubated with a specific NOS2 inhibitor, GW274150 (1). While the results suggest a trend (P = 0.08) toward a lowering of the ROS production in the presence of the NOS2 inhibitor, these data suggest that the majority of the ROS production is attributable to NOS3 and NOS1. To determine whether the concomitant decrease in NOS activity and increase in ROS signaling is associated with a loss of NOS dimerization, NOS dimer blot analysis is performed in myocytes from the hearts of 28-mo-old mice exposed to FA or CB (Fig. 4C). In the myocytes of mice exposed to CB, there is a marked reduction in NOS dimer with a concomitant increase in NOS3 monomer. A summary analysis of NOS dimer blots demonstrates a significantly lower NOS3 dimer-to-monomer ratio in CB-exposed 28-mo-old mice (0.23 ± 0.16) compared with mice exposed to FA (1.2 ± 0.3; P < 0.05), suggesting that a physical uncoupling of the NOS3 dimer occurs with CB exposure.
The age-dependent increase in ROS generation following CB exposure is further evaluated by assessing the NT concentration as a footprint of ONOO− formation. These results show that a significantly (P < 0.01) greater NT production (Fig. 4D) after CB exposure occurs in both age groups, but NT production is significantly higher (P < 0.05) in the older mice. As an index of second messenger activation by NOS or natriuretic peptide release, PKG-1 activity is also evaluated. Exposure to CB results in a significant (P < 0.05) reduction in PKG-1 activity that is unique to 28-mo-old mice (Fig. 4E). Taken together, these results support the hypothesis that NOS-uncoupling acts to increase ROS production following CB exposure, and senescent mice are more susceptible to this source of cardiac oxidative stress response than younger mice.
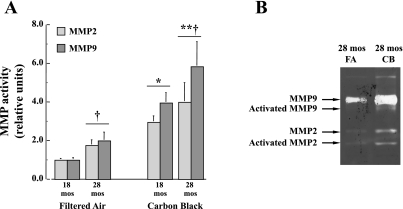
The effect of CB exposure on MMP2 and MMP9 activation is studied because of previous evidence linking MMP activation to increased ROS production. In Fig. 5, the effects of age and CB exposure on the gelatinase activity of MMP2 and MMP9 are shown. Both MMP2 and MMP9 activities are significantly (P < 0.05) increased in 28-mo-old mice, and acute CB exposure results in an incrementally greater (P < 0.01) increase for both age groups. In Table 4, corresponding results in the MMP2 and MMP9 activities for the 28-mo-old mice exposed to either FA or CB are also observed in isolated cardiac myocytes. These results reflect the effects of aging and CB exposure to initiate cardiac remodeling. Fig. 5B shows a representative zymogram comparing the effect of FA or CB exposure on total and activated MMP2 and MMP9 in isolated cardiac myocytes from 28-mo-old mice. This assay demonstrates that both total and activated forms of MMP2 and MMP9 are increased with exposure to CB.
Fig. 5.
A: activity levels of MMP2 and MMP9 are increased in heart tissue samples with age after FA exposure and in both 18- and 28-mo-old mice exposed to CB (n = 10 mice per age group). *P < 0.05, CB vs. FA exposure; †P < 0.05, 28 mo vs. 18 mo. B: representative zymogram performed comparing 28-mo-old mice exposed to FA and mice exposed to CB. These data suggest that both total and activated MMP9 and MMP2 are increased following exposure to CB.
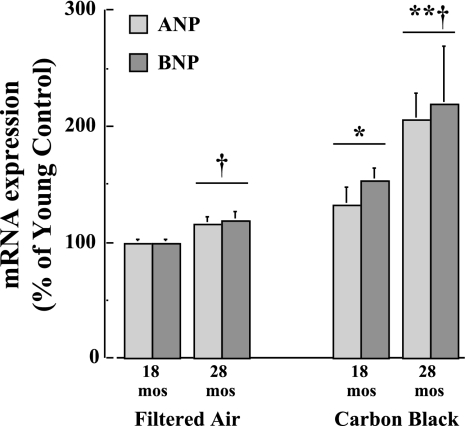
In Fig. 6, the effects of age and CB exposure on RNA expression levels of ANP and BNP are shown. Both ANP and BNP expression levels are significantly (P < 0.05) greater at 28 mo relative to FA-exposed 18-mo-old mouse hearts. With acute CB exposure, ANP and BNP expression levels are significantly (P < 0.01) increased further at 28 mo relative to 18-mo-old mice. In addition, results demonstrate a correspondingly significant (P < 0.05) elevation in plasma BNP concentrations in the 28-mo-old mice exposed to either FA (30.4 ± 8.3 pg/ml) or CB (95.3 ± 19.8 pg/ml) relative to FA-exposed 18-mo-old mice (18.6 ± 5.6 pg/ml). These results suggest that the hearts from older mice are subjected to greater CB-induced cardiac stress compared with younger mice.
