Abstract
Visceral inflammation, including that arising from bladder inflammation, reduces the threshold to sensation of innocuous or noxious stimuli applied to peripheral structures (referred hyperalgesia). Cystitis may induce transient or persistent plastic changes mediated by neurotrophins, particularly nerve growth factor (NGF), which contribute to increased nociceptive input. In this study, acute or subacute cystitis was induced in female rats by one or three (at 72-h intervals) 400-μl intravesical instillations of 1 mM acrolein. Sensitivity of the hindpaws to mechanical and thermal stimuli was determined before and 4, 24, 48, 72, and 96 h after treatment. Other groups of rats were treated with intravesical or intrathecal k252a [a nonspecific antagonist of tyrosine kinase (trk) receptors, including trkA, the high-affinity receptor for NGF] before the first or third acrolein instillation. Some rats were intraperitoneally injected with specific NGF-neutralizing antiserum or normal serum before acrolein instillation. Acute and subacute cystitis induced mechanical, but not thermal, referred hyperalgesia that was attenuated by intravesical pretreatment with k252a. Systemic treatment with NGF-neutralizing antiserum before instillation of acrolein suppressed subsequent mechanical referred hyperalgesia. Expression of NGF was increased within the bladder by acute or subacute cystitis and in L6/S1 dorsal root ganglia by subacute cystitis. These results suggest that the bladder-derived NGF acting via trk receptors at least partially mediates peripheral sensitization to mechanical stimuli associated with acute and subacute acrolein-induced cystitis.
Keywords: k252a, peripheral nociception, visceral inflammation, hyperalgesia
the neurotrophin nerve growth factor (NGF) is a key molecule in signaling pain during inflammation, and NGF is increased in peripheral tissues during inflammation (1, 5, 12, 13, 16). NGF can sensitize afferent nociceptors directly by binding to tyrosine kinase (trk) A (trkA) receptors (the high-affinity receptor for NGF) expressed on primary afferent neurons and indirectly by triggering mast cell degranulation (48). NGF binding to trkA receptors can also contribute to inflammatory pain by stimulating increased neuropeptide expression (6) and increased transcription of nociceptive neurotransmitters and ion channels and by promoting posttranslational changes in the biochemical composition of nociceptors (7, 26, 32, 56, 57).
Visceral inflammation is accompanied by increased sensitivity of somatic structures to noxious stimuli, which is commonly called referred hyperalgesia (4, 53). Direct measurement of visceral pain is difficult, and referred hyperalgesia has been used as a surrogate measurement thought to reflect visceral pain arising from experimental cystitis (4, 25), pancreatitis (53), and colitis (14). It has been suggested that referred hyperalgesia associated with dextran sodium sulfate-induced colitis is part of a generalized neuronal sensitization process that is centrally mediated, and referred hyperalgesia in these animals paralleled other indicators of visceral pain, such as posturing and reluctance to stretch, which are assumed to indicate visceral pain (14).
The hyperalgesic effect of NGF has been confirmed by studies demonstrating that administration of exogenous NGF replicates inflammatory hyperalgesia (11, 55). We reported previously that intravesical instillation of NGF significantly lowered the threshold of the hindpaws of mice to mechanical stimulation. In addition, we showed that peripheral mechanical hypersensitivity after cyclophosphamide-induced cystitis was inhibited by systemic treatment with NGF antiserum or the tyrosine kinase (trk) receptor blocker k252a before induction of cystitis (20). These findings strongly suggest that NGF produced by the bladder modulates peripheral mechanical nociception in the presence of cystitis. Other reports describe an increase in the trkB receptor [the high-affinity receptor for brain-derived neurotrophic factor (BDNF)] in the dorsal horn of the spinal cord after peripheral inflammation, and this may also contribute to increased pain sensation during the early phases of inflammation (18, 19, 33).
The effects of chronic cystitis on peripheral nociception and the relative roles of nerve fibers within the bladder, dorsal root ganglia (DRG), and spinal cord remain unclear. In the present study, we evaluated the effects of acute or subacute cystitis on peripheral sensitivity and further investigated whether blockade of trk receptors in the spinal cord or bladder would inhibit increased peripheral sensitivity to mechanical stimuli that accompany bladder inflammation. We also analyzed the effects of acute or subacute cystitis on expression of NGF and trkA and trkB receptors in the bladder, DRG, and spinal cord. We found that acute or subacute cystitis induced by intravesical instillation of acrolein was accompanied by increased mechanical, but not thermal, referred hyperalgesia of the hindpaws. We further observed that intravesical, but not intrathecal, instillation of k252a before acrolein treatment blocked subsequent referred mechanical hyperalgesia. Acute and subacute cystitis were accompanied by increased NGF in the bladder. Systemic treatment of rats with specific NGF antiserum before intravesical infusion of acrolein inhibited mechanical referred hyperalgesia. These findings are consistent with our previous observation that expression of NGF by the bladder plays a key role in peripheral mechanical hypersensitivity associated with cystitis.
METHODS
Animals.
Eight- to 10-wk-old female Wistar rats (180–250 g body wt) were housed in groups of two per cage and maintained on a 12:12-h light-dark cycle, with food and water available ad libitum. Animals were allowed to adapt to their environment for 4 days before any testing or treatment. The guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain were followed (1a). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin, Madison.
Acrolein-induced acute or subacute cystitis.
Cystitis was induced by one (acute cystitis) or three (at 72-h intervals; subacute cystitis) 400-μl intravesical instillations of 1 mM acrolein (Ultra Scientific, Kingstown, RI). Rats used as controls received one or three 400-μl intravesical doses of 0.9% saline. Before instillation of acrolein or saline, rats were anesthetized by inhalation of isoflurane (2–5%) in oxygen. Bladders were catheterized transurethrally with lubricated PE-10 tubing (0.61 mm OD; Intramedic, Sparks, MD). After catheterization, bladders were emptied by light abdominal compression before instillation of acrolein or saline. Rats remained anesthetized, and the catheter was left in place for 30 min after intravesical instillation of acrolein or saline.
Intravesical and intrathecal k252a.
k252a is a nonspecific antagonist of trk receptors, in particular trkA and trkB. Rats were anesthetized with isoflurane, catheters were placed transurethrally, bladders were emptied, and k252a (2 μg diluted in a final concentration of 10% DMSO, 200 μl total volume; Calbiochem, San Diego, CA) or saline (0.9% in a final concentration of 10% DMSO, 200 μl total volume) was instilled intravesically 15 min before the first or third intravesical instillation of acrolein or saline. This dose of k252a was selected on the basis of other investigators' observations that intrathecal administration of 2 μg of k252a inhibited cAMP response element-binding protein phosphorylation associated with sciatic nerve ligation in rats (36). Bladders were emptied by light abdominal compression before intravesical instillation of acrolein or saline, and rats remained anesthetized until catheters were removed 30 min after intravesical instillation of acrolein or saline.