Fig. 6.
Gene expression levels of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are increased in heart tissue samples with age after FA exposure and in both 18- and 28-mo-old mice exposed to CB (n = 10 mice per age group). *P < 0.05, CB vs. FA exposure; †P < 0.05, 28 mo vs. 18 mo.
Effects of BH4-treatment on cardiac function and NOS-ROS coupling.
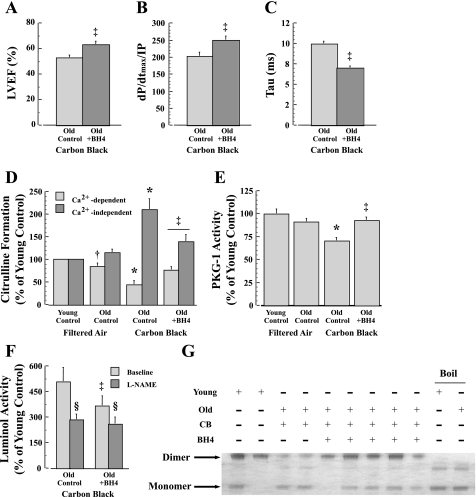
The effects of BH4 treatment on LV (Fig. 7A) and RV (Fig. 7, B and C) function are shown for 28-mo-old mice following CB exposure. With BH4 treatment, the LVEF response is significantly (P < 0.05) greater than control, whereas RV contractility (dP/dtmax/IP, where IP is instantaneous pressure) and isovolumic relaxation time constant (Tau) are also significantly (P < 0.05) improved following CB exposure. There are also significant (P < 0.05) reductions in pulmonary arterial pressure (29%) and pulmonary vascular resistance (44%) following CB exposure with BH4 treatment relative to control (data not shown).
Fig. 7.
Effects of tetrahydrobiopterin (BH4) treatment significantly improved cardiac function in CB-exposed 28-mo-old mice as shown by left ventricular ejection fraction (LVEF; A) and right ventricular contractility (dP/dtmax/IP, where IP is instantaneous pressurse; B) and relaxation time (Tau; C). Improvements in LV and RV function with BH4 treatment were coincident with reversing a fall in Ca2+-dependent and a rise in Ca2+-independent NOS activity (D), and a fall in PKG-1 activity (E). Likewise, BH4 treatment led to a lowered ROS generation derived from NOS-uncoupling in cardiac tissue from older CB-exposed mice (F). G: representative NOS3 dimer blot from isolated cardiac myocytes from 18- and 28-mo-old mice exposed to either FA or CB shows a preservation of the NOS3 dimer in older mice following CB exposure. ‡P < 0.05, BH4 treatment vs. control; *P < 0.05, CB vs. FA exposure; †P < 0.05, 28 mo vs. 18 mo; §P < 0.05, l-NAME vs. baseline.
Coincident with dramatic improvements in LV and RV function with BH4 treatment, there is also a significant decline in ROS generation (Fig. 7F) and a reduction in the percent of luminal activity that is inhibitable by the NOS inhibitor (l-NAME; Fig. 7F). BH4 treatment is associated with an increase in constitutive NOS activity (Ca2+-dependent activity; Fig. 7D) in heart tissue from older CB-exposed mice. Moreover, there is a significant reduction in inducible NOS activity (Ca2+-independent activity; Fig. 7D). These events appear to lead to a significant (P < 0.05) rise in PKG-1 activity from depressed levels observed in CB-exposed controls (Fig. 7E). Finally, there is a significantly greater preservation of the NOS3 dimer in cardiac myocytes from CB-exposed older mice with BH4 treatment, which is similar to levels observed in FA-exposed young mice (Fig. 7G).
DISCUSSION
The major results from the present study demonstrate that acute CB exposure leads to impaired cardiac function in senescent mice. The age-dependent cardiac changes include reductions in FS (Fig. 3B), presumably as a result of diminished myocardial contractility. The adverse fall in FS following CB exposure in senescent mice occurs as a result of LV dilation at both end-diastole and end-systole (Fig. 2). These echocardiographic results are supported by in vivo hemodynamic results showing that ejection fraction and dP/dtmax are diminished with CB in 28-mo-old mice (Table 3). Coincident with the adverse changes in myocardial contractility, CB-exposed senescent mice show elevated right atrial and pulmonary vascular pressures accompanied by an 86% increase in pulmonary vascular resistance. These hemodynamic results suggest that adverse changes in RV function results from an acute and robust CB-induced pulmonary vasoconstriction. There is also evidence of RV dilation in 28-mo-old mice that is not observed in younger mice (Fig. 1). These right-sided changes in senescent mice are associated with increases in RV pressures with CB exposure. These acute CB-induced changes in cardiac function observed in 28-mo-old mice are not seen following FA exposures.
Another set of major results from the present study show an important association between NOS-uncoupling in ROS generation and the acute CB-induced LV dilation and loss of cardiac contractility. Typically, NOS activity results in greater NO production, which generally opposes myocardial dilation (22, 51, 54). In the event that NOS activity is uncoupled from NO production, the result may lead to ROS generation and elevated myocardial oxidant stress (54). To test this mechanism, a series of experiments has led to the conclusion that a primary source of CB-induced increase in ROS production occurs via uncoupling of NOS3 in the aged cardiac myocytes. In the first series, mouse hearts from 28-mo-old mice exposed to FA show substantially lower Ca2+-dependent NOS activity but similar Ca2+-independent NOS activity compared with younger mice. This finding suggests that NO production via NOS3 activity is depressed with aging (Fig. 4A). This age-dependent loss in NOS3 activity is exacerbated with CB exposure in older mice, while Ca2+-independent NOS (i.e., NOS2) activity is elevated. These observations are evident in both heart tissue homogenates and isolated cardiac myocytes (Table 4).
In a second set of studies, luminol activity is notably elevated in hearts of 28-mo-old mice exposed to FA compared with younger mice (Fig. 4B). Likewise, effects of acute CB exposure substantially increase ROS generation in both 18- and 28-mo-old mice; however, hearts from senescent mice show amplified levels of ROS generation compared with the younger group. In the presence of l-NAME, luminol activity is markedly depressed in both age groups exposed to either FA or CB. Again, the attenuating effects of l-NAME are more dramatic in senescent mice, especially after CB exposure (Fig. 4B). The selective NOS2 inhibitor, GW274150 (1), is used to determine the source of NOS-derived ROS production in CB-exposed senescent mice (Fig. 4B). These results suggest that NOS2 is not the primary source of ROS production. Alternatively, the results suggest that NOS1 and/or NOS3 play a more significant role in ROS generation than any potential role of NOS2.
These mechanistic results central to altered cardiac myocyte function are consistent with a third set of observations demonstrating a loss of NOS3 dimer (Fig. 4C) and an accentuated increase in NT production in CB-exposed 28-mo-old mice (Fig. 4D). Finally, PKG-1 activity is depressed in only hearts of 28-mo-old mice exposed to CB, suggesting that second messenger activation of NOS signaling is reduced (Fig. 4E). Although the ∼25% reduction in PKG-l activity appears modest, opposing effects of reduced NOS signaling and elevated ANP/BNP activation may be counterbalancing the end result in PKG-1 activity seen in heart tissue homogenates of 28-mo-old mice exposed to CB. It is known that PKG-1 is highly compartmentalized within a specific subcellular area of the cardiac myocyte (54). Therefore, small changes in PKG activity may be quite meaningful. Currently, it is difficult to address these questions due to an inability to differentiate between sources of PKG-1 activity. Nevertheless, these collective results support the hypothesis that acute CB exposure induces exaggerated ROS production derived from NOS3-uncoupling in heart tissue and isolated cardiac myocytes of senescent mice.