Different groups of rats were anesthetized by inhalation of isoflurane and placed in ventral recumbency on a flat surface. A 27-gauge -inch needle connected to a 25-μl Hamilton syringe was placed perpendicular to the skin between the L5 and L6 vertebrae. k252a (2 μg diluted in a final concentration of 10% DMSO, 20 μl total volume; Calbiochem) or saline (0.9% in a final concentration of 10% DMSO, 20 μl total volume) was injected into the intrathecal space 15 min before the first or third intravesical instillation of acrolein or saline. Rats remained anesthetized for bladder catheterization (PE-10 tubing, Intramedic), and acrolein or saline was instilled intravesically as described previously.
Treatment with NGF-neutralizing antiserum.
Several studies have shown that systemic treatment with specific NGF-neutralizing antibody inhibits mechanical hypersensitivity associated with inflammation (9, 20) and neuropathic pain (52). Two groups of rats were used for studies of acute cystitis and another two groups were used for subacute studies. Rats were anesthetized with isoflurane, catheters were placed transurethrally, and bladders were emptied. A specific NGF-neutralizing antiserum (500 μl/kg; Chemicon, Temecula, CA) or the same volume of normal rabbit serum was given to rats intraperitoneally 15 min before the first (acute) or third (subacute) instillation of acrolein.
Referred hyperalgesia.
Mechanical nociception of the hindpaws was assessed by determination of the median 50% withdrawal threshold with Von Frey monofilaments using the up-down method (10). Rats were placed in individual chambers (20 × 12 × 12 cm) with a wire mesh floor and allowed to acclimate for ≥15 min or until cage exploration stopped. The hindpaws were touched with one of a series of five Von Frey monofilaments (rated at 2, 4, 6, 8, and 15 g). Von Frey monofilaments were calibrated before and after testing by determination of bending force using an electronic scale. Von Frey monofilaments were applied perpendicularly to the plantar surface with sufficient force to cause the monofilament to bend slightly. Testing was initiated with the 2-g monofilament, and other monofilaments were applied in a consecutive fashion; if a negative response was observed, the next-stronger monofilament was applied or, in the presence of a positive response, the next-weaker stimulus was applied. A positive response was recorded when the animal withdrew the paw sharply or licked the tested limb. The median 50% withdrawal threshold was then determined.
Thermal nociception of the hindpaws was measured in acute and subacute acrolein- or saline-treated groups using the method described by Hargreaves et al. (21). Rats were placed in individual chambers (12 × 20 × 17 cm) with a glass floor and allowed to acclimate for 15 min or until cage exploration stopped. Latencies were determined by placement of a movable focused beam of radiant light under the plantar surface of the hindpaw. The lamp and the timer were activated simultaneously, and the stimulus and timer were turned off on withdrawal of the paw. Four measurements separated by 5-min intervals were recorded and averaged. If the animal did not respond within 20 s, the lamp turned off automatically to prevent tissue damage.
Sensitivity of the hindpaws to mechanical or thermal stimuli was determined before and 4, 24, and 48 h after the first or third intravesical administration of acrolein or saline. Two additional groups of animals were tested every 24 h after the first or third acrolein instillation until mechanical withdraw latency was similar to baseline (recovery groups; 72 h for acute cystitis and 96 h for subacute cystitis). Groups pretreated with k252a were only tested for mechanical nociception.
Tissue collection and bladder histology.
Animals were euthanized 48 h after one or three intravesical instillations of acrolein or saline for histological evaluation of the bladder and measurement of NGF and trkA and trkB receptors within the bladder, L6/S1 DRG, and dorsal horn of the lumbar spinal cord. For the recovery groups, animals were euthanized after the last mechanical test (72 h for acute cystitis and 96 h for subacute cystitis). All rats were weighed and anesthetized with pentobarbital sodium (50 mg/kg ip). Bladders were emptied by light abdominal compression. The thorax was opened, and rats were perfused through the left ventricle of the heart with saline (0.9%) for 7 min.
Bladders were weighed, and bladder weight (mg) was normalized to body weight (g). The dome of the bladder was used for histological evaluation. It was removed, fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Three transverse sections of each bladder were obtained at 100-μm intervals and analyzed for evidence of inflammatory cell infiltration and edema. Each bladder section received an edema score (scale of 0–4) and an inflammatory cell score (scale of 0–4). Edema was scored as follows: 0 = no edema evident; 1 = edema present, limited to submucosal region, and width of submucosal region did not exceed combined width of urothelium and detrusor; 2 = edema present in bladder wall but not detrusor, and width of submucosal region greater than combined width of urothelium and detrusor but less than two times this distance; 3 = edema present in bladder wall, possibly including occasional areas of detrusor, and width of submucosal region two to four times width of urothelium and detrusor; and 4 = edema present throughout bladder wall, including detrusor, and width of submucosal region greater than four times width of urothelium and detrusor (however, urothelium was occasionally lost in these tissues because of severity of injury). Leukocytic infiltration was scored by dividing the section into quadrants and assigning a score to each quadrant as follows: 0 = no inflammatory cells, and 1 = leukocytic infiltration. The inflammatory cell score was the average of the total score for each of the three sections examined (ranging from a possible minimum of 0 to a possible maximum of 12 for the total score or 0–4 for the averaged scores).
The body and neck of the bladders were cut in half longitudinally and stored at −80°C. After bladder collection, a laminectomy was performed to expose the lumbar spinal cord and L6/S1 DRG. The L6/S1 DRG were removed, and the corresponding spinal cord was excised and cut into dorsal and ventral halves. Tissues were stored at −80°C until protein extraction for immunoblotting or determination of NGF content by ELISA.
Quantitative RT-PCR.
Additional rats were euthanized 2 h after one or three intravesical instillations of acrolein or saline, and bladders, L6/S1 DRG, and spinal cord were collected for determination of mRNA by RT-PCR. Tissues were placed in TRIzol (1 ml per 50 mg of tissue; Invitrogen, Carlsbad, CA), homogenized, and extracted in chloroform. RNA was precipitated in isopropanol at −20°C overnight, washed with 70% ethanol, and resuspended in water. RNA was treated with DNase (DNA-free reagent, Ambion, Austin, TX), and RNA concentration was measured at 260 nm with a spectrophotometer (SmartSpec 3000, Bio-Rad Laboratories, Hercules, CA). cDNA was prepared from total RNA (1 μg from each sample) using SuperScript III (Invitrogen) according to the manufacturer's instructions. PCR primers were provided by Integrated DNA Technologies (Coralville, IA); the primer sequences were as follows: GATCGGCGTACAGGCAGAAC (forward) and TCTCCCTCTGGGACATTGCT (reverse) for NGF, AGGAGCAAATTTGGGATCAACCGC (forward) and AAGAGAACTGCCACCCAGTGTCAT (reverse) for trkA receptor, GCATTGACCCAGAGAACATCAC (forward) and ACCTTTTCTGGTTTGCAATGAGA (reverse) for trkB receptor, and TGCACCACCAACTGCTTAGC (forward) and GGCATGGACTGTGGTCATGAG (reverse) for GAPDH. Real-time PCR was performed using a thermocycler (model 7300, ABI, Foster City, CA). Briefly, amplification was carried out in a volume of 25 μl containing 1× SYBR Green PCR Master Mix, each primer at 400 nM, and 1 μl of cDNA. Samples were amplified in duplicate using the following thermal cycling conditions: 94°C for 10 min followed by 40–45 cycles of amplification at 94°C for 30 s and then 60°C for 1 min to allow for denaturation and annealing-extension. Abundance of PCR product was determined semiquantitatively using a standard curve for each gene. Expression of each gene was normalized to abundance of mRNA for GAPDH, a relatively constitutively expressed protein.