The notion that NOS3 might serve as a dominant source for ROS production, and, therefore, play a role in cardiac dysfunction in response to CB exposure is somewhat unexpected; however, these results are supported by prior research in vascular tissue. The function of NOS activity in vascular tissue is beneficial under normal conditions, but exposure to mechanical or oxidant stress can shift the role of NOS to a ROS generator and a contributor to vascular disease (25, 66). For example, hypertension induced by deoxycorticosterone acetate-salt administration stimulates endothelial-dependent ROS generation, which is markedly blunted by l-NAME and absent in vessels from mice lacking NOS3 (25). In studies by Zou et al. (66), NOS uncoupling occurs directly by oxidation of a zinc-thiolate complex, which is stimulated by the presence of ONOO−. Catalytic activity of NOS is disrupted by protein structural alterations due to zinc release from the complex, and ROS production is increased while NO synthesis is decreased (66).
More recent studies have shown that NOS uncoupling also plays a role in the myocardium under pressure-overload conditions (33, 37, 55). As an illustration, ROS generation is associated with BH4 oxidation resulting in NOS uncoupling, and NADPH oxidases may provide an important initial trigger in stimulating NOS uncoupling mechanisms (55). In the present study, the primary sources of NOS uncoupling may originate from substrate and/or cofactor limitations (16). Alternatively, ROS production may contribute to cleaving the NOS dimer. These possibilities are examined in a final series of experiments using BH4 treatment to prevent NOS3 uncoupling, and, therefore, ROS generation. The results from these studies further establish the critical link between the basic cellular events and adverse changes in cardiac function associated with aging and CB exposure (Fig. 7). In general, CB-induced alterations in senescent mice observed at the cellular level are prevented by BH4 treatment. For example, there is a reversal in the balance between activities of NOS2 and NOS3 leading to the preservation of PKG-1 activity. Likewise, BH4 treatment also results in a lowering of ROS generation derived from NOS-uncoupling and an apparent protection of the NOS3 dimer in senescent mice exposed to CB. In parallel, BH4-treated mice show improved cardiac function as demonstrated by a greater LVEF and enhanced RV capability to contract and relax. Pulmonary arterial pressures and resistance are also ameliorated with BH4 treatment in CB-exposed senescent mice. Therefore, these results suggest that adverse cardiac effects of PM exposure with senescence are likely mediated, at least in part, by cellular mechanisms involving NOS3-uncoupled ROS generation.
It appears from these results that hearts of CB-exposed older mice are subjected to greater overload conditions. As shown in Table 2, the in vivo cardiac functional results demontrate that the pressures of the right side of the heart are increased following CB exposure in 28-mo-old mice. The increased RV pressure is associated with elevated pulmonary arterial pressures and vascular resistances. These in vivo results are consistent with the effects of CB exposure on the increased gene expression levels of ANP and BNP in the hearts of older mice (Fig. 6), and support the hypothesis that CB-exposed older mice are subjected to greater overload conditions. Right ventricular dilation may also be occurring with age and acute CB exposure (Fig. 1). Exposure to CB appears to adversely affect myocyte function rather than preload-dependent factors, such as a shift in blood volume to the pulmonary circulation. A significant depression in dP/dtmax/IP and PWRmax/EDV (indices that are largely preload independent) seen in senescent mice implies that the CB effect is at the level of myocardial contractility. Future studies are required to determine specific mechanisms mediating the sources of stress on cardiac function. One such study might include the determination of lung wet-to-dry weight ratios following CB exposure to evaluate the effects of increased lung edema. An alternative study of great interest would be to use tissue Doppler imaging to differentiate between physiological and pathological changes in LV function (13).