Immunoblotting.
Bladder, L6/S1 DRG, and dorsal lumbar segments of the spinal cord were weighed and homogenized in ice-cold lysis buffer (12.5 μl/mg tissue; T-PER, Pierce Biotechnology, Rockford, IL) containing a cocktail of protease inhibitors (Roche, Indianapolis, IN). Homogenates were centrifuged for 30 min at 15,000 rpm at 4°C, and protein concentration of the supernatants was determined using the bicinchoninic acid protein assay (Pierce Biotechnology). Tissue homogenates (2 μg/μl) were mixed 1:1 with Laemmli sample buffer (Bio-Rad) and heated (100°C, 5 min) before they were loaded on SDS-polyacrylamide gels (12.5% for NGF or 7.5% for trkA and trkB receptors). Separated proteins were transferred to nitrocellulose membranes and incubated in blocking solution [5% nonfat milk in 20 mM Tris·HCl, 150 mM NaCl, 0.1% Tween 20 (TBS-T)] at room temperature for 1 h and then overnight (4°C) with primary antibody for NGF (1:3,000 dilution in TBS-T; Chemicon), trkA receptor (1:5,000 dilution in TBS-T; Chemicon), or trkB receptor (1:5,000 dilution in TBS-T; Upstate Biotechnology, Lake Placid, NY). Membranes were washed with TBS-T and incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution in TBS-T; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. Antibody detection was performed using chemiluminescence substrate (Pierce Biotechnology). Membranes were stripped and reblotted with α-tubulin antibody (1:10,000 dilution in TBS-T; Santa Cruz Biotechnology) to assess protein loading. Protein levels were estimated from optical density measurements using the Image J program (NIH, Bethesda, MD).
ELISA.
Bladder, L6/S1 DRG, and dorsal lumbar segments of the spinal cord were homogenized in cold lysis buffer [137 mM NaCl, 20 mM Tris·HCl (pH 8.0), 1% NP-40, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml leupeptin, and 0.5 mM sodium vanadate]. Homogenates were centrifuged for 30 min at 15,000 rpm at 4°C, and supernatants were acidified with 1 N HCl (1 μl/50 μl of homogenates) to pH 2.0–3.3 for 15 min at room temperature and then neutralized with 1 N NaOH (1 μl/50 μl of homogenates) to pH 7.6. Protein concentrations of samples were determined using the bicinchoninic acid protein assay (Pierce Biotechnology). NGF protein content was measured by commercially available NGF Emax ImmunoAssay Systems ELISA kits (Promega, Madison, WI). NGF content was normalized to sample protein concentrations and expressed as picograms per milligram of protein. Protein samples obtained from time- and treatment-control animals were used entirely for immunoblotting; therefore, protein samples obtained from rats that were killed 4 h after receiving intravesical saline, intravesical k252a followed by intravesical saline, or intrathecal k252a followed by intravesical saline were used as controls for NGF content determined by ELISA.
Immunohistochemistry.
Bladder tissue sections were deparaffinized in xylene and rehydrated through descending concentrations of alcohol. For antigen retrieval, sections were incubated in 0.01 M citric buffer (pH 6.0) and heated in a boiling water bath for 15 min. Endogenous peroxidase activity was inhibited by incubation with 1% hydrogen peroxide for 30 min. After 1 h of blocking with 10% normal goat serum at room temperature, tissue sections were incubated with specific antibody (rabbit anti-NGF, 1:500 dilution) overnight at 4°C. The ABC method was used to reveal staining following the manufacturer's instructions (Vector Laboratories, Burlingame, CA). Color development was accomplished by treatment of slides with a mixture of diaminobenzidine hydrochloride (DAB) and hydrogen peroxide (DAB Substrate Kit, Vector Laboratories). Control tissue sections, using normal rabbit IgG, instead of specific antibody, were processed in the same manner.
Statistical analysis.
Values, including percent changes, are means ± SE and analyzed using GraphPad Prism 4.01 statistical software (Graph-Pad Software, San Diego, CA). Unpaired parametric t-test (2-tailed) or one- or two-way ANOVA was performed where appropriate. When significant differences in groups were observed by ANOVA, individual means were compared using Dunnett's test or Bonferroni's multiple comparison test, with significance set at P < 0.05. Bladder weight was normalized to body weight, relative protein content determined by immunoblotting was normalized to α-tubulin, NGF content determined by ELISA was normalized to total protein concentration, and target mRNA abundance in samples was normalized to abundance of GAPDH mRNA.
RESULTS
Acute and subacute cystitis induced referred mechanical hyperalgesia.
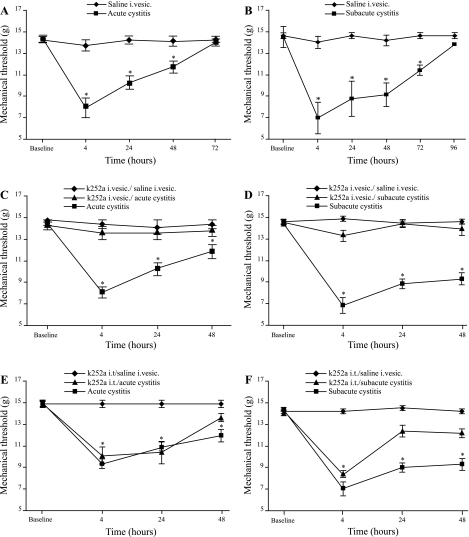
Sensitivity to peripheral mechanical stimuli before treatment was similar in all animals, and acute and subacute cystitis induced referred mechanical hyperalgesia. The threshold for mechanical sensitivity was significantly reduced at 4, 24, and 48 h in rats with acute cystitis and at 4, 24, 48, and 72 h in rats with subacute cystitis compared with saline-treated rats (Fig. 1, A and B). The maximal mechanical sensitivity response was observed at 4 h for acute and subacute cystitis. The mechanical withdrawal threshold returned to baseline 72 h after instillation of a single dose of acrolein (acute cystitis) and 96 h after the third instillation of acrolein (subacute cystitis). Neither acute nor subacute cystitis had an effect on peripheral thermal sensitivity (data not shown).
Fig. 1.
Referred hyperalgesia in the presence of acute and subacute acrolein-induced cystitis. A: acute acrolein-induced cystitis increased mechanical sensitivity 4, 24, and 48 h after intravesical (i.vesc) administration of acrolein. B: subacute acrolein-induced cystitis showed long-lasting referred hyperalgesia that persisted for ≥72 h after the 3rd acrolein instillation. C and D: referred hyperalgesia associated with acute and subacute acrolein-induced cystitis was reversed by administration of intravesical k252a. E: intrathecal (it) administration of k252a did not prevent referred hyperalgesia in rats with acute cystitis. F: intrathecal administration of k252a in rats with subacute cystitis did not prevent referred hyperalgesia 4 h after the 3rd intravesical instillation of acrolein. Values (means ± SE) are presented as latency for withdrawal (n = 6–12). *P < 0.05 (2-way ANOVA followed by Bonferroni's post hoc test).