Another important mechanism is the influence of increased ROS generation on myocardial remodeling and reduced contractility. In this regard, ROS generation activates signaling cascades that stimulate the posttranslational expression of MMPs and promote chamber dilation and myocardial structural remodeling. Prolonged exposure to superoxide species leads to matrix turnover attributable to activation of MMPs and the formation of cardiac fibrosis (45). For example, in mice treated with the antioxidant dimethylthiourea (23) or in mice overexpressing glutathione peroxidase (53), remodeling after myocardial infarction is reduced, and is accompanied by a diminution of MMP accumulation. A key role of MMP9 activation has also been shown to be significant in early stages of vascular remodeling in response to hypertensive distending pressures (26). In the present study, an age-dependent increase in MMP2 and MMP9 activity occurred in 28-mo-old mice (Fig. 5), which may signal an age-dependent process of myocardial remodeling. In addition, both 18- and 28-mo-old mice demonstrate dramatic increases in MMP2 and MMP9 activity following acute CB exposure. Although these findings are novel in explaining acute PM-induced cardiac dysfunction, several previous studies demonstrate parallel findings in in vitro cytotoxicity systems (2, 43). Okayama et al. (43) showed effects of DEP on rat myocardial cell viability. While cell viability was incrementally reduced by DEP in a concentration-dependent manner, superoxide production was increasing by two- to threefold above control levels. The attenuated cell viability in response to DEP was ameliorated by coincubation with antioxidant enzymes, including catalase and superoxide dismutase. Similar reductions in PM-induced cell viability was shown in a pulmonary endothelial cytotoxicity study by Bai et al. (2). Using similar concentrations of DEP as the previous study (25), investigators demonstrated that pretreatment with NOS inhibitors (e.g., l-NAME) and BH4 also ameliorated cell viability, suggesting that DEP induces ROS production by NOS-related mechanisms. The results presented here are consistent with these cytotoxity studies.
The public health impact of the current results requires perspective related to previous human and animal exposure studies with PM. For example, Gurgueira et al. (21) showed increased ROS production in the lungs and hearts of rats exposed to concentrated ambient particulate, but no change in ROS when rats were exposed to CB at a concentration of 170 μg/m3. These investigators used CB as a negative control inferring that CB is the carbonaceous core of ambient PM, but is chiefly inert in biological systems. While the use of CB here is intended to circumvent the toxicity associated with ambient PM, the current results strongly suggest that senescence is an essential factor in PM susceptibility. Likewise, CB exposure was expected to be ineffective in younger mice, and, therefore, the inclusion of an age group younger than 18 mo seemed unnecessary. This senescent-dependent susceptibility factor has been consistently shown in epidemiology studies (14, 65), in which only the elderly (i.e., not middle-aged subjects) show adverse cardiac effects of elevated PM levels. Senescence emerges above other susceptibility factors such as genetic determinants, since no detectable differences are observed between inbred mouse strains in the present study.
Epidemiology studies showing associations between PM exposure and adverse cardiovascular outcomes are largely dependent on PM levels measured by outdoor central monitoring sites. Recent studies have considered the possibility that indoor or personal levels would be a more improved method for monitoring PM exposure (6, 12, 32, 59). The EPA also uses these central monitoring sites to determine whether the National Ambient Air Quality Standards are exceeded. For PM10 and PM2.5, 24-h averages are 150 and 35 μg/m3, respectively. The PM10 and PM2.5 concentrations used here (∼550 and 400 μg/m3) represent the upper limits of real world human exposure levels. Although exceedances are rare and episodic throughout the US, other industrial nations, such as India (20), show PM10 concentrations frequently exceeding the current US standards by two- to threefold.
Perspectives and Significance
The results of the present study clearly demonstrate that both aging and acute PM exposure lead to impaired cardiac function in terms of both LV and RV dilation and loss of myocardial contractility. The age-dependent fall in LVEDD seen in 28- vs. 18-mo-old mice (Table 2) suggests that senescent mice undergo diastolic dysfunction that hinders LV filling. One potential mechanism considers the role of age-dependent changes in Ca2+ handling in the cardiac myocyte and the proteins involved in Ca2+ sequestration (24). Another age-dependent change suggests that increased collagen deposition and thickening leads to a decrease in myocardial distensibility and elasticity (11). In general, these changes with aging lead to prolonged relaxation times and lower LVEDD. The adverse cardiac functional changes induced by PM are linked to altered cellular mechanisms associated with NOS uncoupling and subsequent increased ROS production. These findings mark an important new step in clarifying the adverse effects of PM air pollution on the heart, particularly related to senescent-dependent susceptibility. Moreover, the demonstration of this process in animal exposure studies forms the basis for future clinical studies related to aged populations exposed to PM.