Intravesical pretreatment with k252a attenuated referred mechanical hyperalgesia associated with acute and subacute acrolein-induced cystitis.
We previously observed that pretreatment of mice with intravenous k252a prevented referred mechanical hyperalgesia after cyclophosphamide-induced cystitis (20). To further localize the site of action of k252a in producing this effect, we treated rats with intravesical or intrathecal k252a before intravesical instillation of acrolein. Instillation of intravesical k252a before acrolein treatment ablated increased sensitivity to peripheral mechanical stimuli in rats with acute or subacute acrolein-induced cystitis (Fig. 1, C and D). In contrast, intrathecal k252a given before instillation of acrolein had no effect on the threshold to peripheral mechanical stimulation 4 and 24 h after acute cystitis (Fig. 1E). Intrathecal administration of k252a before the third acrolein instillation did not affect the threshold to peripheral mechanical stimulation 4 h after instillation of acrolein (Fig. 1F). However, the threshold of response to peripheral application of mechanical stimuli was not significantly different from that of controls 24 and 48 h after the third instillation of acrolein in animals pretreated with intrathecal k252a.
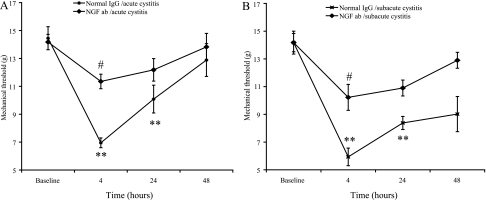
Systemic treatment with NGF-neutralizing antiserum attenuated referred mechanical hyperalgesia associated with acute and subacute acrolein-induced cystitis.
In rats treated with normal rabbit serum, acrolein increased sensitivity to peripheral mechanical stimuli as described previously (Fig. 2). Treatment with NGF-neutralizing antiserum before acrolein instillation inhibited increased sensitivity to peripheral mechanical stimuli in rats with acute or subacute acrolein-induced cystitis (Fig. 2).
Fig. 2.
Referred hyperalgesia associated with acute (A) and subacute (B) acrolein-induced cystitis was reversed by systemic treatment with nerve growth factor (NGF)-neutralizing antiserum [NGF antibody (Ab)]. Treatment with normal rabbit serum did not affect referred hyperalgesia. Values are means ± SE (n = 4–6). **P < 0.01 vs. baseline. #P < 0.05 vs. normal serum.
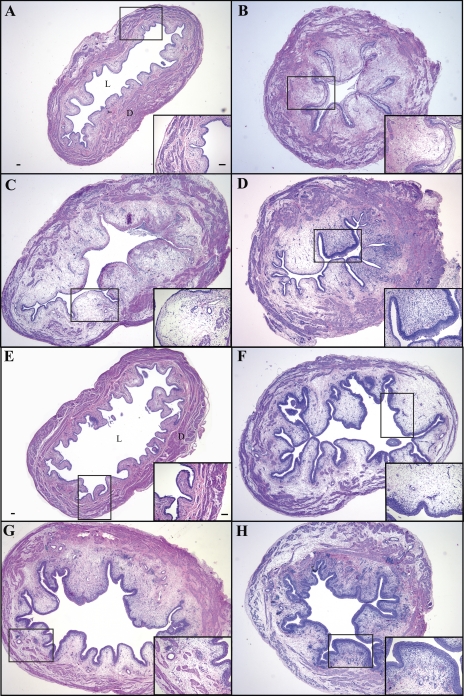
Acrolein induced increased bladder weight, edema, and inflammation scores, regardless of pretreatment with intravesical or intrathecal k252a.
Intravesical instillation of saline (acute or subacute) or 10% DMSO had no apparent effect on bladder histology, but instillation of acrolein consistently induced histological evidence of edema and leukocyte infiltration within the bladder wall (Fig. 3, Tables 1 and 2). A significant increase was observed in bladder weight, edema, and inflammation scores in acrolein-treated animals compared with control animals (Tables 1 and 2), but pretreatment with intravesical or intrathecal k252a did not attenuate increased bladder weight, edema, or inflammation scores after acrolein instillation (data not shown).
Fig. 3.
Histological appearance of bladder tissue 48 h after 1st (A) or 3rd (E) intravesical instillation of saline, 4 (B), 48 (C), and 72 (D) h after acrolein induction of acute cystitis, and 4 (F), 48 (G), and 96 (H) h after acrolein induction of subacute cystitis. Edema and some loss of urothelium were observed 4 h after acute cystitis (B). At 4 h after subacute cystitis, edema was present, but urothelium appeared intact (F). Edema, inflammatory cell infiltration, and vasodilation were observed 48 and 72 h after acute (C and D, respectively) or 4, 48, and 72 h after subacute (F, G, and H, respectively) acrolein-induced cystitis. L, lumen; D, detrusor. Original magnification ×20 and ×100. Scale bar, 100 μm.
Table 1.
Effects of first acrolein instillation on bladder weight and histology scores
| n | Bladder Wt/Body Wt, mg/g | Edema Score | Inflammation Score | |
|---|---|---|---|---|
| Control | 18 | 0.5±0.03 | 0.93±0.58 | 0.50±0.46 |
| Acrolein | ||||
| 2 h | 12 | 1.1±0.06† | 3.44±0.62† | 1.39±0.51† |
| 4 h | 10 | 0.8±0.1† | 3.67±0.37† | 1.11±0.27* |
| 48 h | 13 | 1.0±0.03† | 3.49±0.48† | 3.72±0.45† |
| 72 h | 6 | 0.9±0.04† | 3.83±0.41† | 3.78±0.40† |
Values are means ± SE.
P < 0.01;
P < 0.05 vs. control (1-way ANOVA followed by Dunnett's post hoc test).
Table 2.
Effects of third acrolein instillation (n = 7 for each group) on bladder weight and histology scores
| n | Bladder Wt/Body Wt, mg/g | Edema Score | Inflammation Score | |
|---|---|---|---|---|
| Control | 12 | 0.7±0.06 | 0.69±0.61 | 0.17±0.39 |
| Acrolein | ||||
| 2 h | 8 | 1.2±0.09† | 3.72±0.44† | 3.61±0.49† |
| 4 h | 8 | 1.2±0.06† | 3.22±0.34† | 3.22±0.50† |
| 48 h | 17 | 1.3±0.06† | 2.94±0.34† | 2.94±0.50† |
| 96 h | 6 | 1.1±0.1* | 3.13±0.36† | 3.13±0.69† |
Values are means ± SE.
P < 0.01;
P < 0.05 vs. control (1-way ANOVA followed by Dunnett's post hoc test).
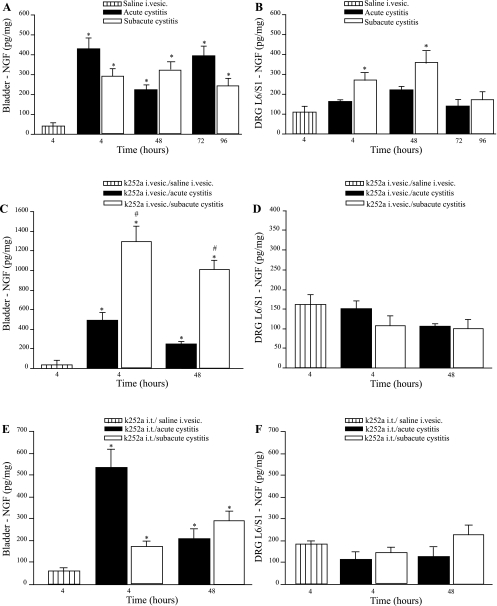
Acrolein-induced cystitis is accompanied by increased NGF within the bladder wall and L6/S1 DRG.
NGF content of the bladder measured by ELISA was significantly increased 4 and 48 h after a single intravesical acrolein instillation (428.7 ± 48.4 and 217.6 ± 9.6 pg/mg, respectively) compared with NGF content of bladders from animals treated with intravesical saline (39.3 ± 3.5 pg/mg), and bladder NGF content remained increased 72 h after a single acrolein instillation (394.1 ± 41.4 pg/mg; Fig. 4A), even though these animals did not show peripheral mechanical sensitization. NGF content of the bladder was also increased 4, 48, and 96 h after the third instillation of acrolein (286.2 ± 39.5, 324.7 ± 35.2, and 264.7 ± 38.8 pg/mg, respectively) compared with control bladders (39.3 ± 3.5 pg/mg). NGF protein of L6/S1 DRG was not increased 4, 48, and 72 h (137.6 ± 4.5, 184.5 ± 14.5, and 115.6 ± 8.9 pg/mg, respectively) after one acrolein instillation compared with control values (113.2 ± 22.9 pg/mg; Fig. 4B). However, NGF protein content of L6/S1 was increased 4 and 48 h (275.9 ± 42.8 and 354.2 ± 49.9 pg/mg, respectively), but not 96 h (155.5 ± 42.7 pg/mg), after the third acrolein instillation compared with control values (113.2 ± 22.9 pg/mg; Fig. 4B). Neither acute nor subacute acrolein-induced cystitis affected NGF protein content of the dorsal horn of the lumbar spinal cord (data not shown).
Fig. 4.
Measurement of NGF protein content in the bladder and L6/S1 dorsal root ganglia (DRG) by ELISA after acute or subacute acrolein-induced cystitis. A: bladder NGF was increased 4, 48, and 72 h after acute cystitis and 4, 48, and 96 h after subacute cystitis compared with control group. B: L6/S1 DRG NGF protein content was increased 4 and 48 h after subacute cystitis, but not 96 h after subacute cystitis or at any time after acute cystitis. C: intravesical k252a had no effect on bladder NGF after acute cystitis, but the increase in bladder NGF 4 and 48 h after subacute cystitis was greater than that observed at these time points after acrolein alone. D: intravesical instillation of k252a prevented increased NGF protein in L6/S1 DRG after subacute acrolein-induced cystitis. E: intrathecal k252a had no effect on bladder NGF after acute cystitis or 48 h after subacute cystitis. F: intrathecal k252a prevented increased NGF in L6/S1 DRG after subacute acrolein-induced cystitis. Values are means ± SE. *P < 0.05, acute or subacute cystitis vs. saline (control). #P < 0.05, subacute cystitis in animals treated with k252a vs. subacute cystitis in animals that did not receive k252a at the same time point.
Results of analysis of protein content of the bladder, L6/S1 DRG, and the dorsal horn of the lumbar spinal cord by immunoblotting were consistent with those obtained with ELISA (Table 3). NGF was increased in the bladder 48 h after acute and subacute instillation of acrolein. NGF was also increased in L6/S1 DRG 48 h after initiation of subacute cystitis, but not in L6/S1 DRG 48 h after acute cystitis or in the dorsal horn of the spinal cord.
Table 3.
NGF protein levels 48 h after first and third acrolein instillations
| 1st Acrolein Instillation | 3rd Acrolein Instillation | |
|---|---|---|
| Bladder | 131±4† | 148±4† |
| L6/S1 DRG | 92±6 | 153±8* |
| Lumbar spinal cord | 117±6 | 91±10 |
Values are means ± SE (n = 7 for each group), expressed as average density of protein bands compared with control (where control = 100%). NGF, nerve growth factor; DRG, dorsal root ganglion.
P < 0.001;
P < 0.05 vs. control (2-tailed t-test).
Abundance of NGF mRNA was greater in the bladder wall 2 h after acute (1.65 ± 0.3) or subacute cystitis (5.38 ± 0.5) compared with controls (0.65 ± 0.06 and 2.01 ± 0.3). At 2 h after the first or third instillation of acrolein, there were no differences in NGF mRNA abundance in L6/S1 DRG and the dorsal horn of the lumbar spinal cord when treated animals were compared with controls (data not shown).
Effects of pretreatment with intravesical or intrathecal k252a on NGF content within the bladder, L6/S1 DRG, and dorsal horn of the spinal cord.
We also evaluated the effects of treatment with intravesical or intrathecal k252a before intravesical acrolein on NGF content determined by ELISA. After pretreatment with intravesical k252a, an increase in NGF content within the bladder was observed 4 and 48 h after acute cystitis (495.1 ± 75.1 and 247.7 ± 22.8 pg/mg, respectively) compared with control values (22.9 ± 4.8 pg/mg), but intravesical k252a had no effect on bladder NGF relative to that observed after acute cystitis in rats that received intravesical acrolein (Fig. 4C). Intravesical k252a increased NGF content within the bladder 4 and 48 h after subacute cystitis (1,285.3 ± 157.5 and 914.3 ± 38.7 pg/mg, respectively; Fig. 4C) compared with controls and rats that received three intravesical instillations of acrolein.
Intrathecal k252a had no effect on bladder NGF 4 and 48 after acute cystitis (534.4 ± 83.1 and 202.6 ± 26.9 pg/mg, respectively; Fig. 4E) relative to animals evaluated 4 and 48 h after treatment with intravesical acrolein alone (428.7 ± 48.4 and 217.6 ± 9.6 pg/mg, respectively). Similarly, intrathecal k252a had no effect on bladder NGF 4 and 48 h after subacute cystitis (176.9 ± 25.9 and 257.2 ± 29.5 pg/mg, respectively; Fig. 4E) compared with 4 and 48 h after subacute cystitis alone (286.2 ± 39.5 and 324.7 ± 35.2 pg/mg, respectively; Fig. 4E).
Intravesical or intrathecal k252a inhibited the increase in DRG NGF content 4 and 48 h after the third intravesical instillation of acrolein (subacute cystitis; Fig. 4, D and F). Pretreatment with intravesical or intrathecal k252a had no effect on NGF content of the dorsal horn of the lumbar spinal cord after acute or subacute cystitis (data not shown).
Acrolein-induced cystitis had no effect on trkA or trkB receptor protein content or abundance of trkA or trkB receptor mRNA.