GRANTS
This study was supported in part by National Institute on Aging Grant AG-21057 (to C. G. Tankersley), an American Heart Association Scientist Development Grant, a W. W. Smith Charitable Trust Grant, a Shih-Chun Wang Young Investigator Award; a Giles F. Filley Award of the American Physiological Society; and the Bernard Family Foundation and National Heart, Lung, and Blood Institute P50-HL-084946 (to H. C. Champion).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alderton WK, Angell AD, Craig C, Dawson J, Garvey E, Moncada S, Monkhouse J, Rees D, Russell LJ, Russell RJ, Schwartz S, Waslidge N, Knowles RG. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br J Pharmacol 145: 301–312, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y, Suzuki AK, Sagai M. The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species. Free Radic Biol Med 30: 555–562, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Batalha JR, Saldiva PH, Clarke RW, Coull BA, Stearns RC, Lawrence J, Murthy GG, Koutrakis P, Godleski JJ. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ Health Perspect 110: 1191–1197, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi G, Di Giulio C, Rapino C, Rapino M, Antonucci A, Cataldi A. p53 and p66 proteins compete for hypoxia-inducible factor 1 alpha stabilization in young and old rat hearts exposed to intermittent hypoxia. Gerontology 52: 17–23, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, Stull LB, Kass DA, Murphy AM. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol 292: H318–H325, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, Kanchanaraksa S, Swartz LJ, Callahan KA, Butz AM, Rand CS, Diette GB, Krishnan JA, Moseley AM, Curtin-Brosnan J, Durkin NB, Eggleston PA. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res 98: 167–176, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105: 1534–1536, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 109: 2655–2671, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Creason J, Neas L, Walsh D, Williams R, Sheldon L, Liao D, Shy C. Particulate matter and heart rate variability among elderly retirees: the Baltimore 1998 PM study. J Expo Anal Environ Epidemiol 11: 116–122, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Davidson SM, Duchen MR. Effects of NO on mitochondrial function in cardiomyocytes: pathophysiological relevance. Cardiovasc Res 71: 10–21, 2006. [DOI] [PubMed] [Google Scholar]
- 11.de Souza RR Aging of myocardial collagen. Biogerontology 3: 325–335, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Delfino RJ, Quintana PJ, Floro J, Gastanaga VM, Samimi BS, Kleinman MT, Liu LJ, Bufalino C, Wu CF, McLaren CE. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect 112: 932–941, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, Henry JP, Thuillez C. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation 105: 1602–1608, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J 40: 76s–80s, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Dockery DW Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Perspect 109, Suppl 4: 483–486, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Frampton MW, Stewart JC, Oberdorster G, Morrow PE, Chalupa D, Pietropaoli AP, Frasier LM, Speers DM, Cox C, Huang LS, Utell MJ. Inhalation of ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ Health Perspect 114: 51–58, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol Heart Circ Physiol 274: H1416–H1422, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation 101: 1267–1273, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Gupta AK, Nag S, Mukhopadhyay UK. Characterisation of PM10, PM2.5 and benzene soluble organic fraction of particulate matter in an urban area of Kolkata, India. Environ Monit Assess 115: 205–222, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect 110: 749–755, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichinose F, Hataishi R, Wu JC, Kawai N, Rodrigues AC, Mallari C, Post JM, Parkinson JF, Picard MH, Bloch KD, Zapol WM. A selective inducible NOS dimerization inhibitor prevents systemic, cardiac, and pulmonary hemodynamic dysfunction in endotoxemic mice. Am J Physiol Heart Circ Physiol 285: H2524–H2530, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res 87: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol 132: 699–721, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation 109: 1041–1047, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang X, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell 4: 57–64, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol 37: 671–680, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Liao D, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect 107: 521–525, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao D, Duan Y, Whitsel EA, Zheng ZJ, Heiss G, Chinchilli VM, Lin HM. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol 159: 768–777, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Linn WS, Gong H Jr, Clark KW, Anderson KR. Day-to-day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manag Assoc 49: 108–115, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Liu LJ, Box M, Kalman D, Kaufman J, Koenig J, Larson T, Lumley T, Sheppard L, Wallace L. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ Health Perspect 111: 909–918, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malinski T Understanding nitric oxide physiology in the heart: a nanomedical approach. Am J Cardiol 96: 13i–24i, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Mar TF, Koenig JQ, Jansen K, Sullivan J, Kaufman J, Trenga CA, Siahpush SH, Liu LJ, Neas L. Fine particulate air pollution and cardiorespiratory effects in the elderly. Epidemiology 16: 681–687, 2005. [DOI] [PubMed] [Google Scholar]