Cystitis had no effect on expression of trkA or trkB receptors, and, similarly, treatment with intrathecal or intravesical k252a failed to alter expression of trk receptors in the tissues (data not shown).
Immunohistochemistry.
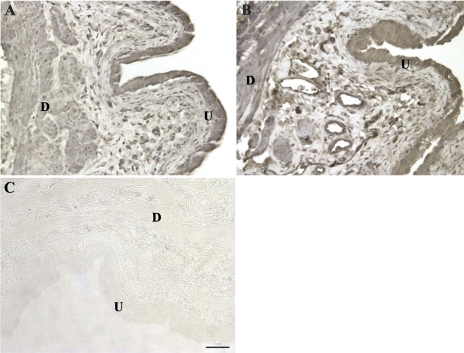
Staining for NGF in the bladder wall was observed primarily in the urothelium, detrusor, and endothelium of vessels within the submucosa (Fig. 5). It is difficult to quantify abundance of protein accurately using immunohistochemistry, but in sections processed under identical conditions, NGF staining appeared subjectively to be more intense subsequent to inflammation (acute or subacute).
Fig. 5.
Immunohistochemical staining of NGF in the bladder wall 4 h after a single intravesical infusion of saline (A) or acrolein (B; acute cystitis). Specific staining of NGF was observed in the urothelium (U) and detrusor smooth muscle (D). Sporadic positive staining was also observed in leukocytes in the submucosal region of the bladder between the urothelium and detrusor. In the absence of specific antibody, no positive staining was observed (C). Staining for NGF was similar in all sections from control rats and rats with cystitis (acute and subacute). Original magnification ×20. Scale bar, 50 μm.
DISCUSSION
The results of these experiments demonstrate that acute or subacute cystitis resulted in increased peripheral mechanical sensitivity that persisted up to 48 h after acute cystitis and 72 h after subacute cystitis. This effect was blocked by intravesical, but not intrathecal, instillation of k252a before acrolein treatment. Systemic treatment with NGF-neutralizing antiserum inhibited increased referred peripheral mechanical sensitivity subsequent to acrolein-induced cystitis. Bladder NGF was consistently increased after cystitis, and intravesical treatment with k252a did not prevent increased bladder NGF 4 h after acute or subacute cystitis and actually increased bladder NGF content 4 and 48 h after subacute cystitis. None of the treatments appeared to affect expression of trk receptors.
As we and others observed previously, visceral inflammatory pain arising from cystitis is accompanied by referred hyperalgesia in response to noxious and nonnoxious stimuli (28, 34, 35, 44). The present study provides further evidence that acute and subacute cystitis induce referred mechanical hyperalgesia that appears to be at least partly mediated by NGF. The observation that intravesical, but not intrathecal, administration of the trk receptor antagonist k252a blocked increased referred mechanical hyperalgesia associated with cystitis provides intriguing evidence that neurotrophins, particularly NGF, produced in the bladder may be primarily responsible for the effects of cystitis on referred mechanical hyperalgesia. The effects of intravesical instillation of k252a are especially interesting, because although one would assume that these effects may be relatively short-lived locally, suppression of referred mechanical hyperalgesia persisted, despite the development of histological evidence of severe inflammation.
The withdrawal threshold of the hindpaws in response to mechanical stimuli returned to baseline values 72 and 96 h after acute and subacute acrolein-induced cystitis, respectively. Despite restoration of peripheral sensitivity to normal values at these intervals, the bladders still appeared inflamed. These observations suggest that the processes that result in the referred hyperalgesia that accompanies visceral inflammation can resolve within days of initiation of inflammation in these models. In a model of colon inflammation induced with 2,4,6-trinitrobenzenesulfonic acid (TNBS) in mice, referred mechanical and thermal hyperalgesia were noted 7 days, but not 14 days, after induction of colitis. This was associated with histological evidence of colonic inflammation 7 days after induction of colitis that was resolved 14 days after TNBS administration (29). In this study, referred hyperalgesia was only observed in the presence of inflammation; however, the progression or resolution of referred mechanical and thermal hyperalgesia or inflammation was not evaluated between 7 and 14 days after TNBS administration.
Another question that remains unanswered by these studies, including the present investigation, is whether repeated episodes of inflammation subsequent to the time points studied in these experiments could result in referred hyperalgesia or allodynia that persists for longer periods of time or even fails to resolve completely (i.e., peripheral thresholds to nonnoxious or noxious stimuli never return to normal). Repeated episodes of inflammation could lower the threshold for nociceptor activation, contributing to a centrally mediated process referred to as the wind-up phenomenon. In the wind-up phenomenon, neurons within the dorsal horn of the spinal cord responsible for nociceptive input become sensitized via reduction of the activation threshold and prolonged discharge following brief stimuli (27, 49). This process intensifies pain perception and is mediated by the release of a large amount of glutamate and neurotransmitters (particularly substance P) by sensitized afferent fibers, activating N-methyl-d-aspartate and neurokinin 1 receptors, respectively (15, 45). This process (e.g., wind-up) is further augmented by suppression of descending pathways within the spinal cord that function to inhibit transmission of nociceptive input to higher centers within the central nervous system (22). It is possible that inhibition of cystitis-associated referred mechanical hyperalgesia by intravesical k252a may be the result of suppression of wind-up arising from inflammatory pain in this model.
Previous studies have reported conflicting observations regarding referred hyperalgesia in the presence of cystitis. For example, cyclophosphamide (50 mg/kg ip given twice, separated by 72 h) in mice increased sensitivity to mechanical and thermal stimulation of the hindpaws compared with controls (2). Bladder inflammation induced by intravesical instillation of 50% turpentine oil was associated with thermal (25) and mechanical hyperalgesia in rats (24) that persisted for ≥24 h. However, intravesical 1% zymosan did not affect mechanical or thermal sensitivity of the hindpaws in rats (46). We failed to detect any differences in peripheral thermal nociception between controls and cystitis groups. Although we have no conclusive explanation for these differences, this disparity in results may be due to differences in methods used to induce cystitis, testing methods or intervals, strains of animals, or animal manipulation and stress. It is interesting to note that a study of referred hyperalgesia in humans with spontaneous cystitis of noninfectious causes found increased mechanical, but not thermal, hyperalgesia in these patients (40).
Immunohistochemical staining indicated that, in the bladder, NGF was primarily associated with the urothelium and detrusor. We reported previously that cystitis arising from intravesical instillation of Escherichia coli lipopolysaccharide caused increased NGF in the urothelium and detrusor (3). Increased NGF staining was also observed in the endothelium of vessels in the submucosal region of inflamed bladders, and inflammation has been reported to increase NGF expression by the endothelium (37).
A significant finding from the present study is that a single intravesical instillation of k252a before infusion of acrolein blocked sensitization of the peripheral nociceptive system in acute and subacute acrolein-induced cystitis, despite having no apparent effect on bladder histology. The lack of an effect of k252a on inflammation is consistent with results of a previous study using a model of allergic asthma in the guinea pig. In that study, intranasal administration of k252a did not prevent influx of inflammatory cells into the airways; however, administration of k252a prevented airway hyperresponsiveness (10). Interestingly, we observed that intrathecal administration of k252a did not prevent sensitization of the peripheral nerve system after acute cystitis. Treatment of rats with intrathecal k252a before the third instillation of acrolein did not prevent reduction in mechanical threshold 4 h after acrolein instillation; however, we did not observe peripheral mechanical sensitization 24 and 48 h after the third acrolein instillation compared with saline-treated animals. Mechanical thresholds were numerically lower than baseline values at these time points, but the differences did not reach the level of statistical significance. The observation that intrathecal k252a appeared to prevent increased sensitivity to mechanical stimuli 24 and 48 h after subacute cystitis is potentially interesting and suggests that the processes that mediate changes in mechanical nociception associated with subacute inflammation may differ from those associated with acute inflammation. There also appeared to be no relationship between these findings and abundance of NGF in the tissues. These observations could simply be due to biological variation among individuals; however, failure of intrathecal k252a to prevent peripheral mechanical sensitization in conjunction with acute cystitis and 4 h after the third acrolein instillation suggests that a local action of k252a is necessary to block visceral-somatic interactions induced by acute and subacute bladder inflammation. The results obtained in rats treated with NGF antiserum support the conclusion that the effects of k252a are due to blockade of trkA receptors; however, conclusive support for this observation would require use of a compound that acts very specifically to block only trkA receptors.
Numerous endogenous agents induced by inflammation may contribute to viscerosomatic pain. It has been suggested that NGF binding to the trkA receptor is a key event in this process (39). In the present study, NFG mRNA and protein content within the bladder wall were consistently increased after acute and subacute acrolein-induced cystitis. Previous studies have indicated that increased NGF in the bladder that accompanies cystitis may be responsible for sensitization of sensory pathways and development of somatic hypersensitivity (2, 31, 54). Although our laboratory and others have reported that the urothelium (51) and detrusor (3, 50) can produce NGF, it is well known that mast cells, macrophages, keratinocytes, and T cells can release NGF, particularly in the presence of tissue trauma or inflammation. On the basis of previous descriptions of production and transport of NGF, our conclusion is that the bladder is the source of NGF responsible for peripheral sensitization.
We also found increased NGF protein content in L6/S1 DRG in the presence of subacute cystitis, but we did not observe changes in NGF content in the dorsal horn of the spinal cord after acute or subacute cystitis. These findings do not conclusively disprove the hypothesis that NGF localized in these structures may participate in increased neuronal activity in the spinal cord. Still, our results and those of other investigators strongly indicate that peripheral NGF, acting primarily via trkA receptors, initiates a process that results in sensitization of afferent nociceptors (41).
In the present study, we were unable to detect changes in trkA or trkB receptor protein content or abundance of trkA or trkB receptor mRNA within the bladder, L6/S1 DRG, or lumbar spinal cord after cystitis. Other investigators reported increased trkA and trkB receptors in the major pelvic ganglia and bladders of rats with cyclophosphamide-induced cystitis (38). We have no conclusive explanation for the inconsistency of the observations among studies that showed the effects of inflammation on neurotrophins and trk receptor expression, but our findings suggest that variability in results may relate to the relatively low amount of these agents in the tissues analyzed.
We analyzed the effects of intravesical and intrathecal k252a on NGF protein content followed by acute or subacute cystitis. In subacute cystitis, we observed that NGF within the bladder after intravesical k252a was higher than NGF after subacute cystitis without pretreatment with k252a; however, animals did not show peripheral sensitization. This event was not observed with intrathecal k252a. Intravesical or intrathecal k252a inhibited the increase in L6/S1 DRG NGF after subacute cystitis that was observed in the absence of pretreatment with k252a. The effects of k252a on abundance of NGF, either that formed before exposure of the tissues to k252a or an effect on NGF synthesis, are unclear. Intravesical instillation of k252a presumably prevented binding of NGF to trkA receptors, and this may be responsible for increased bladder NGF in rats with subacute cystitis. NGF content of the DRG was also increased 4 and 48 h after the third infusion of acrolein in rats, and this effect was inhibited by prior treatment with intravesical or intrathecal k252a. NGF synthesis within the DRG is limited under normal circumstances, and we found no evidence of increased NGF mRNA in the DRG subsequent to cystitis. NGF content of the DRG is primarily dependent on transport of NGF from the periphery to the DRG, which is the result of NGF binding to trkA receptors and subsequent antegrade migration of homodimers within endosomes (8). The effects of k252a on NGF transport have not been described. Intravesical k252a may have prevented retrograde movement of NGF from the bladder to the DRG by preventing NGF binding to trkA receptors. The trkA receptors are expressed by the DRG and spinal cord, and it is assumed that intrathecal instillation of k252a would prevent NGF binding to these receptors. Whether intrathecal k252a inhibits trkA receptor-NGF binding in a retrograde manner is completely speculative but theoretically possible. An alternative explanation for suppression of increased NGF in the DRG in the presence of cystitis is inhibition by k252a of some as yet unidentified process regulating NGF transport that is dependent on local binding of NGF to trkA receptors.
The natural alkaloid k252a acts as a kinase inhibitor by competing with binding of ATP to the catalytic domain (47). It has been described as a nonspecific trk receptor blocker with the capacity to block a variety of trk receptors that recognize growth factors, although a relative selectivity of k252a for the trkA receptor has been described (42). In PC12 cells, k252a was shown to be a potent inhibitor of signal transduction induced by NGF/trkA receptors (30). However, in rats, treatment with k252a before administration of BDNF into the hippocampus blocked BDNF-induced release of glutamate and dopamine (43). Little is known about the pharmacokinetics of k252a in vivo and the stability of the molecule. The duration of k252a association with trk receptors may be relevant in an explanation of the effects of intravesical instillation of k252a: k252a appears to have a long-lasting action in vitro, and a single application of k252a to rat embryonic striatal and basal forebrain cultures prevented cell death for 5 days (17). It is unclear whether the effects of k252a on cystitis-induced changes in peripheral sensitization and concentrations of NGF are the result of transient or persistent action of this compound, and additional information on the in vivo pharmacokinetics and duration of action of k252a is required.