- 35.McConnell BK, Fatkin D, Semsarian C, Jones KA, Georgakopoulos D, Maguire CT, Healey MJ, Mudd JO, Moskowitz IP, Conner DA, Giewat M, Wakimoto H, Berul CI, Schoen FJ, Kass DA, Seidman CE, Seidman JG. Comparison of two murine models of familial hypertrophic cardiomyopathy. Circ Res 88: 383–389, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci 55: B117–B123, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26: 2439–2444, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation 107: 1202–1208, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Nemmar A, Hoet PH, Vermylen J, Nemery B, Hoylaerts MF. Pharmacological stabilization of mast cells abrogates late thrombotic events induced by diesel exhaust particles in hamsters. Circulation 110: 1670–1677, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Nemmar A, Hoylaerts MF, Hoet PH, Dinsdale D, Smith T, Xu H, Vermylen J, Nemery B. Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am J Respir Crit Care Med 166: 998–1004, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect 112: 1299–1306, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect 114: 412–419, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okayama Y, Kuwahara M, Suzuki AK, Tsubone H. Role of reactive oxygen species on diesel exhaust particle-induced cytotoxicity in rat cardiac myocytes. J Toxicol Environ Health A 69: 1699–1710, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med 40: 2214–2222, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci 26: 302–310, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peel JL, Metzger KB, Klein M, Flanders WD, Mulholland JA, Tolbert PE. Ambient air pollution and cardiovascular emergency department visits in potentially sensitive groups. Am J Epidemiol 165: 625–633, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103: 2810–2815, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, Pepys MB, Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J 22: 1198–1204, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 109: 71–77, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Pope CA, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am Heart J 138: 890–899, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Sebag IA, Handschumacher MD, Ichinose F, Morgan JG, Hataishi R, Rodrigues AC, Guerrero JL, Steudel W, Raher MJ, Halpern EF, Derumeaux G, Bloch KD, Picard MH, Scherrer-Crosbie M. Quantitative assessment of regional myocardial function in mice by tissue Doppler imaging: comparison with hemodynamics and sonomicrometry. Circulation 111: 2611–2616, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation 109: 544–549, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, Dostmann WR, Smith CJ, Kass DA. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res 96: 100–109, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 115: 1221–1231, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tankersley CG, Campen M, Bierman A, Flanders SE, Broman KW, Rabold R. Particle effects on heart-rate regulation in senescent mice. Inhal Toxicol 16: 381–390, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 113: 1052–1055, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vermylen J, Nemmar A, Nemery B, Hoylaerts MF. Ambient air pollution and acute myocardial infarction. J Thromb Haemost 3: 1955–1961, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Wallace LA, Mitchell H, O'Connor GT, Neas L, Lippmann M, Kattan M, Koenig J, Stout JW, Vaughn BJ, Wallace D, Walter M, Adams K, Liu LJ. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect 111: 1265–1272, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wellenius GA, Batalha JR, Diaz EA, Lawrence J, Coull BA, Katz T, Verrier RL, Godleski JJ. Cardiac effects of carbon monoxide and ambient particles in a rat model of myocardial infarction. Toxicol Sci 80: 367–376, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol 161: 1030–1036, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United States cities. Am J Cardiol 97: 404–408, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu M, Sreejayan N, Quinn MT, Ren J. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: role of oxidative stress. FASEB J 20: 1024–1026, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 277: H1967–H1974, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect 113: 978–982, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]