Perspectives and Significance
The findings of the present study support a role for NGF-trkA receptor interaction within the bladder in mediation of peripheral sensitization to mechanical stimuli associated with acute and subacute acrolein-induced cystitis. The present study strongly suggests that local blockade of trk receptors before induction of cystitis blocks sensitization of the peripheral nerve system. A more clinically relevant issue may be the potential of intravesical treatment to suppress increased somatic sensitization to noxious and nonnoxious stimuli in the presence of established inflammation. The results of the present study also raise intriguing questions about the role of BDNF at the level of the DRG and spinal cord in these processes, and future studies will be directed toward developing a more explicit understanding of the processes resulting in peripheral sensitization that accompanies visceral inflammation and how this sensitization, once established, can be suppressed or eliminated.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK-57258 and R01 DK-066349 and Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior fellowship BEX 115102-9 (to S. Guerios).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aloe L, Tuveri MA, Levi-Montalcini R. Studies on carrageenan-induced arthritis in adult rats: presence of nerve growth factor and role of sympathetic innervation. Rheumatol Int 12: 213–216, 1992. [DOI] [PubMed] [Google Scholar]
- 1a.Anonymous. Ethical standards for investigations of experimental pain in animals. Pain 9: 141–143, 1980. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 291: G658–G665, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bjorling DE, Jacobsen HE, Blum JR, Shih A, Beckman M, Wang ZY, Uehling DT. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. Br J Urol Int 87: 697–702, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain in the mouse: species and strain differences. J Urol 170: 1008–1012, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chien CC, Fu WM, Huang HI, Lai YH, Tsai YF, Guo SL, Wu TJ, Ling QD. Expression of neurotrophic factors in neonatal rats after peripheral inflammation. J Pain 8: 161–167, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Cho HJ, Kim JK, Zhou XF, Rush RA. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res 764: 269–272, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Cruz CD, McMahon SB, Cruz F. Spinal ERK activation contributes to the regulation of bladder function in spinal cord injured rats. Exp Neurol 200: 66–73, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci USA 104: 13666–13671, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delafoy L, Raymond F, Doherty AM, Eschalier A, Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain 105: 489–497, 2003. [DOI] [PubMed] [Google Scholar]
- 10.De Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for trkA. Clin Exp Allergy 36: 1192–1200, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Dixon WJ Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20: 441–462, 1980. [DOI] [PubMed] [Google Scholar]
- 12.Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain 66: 87–97, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449–459, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Eijkelkamp N, Kavelaars A, Elsenbruch S, Schedlowski M, Holtmann G, Heijnen CJ. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am J Physiol Gastrointest Liver Physiol 293: G749–G757, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Flossos A An introduction to the neurobiology of pain. Greek E-Journal Perioperative Med 2: 2–10, 2004. [Google Scholar]
- 16.Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, Buchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 230: 615–624, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glicksman MA, Forbes ME, Prantner JE, Neff NT. K-252a promotes survival and choline acetyltransferase activity in striatal and basal forebrain neuronal cultures. J Neurochem 64: 1502–1512, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB prevent inflammation-induced hyperalgesia. Pain 100: 171–181, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Groth RD, Coicou LG, Mermelstein PG, Seybold VS. Neurotrophin activation of NFAT-dependent transcription contributes to the regulation of pro-nociceptive genes. J Neurochem 102: 1162–1174, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77–88, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Herrero JF, Cervero F. Supraspinal influences on the facilitation of rat nociceptive reflexes induced by carrageenan monoarthritis. Neurosci Lett 209: 21–24, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Jaggar SI, Scott HC, James IF, Rice AS. The capsaicin analogue SDZ249–665 attenuates the hyper-reflexia and referred hyperalgesia associated with inflammation of the rat urinary bladder. Pain 89: 229–235, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83: 442–448, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36: 57–68, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Katz WA, Rothenberg R. The nature of pain: pathophysiology. J Clin Rheumatol 11: S11–S15, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Kawabata A, Matsunami M, Tsutsumi M, Ishiki T, Fukushima O, Sekiguchi F, Kawao N, Minami T, Kanke T, Saito N. Suppression of pancreatitis-related allodynia/hyperalgesia by proteinase-activated receptor-2 in mice. Br J Pharmacol 148: 54–60, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol 290: G451–G457, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lazarovici P, Levi BZ, Lelkes PI, Koizumi S, Fujita K, Matsuda Y, Ozato K, Guroff G. K-252a inhibits the increase in c-fos transcription and the increase in intracellular calcium produced by nerve growth factor in PC12 cells. J Neurosci Res 23: 1–8, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Liang R, Ustinova EE, Patnam R, Fraser MO, Gutkin DW, Pezzone MA. Enhanced expression of mast cell growth factor and mast cell activation in the bladder following the resolution of trinitrobenzenesulfonic acid (TNBS) colitis in female rats. Neurourol Urodyn 26: 887–893, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matayoshi S, Jiang N, Katafuchi T, Koga K, Furue H, Yasaka T, Nakatsuka T, Zhou XF, Kawasaki Y, Tanaka N, Yoshimura M. Actions of brain-derived neurotrophic factor on spinal nociceptive transmission during inflammation in the rat. J Physiol 569: 685–695, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGaraughty S, Jarvis MF. Antinociceptive properties of a non-nucleotide P2X3/P2X2/3 receptor antagonist. Drug News Perspect 18: 501–507, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Meotti FC, Carqueja CL, Gadotti Vde M, Tasca CI, Walz R, Santos AR. Involvement of cellular prion protein in the nociceptive response in mice. Brain Res 1151: 84–90, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Miletic G, Hanson EN, Miletic V. Brain-derived neurotrophic factor-elicited or sciatic ligation-associated phosphorylation of cyclic AMP response element binding protein in the rat spinal dorsal horn is reduced by block of tyrosine kinase receptors. Neurosci Lett 361: 269–271, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Moser KV, Reindl M, Blasig I, Humpel C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res 1017: 53–60, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172: 2434–2439, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology 69: 24–33, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Ness TJ, Powell-Boone T, Cannon R, Lloyd LK, Fillingim RB. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol 173: 1983–1987, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv 7: 26–41, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Ohmichi M, Decker SJ, Pang L, Saltiel AR. Inhibition of the cellular actions of nerve growth factor by staurosporine and K252A results from the attenuation of the activity of the trk tyrosine kinase. Biochemistry 31: 4034–4039, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Paredes D, Granholm AC, Bickford PC. Effects of NGF and BDNF on baseline glutamate and dopamine release in the hippocampal formation of the adult rat. Brain Res 1141: 56–64, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Racz I, Schutz B, Abo-Salem OM, Zimmer A. Visceral, inflammatory and neuropathic pain in glycine receptor α3-deficient mice. Neuroreport 16: 2025–2028, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Ramos-Zepeda G, Herrero JF. Enhancement of wind-up by the combined administration of adenosine A1 receptor ligands on spinalized rats with carrageenan-induced inflammation. Neurosci Lett 384: 177–182, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain 7: 469–479, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Ross AH, McKinnon CA, Daou MC, Ratliff K, Wolf DE. Differential biological effects of K252 kinase inhibitors are related to membrane solubility but not to permeability. J Neurochem 65: 2748–2756, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Skaper SD, Pollock M, Facci L. Mast cells differentially express and release active high molecular weight neurotrophins. Brain Res Mol Brain Res 97: 177–185, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Solano R, Mazario J, Orellana JM, Herrero JF. Male Wistar rats show uniform wind-up responses in carrageenan-induced inflammation but not in the normal situation. Lab Anim 37: 207–214, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 3: 101–110, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Teng J, Wang ZY, Bjorling DE. Estrogen-induced proliferation of urothelial cells is modulated by nerve growth factor. Am J Physiol Renal Physiol 282: F1075–F1083, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Wild KD, Bian D, Zhu D, Davis J, Bannon AW, Zhang TJ, Louis JC. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther 322: 282–287, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Paricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 133: 1282–1292, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847–10855, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology 59: 61–67, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 24: 8300–8309, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol 192: 46–59, 2005. [DOI] [PubMed] [Google Scholar]